Submitted:
28 May 2025
Posted:
29 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Synthesis of the new α-benzothiophenyl-α-hydroxy-ethylphosphonates
2.2. Spectroscopic Characterization
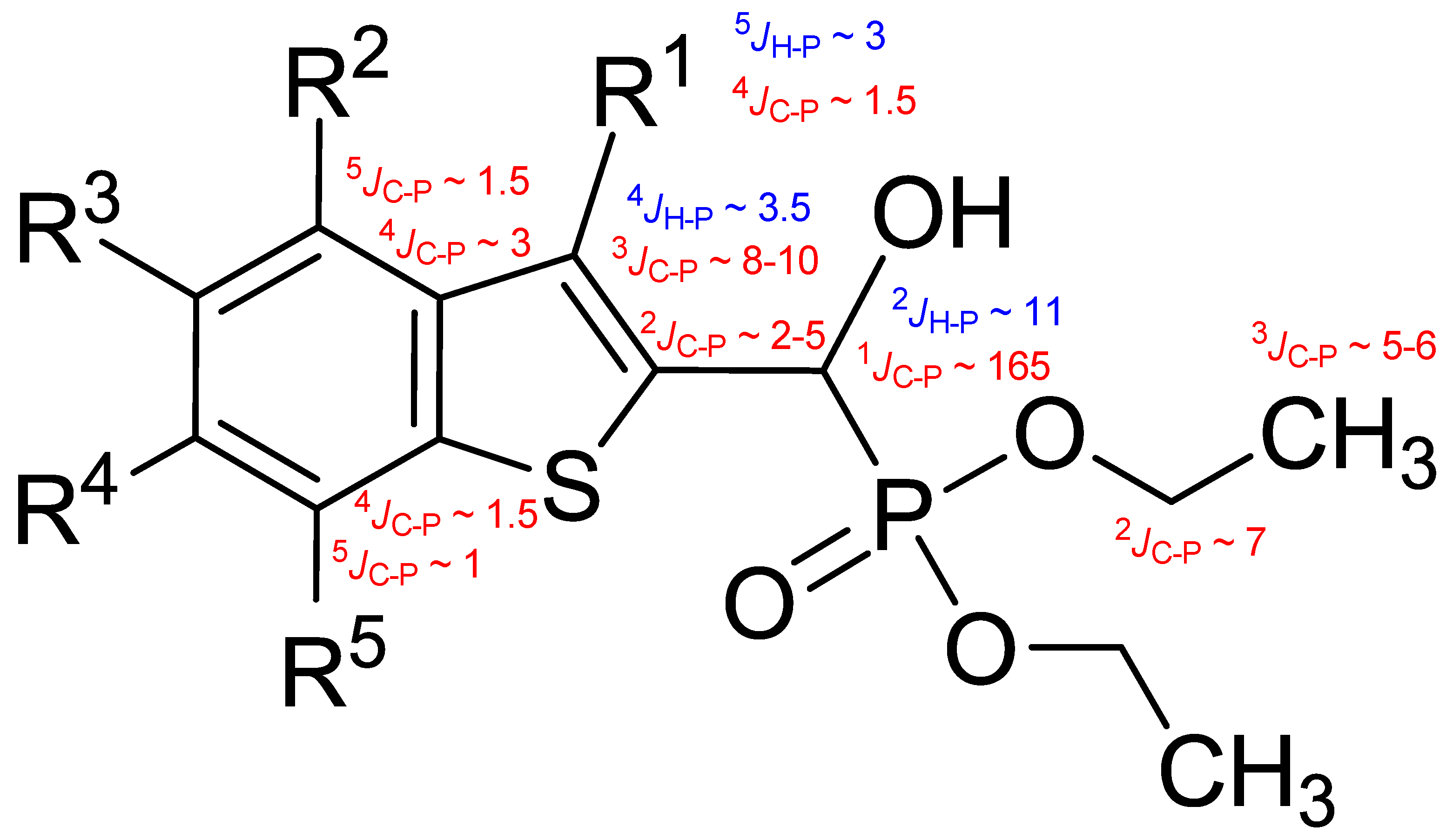
2.2.1. 13C NMR Spectroscopy
2.2.2. 1H NMR Spectroscopy
2.2.3. 31P NMR Spectroscopy
2.2.4. IR Spectroscopy
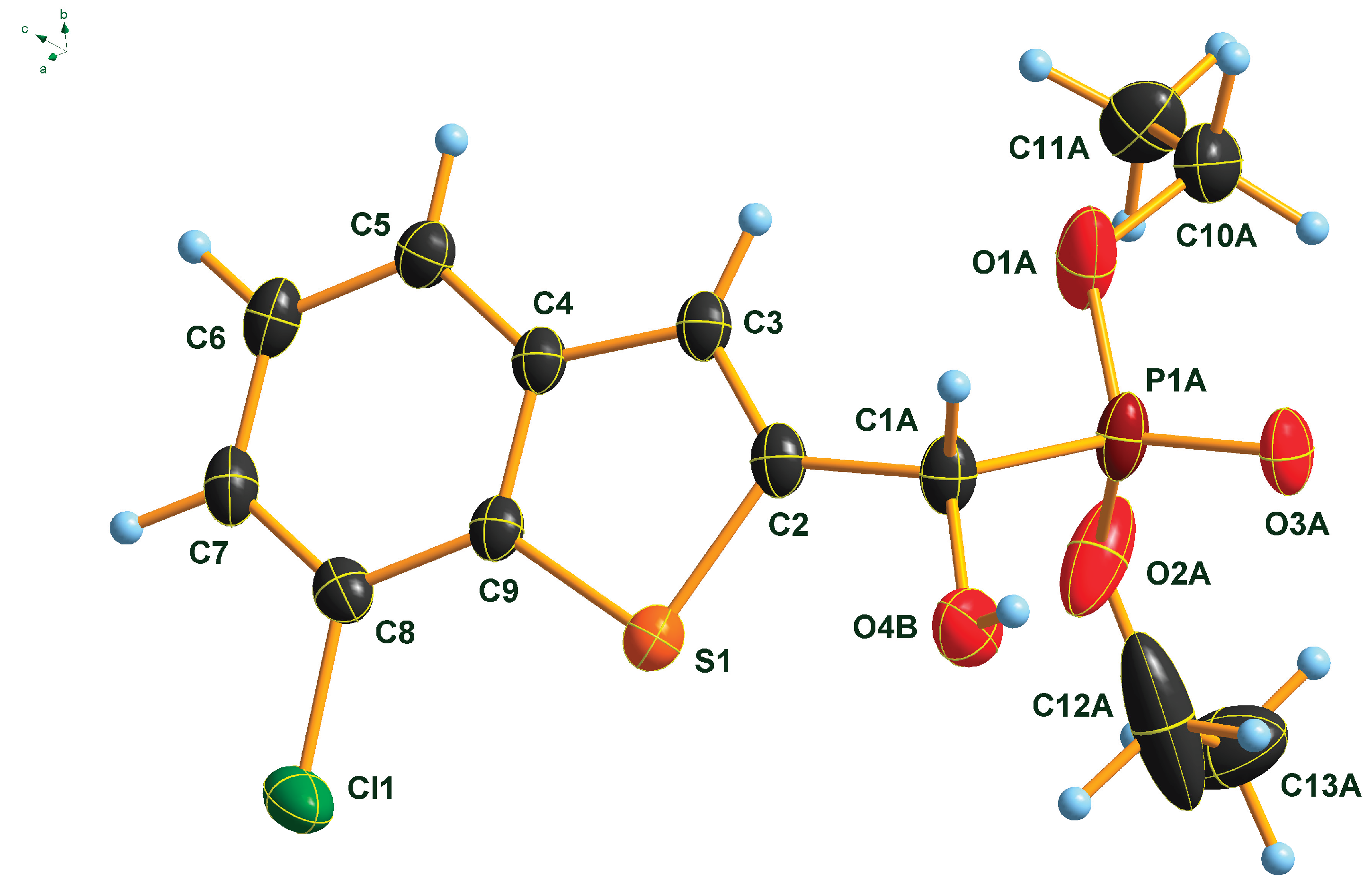
2.3. Single Crystal X-ray analysis of diethyl α-(7-chlorobenzothiophenyl-)α-hydroxy-methylphosphonate (2f)
2.4. Bioactivity study of the α-benzothiophenyl-α-hydroxy-methylphosphonates (2a-w)
3. Experimental
3.1. General
3.2. General procedure for the synthesis of diethyl [(1-benzothiopen-2-yl)(hydroxy)methyl]phosphonates 2
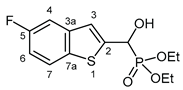
3.2.1. Diethyl [(5-fluoro-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2a)
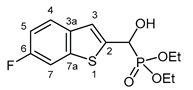
