1. Introduction
Waste from agricultural activities leads to reduced productivity and damage to the environment. Globally, over one third of all food produced, approximately 3 million tonnes, goes to waste. Fruit and vegetables have the highest wastage rates, often going to waste before even leaving the farm [
1]. Plants, including those grown as crops, produce a wide variety of bioactive compounds, many of which have beneficial health effects. However, when plants are harvested for market, the processing often generates waste. This plant-derived waste also remains high in health-promoting bioactive compounds such as polyphenols [
2], meaning that upcycling plant waste holds potential to drive production of innovative, inexpensive, sustainable, and healthy foods.
Polyphenols are phytochemicals produced by plants and found in many fruits and vegetables. In plants, polyphenols play crucial roles acting as antioxidants to quench free radicals [
3], defending against infection by pathogens and absorbing ultraviolet light to protect tissues from damage. Polyphenols are consumed by humans and animals through the diet, and dietary polyphenols are gaining scientific and consumer attention due to their health benefits. Studies have found that polyphenols have protective effects against cancer, cardiovascular disease, and neurodegenerative diseases [
4,
5]. Anthocyanins are a subclass of polyphenols that act as pigments that give plants vibrant colours (purple, blue, and red) attracting pollinators and are known for their antioxidant, anti-inflammatory, and cardiovascular benefits. Their chemical structure means that they can act as free radical scavengers against harmful oxidants such as reactive oxygen and nitrogen species, quenching reactive radical species by single electron transfer reactions and through hydrogen atom abstraction from phenolic groups [
6]. Epidemiological studies have long suggested that diets rich in anthocyanins and other polyphenols slow age-related cognitive decline and reduces the risk of dementia. Thus, consumption of anthocyanin-rich foods could play a role in cognitive health and disease prevention [
7,
8]. Major dietary sources of anthocyanins include dark-coloured fruits, vegetables, grains, and legumes, e.g.
, blackcurrants, blackberries and blueberries (300-500mg/100g of fresh product), red cabbage (300-500mg/100g) and black rice (200-400mg/100g). Cherries also contain relatively high levels of anthocyanins (50-100mg/100g) and are widely popular, making them an important source of anthocyanins [
5,
9,
10].
Cherry production is an important horticultural industry globally, with the major producing countries being Turkey, the United States, Chile, and Uzbekistan. In 2022, the estimated total world production was 4.36 million metric tonnes [
11]. Cherry production generates a significant amount of agricultural waste, including fruit that is discarded for not meeting market standards. Most markets require fruits that are of uniform size, shape, colour and ripeness, and fruits that are not within these standards are typically sent to landfill. Although there are variations between regions and market standards, it is estimated that approximately 10% of cherries are discarded before they reach consumers due to not meeting quality standards, negatively impacting productivity, carbon emissions and sustainability [
12]. Although cosmetically unattractive, waste cherries are a potential source of natural compounds with beneficial effects on health. Converting cherry waste into food products could lead to increased agricultural sustainability and productivity while at the same time increasing availability of inexpensive, healthy foods.
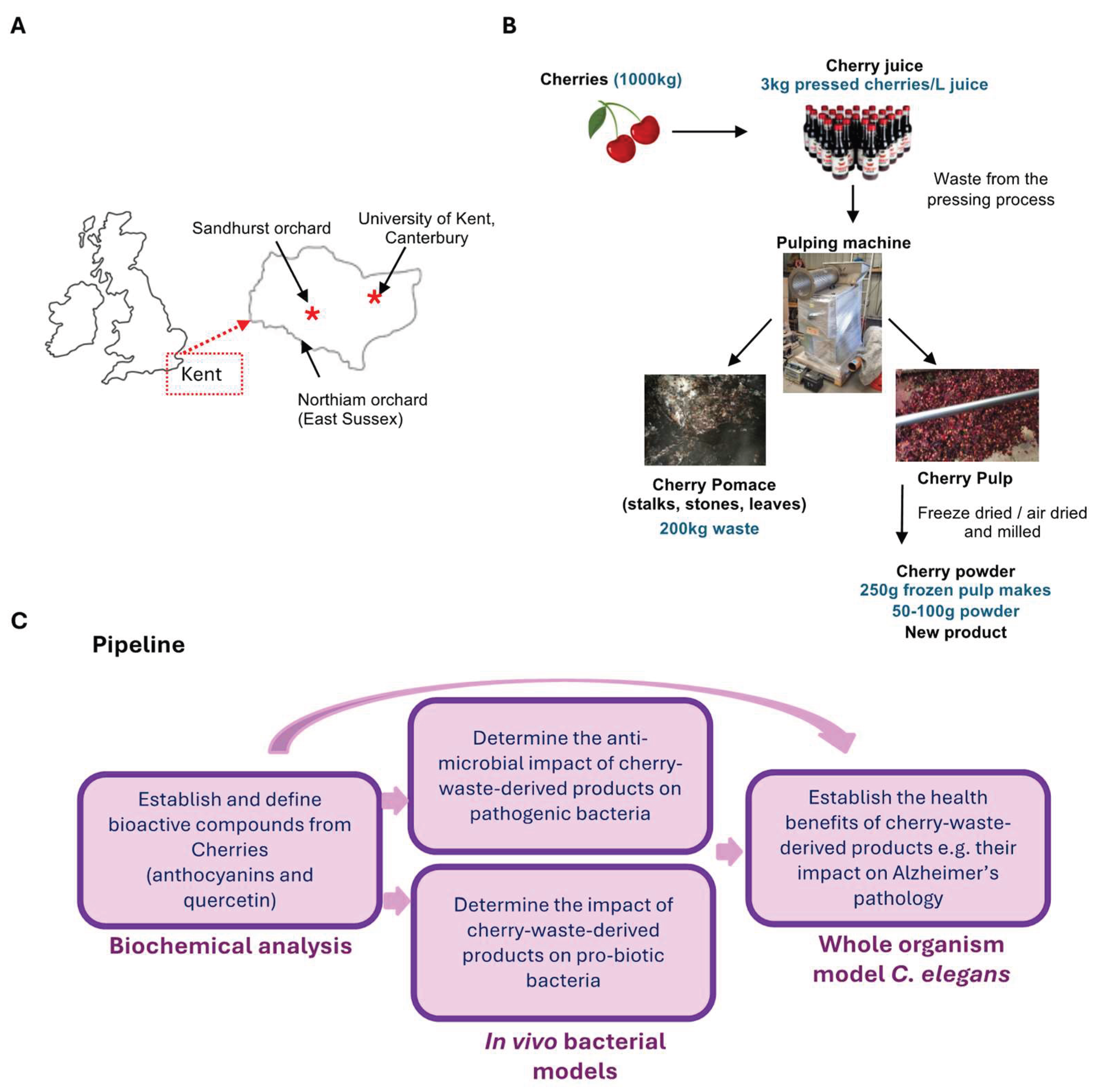
Kent in the South East of England (
Figure 1A), is the largest cherry producer in the UK and also generates large amounts of waste cherries. 60 tonnes of Kent cherries that are bruised, soft or discoloured are transported to landfill every year (Michael Dallaway, personal communication). These cherries have the potential to be developed into new innovative foods with beneficial health and environmental profiles. In this study, researchers in Kent collaborated with a small local horticultural business to analyse anthocyanin content, and health properties, in products made from cherry waste (
Figure 1B-C). Our goal was to demonstrate value of cherry waste as means to transform the waste into new foods. Here, we quantify the anthocyanin content of a cherry juice made by pressing waste cherries, as well as the pulp and pomace remaining after juice pressing (
Figure 1B-C). We also analyse the content of quercetin, a polyphenol in the flavanol subclass, which like anthocyanins, is associated with health benefits [
13]. We find that all these cherry products are rich sources of several different anthocyanins, including Cyanidin-3-O-rutinoside, and have low, but detectable, levels of quercetin. To examine the importance of these bioactive containing products to organismal health, we used the model organism
Caenorhabditis elegans, a valuable tool for studies of health outcomes in vivo (
Figure 1C). We find that supplementing
C. elegans food with cherry pulp powder, a product rich in anthocyanins, is protective in a
C. elegans model of Alzheimer’s disease pathology.
Our work shows a high anthocyanin content of bioactives in a specific stream of cherry waste products, and demonstrates its potential health benefits. The study provides scientific evidence for innovative approaches that can be adopted at scale in Kent, and other agricultural regions and generates information relevant to consumers, markets and growers.
