1. Introduction and Aims
Upright exercise stress echocardiography (ESE) has been shown to induce significant intraventricular pressure gradients (IVPGs) in a substantial proportion of children presenting with exercise-related symptoms [
1]. However, the clinical relevance of this finding remains unclear and warrants further investigation. Given that IVPGs are associated with a hyperkinetic left ventricular response [
2,
3,
4,
5], there is a rationale for using β-blockers as targeted negative inotropic therapy in these cases. This therapeutic approach has been described in the management of cardiac Syndrome X and in athletic populations [
5]. To date, however, there is limited evidence supporting the initiation of β-blocker therapy in children based on ESE findings [
6].
Stress echocardiography—particularly treadmill exercise stress echocardiography (ESE)—has been employed by the authors since 1996 for the evaluation of suspected or confirmed cardiac conditions in adults. Based on this experience, the authors propose that ESE may offer comparable diagnostic value in pediatric patients. However, its use in children remains limited, possibly due to concerns regarding the method’s learning curve, as well as questions about its safety and applicability in the pediatric population, given the relatively scarce literature on this topic [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19].
In this study, a team of highly experienced adult cardiologists, performing an average of 500 exercise stress echocardiograms annually over the past 20 years (totaling 1,887 exercise stress echocardiograms in 2024), conducted exercise stress echocardiography on a group of children, both with and without beta-blocker therapy.
In the evaluation of chest pain in children—whether exercise-related or not—clinical assessment is typically supplemented by a range of diagnostic tools, including electrocardiograms (ECGs), exercise stress tests, transthoracic echocardiography, 24-hour Holter monitoring, and more recently, advanced imaging modalities such as exercise stress echocardiography, nuclear imaging, cardiac magnetic resonance imaging (MRI), and coronary computed tomography angiography (CTA).
Over the past two decades, considerable efforts have been made to optimize the diagnostic approach for evaluating symptoms in pediatric patients. Notably, standard exercise stress testing without echocardiographic imaging rarely yields conclusive information regarding the etiology of chest pain in children [
1].
We emphasize the value of initiating the diagnostic workup with exercise stress echocardiography (ESE) in children presenting with exercise-related symptoms. This approach not only reduces the need for radiation-based imaging modalities but may also play a critical role in guiding targeted treatment strategies—as illustrated by the cases presented in
Figure 1 and
Figure 2, where β-blocker therapy was initiated based on ESE findings [
1].
Given its diagnostic accuracy, ability to evaluate cardiac function dynamically, and absence of ionizing radiation [
1], ESE should be considered a first-line diagnostic tool in the clinical assessment of pediatric patients with exertional symptoms.
It is important to recognize that, while imaging studies are essential in clinical practice, those involving ionizing radiation may contribute to an increased lifetime risk of cancer. In children with congenital heart disease, exercise stress echocardiography (ESE) offers a valuable alternative by reducing the need for radiation-based imaging modalities such as computed tomography (CT) and nuclear scintigraphy. This consideration is particularly critical, as a child aged 15 to 20 years with congenital heart disease may have already accumulated radiation exposure equivalent to approximately 2,000 chest X-rays [
1].
Children with congenital or acquired heart disease are especially vulnerable from an ethical standpoint. They require continuous medical care, which places a greater responsibility on healthcare professionals, particularly physicians. This heightened responsibility arises because children lack the ability to make fully informed decisions and do not have sufficient knowledge to choose the most appropriate medical options. Therefore, healthcare professionals must make protective decisions that prioritize their patients’ well-being, selecting the most appropriate diagnostic and therapeutic approaches that will impact both their immediate and long-term quality of life.
It is essential to rigorously justify any radiological procedure involving ionizing radiation and to prioritize non-ionizing imaging modalities whenever feasible. The primary objective of radiation protection is to optimize imaging protocols and implement safety measures that ensure acquisition of the required diagnostic information with the lowest possible radiation dose, without compromising image quality.
In pediatric imaging, a critical consideration is the capability of imaging equipment to adjust radiation doses according to the child’s size and weight. In summary, while acknowledging the indispensable diagnostic and therapeutic roles of medical imaging, it is imperative to minimize radiation exposure by employing the lowest effective doses that maintain diagnostic accuracy and patient safety [
1].
Beta-adrenergic receptor antagonists, commonly known as beta-blockers, are widely used in the management of various pediatric conditions, including arrhythmias, hypertension, heart failure, hypertrophic cardiomyopathy, migraine, hyperthyroidism, and hemangiomas [
20]. These agents are classified into three generations based on their receptor selectivity profiles. First-generation beta-blockers, such as propranolol, are non-selective and inhibit both β₁- and β₂-adrenergic receptors. Second-generation agents, exemplified by metoprolol, demonstrate relative selectivity for β₁-adrenergic receptors. Third-generation beta-blockers, including carvedilol, possess broader activity by antagonizing β₁-, β₂-, and α₁-adrenergic receptors.
Beta-blockers are commonly used to treat adult cardiac conditions such as hypertension, atrial arrhythmias, and chronic heart failure. Similarly, they are employed in managing various pediatric tachyarrhythmias, both in non-operative and peri-operative settings [
21]. However, despite their widespread use in children, there remains a notable lack of pediatric-specific data to guide precise dosing and individualized treatment. Consequently, most pediatric management strategies are extrapolated from adult studies. Commonly prescribed oral beta-blockers in the pediatric population include atenolol, carvedilol, metoprolol, propranolol, and bisoprolol [
22]. In adult patients with exercise-induced intraventricular pressure gradients (IVPGs), with or without hypertrophic cardiomyopathy, beta-blocker therapy is recommended [
23,
24,
25,
26,
27,
28]. Current literature suggests that children may similarly benefit from this treatment approach [
21].
A central bioethical challenge in pediatric and adolescent healthcare is the assessment of autonomy, specifically, whether a minor possesses the capacity to make informed decisions regarding their treatment based on personal beliefs and values. Traditionally, such decisions have been made by parents or caregivers, as minors are often deemed by law to lack the full cognitive and psychosocial maturity required for autonomous medical decision-making [
29].
However, it is crucial to emphasize that children have an unequivocal right to the highest attainable standard of health care. This right extends beyond mere access to treatment and includes the right to receive clear, developmentally appropriate information and to actively participate in decisions concerning their health. Information must be communicated in a manner tailored to the child's developmental stage to ensure understanding and meaningful engagement. Once adequately informed, children should be empowered to express their views freely on matters affecting their well-being, with their opinions given due weight in accordance with their age and maturity [
30].