3.2.2. Diethyl [(6-fluoro-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2b)
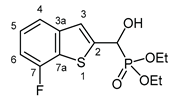
3.2.3. Diethyl [(7-fluoro-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2c)
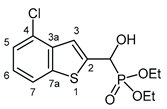
3.2.4. Diethyl [(4-chloro-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2d)
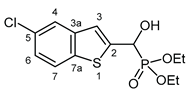
3.2.5. Diethyl [(5-chloro-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2e)
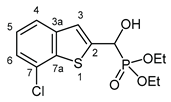
3.2.6. Diethyl [(7-chloro-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2f)
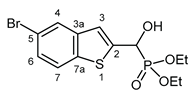
3.2.7. Diethyl [(5-bromo-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2g)
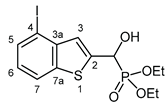
3.2.8. Diethyl [hydroxy(4-iodo-1-benzothiophen-2-yl]methyl]phosphonate (2h)Yield: 283 mg (66%). White crystals. Mp 94‒95 °C (hexane–EtOAc). IR (KBr): 3188, 2979, 1522, 1441, 1404, 1255, 1238, 1196, 1022, 976 cm-1. 1H NMR (CDCl3, 600 MHz): δ 7.77 (d, J = 8.0 Hz, 1H, H-7), 7.75 (dd, J1 = 7.6 Hz, J2 = 0.7 Hz, 1H, H-5), 7.46 (d, J = 3.5 Hz, 1H, H-3), 7.02 (t, J = 8.0 Hz, 1H, H-6), 5.35 (d, J = 11.7 Hz, 1H, CH), 4.24–4.14 (m, 5H, 2×CH2, OH), 1.35 (t, J = 7.1 Hz, 3H, CH3), 1.32 (t, J = 7.1 Hz, 3H, CH3) ppm. 13C NMR (CDCl3, 150 MHz): δ 142.3 (d, J = 2.6 Hz, C-3a), 141.2 (d, J = 2.5 Hz, C-2), 138.9 (d, J = 1.5 Hz, C-7a),134.3 (C-5), 126.3 (d, J = 8.3 Hz, C-3), 125.5 (d, J = 0.8 Hz, C-6), 122.3 (C-7), 90.1 (d, J = 1.5 Hz, C-4), 67.7 (d, J = 164.8 Hz, CH), 64.0 (d, J = 7.0 Hz, CH2), 63.6 (d, J = 7.3 Hz, CH2), 16.5 (d, J = 5.4 Hz, CH3), 16.4 (d, J = 5.8 Hz, CH3) ppm. 31P NMR (CDCl3, 242 MHz): δ 18.7 ppm. HRMS: [M]+ Calcd for C13H16IO4PS 425.9552; Found 425.9553.