2. Materials and Methods
Ultra-Performance Liquid Chromatography (UPLC-MS) coupled with Quadrupole Time-of-Flight Mass Spectrometry (UPLC-QToF)
Samples were analysed by UPLC-MS to identify target anthocyanins, then quantified using triple quadrupole. Liquid samples were analysed directly, and solid samples were extracted with solvent prior to analysis. Liquid samples were additionally diluted with extraction solvent 10-fold to account for compounds present in high concentrations.
Sample Preparation
For comparison of cherry juice batches, cherry juice was centrifuged at 13,200 RPM (14,220 × g) for 10 minutes. Supernatants were then filtered (0.2 µm pore size filter) and stored at −80 °C.
Standards
Individual standards were diluted with formic acid/methanol/water (1/14/35 v/v/v) such that the calibration range of the anthocyanin was 50, 10, 5, 2, 1, 0.5, 0.2 µg/mL in solution. Quercetin was prepared at 10 – 0.2 µg/mL. Samples were stored at -20°C until analysis. Cyanidin 3-o-glucoside standard was from Extrasynthase, France (code 0915 S). Quercetin (95%) was from Merck (code Q4951). Anthocyanins were measured against a cyanidin-3-O-glucoside standard and reported as equivalent concentrations
UPLC-QToF
Instrumental analysis was performed using a Waters (Wilmslow, UK) Acquity UPLC coupled to a Select Series Cyclic QToF mass spectrometer. The analytical column was a Waters HSS T3 C18 100 x 2.1 mm x 1.7µm at 35°C. Mobile phases were solvent A: water with 1% formic acid and solvent B: acetonitrile with 1% formic acid with a constant flow of 0.4 mL/min. A gradient was applied as 5%B at injection for 1 min, 10%B at 5 min, 16%B at 15 min, 95%B at 40 min, then back to initial conditions for 3 min. Sample injections were 2 µL. To identify each anthocyanin, the instrument was operated in positive electrospray ionisation mode in full scan to determine the molecular ion (low fragmentation energy) and fragments (high fragmentation energy), particularly the associated anthocyanin aglycone fragment. The identified anthocyanins were then analysed by a triple quadrupole instrument, looking at the molecular ion and fragment ion only for selectivity and sensitivity.
UPLC-MS/MS
Instrumental analysis was performed using a Waters Acquity UPLC coupled to a TQ-Absolute triple quadrupole mass spectrometer. The analytical column was a Waters HSS T3 C18 100 x 2.1 mm x 1.7µm at 35°C. Mobile phases were solvent A: water with 1% formic acid and solvent B: acetonitrile with 1% formic acid with a constant flow of 0.4 mL/min. A gradient was applied as 5%B at injection for 1 min, 10%B at 5 min, 16%B at 15 min, 95%B at 16 min and held for 2 minutes, then back to initial conditions for 3 min. Sample injections were 1 µL.
Preparation of Cherry Product Stock Solutions for Bioactivity Assays
Cherry Juice
Dallaways’ Kentish Cherry Juice was freeze dried for 48 hours to remove all moisture. The residue was resuspended in 100% DMSO at a 1g:2mL ratio, vortexed, then incubated with rocking at room temperature for 1 hour to resuspend. The sample was centrifuged at 14,220 × g for 10 minutes. Supernatant was taken and filtered (0.2µm pore size), split into aliquots and stored at -20°C.
Cherry Pulp Powder
Cherry pulp powder was produced using cherry waste from the Dallaways juice pressing process. Waste cherry pulp was filtered using a pulping machine then freeze dried and milled into a fine powder. The powder was resuspended in 100% DMSO at a 1g:4mL ratio, vortexed then incubated with rocking at room temperature for 1 hour to resuspend. The sample was centrifuged at 14,220 × g for 10 minutes. Supernatant was taken and filtered (0.2µm pore), split into aliquots then stored at -20°C.
Blackberry powder
Blackberry extract powder was sourced from Natural Products of Worth (NPOW). The powder was resuspended in 100% DMSO at a 1g:20mL ratio, vortexed and incubated with rocking at room temperature for 1 hour to resuspend. The sample was centrifuged at 14,220 × g for 10 minutes. The supernatant was taken and filtered (0.2µm pore size), split into aliquots then stored at -20°C.
Kirby-Bauer Analysis
Bacterial cultures of E. coli K-12, Staphylococcus albus, and Bacillus megaterium were inoculated into LB and incubated overnight at 37°C (30°C for B. megaterium) with shaking at 180 RPM. The optical density at 600 nm (OD₆₀₀) of each culture was measured and diluted to 0.1 using LB. 100µL was evenly spread on to LB agar plates, which were left to dry at room temperature for 30 mins. Sterile filter paper discs were treated with 15µL of either anthocyanin solution (in DMSO), a negative control (sterile Milli-Q® water or DMSO) or a positive control (50µg/mL kanamycin or 100µg/mL ampicillin), then left to dry for a minimum of 2 hours. Discs were placed on to the relevant bacterial lawns and incubated at 37°C (30°C for B. megaterium) for 24 hours. The diameter of the zones of inhibition were measured in mm.
Growth Measurements of E. coli OP50
E. coli OP50 was inoculated into LB and incubated overnight at 37°C with shaking at 180 RPM. The overnight culture was diluted 1000x in LB. A sterile 96-well plate was set up to include 100 µL per well of the following negative controls diluted in LB: (1) 1% DMSO (2) Anthocyanins only (3) OP50 bacteria only and (4) OP50 with 1% DMSO. OP50 bacteria were grown in the presence of anthocyanins of varying concentrations. The relevant values for the ‘Anthocyanin only’ control were subtracted from each OP50 growth curve to account for the strong pink/red colour of anthocyanins. 100 µL total volume was added to each well of a sterile 96-well plate and each condition was performed in triplicate to give 3 technical replicates per plate. The OD600 was measured using a BMG Labtech SPECTROstar Nano plate reader where the plate was incubated at 37°C for a minimum of 18 hours with shaking at 400 RPM. Each experiment was also repeated to give 3 biological repeats.
Growth Measurement of Lactobacillus Strains
The following Lactobacillus strains were sourced from the DSMZ (German Collection of Microorganisms and Cell Cultures): L. acidophilus (#20079), L. brevis (#20054), L. helveticus (#20075), L. paracasei (#28675), L. plantarum (#20174), L. rhamnosus (#111733) and L. salivarius (#20555). All strains were streaked on to MRS (Fisher Scientific #11713553) agar plates and incubated at 37°C for 48 hours with 5% CO2. Colonies were inoculated into MRS liquid media and incubated at 37°C with 5% CO2 for 48 hours. For growth of L. acidophilus and L. helveticus, the MRS media was supplemented with 0.05% cysteine hydrochloride. The overnight cultures were diluted 1000x in MRS (+/- 0.05% cysteine hydrochloride). Sterile 96-well plates were set up to include 100 µL per well of the following negative controls diluted in MRS: (1) 1% DMSO and (2) Anthocyanins only. Bacteria were grown in the presence of either 1% DMSO or 100 µg/mL of the relevant anthocyanin. The values for the ‘Anthocyanin only’ controls were subtracted from each bacterial growth curve to account for the strong pink/red colour of anthocyanins. 100 µL total volume was added to each well of a sterile 96-well plate and each condition was performed in triplicate to give 3 technical replicates per plate. The OD600 was measured using a BMG Labtech SPECTROstar Nano plate reader where the plate was incubated at 37°C for a minimum of 48 hours with shaking at 400 RPM. Each experiment was also repeated to give 3 biological replicates.
The maximum rate of growth was calculated by subtracting the average values of the sample media controls from each technical replicate of the growth data, then plotting the graph of each subtracted technical replicate density against time. The exponential phase of the growth curve was used to calculate the maximum rate of growth of the given bacteria, OD600/hour. The maximum growth rate was then standardised to the respective solvent control.
C. elegans Culture Methods and Strains
C. elegans were maintained at 20 °C on Nematode Growth Medium (NGM) plates seeded with Escherichia coli OP50. C. elegans strains used in this study were N2 (Wild-type) and GMC101 dvIs100 [unc-54p::A-beta-1-42::unc-54 3′-UTR + mtl-2p::GFP].