Caring for a child whose autonomy is limited due to developmental immaturity requires a professional approach rooted in the principle of beneficence, which obligates healthcare providers to act in the child’s best interests. This principle not only requires the avoidance of harm but also mandates that, when harm cannot be entirely prevented, it must be minimized to the greatest extent possible, with an emphasis on maximizing benefit [
31].
In the context of pediatric research, there has historically been a strong reluctance to include children in clinical trials—a stance largely motivated by the ethical imperative to protect vulnerable populations. However, this protective approach has inadvertently limited access to rigorously evaluated and age-appropriate pharmacological therapies. As a result, pediatric medicine continues to face a critical gap in evidence-based treatment options, compelling clinicians to rely on suboptimal or extrapolated therapies. This not only compromises individual patient outcomes but also hampers progress in the development of targeted and effective pediatric care [
29,
30,
31].
Beta-blocker administration in children is well-established for a range of clinical indications. However, their effectiveness in managing intraventricular pressure gradients (IVPG) remains inadequately studied. Despite this lack of specific evidence, the ethical rationale for their use in pediatric patients is strong, particularly in the absence of validated, effective, and safe therapeutic alternatives. Limiting pediatric patients to medications formally approved only for adults—when such treatments may offer substantial clinical benefit—raises significant ethical concerns. Ensuring equitable access to appropriate therapeutic interventions is essential to upholding the child’s best interests and affirming their right to equal standards of care [
28,
30,
31].
The aim of this open-label, prospective, non-randomized study was to assess the feasibility of using exercise stress echocardiography (ESE) to guide individualized beta-blocker therapy in children who present with symptoms or abnormal findings on electrocardiography or exercise stress testing, accompanied by IVPG—with or without systolic anterior motion (SAM) of the mitral valve—during physical exertion.
2. Methods
2.1. Sample
We performed stress echocardiography on a group of 66 children, 15 of whom (23%) were female, with a mean age of 14.6 ± 1.0 years (ranging from 11 to 17 years). These children developed a significant intraventricular gradient (greater than 30 mmHg) without beta-blocker therapy and underwent ESE again after being treated with beta-blockers. The 66 children were part of a larger cohort of 101 children who developed IVPG during ESE [
1] received beta-blocker treatment prescribed by their physicians, and underwent repeated ESE, as requested by their doctors, in our department between 2002 and 2019.
These children do not have congenital heart disease, hypertrophic cardiomyopathy, or any abnormal findings on their echocardiograms. Exclusion criteria included the presence of left ventricular (LV) hypertrophy, mitral valve prolapse, or any other structural heart conditions.
They have unexplained exercise-related symptoms (such as chest pain in 22 children, abnormal fatigue in 6 children, dizziness/syncope in 21 children), and/or alterations in the ECG or exercise stress test. 7 children. The doctors of the children prescribed the beta-blockers after the result of the first exercise stress echocardiogram. The children first underwent exercise stress echocardiography without beta-blockers and later repeated the test – the present study, while on beta-blocker therapy within the following year. The methodology used [
1,
5] included evaluating wall motion during treadmill exercise, along with pulsed and continuous wave Doppler assessments to detect IVPG, as well as color Doppler imaging. Mitral valve motion was also analyzed, particularly for systolic anterior motion (SAM).
In all cases, beta-blockers were taken at breakfast on the morning of the exam.
At the time of the ESE performed during therapy, the beta-blockers prescribed by the children's physicians were: atenolol 50 mg for two children and bisoprolol for 64 athletes (2.5 mg for 14, 10 mg for one, and 5 mg for 49 children).
This study was approved by the Ethics Committee of the Heart Center at the Red Cross Hospital in Lisbon. Children who underwent an ESE performed by our team at the Red Cross Hospital, Ucardio, and Hospital Particular of Algarve were identified through a review of the internal EchoLabs database. Medical records were examined to collect patient data, including demographics, clinical diagnoses, indications for ESE, and test results.
Written informed consent was obtained from parents or guardians, along with written informed assent from all study participants [
29].
In the initial years of the study, the entire exam was recorded on video, while in later years, recordings were partially captured using the DICOM format.
An intraventricular pressure gradient was considered significant if it exceeded 30 mmHg at the end of systole, either before, during, or immediately after exercise. The Doppler echocardiographic parameters represent the average of three measurements taken from consecutive high-quality recordings.
2.2. Exercise Stress Echocardiography
A comprehensive echocardiographic evaluation was performed, including measurements of the left ventricular (LV) outflow tract, LV mass index, relative wall thickness, and LV end-diastolic volume at rest. The maximum flow velocity in the LV outflow tract was assessed using continuous-wave Doppler from an apical five-chamber view to calculate the intraventricular pressure gradient (IVPG) before, during, and after exercise, all conducted in the upright position. This methodological approach is particularly relevant, as most physical activities performed by children in daily life occur in an upright posture, thus providing physiologically meaningful insights [
5]. The occurrence of IVPG during exertion is a frequently observed phenomenon in symptomatic children or those with ST/T wave abnormalities on resting ECG or a positive exercise stress test, especially when actively investigated [
1,
5]. The cases reported in this study are of particular interest because post-exercise recovery was also conducted in an upright position—closely mimicking real-life conditions—and the observed gradients were comparable to those seen in patients with hypertrophic cardiomyopathy. Notably, the exercise stress echocardiograms were completed in the presence of symptoms such as fatigue, dizziness, or chest pain.
2.3. Ergometric Parameters
During ESE, the following parameters were evaluated: test duration (in seconds), systolic blood pressure at rest and peak exercise, heart rate at rest and at peak exercise, peak double product, and the presence of ST segment changes—specifically, ST depression of 1 mm occurring 80 ms after the J point. Additionally, any symptoms experienced during the test that resembled those that initially led to the patient's evaluation were documented.
2.4. Statistical Analysis
The results are presented as means ± standard deviation for continuous variables, and frequencies and percentages for categorical variables. The comparison between the two assessments was performed by using paired t-tests, when the assumptions of size and distribution were met, or Wilcoxon signed-rank tests, otherwise. The McNemar test was used to assess changes in paired proportions, enabling the evaluation of differences in dichotomous outcomes before and after intervention. We have some patients who are missing the IVG measurement under BB therapy. For those cases, we considered only the patients with two complete measurements, without and with BB therapy. The analyses were performed using SPSS (version 30). All statistical tests were two-tailed and used a type 1 error rate of 0.05.