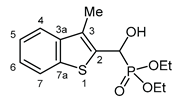
3.2.9. Diethyl [(hydroxy(3-methyl-1-benzothiophen-2-yl)methyl]phosphonate (2i)
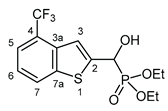
3.2.10. Diethyl {hydroxy [4-(trifluoromethyl)-1-benzothiophen-2-yl]methyl}phosphonate (2j)
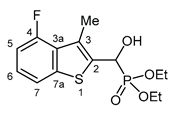
3.2.11. Diethyl [(4-fluoro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2k)

3.2.12. Diethyl [(5-fluoro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2l)
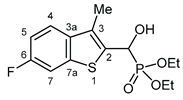
3.2.13. Diethyl [(6-fluoro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2m)
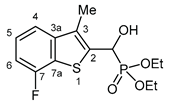
3.2.14. Diethyl [(7-fluoro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2n)

3.2.15. Diethyl [(5,7-difluoro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2o)
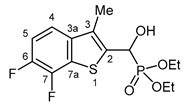
3.2.16. Diethyl [(6,7-difluoro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2p)
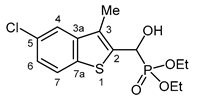
3.2.17. Diethyl [(5-chloro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2q)
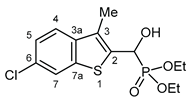
3.2.18. Diethyl [(6-chloro-3-methyl-1-benzothiophen-2-yl)(hydroxy)methyl]phosphonate (2r)
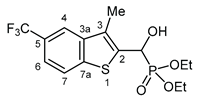
3.2.19. Diethyl {hydroxy [3-methyl-5-(trifluoromethyl)-1-benzothiophen-2-yl]methyl}phosphonate (2s)
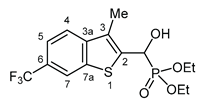
3.2.20. Diethyl {hydroxy [3-methyl-6-(trifluoromethyl)-1-benzothiophen-2-yl]methyl}phosphonate (2t)
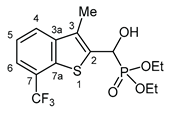
3.2.21. Diethyl {hydroxy [3-methyl-7-(trifluoromethyl)-1-benzothiophen-2-yl]methyl}phosphonate (2u)
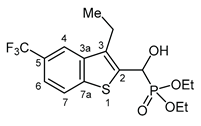
3.2.22. Diethyl [(3-ethyl-5-trifluoromethyl-1-benzothiophen-2-yl)](hydroxy)methyl]phosphonate (2v)
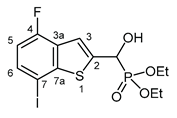
3.2.23. Diethyl [(4-fluoro-7-iodo-1-benzothiopen-2-yl)(hydroxy)methyl]phosphonate (2w)
3.3. X-Ray Experimental
3.3.1. Single Crystal X-Ray Diffraction Studies
| 2f | |
|---|---|
| Empirical formula | C13H16ClO4PS |
| Formula mass | 334.74 |
| T[K] | 123(2) |
| Crystal size [mm] | 0.40 × 0.25 × 0.20 |
| Crystal description | colorless block |
| Crystal system | triclinic |
| Space group | P-1 |
| a [Ǻ] | 7.6579(4) |
| b [Ǻ] | 7.8950(4) |
| c [Ǻ] | 13.9491(6) |
| α [°] | 78.234(4) |
| β [°] | 85.603(4) |
| γ [°] | 65.219(5) |
| V [Ǻ3] | 749.57(7) |
| Z | 2 |
| ρcalcd. [g cm-3] | 1.483 |
| μ [mm-1] | 0.509 |
| F(000) | 348 |
| Θ range [°] | 2.89 – 25.24 |
| Index ranges | -10 ≤ h ≤ 10 |
| -10 ≤ k ≤ 10 | |
| -18 ≤ l ≤ 18 | |
| Reflns. collected | 12988 |
| Reflns. obsd. | 3270 |
| Reflns. unique | 3718 (Rint = 0.0204) |
| R1, wR2 (2σ data) | 0.0405, 0.1050 |
| R1, wR2 (all data) | 0.0471, 0.1111 |
| GOOF on F2 | 1.029 |
| Peak/hole [e Ǻ-3] | 0.754 / -0.457 |
3.4. Bioactivity Experimental
3.4.1. Cell Culturing
3.4.2. Cell Viability Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.B.; Shi, D.Q. Synthesis and biological activity of novel phosphonate derivatives containing of pyridyl and 1,2,3-triazole rings. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1134–1144. [Google Scholar] [CrossRef]
- Kolodiazhnyi, O.I. Chiral hydroxyl phosphonates: Synthesis, configuration and biological properties. Russ. Chem. Rev. 2006, 75, 227–253. [Google Scholar] [CrossRef]