Preparation of Experimental NGM Plates
35mm diameter NGM plates were seeded with 150 µL
E. coli OP50 then incubated for a minimum of 48 hours at room temperature before use. Anthocyanins (prepared in DMSO) were added to the plates then incubated in the dark at room temperature for 12–24 hours before use. Concentration of each sample shown in
Table S1.
Developmental Assays
Fifteen adult C. elegans were placed on experimental plates and allowed to lay eggs for 2 h at 20°C. After 68 h, the number of animals at L4 and adult stages was counted to evaluate effects on developmental rate. The experiment was repeated four times.
Proteotoxicity Assay
GMC101 animals were allowed to lay eggs for 4 hours on supplemented plates to synchronise the population. ~54 h after the egg lay, L4 stage animals were transferred to new plates and shifted to 25 °C to induce expression of amyloid-β42. Animals were scored every day for four days, and the numbers of paralysed and non-paralysed animals counted. C. elegans were counted as paralysed if they were alive but failed to move forwards or backwards after stimulation with a platinum wire. 3-4 biological replicates were performed with three plates containing 30-40 animals each per condition.
Statistical Analysis
Data processing and statistical evaluation were performed with GraphPad Prism, version 10.1.1 (270) (San Diego, CA, USA). Statistical tests stated in figure legends.
3. Results
Quantification and Identification of Anthocyanins in Kent Cherry Products
Juicing cherries is an economically viable way of using up fruit that is not sold directly to consumers. As it is reported that cherries have high levels of anthocyanins [
5,
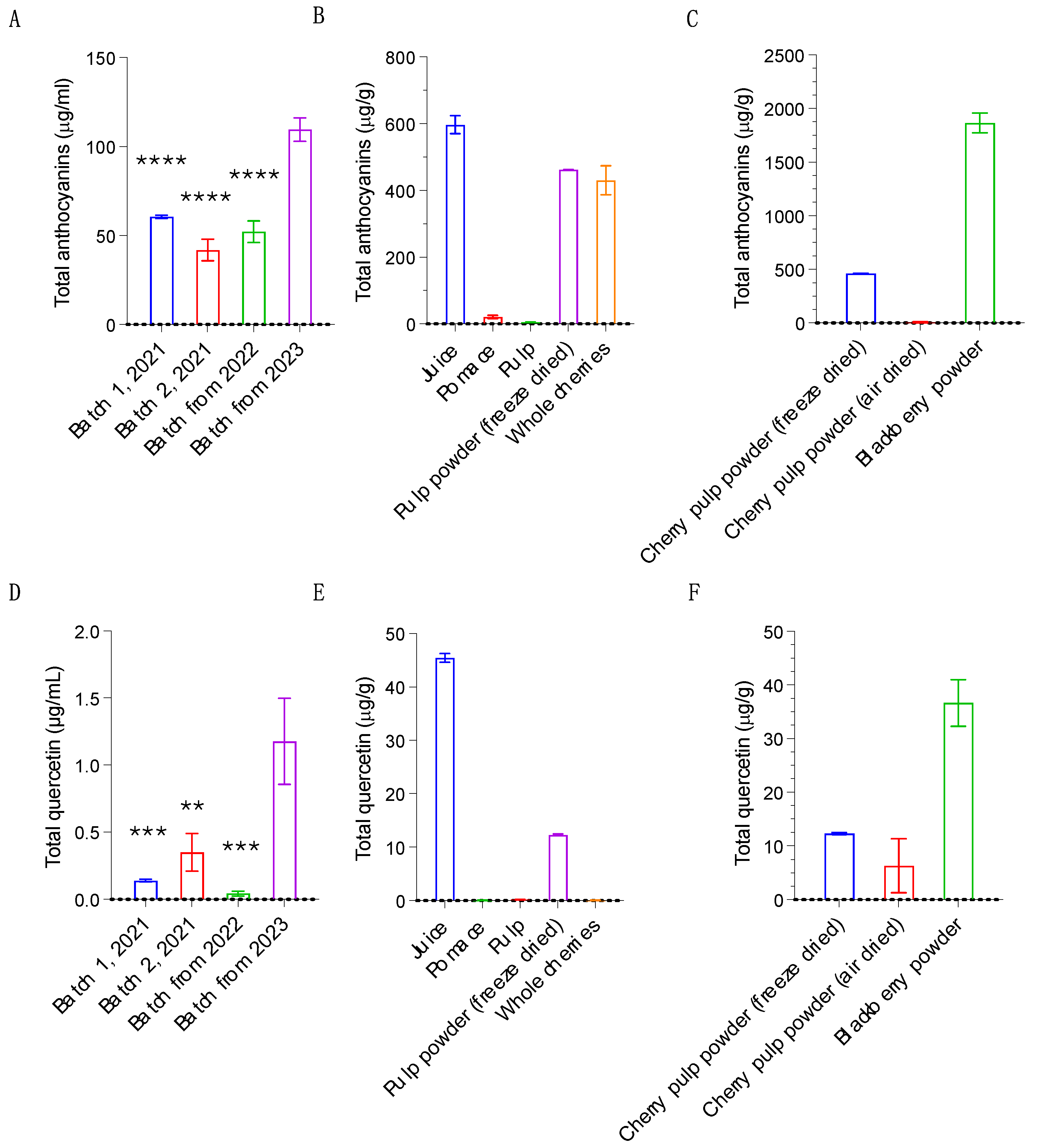
9], we determined the levels of anthocyanins in cherry juice made from waste cherries. We first quantified total anthocyanin content in juice made from three independent harvests (2021, 2022 and 2023). For all batches, the juice was bottled straight after pressing and stored identically until analysis. Juice samples were compared to a known panel of standards using Liquid Chromatography and Mass Spectrometry (LC-MS). This identified high levels of total anthocyanins in all batches of cherry juice ranging from 42-110 mg/L (
Figure 2A) with the 2023 batch having the highest anthocyanin content. Anthocyanin levels in plants are increased by high temperatures and exposure to sunlight, and degrade at room temperature [
14] thus the higher anthocyanin content in the 2023 batch is likely either to be a result of a shorter storage time, or the higher than average temperatures in 2023 (e.g., record-breaking temperatures in June 2023) [
15].
Waste from the production of cherry juice was taken and processed through a pulping machine to give cherry pulp and pomace samples (
Figure 1B). To determine whether these by-products contained valuable bioactives, we examined total anthocyanin levels in pulp and pomace produced from juice pressing of the 2022 and 2023 cherry harvests. We also generated two new products, powders made from cherry pulp from the 2022 harvest. The powders were made by either freeze-drying or air-drying pulp and then milling into a fine powder. We measured anthocyanin levels in the powders, whole fresh cherries and in the cherry juice (
Figure 2B). In addition, we included a commercially available blackberry extract powder for comparison (
Figure 1C). The samples were prepared using ethanol-based extraction with acidified conditions [
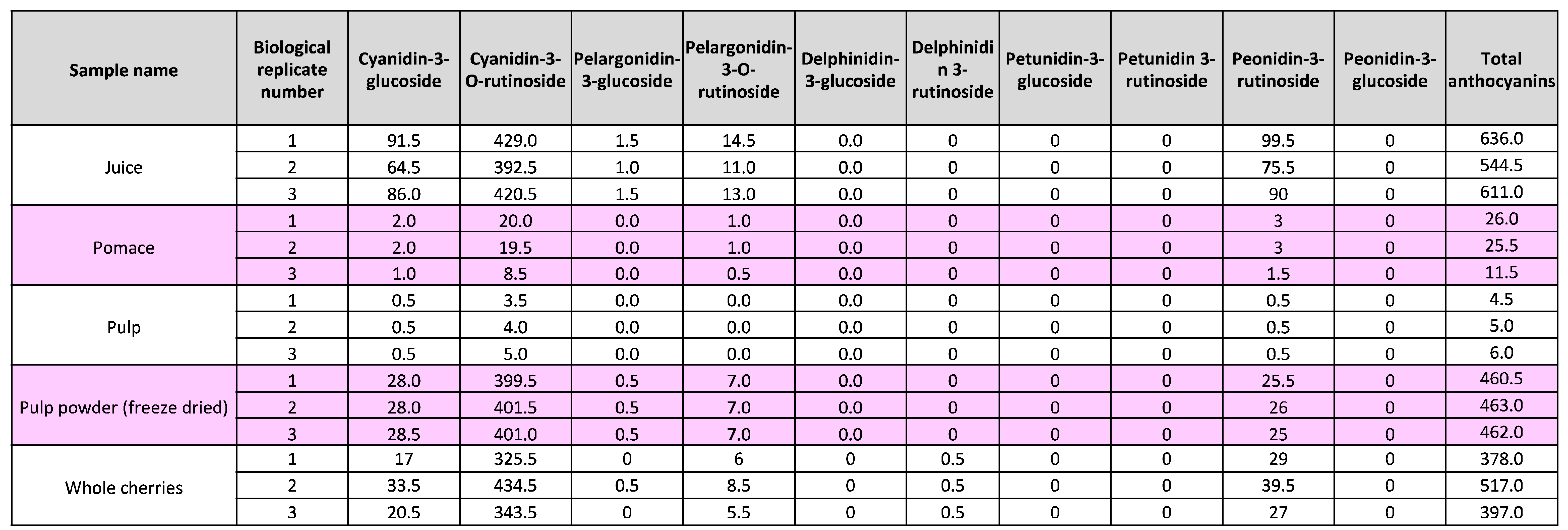
16] and analysed by LCMS. We found that while whole cherries contained a total of 431 μg/g anthocyanins, levels in cherry juice were higher at 598 μg/g (
Figure 2B). Cherry pulp contained 5 μg/g total anthocyanin and pomace contained 21 μg/g, likely as a result of pomace containing the skin, which is where most of the anthocyanins reside. The air-dried pulp powder had a lower anthocyanin content of 7.5 μg/g, while the freeze-dried pulp powder had a relatively high content of 462 μg/g (
Figure 2B). This is consistent with other studies showing improved retention of anthocyanins in freeze dried fruits compared to hot-air drying due to instability at higher temperatures [
17]. The levels of anthocyanins in our samples were comparable to a commercially available blackberry powder enriched with anthocyanin extracts, which contained 1864 μg/g anthocyanins (
Figure 2C).