3. Results
Of the 66 children enrolled in the study, 15 (23%) were female. The mean age was 14,6, 1,7 years old (11 to 17). On the resting echocardiogram, all the exams were considered normal with and without beta blockers. No wall motion abnormalities were detected in any of the exams, with or without beta blockers. IVPG (
Figure 1) at peak exercise on the first assessment was 105 ± 38 mmHg, with mitral valve SAM in 28 children (
Figure 2) in the complete group and 58
+ 32 mmHg in only 29 children treated with beta-blockers, P< 0.0001.
During the first examination, 47 children (71%) experienced symptoms that had initially prompted the ESE. These symptoms included unexplained exercise-related issues, such as chest pain in 22 children, abnormal fatigue in 6 children, and dizziness/syncope in 21 children, along with ECG abnormalities or exercise stress test alterations in 7 children. Under beta-blocker treatment, only 7 children (11%) reproduced the symptoms that had necessitated the initial ESE: chest pain in 3 children, dizziness in 3 children, and abnormal fatigue in 1 child.
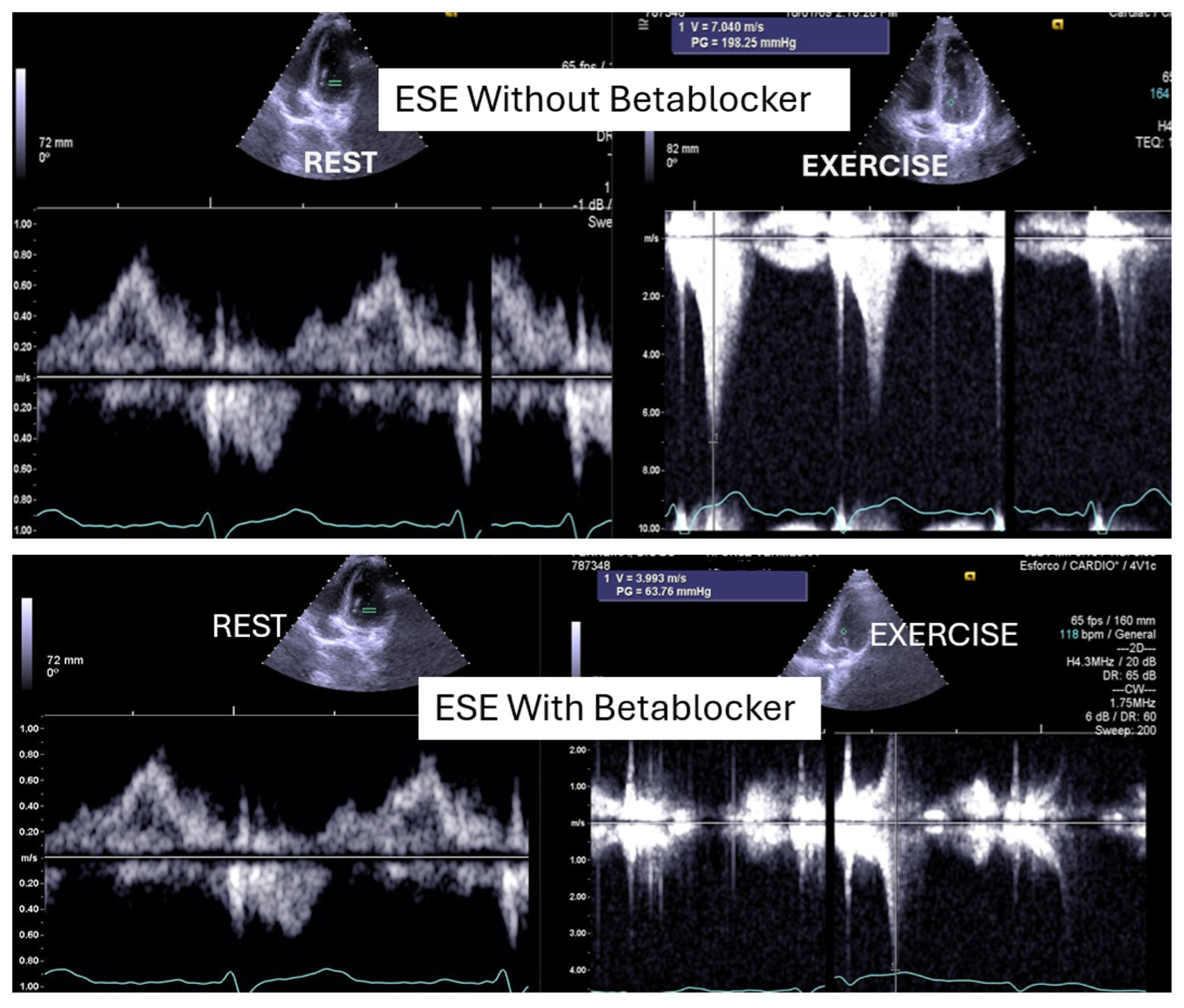
Figure 1.
This figure shows an intraventricular gradient during exercise in a child experiencing exercise-related chest pain and syncope, accompanied by elevated troponin levels (top). A significant reduction in the gradient is observed under beta-blocker therapy (bottom).
Figure 1.
This figure shows an intraventricular gradient during exercise in a child experiencing exercise-related chest pain and syncope, accompanied by elevated troponin levels (top). A significant reduction in the gradient is observed under beta-blocker therapy (bottom).