- Song, H.; Mao, H.; Shi, D. Synthesis and Herbicidal Activity of α-Hydroxy Phosphonate Derivatives Containing Pyrimidine Moiety. Chin. J. Chem. 2010, 28, 2020–2024. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L. P.; Ning, B. K.; Mao, M. Z.; Xue, C.; Wang, H. Y. Synthesis and insecticidal activities of O,O-dialkyl-2-[3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carbonyloxy] (aryl)methylphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1362–1367. [Google Scholar] [CrossRef]
- Lorenz, W.; Henglein, A.; Schrader, G. The New Insecticide O,O-Dimethyl 2,2,2-Trichloro-1-hydroxyethylphosphonate. J. Am. Chem. Soc. 1955, 77, 2554–2556. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Y.; Peng, H.; He, H. W.; Lu, X. T. Synthesis and herbicidal activity of α-[(substituted phenoxybutyryloxy or valeryoxy)]alkylphosphonatesand 2-(substituted phenoxybutyryloxy)alkyl-5,5-dimethyl-1,3,2-dioxaphosphinan-2-one containing fluorine. J. Fluorine Chem. 2017, 193, 8–16. [Google Scholar] [CrossRef]
- Pokalwar, R.U.; Hangarge, R.V.; Maske, P.V.; Shingare, M.S. Synthesis and antibacterial activities of α-hydroxyphosphonates and α-acetyloxyphosphonates derived from 2-chloroquinoline-3-carbaldehyde. Arkivoc 2006, 11, 196–204. [Google Scholar] [CrossRef]
- Kategaonkar, A.H.; Pokalwar, R.U.; Sonar, S.S.; Gawali, V.U.; Shingate, B.B.; Shingare, M.S. Synthesis, in vitro antibacterial and antifungal evaluations of new α-hydroxyphosphonate and new α-acetoxyphosphonate derivatives of tetrazolo [1, 5-a] quinoline. Eur. J. Med. Chem. 2010, 45, 1128–1132. [Google Scholar] [CrossRef]
- Reddy, G. S.; Sundar, C.S.; Prasad, S.S.; Dadapeer, E.D.; Raju, C.N.; Reddy, C.S. Synthesis, spectral characterization and antimicrobial activity of α-hydroxyphosphonates. Der Pharma Chemica 2012, 4, 2208–2213, http://derpharmachemica.com/vol4-iss6/DPC-2012-4-6-2208-2213.pdf. [Google Scholar]
- Sampath, S.C.; Raju, N.C.; Rao, V. An efficient synthesis, spectral characterization, antimicrobial, and antioxidant activities of novel α-hydroxyphosphonates and α-hydroxyphosphinates. Phosphorus Sulfur Silicon 2016, 191, 95–99. [Google Scholar] [CrossRef]
- Patil, N.S.; Deshmukh, G.B.; Patil, S.V.; Bholay, A.D.; Gaikwad, N.D. Synthesis and biological evaluation of novel N-aryl maleimide derivatives clubbed with α-hydroxyphosphonates. Eur. J. Med. Chem. 2014, 83, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.U.M.; Sundar, C.S.; Prasad, S.S.; Rani, C.R.; Reddy, C.S. Neat Synthesis and Anti-oxidant Activity of α-Hydroxyphosphonates. Bull. Korean Chem. Soc. 2011, 32, 3343–3347. [Google Scholar] [CrossRef]
- Naidu, K.R.M.; Kumar, K.S.; Arulselvan, P.; Reddy, C.B.; Lasekan, O. Synthesis of α-Hydroxyphosphonates and Their Antioxidant Properties. Arch. Pharm. Chem. Life Sci. 2012, 345, 957–963. [Google Scholar] [CrossRef]
- Yang, J.; Ma, J.; Che,W.; Li, M.; Li, G.; Song, B. Microwave-assisted synthesis and antitumor activity of salicyl acyloxy phosphonate derivatives. Chin. J. Org. Chem. 2014, 34, 2566–2571. [CrossRef]
- Bagchi, S.; Rathee, P.; Jayaprakash, V. Banerjee, S. Farnesyl Transferase Inhibitors as Potential Anticancer Agents. Mini-Rev. Med. Chem. 2018, 18, 1611–1623. [Google Scholar] [CrossRef]