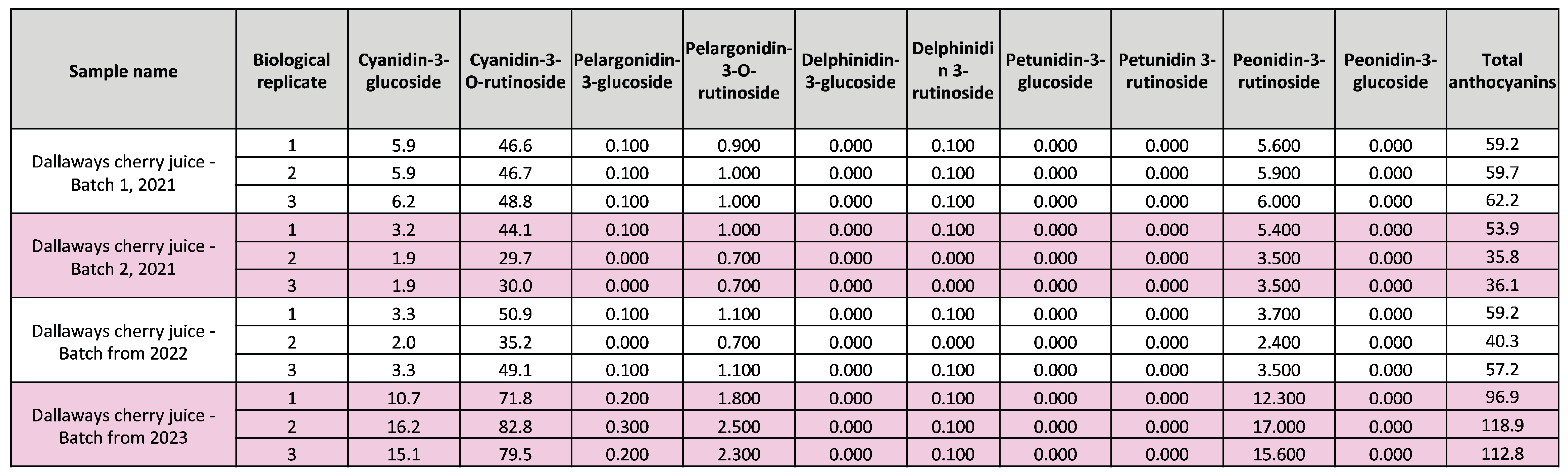
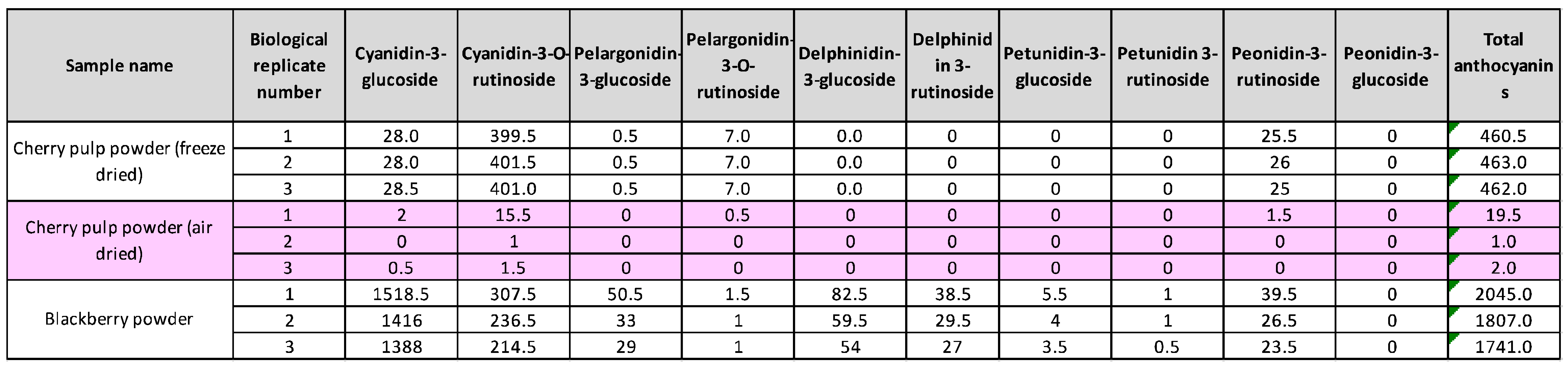
Next, we identified and quantified the anthocyanin species present in the samples. We detected a mixture of eight different anthocyanins, with Cyanidin-3-O-rutinoside being the predominant species across all cherry harvests and sample types, constituting 69-87% of total anthocyanins (
Table 1,
Table 2). In contrast, in the blackberry powder Cyanidin-3-O-glucoside was the main anthocyanin component (
Table 3). Taken together, these data show that cherry juice made from waste fruit is rich in anthocyanins and anthocyanins remain in the leftover pomace and pulp, and in powder produced from of freeze-dried pulp. Due to the negligible amounts of anthocyanins in air-dried pulp powder, we only proceeded with the freeze-dried pulp powder (
Figure 2C).
In addition to anthocyanins, cherries also contain quercetin, a flavonoid with antioxidant and antibacterial functions. To determine the presence and concentration of quercetin in Kent cherries, we analysed our samples using LC-MS against quercetin standards. As for the anthocyanins, we detected a higher quercetin content in the juice from the 2023 batch compared to the 2021 and 2022 batches (8-, 3- and 25-fold higher levels respectively;
Figure 2D). In comparison to anthocyanins, overall quercetin content was low, ranging from 0.04 to 1.18 mg/L. We also measured quercetin in the cherry waste products and found low but detectable levels in pomace and pulp (0.2 μg/g and 0.1 μg/g respectively), while the freeze-dried powder contained quercetin levels similar to the juice at 12.3 μg/g (
Figure 2E). Cherry juice was freeze dried to make a solid for direct comparison with cherry pomace, pulp and powders and this was shown to contain 45 μg/g quercetin (
Figure 2F) confirming the presence of quercetin in cherry juice and pulp powder.