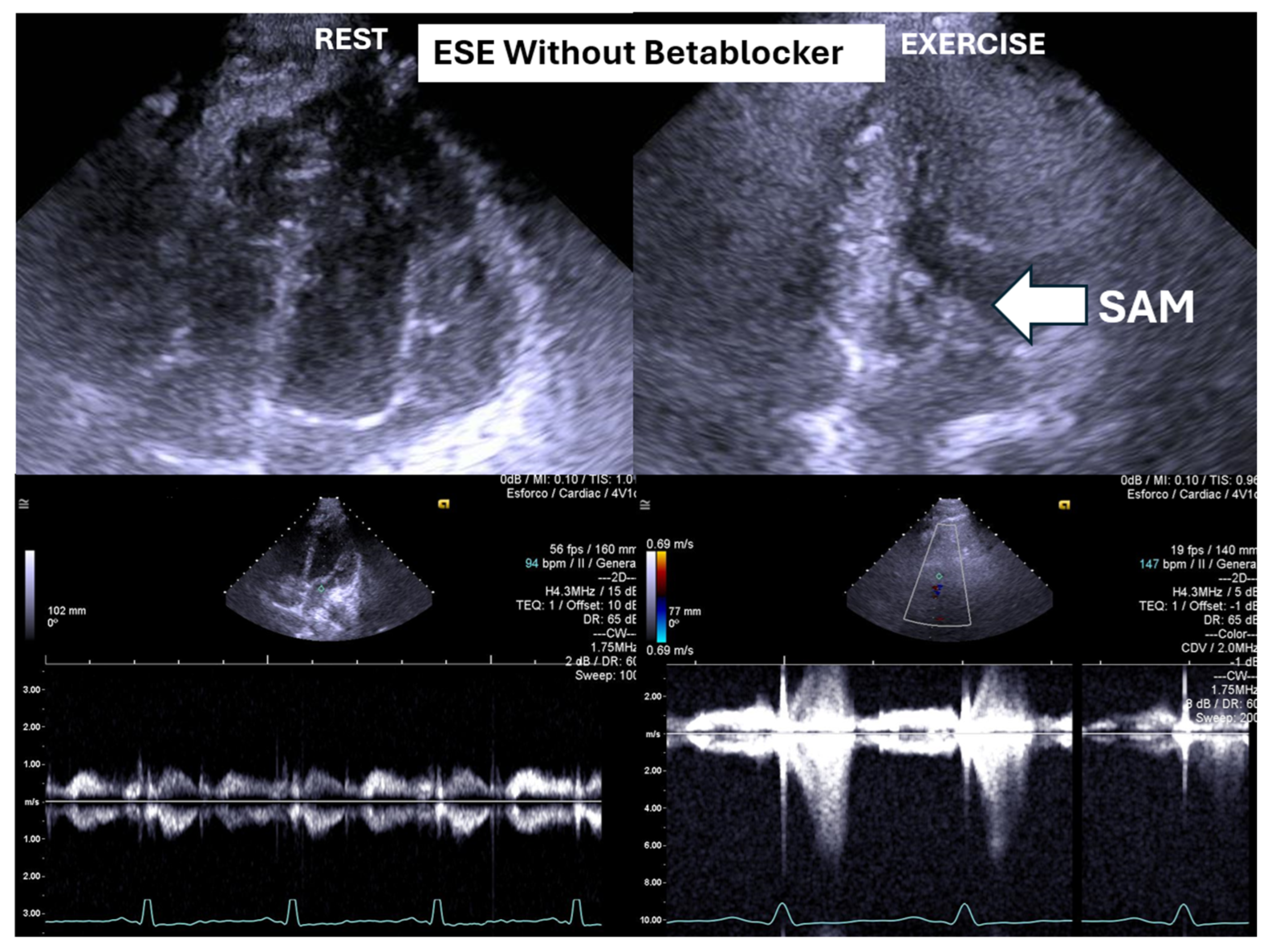
Figure 2.
This figure depicts an intraventricular pressure gradient observed during exercise in a child presenting severe exertional dyspnea, accompanied by pronounced systolic anterior motion (SAM) of the mitral valve.
Figure 2.
This figure depicts an intraventricular pressure gradient observed during exercise in a child presenting severe exertional dyspnea, accompanied by pronounced systolic anterior motion (SAM) of the mitral valve.
At the time of the second ESE, fifty-nine children (89%) demonstrated clinical improvement and are currently without experiencing the symptoms that had led to their inclusion in the study. The main results of the variables studied are shown in
Table I.
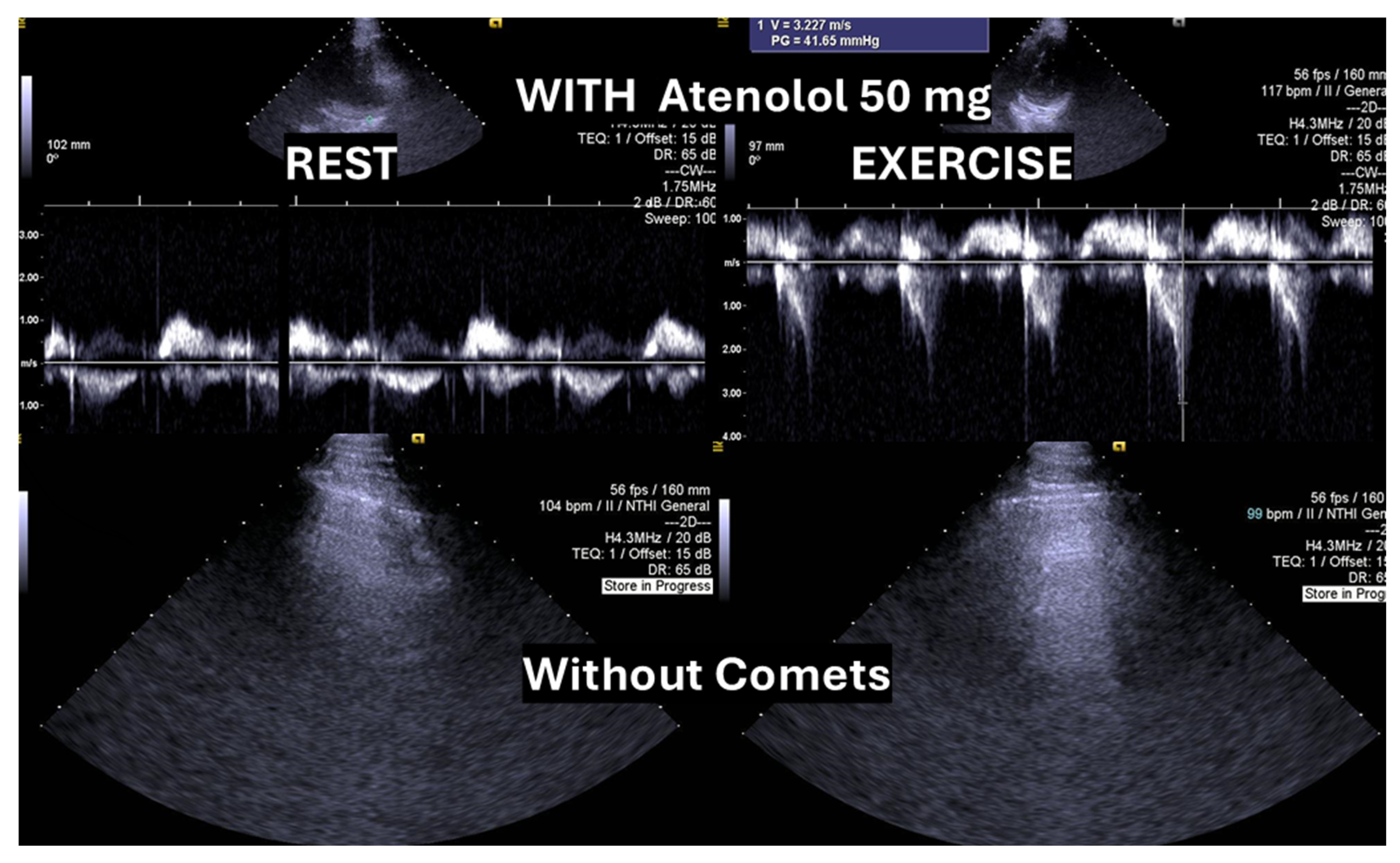
One of these children also developed “lung comets” [
32,
33,
34,
35,
36], which were investigated due to severe dyspnea associated with marked systolic anterior motion (SAM) and a significant intraventricular pressure gradient (IVPG) (
Figure 3). To the best of our knowledge, this is the first report describing the presence of lung comets during exercise stress echocardiography (ESE) in pediatric patients, specifically in association with severe exercise-induced IVPG.