- Al-Kali, A.; Gandhi, V.; Ayoubi, M.; Keating, M.; Ravandi, F. Forodesine: Review of Preclinical and Clinical Data. Future Oncol. 2010, 6, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Yokomatsu, T.; Abe, H.; Sato, M. Suemune, K.; Kihara, T.; Soeda, S.; Shimeno, H.; Shibuya, S. Synthesis of 1,1-difluoro-5-(1H-9-purinyl)-2-pentenylphosphonic acids and the related methano analogues. Remarkable effect of the nucleobases and the cyclopropane rings on inhibitory activity toward purine nucleoside phosphorylase. Bioorg. Med. Chem. 1998, 6, 2495–2505. [CrossRef]
- Kalla, R.M.N.; Lee, H.R.; Cao, J.; Yoo, J.W.; Kim, I. Phospho sulfonic acid: an efficient and recyclable solid acid catalyst for the solvent-free synthesis of α-hydroxyphosphonates and their anticancer properties. New J. Chem. 2015, 39, 3916–3922. [Google Scholar] [CrossRef]
- Patel, D.V.; Rielly-Gauvin, K.; Ryono, D. E.; Free, C.A.; Rogers, W.L.; Smith, S.A.; DeForrest, J.M.; Oehl, R.S.; Petrillo Jr., E. W..alpha.-Hydroxy Phosphinyl-Based Inhibitors of Human Renin. J. Med. Chem. 1995, 38, 4557–4569. [Google Scholar] [CrossRef]
- Stowasser, B.; Budt, K.-H.; Jian-Qi, L.; Peyman, A.; Ruppert, D. New hybrid transition state analog inhibitors of HIV protease with peripheric C2-symmetry. Tetrahedron Lett. 1992, 33, 6625–6628. [Google Scholar] [CrossRef]
- Prior, A.M.; Kim, Y.; Weerasekara, S.; Moroze, M.; Alliston, K.R.; Uy, R.A.; Groutas, W.C.; Chang, K.O.; Hua, D.H. Design, synthesis, and bioevaluation of viral 3C and 3C-like protease inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6317–6320. [Google Scholar] [CrossRef] [PubMed]
- Pompliano, D.L.; Rands, E.; Schaber, M.D.; Mosser, S.D.; Anthony, N.J.; Gibbs, J.B. Steady-state kinetic mechanism of ras farnesyl:protein transferase. Biochemistry 1992, 31, 3800–3807. [Google Scholar] [CrossRef] [PubMed]
- Frechette, R.F.; Ackerman, C.; Beers, S.; Look, R.; Moore, J. Novel hydroxyphosphonate inhibitors of CD-45 tyrosine phosphatase. Bioorg. Med. Chem. Lett. 1997, 7, 2169–2172. [Google Scholar] [CrossRef]
- Desai, J.; Wang, Y.; Wang, K.; Malwal, S.R.; Oldfield, E. Isoprenoid Biosynthesis Inhibitors Targeting Bacterial Cell Growth. Chem. Med. Chem. 2016, 11, 2205–2215. [Google Scholar] [CrossRef]
- Forlani, G.; Occhipinti, A.; Berlicki, Ł.; Dziedzioła, G.; Wieczorek, A.; Kafarski, P. Tailoring the Structure of Aminobisphosphonates To Target Plant P5C Reductase. J. Agric. Food Chem. 2008, 56, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Pudovik, A.N.; Zametaeva, G.A. New synthesis of esters of phosphonic and thiophosphonic acids. XIII. Addition of diethyl thiophosphite to ketones and aldehydes. Izv. Akad. Nauk SSSR Ser. Khim. 1952, 1952, 932–939. [Google Scholar]
- Pudovik, A.N.; Konovalova, I.V. Addition reactions of esters of phosphorus(III) acids with unsaturated systems. Synthesis 1979, 2, 81–96. [Google Scholar] [CrossRef]
- Rádai, Z.; Keglevich, G. Synthesis and Reactions of α-Hydroxyphosphonates. Molecules 2018, 23, 1493. [Google Scholar] [CrossRef]
- Keglevich, G.; Tóth, V.R.; Drahos, L. Microwave-Assisted Synthesis of α-Hydroxybenzylphosphonates and -benzylphosphine Oxides. Heteroatom Chem. 2011, 22, 15–17. [Google Scholar] [CrossRef]
- Texier-Boullet, F.; Foucaud, A. Synthesis of 1-Hydroxyalkanephosphonic Esters on Alumina. Synthesis 1982, 916, 25. [Google Scholar] [CrossRef]
- Keglevich, G.; Rádai, Z.; Kiss, N.Z. To date the greenest method for the preparation of α-hydroxyphosphonates from substituted benzaldehydes and dialkyl phosphites. Green Process Synth. 2017, 6, 197–201. [Google Scholar] [CrossRef]
- DIAMOND, Crystal Impact GbR. Version 3.2i; Bonn, Germany. 2014.