Anti-Bacterial and Probiotic Properties of Cherry Juice and Cherry Pulp Powder
Anthocyanins have been shown to alter bacterial growth [
18]. Biologically, this would be beneficial to health if they inhibit the growth of pathogenic bacteria or promote the growth of probiotic bacteria. Given the levels of anthocyanins and quercetin identified in the cherry products (
Figure 2A-D), we assessed their impact on bacterial growth. First, we tested their antimicrobial properties using the Kirby-Bauer test, a microbiological assay used to determine how sensitive bacteria are to antimicrobials [
19]. We first determined how 100ug/mL of individual anthocyanins (Cyanidin-3-O-rutinoside, Cyanidin-3-O-glucoside, Pelargonidin-3-O-rutinoside, Peonidin-3-O-rutinoside) impacted growth of the Gram-negative bacteria
E. coli OP50 and K-12 and the Gram-positive bacteria
S. albus, and
B. megaterium on agar. While Kanamycin control (50ug/mL) resulted in inhibition zones ranging between 12.5 and 19.0 mm for the different bacteria, none of the anthocyanins impacted on bacterial growth. In contrast, 100ug/ml quercetin generated inhibition zones of 9.0mm for both
S. albus, and
B. megaterium, confirming the antimicrobial properties of this compound (
Figure 3A). We also quantified the ability of the cherry juice and cherry pulp powder to inhibit growth of bacteria. The DMSO stocks of cherry juice, pulp powder and blackberry extracts were also added to filter discs but found that neither of the cherry product samples nor the blackberry extract inhibited bacterial growth (
Figure 3B).
Next, we tested whether the same samples have probiotic properties by conducting bacterial growth curve assays. Seven probiotic strains from the
Lactobacillus genus (
L. brevis, L. paracasei, L. plantarum, L. helveticus, L. salivarus, L. acidophilus and
L. rhamnosus) were grown for 48 hrs with and without the cherry products, individual anthocyanins or controls, and their growth quantified using OD600 measurements. For
L. salivarus, we found that treatment with the pulp powder resulted in an 8.6% increase in maximum growth rate (
Figure 3C) compared to DMSO only control. In contrast, the pulp powder, as well as the blackberry powder, had a strong inhibitory effect on
L. acidophilus, reducing maximum growth rate by 48% and 52% respectively (
Figure 3D). Growth of
L. rhamnosus was mildly inhibited by Cyanidin-3-O-rutinoside, Cyanidin-3-O-glucoside, and cherry juice (7-16%,
Figure 3D), and by 22% with the blackberry powder (
Figure 3E). Treatment with cherry products or anthocyanins did not affect growth of
L. brevis, L. paracasei, L. plantarum or
L. helveticus (
Figure S1A-C). Quercetin at 100ug/mL inhibited
L. rhamnosus (
Figure 3C) and
L. paracasei (
Figure S1D). Except for the slight beneficial effect of the pulp powder on growth of
L. salivarius, our findings are not consistent with cherry products or individual anthocyanins having probiotic properties.
Cherry Products Do Not Have a Negative Impact on C. elegans Growth or Development
We next asked whether the cherry products impact organismal health in the whole-organism model
C. elegans. The ease of cultivation, rapid generation time, genetic tractability and extensive toolbox of
C. elegans enables high-throughput testing to determine bioactivity across a wide range of health outcomes [
20].
C. elegans is a bacterivore, and under laboratory conditions feeds on
E. coli OP50. To rule out the possibility that cherry products inhibit the growth of OP50 and thereby impact food availability or quality, we quantified OP50 growth in the absence and presence of individual anthocyanins, cherry juice or pulp powder. We found that both the cherry juice and cherry pulp powder increased bacterial growth, increasing the maximum growth rate and the final culture density. In contrast, blackberry powder reduced the growth rate of OP50 (
Figure 4A,
Figure S2A). Treatment with individual anthocyanins at 100 µg/mL did not alter maximum growth rate while quercetin resulted in a small but significant decrease in maximum growth rate (
Figure 4B-C;
Supplementary Figure S2B-C).
This demonstrates that none of the samples inhibit growth of OP50 and supplementing the
C. elegans OP50 growth plates with them will not negatively impact their nutrient source. Some plant compounds can be toxic, so we next asked if the cherry samples have detrimental effects on
C. elegans growth and development, both of which are good readouts of overall health. After hatching,
C. elegans pass through four larval stages before becoming reproductively capable adults (
Figure 4D). We measured the time it took for
C. elegans treated with cherry products, blackberry powder, individual anthocyanins (100ug/mL) or quercetin (10ug/mL and 100ug mg/mL) to pass through larval development and reach adulthood compared to controls. We found that after 72 hours ~60% of
C. elegans treated with the solvent control reached adulthood (
Figure 4E, F). However, addition of juice, pulp powder, blackberry extract or individual compounds did not impact
C. elegans development (
Figure 4E, F). Together our data shows that the cherry products do not alter
C. elegans growth, suggesting that they are non-toxic and making this model a viable option for conducting bioactivity assays.
Cherry Pulp Powder Protects Against Amyloid-β Proteotoxicity
Anthocyanins have been reported to protect against neurodegenerative diseases characterised by the progressive loss of neurons and the accumulation of misfolded proteins in the brain, improving cognition and neuroprotection [
5,
21]. Given their chemical composition, we reasoned that the cherry products tested here may also have similar effects. To test this, we utilised a humanised
C. elegans model of proteotoxicity [
22]. In this model, human amyloid-β42 is expressed in the body-wall muscle, leading to aggregation of amyloid-β42 in muscle, ultimately resulting in muscle dysfunction and paralysis of the animal and enabling quantification of proteotoxicity by inspection using a dissection microscope (
Figure 4G). We evaluated proteotoxicity in this model in normal conditions and in the presence of cherry products and blackberry extract by measuring paralysis on day 2 of adulthood in amyloid-β42 expressing animals. As previously shown, under normal conditions 95% of
C. elegans expressing the amyloid are paralysed within 2 days (
Figure 4H). However, supplementation with cherry pulp powder had a protective effect, reducing paralysis by 28% compared to controls, while blackberry extract was not protective (
Figure 4H). Purified anthocyanin supplements are popular health foods. However, we found that treating
C. elegans with individual anthocyanins had no impact on amyloid-induced pathology (
Figure 4I). We also tested supplementation with quercetin at 10ug/mL and 100ug/mL but did not observe any effects (
Figure 4J). These findings show that in the context of pathology related to human disease, whilst supplementation with blackberry extract, an individual anthocyanin or quercetin is not protective, cherry pulp powder is. This implies that more complex interactions between bioactive compounds in a food matrix is required to improve health.
4. Discussion
Insights From Analysing Waste Cherry Products
Here, we describe the anthocyanin content and bioactive properties of products made from cherry waste. Our findings demonstrate the presence of anthocyanins in juice, pulp, and pulp powder made from cherries that would otherwise be destined for landfill. As anthocyanins have established health-promoting properties, these findings strongly suggest that food products made from horticultural waste can be developed into inexpensive, health-promoting foods. Our study also demonstrates beneficial bioactivity of products made from waste and the benefits of working locally to improve grower-researcher collaborations.
We showed that our upcycled cherry pulp powder protects against Alzheimer’s-related Aβ toxicity in a
C. elegans model, implying neuroprotective effects. Clinical trials have shown that anthocyanin-rich extracts have neuroprotective effects across age groups. Dietary supplementation with extracts from blueberries improved cognitive performance in older adults [
23], in elderly adults with cognitive impairment [
24], in healthy adults [
25,
26,
27], and children [
28]. Less is known about using cherry-based supplements, but consumption of anthocyanin-rich cherry juice for 12 weeks has been shown to improve memory and cognition in older adults with mild-to-moderate dementia [
29]. Future research will determine whether cherry products, like blueberries, also have broad effects on cognitive function and have the potential to prevent or delay Alzheimer’s disease (AD) and dementia.
Several studies have shown that anthocyanins have prebiotic properties, enhancing/promoting growth of gut bacteria and bactericidal properties, inhibiting growth of pathogens. In our study we did not find any positive or negative effects on growth of Lactobacilli probiotic strains. We also did not observe antibiotic effects when conducting tests on pathogenic bacteria. Differences between our findings and other studies could be due to the use of different experimental approaches e.g., in vitro or in vivo assays, oxygen concentrations, experimental conditions e.g., solid media or liquid media, as well as the bacterial strains that were tested.