In patients with hypertrophic cardiomyopathy, the presence of lung comets has been associated with diastolic dysfunction, left ventricular outflow tract obstruction, and, in some cases, systolic dysfunction [
32]. The evaluation of B-lines—both at rest and during stress—is increasingly recognized as a valuable tool for the diagnosis of heart failure, assessment of its severity, monitoring of therapeutic efficacy, and enhancement of risk stratification. This clinical relevance has been underscored in recent cardiology guidelines and expert recommendations.
According to the 2021 universal definition of heart failure by the European Society of Cardiology, diagnosis is based on the presence of typical signs and symptoms, corroborated by elevated natriuretic peptide levels and/or objective evidence of cardiogenic pulmonary or systemic congestion [
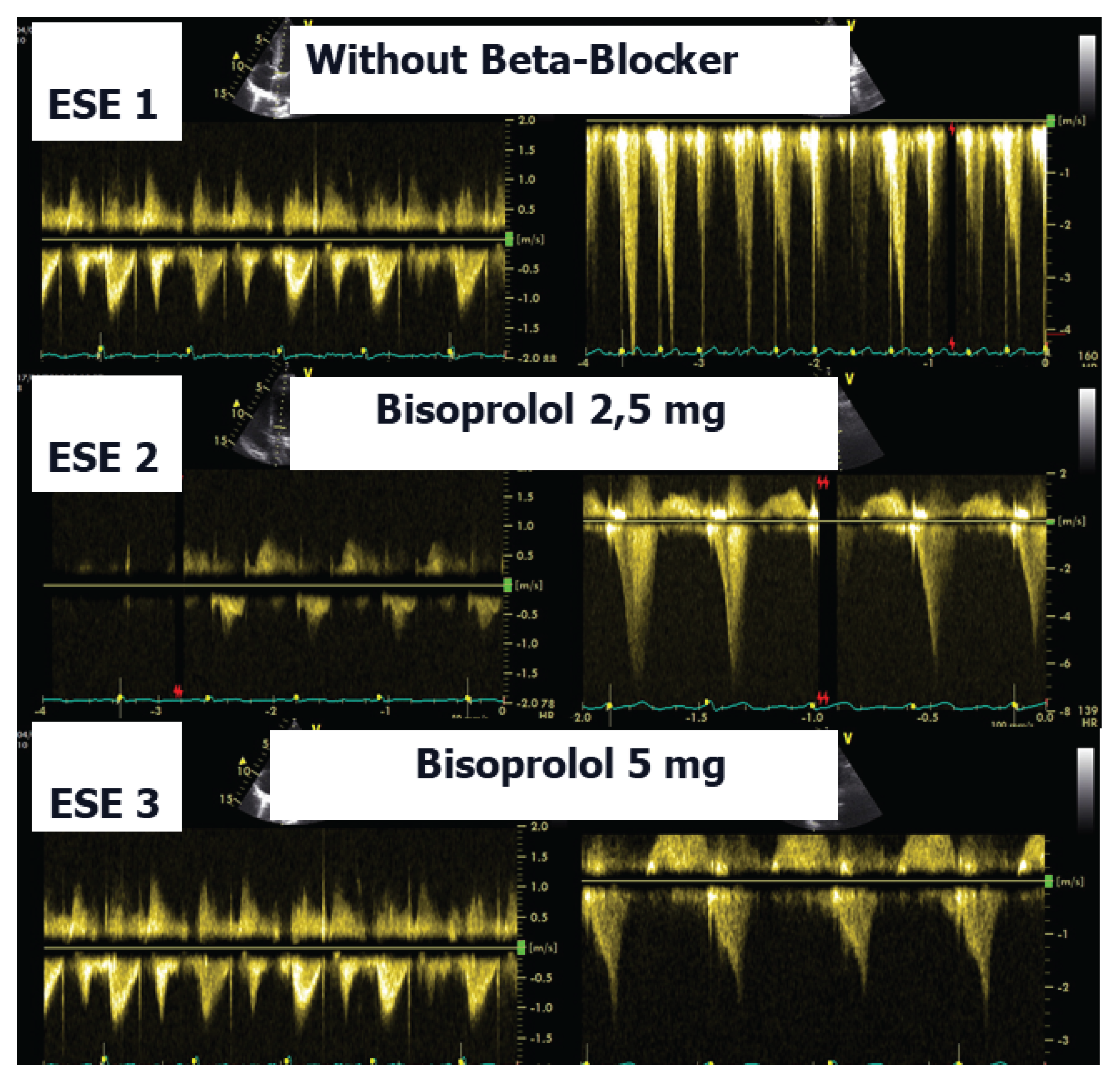
35]. In the present case, following a stepwise titration of beta-blocker therapy—from bisoprolol 2.5 mg, 5 mg, 7.5 mg, and 10 mg to atenolol 50 mg—the child no longer exhibited lung comets (
Figure 4).