- Matsunaga, N,; Kaku, T.; Itoh, F.; Tanaka, T.; Hara, T.; Miki, H.; Iwasaki, M.; Aono, T.; Yamaoka, M.; Kusaka, M.; Tasaka, A. C17,20-Lyase inhibitors I. Structure-based de novo design and SAR study of C17,20-lyase inhibitors. Bioorg. Med. Chem. 2004, 12, 2251–2273. [CrossRef] [PubMed]
- Hur, W.; Rosen, H.; Gray, N.S. A benzo[b]thiophene-based selective type 4 S1P receptor agonist. Bioorg. Med. Chem. Lett. 2017, 27, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shigeno, M.; Fujii, Y.; Kajima, A.; Nozawa-Kumada, K.; Kondo, Y. Catalytic Deprotonative α-Formylation of Heteroarenes by an Amide Base Generated in Situ from Tetramethylammonium Fluoride and Tris(trimethylsilyl)amine. Org. Process. Res. Dev. 2019, 23, 443–451. [Google Scholar] [CrossRef]
- Program package ‘CrysAlisPro 1.171.40.82a (Rigaku OD, 2020)’.
- Sheldrick, G. M. (1997) SHELXS-97: Program for Crystal Structure Solution, University of Göttingen, Germany.
- Sheldrick, G. M. (1997) SHELXL-97: Program for the Refinement of Crystal Structures, University of Göttingen, Germany.
- Spek, A. L. (1999) PLATON: A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands.
- Szalai, Z.; Bednárik, J.; Tóth, BS.; Takács, A.; Tekula, S.; Kőhidai, L.; Karaghiosoff, K.; Drahos, L.; Keglevich, G. Cytotoxic Activity of Bisphosphonic Derivatives Obtained by the Michaelis-Arbuzov or the Pudovik Reaction. Pharmaceuticals (Basel). 2025, 18, 91. [Google Scholar] [CrossRef]
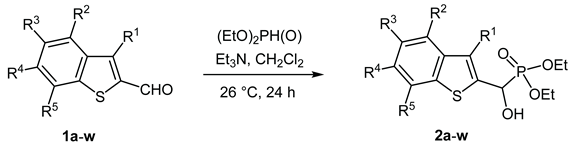 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Product | R1 | R2 | R3 | R4 | R5 | Yield (%) |
| 1 | 2a | H | H | F | H | H | 75 |
| 2 | 2b | H | H | H | F | H | 87 |
| 3 | 2c | H | H | H | H | F | 94 |
| 4 | 2d | H | Cl | H | H | H | 72 |
| 5 | 2e | H | H | Cl | H | H | 62 |
| 6 | 2f | H | H | H | H | Cl | 86 |
| 7 | 2g | H | H | Br | H | H | 73 |
| 8 | 2h | H | I | H | H | H | 66 |
| 9 | 2i | Me | H | H | H | H | 61 |
| 10 | 2j | H | CF3 | H | H | H | 79 |
| 11 | 2k | Me | F | H | H | H | 71 |
| 12 | 2l | Me | H | F | H | H | 79 |
| 13 | 2m | Me | H | H | F | H | 51 |
| 14 | 2n | Me | H | H | H | F | 79 |
| 15 | 2o | Me | H | F | H | F | 75 |
| 16 | 2p | Me | H | H | F | F | 56 |
| 17 | 2q | Me | H | Cl | H | H | 79 |