Mechanisms by Which Cherry Powder May Protect Against Aβ Toxicity
Our finding that cherry powder protects against Aβ-associated toxicity in a
C. elegans Aβ model is consistent with work in other experimental models. Several studies in mouse models show that anthocyanin supplementation has significant potential to prevent AD [
21]. For example, anthocyanin-enriched bilberry and blackcurrant extracts reduce Aβ production, Aβ deposition, Aβ-associated memory loss, and neurodegeneration in transgenic mice expressing human Aβ [
30,
31,
32]. Bilberry-derived anthocyanin mixtures inhibit formation of Aβ peptide fibrils in cell-free in vitro experiments and reduce Aβ toxicity in neuronal cells [
32]. The anthocyanin Cyanidin-3-O-glucoside, found at high levels in blackberries and blueberries, can inhibit aggregation of Aβ into oligomers and Aβ neurotoxicity in human neuronal SH-SY5Y cells [
33]. These positive effects may be due to the antioxidant or anti-inflammatory activity of anthocyanins [
5,
9,
21], but also via anthocyanins directly interfering with Aβ peptide oligomerization and preventing the formation of toxic fibrils [
32].
In our study, we found beneficial effects from supplementation with cherry pulp powder, but not from cherry juice, which has a similar anthocyanin profile to the powder. The blackberry extract, which has a high anthocyanin content but a different anthocyanin profile, did not show bioactivity either, nor did supplementation with an individual anthocyanin. The bioactive properties of the cherry powder could be a result of the precise anthocyanin composition of the product, differences in other components, or due to the food matrix of the powder. The food matrix refers to the intricate interactions between nutrients, fibre, bioactive compounds, and other components that play a crucial role in shaping how a food is digested and metabolised, and its beneficial effects upon consumption [
34]. Studies investigating the bioavailability of anthocyanins in a food matrix compared to anthocyanin-rich extracts suggest that food matrices protect anthocyanins during digestion, leading to increased stability and absorption of anthocyanins [
35]. Thus, consuming anthocyanin-rich cherry powder might impart health benefits that are not observed in extracts or supplements.
An opportunity to Use Waste to Produce Inexpensive Healthy Foods
With the global population increasing, and demographics rapidly shifting towards ageing societies with increasing numbers of people suffering from multiple age-related chronic diseases, there is a growing demand for production of foods that meet our growing health needs, while being sustainable [
36]. Moreover, consumer focus is moving away from foods that simply supply energy and basic nutrition to those that also contain bioactive compounds with health benefits e.g., disease protection. In some markets, there is also a consumer desire to recycle, reuse, and buy sustainable products. Our study demonstrates that agricultural waste streams can be sources for healthy food products, such as pulp powders. Such powders offer other advantages in addition to health benefits; they are rich in flavour, have long shelf lives, are easy to transport and store, and can be incorporated into a variety of foods. As agricultural waste is a major contributor to reduced productivity and negative environmental impact, upcycling plant waste in this way has significant potential to yield a supply of inexpensive, sustainable and healthy foods, increase productivity and build a circular bioeconomy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, ME. and JMAT.; formal analysis, MP, KH, SAB, WGS, ME; investigation, SAB, WGS, AAK, KH, MP; data curation, SAB, WGS; writing—original draft preparation, ME and JMAT; writing—review and editing, ME and JMAT; supervision, ME and JMAT; project administration, MD, ME and JMAT.; funding acquisition, MD, ME and JMAT. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovate UK, grant number 10027661. The APC was funded by The University of Kent.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw data available on request.
Acknowledgments
We acknowledge Dr David Beal (University of Kent) and Prof. Martin Warren (Quadram Institute) for their support with data analysis. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD |
Alzheimer’s disease |
| CGC |
Caenorhabditis Genetics Center |
| DMSO |
Dimethyl sulfoxide |
| DOAJ |
Directory of Open Access Journals |
| LB |
Luria–Bertani broth |
| MDPI |
Multidisciplinary Digital Publishing Institute |
| MRS |
De Man–Rogosa–Sharpe agar |
| NGM |
Nematode Growth Medium |
| QToF |
Quadrupole Time-of-Flight |
| SEM |
Standard error of the mean |
| UPLC-MS |
Ultra-Performance Liquid Chromatography–Mass Spectrometry |
| UPLC-QToF |
Ultra-Performance Liquid Chromatography coupled to Quadrupole Time-of-Flight |
References
- United Nations Environment Programme Think Eat Save Tracking Progress to Halve Global Food Waste; 2024.
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture Waste Valorisation as a Source of Antioxidant Phenolic Compounds within a Circular and Sustainable Bioeconomy. Food Funct 2020, 11, 4853–4877. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Jennings, A.; Thompson, A.S.; Tresserra-Rimbau, A.; O’Neill, J.K.; Hill, C.; Bondonno, N.P.; Kühn, T.; Cassidy, A. Flavonoid-Rich Foods, Dementia Risk, and Interactions With Genetic Risk, Hypertension, and Depression. JAMA Netw Open 2024, 7, e2434136. [Google Scholar] [CrossRef]
- Hole, K.L.; Williams, R.J. Flavonoids as an Intervention for Alzheimer’s Disease: Progress and Hurdles Towards Defining a Mechanism of Action1. Brain Plasticity 2021, 6, 167–192. [Google Scholar] [CrossRef]
- Lakshmikanthan, M.; Muthu, S.; Krishnan, K.; Altemimi, A.B.; Haider, N.N.; Govindan, L.; Selvakumari, J.; Alkanan, Zina. T.; Cacciola, F.; Francis, Y.M. A Comprehensive Review on Anthocyanin-Rich Foods: Insights into Extraction, Medicinal Potential, and Sustainable Applications. J Agric Food Res 2024, 17, 101245. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Characterisation and Bioactive Properties of Prunus Avium L.: The Widely Studied Fruits and the Unexplored Stems. Food Chem 2015, 173, 1045–1053. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Mourtzinos, I.; Goula, A.M. Sweet Cherry and Its By-Products as Sources of Valuable Phenolic Compounds. Trends Food Sci Technol 2024, 145, 104367. [Google Scholar] [CrossRef]
- ECONOMIC ANALYSIS OF POSTHARVEST LOSSES.
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- UK Met Office Weather and Climate Summaries.