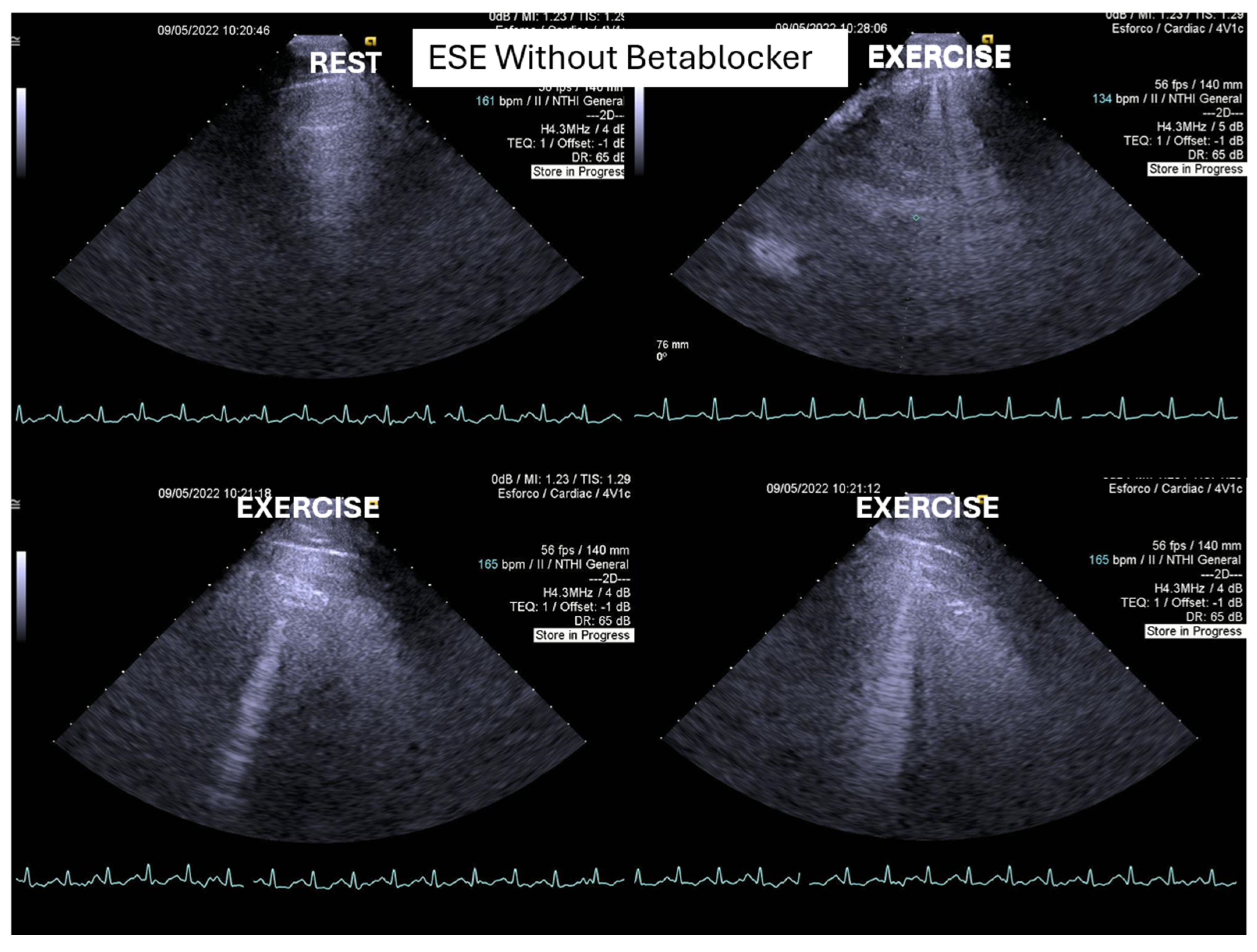
We observed the development of intraventricular pressure gradients (IVPGs) in children without left ventricular hypertrophy upon assuming an upright posture prior to exercise—a phenomenon previously described in patients with hypertrophic cardiomyopathy [
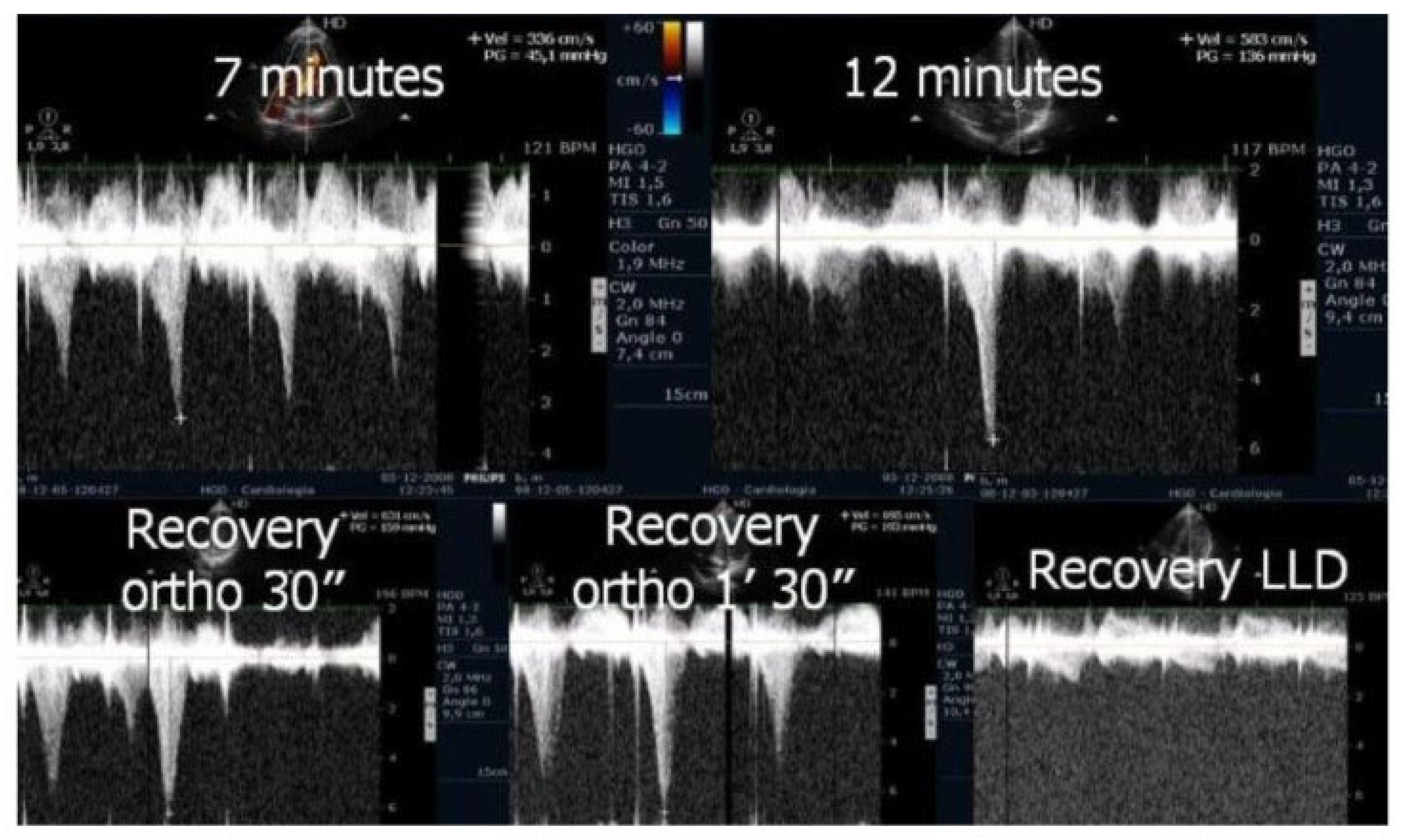
5]. At the onset of exercise (
Figure 5), the IVPG initially decreased, likely due to increased preload resulting from activation of the lower limb musculature. However, as exercise progressed, the IVPG steadily increased (
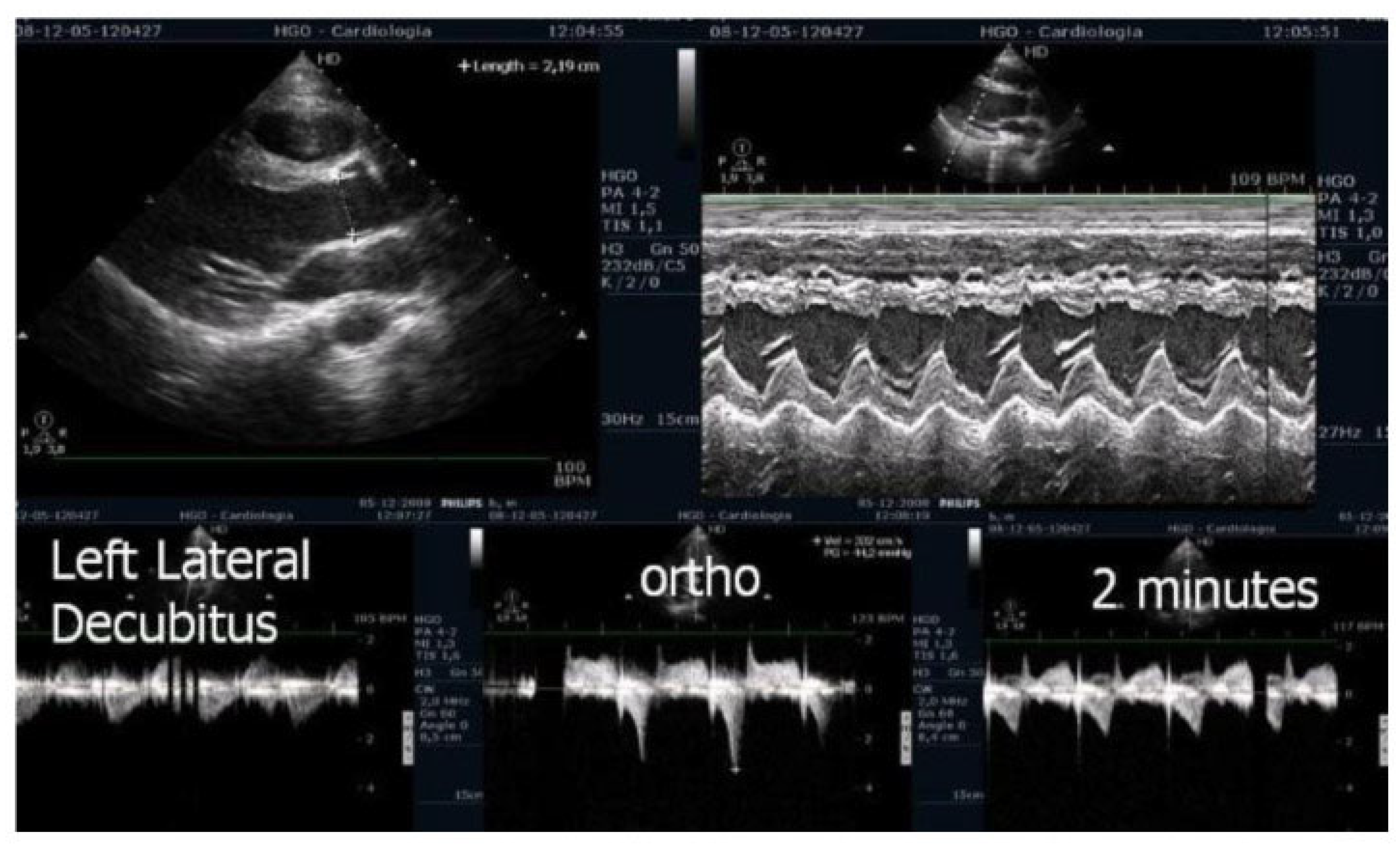
Figure 6). After exercise, maintaining an upright posture led to a more pronounced reduction in preload compared to the supine position. This hemodynamic shift likely accounts for the post-exercise elevation in IVPG observed in most children studied. This postural response may also explain the orthostatic recovery IVPG observed in one highly symptomatic child who presented with exertional angina and ST-segment abnormalities (
Figure 7). Additionally,
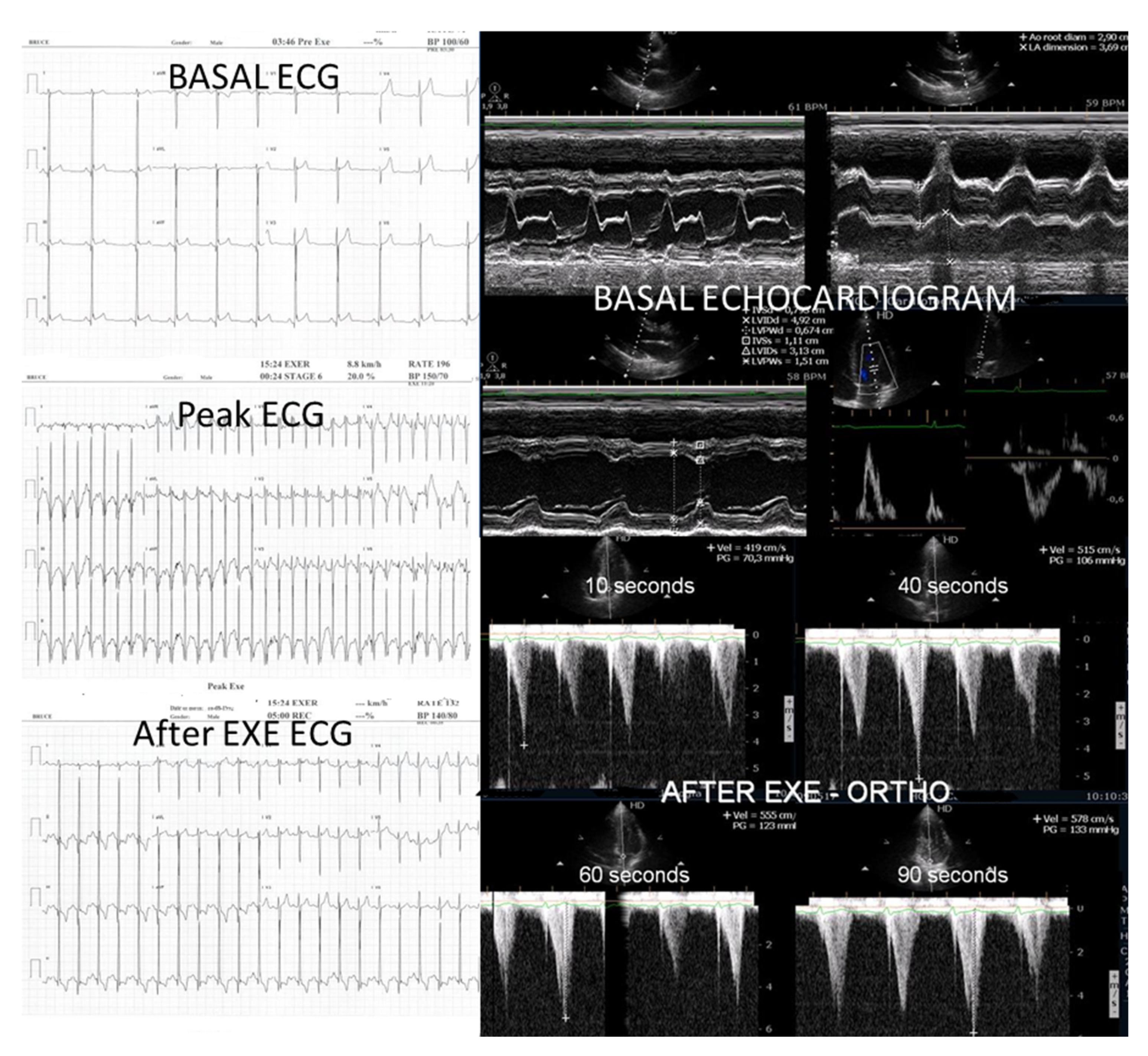
Figure 8 illustrates the impact of beta-blocker therapy on ESE findings in one patient, including the titration of atenolol to a dose of 50 mg, underscoring the clinical relevance of individualized treatment.
4. Discussion
In our study, beta-blocker therapy was associated with a reduction in heart rate, exercise-induced ST-segment alterations, systolic blood pressure, and consequently, peak heart rate–systolic blood pressure product (HR×SBP). The incidence of intraventricular gradients (IVG) (
Figure 1) and systolic anterior motion (SAM) of the mitral valve (
Figure 2) during exertion also decreased significantly. These hemodynamic improvements were accompanied by a notable reduction in symptoms during exercise testing and follow-up (
Figure 3 and
Figure 4).
Lau et al. [
25] were the first to identify exercise-induced intraventricular gradients in a symptomatic adult patient with effort angina. The condition was managed using bisoprolol, which led to clinical improvement and a substantial reduction in the intraventricular pressure gradient (IVPG). It has long been recognized that small intraventricular pressure gradients