| 18 | 2r | Me | H | H | Cl | H | 66 |
| 19 | 2s | Me | H | CF3 | H | H | 66 |
| 20 | 2t | Me | H | H | CF3 | H | 69 |
| 21 | 2u | Me | H | H | H | CF3 | 78 |
| 22 | 2v | Et | H | CF3 | H | H | 77 |
| 23 | 2w | H | F | H | H | I | 68 |
| U266 | EBC-1 | A2058 | HT-29 | |
|---|---|---|---|---|
| Compounds | 100 µM | |||
| Medium | 2.05 ± 0.21*** | 0.92 ± 0.04*** | 1.03 ± 0.04 | 0.87 ± 0.02*** |
| DMSO | 1.00 ± 0.05 | 1.00 ± 0.02 | 1.00 ± 0.07 | 1.00 ± 0.04 |
| 2a | 0.85 ± 0.07*** | 0.93 ± 0.02** | 0.96 ± 0.06** | 0.90 ± 0.02* |
| 2b | 0.76 ± 0.04*** | 0.97 ± 0.01 | 0.97 ± 0.06*** | 0.97 ± 0.02 |
| 2c | 0.78 ± 0.06*** | 0.89 ± 0.02*** | 0.93 ± 0.05** | 0.90 ± 0.01** |
| 2d | 0.55 ± 0.07*** | 0.77 ± 0.01*** | 0.82 ± 0.03*** | 0.83 ± 0.03*** |
| 2e | 0.52 ± 0.05*** | 0.71 ± 0.02*** | 0.90 ± 0.02*** | 0.86 ± 0.02*** |
| 2f | 0.51 ± 0.03*** | 0.66 ± 0.03*** | 0.78 ± 0.11*** | 0.79 ± 0.03*** |
| 2g | 0.46 ± 0.01*** | 0.71 ± 0.01*** | 0.92 ± 0.01*** | 0.91 ± 0.06** |
| 2h | 0.51 ± 0.01*** | 0.66 ± 0.04*** | 0.76 ± 0.01*** | 0.81 ± 0.04*** |
| 2i | 0.94 ± 0.01 | 0.99 ± 0.03* | 1.06 ± 0.01 | 0.98 ± 0.01 |
| 2j | 0.61 ± 0.02*** | 0.76 ± 0.02*** | 0.80 ± 0.02*** | 0.78 ± 0.03*** |
| 2k | 1.08 ± 0.05 | 0.78 ± 0.03*** | 0.83 ± 0.03*** | 0.83 ± 0.02*** |
| 2l | 0.75 ± 0.08*** | 0.93 ± 0.01** | 0.97 ± 0.05* | 0.92 ± 0.03* |
| 2m | 0.87 ± 0.01** | 0.89 ± 0.02*** | 0.90 ± 0.04*** | 0.95 ± 0.04* |
| 2n | 0.81 ± 0.08*** | 0.86 ± 0.03*** | 0.90 ± 0.01*** | 0.91 ± 0.02* |
| 2o | 0.63 ± 0.02*** | 0.93 ± 0.05* | 0.98 ± 0.04* | 0.96 ± 0.02 |
| 2p | 0.69 ± 0.02*** | 0.85 ± 0.01*** | 0.82 ± 0.01*** | 0.91 ± 0.02** |
| 2q | 0.52 ± 0.06*** | 0.91 ± 0.04*** | 0.92 ± 0.02*** | 0.92 ± 0.02** |
| 2r | 0.51 ± 0.05*** | 0.68 ± 0.02*** | 0.89 ± 0.06*** | 0.84 ± 0.04*** |
| 2s | 0.30 ± 0.01*** | 0.71 ± 0.01*** | 1.02 ± 0.01 | 0.86 ± 0.03*** |
| 2t | 0.09 ± 0.02*** | 0.65 ± 0.01*** | 0.95 ± 0.05** | 0.58 ± 0.14*** |
| 2u | 0.55 ± 0.05*** | 0.85 ± 0.01*** | 0.82 ± 0.04*** | 0.87 ± 0.02*** |
| 2v | 0.77 ± 0.02*** | 1.03 ± 0.01 | 1.05 ± 0.01 | 0.99 ± 0.01 |
| 2w | 0.33 ± 0.01*** | 0.69 ± 0.01*** | 0.71 ± 0.01*** | 0.75 ± 0.01*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