- Petreska Stanoeva, Jasmina; Balshikevska, Elena; Stefova, Marina; Tusevski, Oliver; Simic, Sonja G Comparison of the Effect of Acids in Solvent Mixtures for Extraction of Phenolic Compounds From Aronia Melanocarpa. Nat Prod Commun 2020, 15, 1934578X20934675. [CrossRef]
- Kittibunchakul, S.; Temviriyanukul, P.; Chaikham, P.; Kemsawasd, V. Effects of Freeze Drying and Convective Hot-Air Drying on Predominant Bioactive Compounds, Antioxidant Potential and Safe Consumption of Maoberry Fruits. LWT 2023, 184, 114992. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial Activity of Anthocyanins and Catechins against Foodborne Pathogens Escherichia Coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am J Clin Pathol 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis Elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- McColl, G.; Roberts, B.R.; Pukala, T.L.; Kenche, V.B.; Roberts, C.M.; Link, C.D.; Ryan, T.M.; Masters, C.L.; Barnham, K.J.; Bush, A.I.; et al. Utility of an Improved Model of Amyloid-Beta (Aβ1-42) Toxicity in Caenorhabditis Elegansfor Drug Screening for Alzheimer’s Disease. Mol Neurodegener 2012, 7, 57. [Google Scholar] [CrossRef]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlueTM) in Maintenance of Episodic and Working Memory in Older Adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Boespflug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler, M.D.; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced Neural Activation with Blueberry Supplementation in Mild Cognitive Impairment. Nutr Neurosci 2018, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Skelton, M.R.; Summer, S.S.; Shidler, M.D.; Sullivan, P.G. Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Pouchieu, C.; Pourtau, L.; Gaudout, D.; Pallet, V.; Drummond, P.D. Effects of a Polyphenol-Rich Grape and Blueberry Extract (MemophenolTM) on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Study. Front Psychol 2023, 14. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Mesnage, R.; Fernandes, F.; Abhayaratne, N.; Xu, Y.; Zhang, Z.; Bell, L.; Williams, C.; Rodriguez-Mateos, A. Wild Blueberry (Poly)Phenols Can Improve Vascular Function and Cognitive Performance in Healthy Older Individuals: A Double-Blind Randomized Controlled Trial. Am J Clin Nutr 2023, 117, 1306–1319. [Google Scholar] [CrossRef]
- Barfoot, K.L.; May, G.; Lamport, D.J.; Ricketts, J.; Riddell, P.M.; Williams, C.M. The Effects of Acute Wild Blueberry Supplementation on the Cognition of 7–10-Year-Old Schoolchildren. Eur J Nutr 2019, 58, 2911–2920. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of Anthocyanin-Rich Cherry Juice for 12 Weeks Improves Memory and Cognition in Older Adults with Mild-to-Moderate Dementia. Eur J Nutr 2017, 56, 333–341. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Aβ1–42 Mouse Model of Alzheimer’s Disease. Mol Neurobiol 2017, 54, 6490–6506. [Google Scholar] [CrossRef]
- Vepsäläinen, S.; Koivisto, H.; Pekkarinen, E.; Mäkinen, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Haapasalo, A.; Karjalainen, R.O.; Tanila, H.; et al. Anthocyanin-Enriched Bilberry and Blackcurrant Extracts Modulate Amyloid Precursor Protein Processing and Alleviate Behavioral Abnormalities in the APP/PS1 Mouse Model of Alzheimer’s Disease. J Nutr Biochem 2013, 24, 360–370. [Google Scholar] [CrossRef]
- Yamakawa, M.Y.; Uchino, K.; Watanabe, Y.; Adachi, T.; Nakanishi, M.; Ichino, H.; Hongo, K.; Mizobata, T.; Kobayashi, S.; Nakashima, K.; et al. Anthocyanin Suppresses the Toxicity of Aβ Deposits through Diversion of Molecular Forms in in Vitro and in Vivo Models of Alzheimer’s Disease. Nutr Neurosci 2016, 19, 32–42. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Merlicco, A.; Bolondi, C.; Teti, G.; Falconi, M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective Effects of Cyanidin 3-O-Glucopyranoside on Amyloid Beta (25–35) Oligomer-Induced Toxicity. Neurosci Lett 2010, 473, 72–76. [Google Scholar] [CrossRef]
- Aguilera, J.M. The Food Matrix: Implications in Processing, Nutrition and Health. Crit Rev Food Sci Nutr 2019, 59, 3612–3629. [Google Scholar] [CrossRef] [PubMed]
- Kumkum, R.; Aston-Mourney, K.; McNeill, B.A.; Hernández, D.; Rivera, L.R. Bioavailability of Anthocyanins: Whole Foods versus Extracts. Nutrients 2024, 16, 1403. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. The Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Pipeline for analysing products made from cherry waste. A) Location of cherry orchards and University campus in Kent, United Kingdom. B) Fresh (waste) cherries, otherwise destined for landfill, are pressed into juice then the waste is processed using a pulping machine to produce pomace and pulp, the latter of which is dried and milled into cherry powder. C) Experimental pipeline to establish bioactive constituents and health benefits of cherry products.
Figure 1.
Pipeline for analysing products made from cherry waste. A) Location of cherry orchards and University campus in Kent, United Kingdom. B) Fresh (waste) cherries, otherwise destined for landfill, are pressed into juice then the waste is processed using a pulping machine to produce pomace and pulp, the latter of which is dried and milled into cherry powder. C) Experimental pipeline to establish bioactive constituents and health benefits of cherry products.
Figure 2.
Cherry products contain high levels of bioactive compounds. Total levels of anthocyanins in different batches of cherry juice (A) or products (B-C). Total levels of quercetin in different batches of cherry juice (D) or products (E-F). 1 representative of 3 technical replicates are shown for each sample. Error bars represent ± SEM. Statistical analysis performed using One-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons, comparing with Batch from 2023. ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Figure 2.
Cherry products contain high levels of bioactive compounds. Total levels of anthocyanins in different batches of cherry juice (A) or products (B-C). Total levels of quercetin in different batches of cherry juice (D) or products (E-F). 1 representative of 3 technical replicates are shown for each sample. Error bars represent ± SEM. Statistical analysis performed using One-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons, comparing with Batch from 2023. ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Figure 3.
Cherry anthocyanins and products do not impact growth of common pathogens and probiotics. A-B) Kirby-Bauer disk diffusion susceptibility test on different bacterial strains, including common pathogens. Growth of bacteria is inhibited by quercetin but not by anthocyanins (100 µg/mL each) (A) or by cherry products (B). Statistical analysis by multiple unpaired t-tests, in comparison to DMSO control. C-E) Maximum growth rates of probiotic bacteria. Individual anthocyanins at 50 µg /mL or cherry products themselves have minor or no effects on growth rates of L. rhamnosus or L. salivarus (C-D). Effect of anthocyanins, cherry products and quercetin on the growth rate of L. rhamnosus, L. salivarious and L. acidophilus is reduced by blackberry powder and cherry pulp powder. (E). Statistical analysis by Student’s t-test, two-tailed, homoscedastic. Error bars represent ± SEM. (A-B) 1 representative of 3 technical replicates shown. (C-D) combined results from 5-10 biological replicates shown. * p < 0.05, ** p < 0.01, **** p < 0.0001, **** p < 0.0001.
Figure 3.
Cherry anthocyanins and products do not impact growth of common pathogens and probiotics. A-B) Kirby-Bauer disk diffusion susceptibility test on different bacterial strains, including common pathogens. Growth of bacteria is inhibited by quercetin but not by anthocyanins (100 µg/mL each) (A) or by cherry products (B). Statistical analysis by multiple unpaired t-tests, in comparison to DMSO control. C-E) Maximum growth rates of probiotic bacteria. Individual anthocyanins at 50 µg /mL or cherry products themselves have minor or no effects on growth rates of L. rhamnosus or L. salivarus (C-D). Effect of anthocyanins, cherry products and quercetin on the growth rate of L. rhamnosus, L. salivarious and L. acidophilus is reduced by blackberry powder and cherry pulp powder. (E). Statistical analysis by Student’s t-test, two-tailed, homoscedastic. Error bars represent ± SEM. (A-B) 1 representative of 3 technical replicates shown. (C-D) combined results from 5-10 biological replicates shown. * p < 0.05, ** p < 0.01, **** p < 0.0001, **** p < 0.0001.
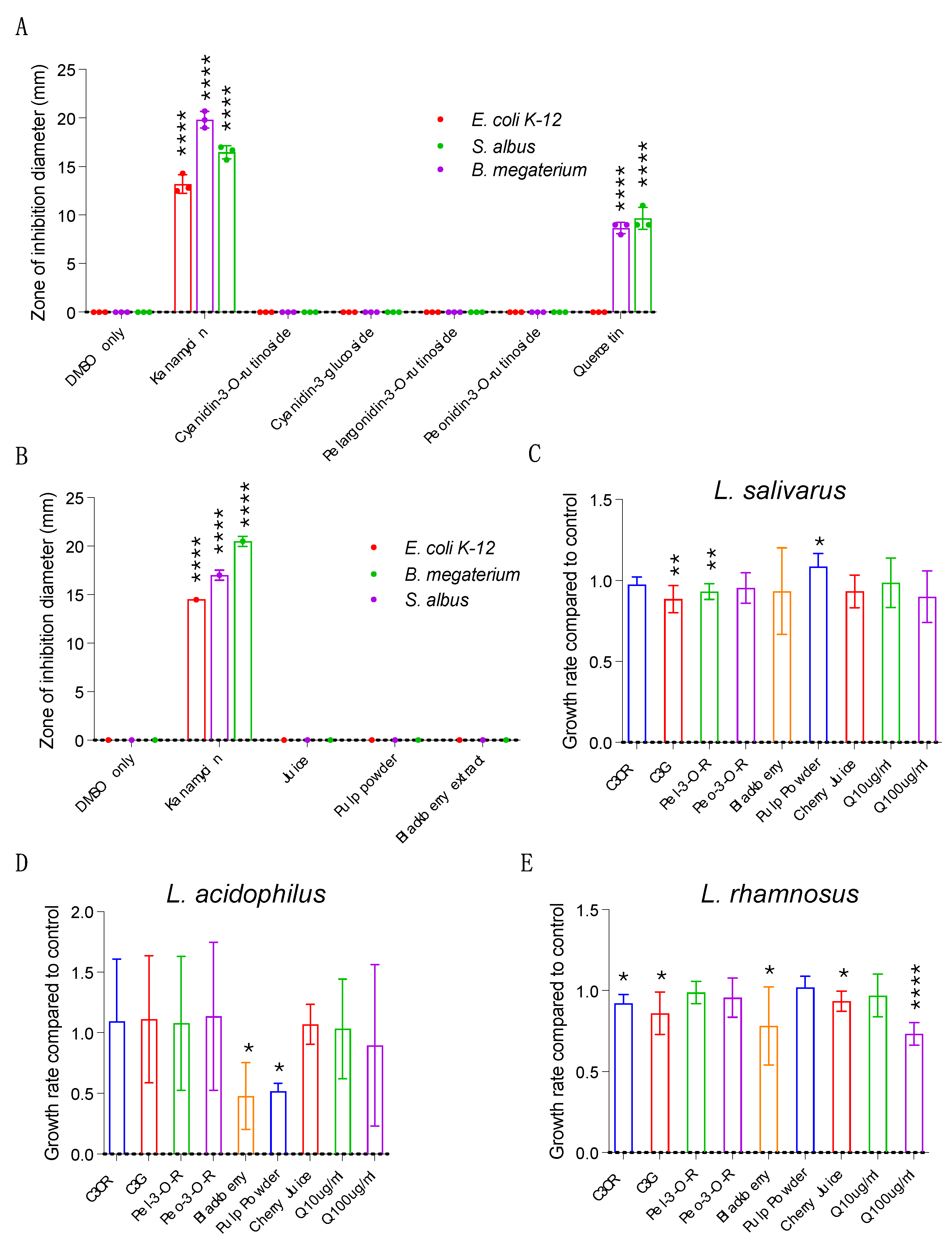
Figure 4.