are a common phenomenon. Three mechanisms have been proposed to explain their significant increase during exercise: (1) an amplification of non-obstructive physiological gradients, (2) end-systolic obstruction due to mid-cavity obliteration of the ventricle, and (3) mid-systolic obstruction caused by systolic anterior motion (SAM) of the mitral valve, which restricts blood ejection.
However, SAM typically occurs when there is an alteration in ventricular chamber geometry or the mitral valve apparatus. This was not the case with our children. Nonetheless, studies have shown that intraventricular gradients can arise due to maneuvers that modify loading conditions in structurally normal hearts, such as those occurring during exercise [
5]. Furthermore, dehydration during physical activity, reducing ventricular volume, may exacerbate the development of significant IVPGs and should be carefully monitored.
Subsequently, we identified the presence of intraventricular pressure gradients (IVPGs) in patients presenting with angina despite having angiographically normal coronary arteries. This observation prompted a study involving 91 patients diagnosed with cardiac Syndrome X, from which 20 individuals exhibiting IVPGs were selected for beta-blocker therapy. Treatment resulted in a significant improvement in clinical symptoms [
5].
Treating pediatric patients, as demonstrated in this study, presents unique challenges, particularly because the use of beta-blockers in children is often based on data extrapolated from adult populations. One major limitation is the absence of well-defined pediatric dosing guidelines during drug development. Historically, regulatory frameworks and pharmaceutical industry practices have largely excluded children from clinical trials, resulting in a paucity of pediatric-specific pharmacological data. Nevertheless, existing evidence supports the clinical efficacy of beta-blockers in children with left ventricular outflow tract obstruction [
26]. Additionally, beta-blockers have been shown to alleviate symptoms potentially associated with exercise-induced intraventricular pressure gradients (IVPGs), both in patients with and without hypertrophic cardiomyopathy [
5,
6,
20,
22,
23,
24,
25,
26,
27,
31]. Based on this body of evidence and ethical considerations, we elected to include beta-blocker therapy in our pediatric patient population.
In the present study, while symptom improvement was observed among participants, no significant change in functional capacity was detected. This finding aligns with current regulations, which generally do not classify beta-blocker use as prohibited in most sports practiced by children—including those who were athletes in this cohort [
37]. The results also suggest that exercise stress echocardiography is a valuable tool for identifying symptomatic children who develop intraventricular pressure gradients (IVPGs) during exertion and may particularly benefit from beta-blocker therapy.
Study Limitations
One limitation of this study is its relatively small sample size. However, all 66 children met strict inclusion criteria, as they were symptomatic and had objective evidence of IVPG during exercise. As a result, they represent a carefully chosen group most likely to benefit from targeted beta-blocker therapy.
Another limitation is the absence of randomization and double-blinding for participants and investigators, which was not feasible due to ethical, logistical, and financial constraints. In our open-label design, the children, their parents or guardians, and the investigators were all aware of the treatment being given. Additionally, the type and dosage of beta-blockers varied significantly, reflecting real-world treatment practices. All post-treatment tests were conducted during the second assessment, which may have affected certain results, such as treadmill test duration and children's symptom ratings. However, this would not have influenced objective measures like the presence and severity of IVPG, which remained the study's primary focus. Further research, ideally through placebo-controlled randomized trials, is needed to assess the potential benefits of beta-blockers for these children. The lack of recommendations for IVPG screening during exercise in children with exercise-related symptoms or positive test results, combined with the absence of clear guidelines for beta-blocker treatment in these cases, highlights the need for additional studies.