Cherry pulp powder improves the health in a C. elegans proteotoxicity model of Alzheimer’s Disease. A-C) Growth of the C. elegans laboratory food source E. coli OP50. OP50 growth is not altered by cherry products (A), individual anthocyanins (B) or quercetin (C). D) Schematic of C. elegans development at 20°C made in Biorender, free version. A-C) n=9 for each sample. E-F) C. elegans developmental rate. Development is not impacted by cherry products (E), individual anthocyanins or quercetin (F) compared to vehicle controls. The solvent DMSO negatively affects development compared to non-DMSO controls. Statistical analysis by Fishers exact test. G) C. elegans model Alzheimer’s disease pathology. Human Aβ peptide is expressed in the C. elegans body wall muscle, leading to paralysis. H-J) Scoring of paralysis in animals supplemented with cherry products, individual anthocyanins or quercetin. Cherry pulp powder reduces paralysis (H). Paralysis is not affected by other products, by anthocyanins (I) or quercetin (J). (H-J) Statistical analysis by two-way ANOVA. Combined data from 4 (H and J) or 3 (I) biological replicates, each containing 100-120 animals. * p < 0.05, **, p < 0.01; ***, p < 0.001.
Figure 4.
Cherry pulp powder improves the health in a C. elegans proteotoxicity model of Alzheimer’s Disease. A-C) Growth of the C. elegans laboratory food source E. coli OP50. OP50 growth is not altered by cherry products (A), individual anthocyanins (B) or quercetin (C). D) Schematic of C. elegans development at 20°C made in Biorender, free version. A-C) n=9 for each sample. E-F) C. elegans developmental rate. Development is not impacted by cherry products (E), individual anthocyanins or quercetin (F) compared to vehicle controls. The solvent DMSO negatively affects development compared to non-DMSO controls. Statistical analysis by Fishers exact test. G) C. elegans model Alzheimer’s disease pathology. Human Aβ peptide is expressed in the C. elegans body wall muscle, leading to paralysis. H-J) Scoring of paralysis in animals supplemented with cherry products, individual anthocyanins or quercetin. Cherry pulp powder reduces paralysis (H). Paralysis is not affected by other products, by anthocyanins (I) or quercetin (J). (H-J) Statistical analysis by two-way ANOVA. Combined data from 4 (H and J) or 3 (I) biological replicates, each containing 100-120 animals. * p < 0.05, **, p < 0.01; ***, p < 0.001.
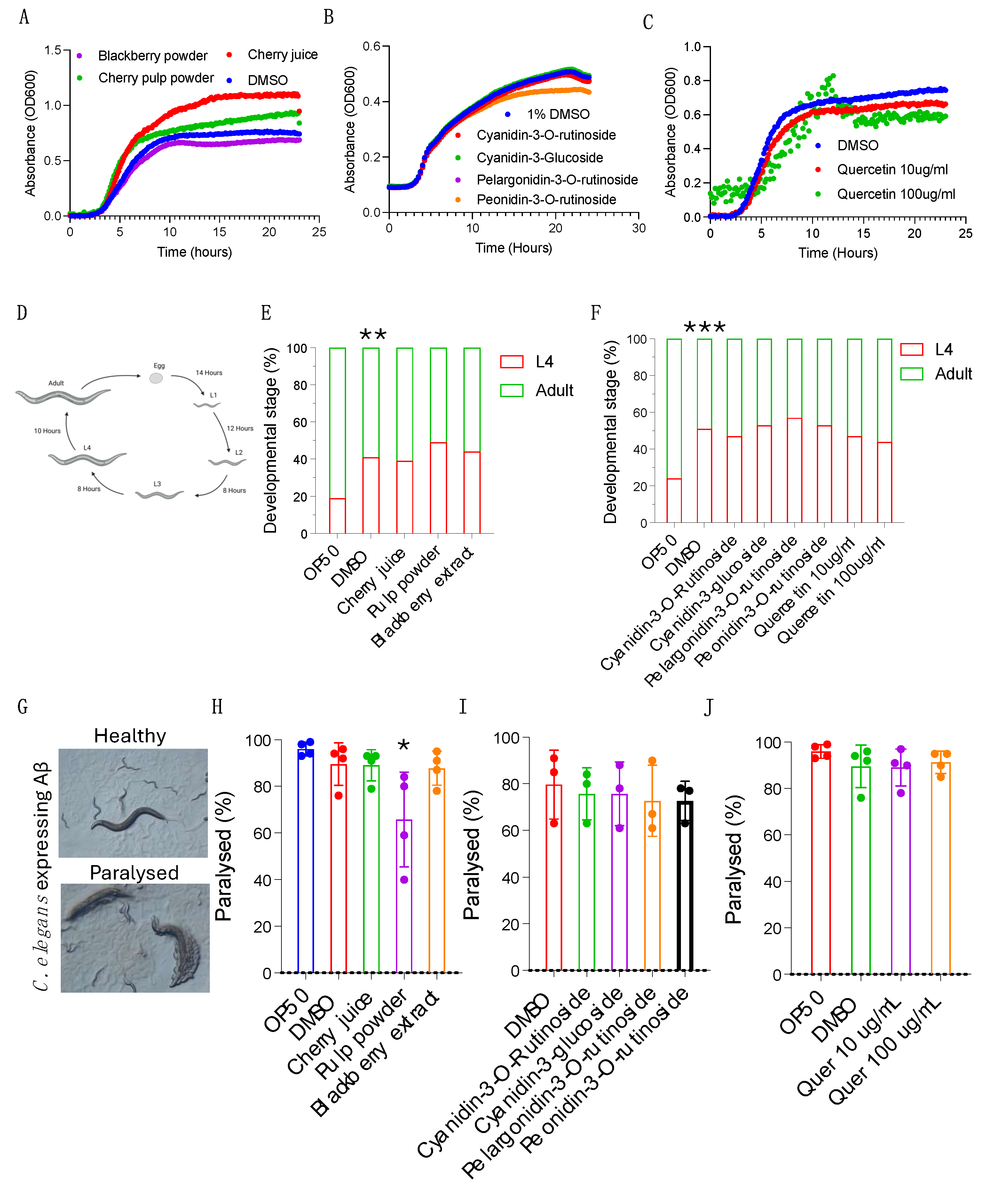
Table 1.
Concentration of anthocyanins (mg/L) in different batches of cherry juice.
Table 1.
Concentration of anthocyanins (mg/L) in different batches of cherry juice.
Table 2.
Concentration of anthocyanins (μg/g) in different cherry sample types.
Table 2.
Concentration of anthocyanins (μg/g) in different cherry sample types.
Table 3.
Concentration of anthocyanins (μg/g) in different powders.
Table 3.
Concentration of anthocyanins (μg/g) in different powders.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).