1. Introduction
Vitamin D deficiency poses a serious public health issue, especially in areas with limited sunlight. According to studies, a substantial portion of the population has below-optimal vitamin D levels, particularly in the winter and spring [
1]. Due to reduced solar exposure, changed levels of albumin and vitamin D-binding protein (VDBP), and malabsorption, patients with gastrointestinal diseases are particularly susceptible to vitamin D insufficiency and deficiency [
2,
3].
Vitamin D is a fat-soluble secosteroid that helps maintain healthy bones by controlling the metabolism of calcium and phosphate. When exposed to ultraviolet B (UVB), it is produced in the skin; alternatively, it can be acquired through diet. The primary circulating form of vitamin D, 25-hydroxyvitamin D (25(OH)D
3), is formed in the liver through hydroxylation of vitamin D. Further hydroxylation in the kidneys creates the biologically active form, 1,25-dihydroxyvitamin D (1,25(OH)
2D
3), which oversees calcium absorption, immune responses, and cell differentiation. Vitamin D deficiency has been associated with a range of diseases, including osteoporosis, cardiovascular issues, and specific cancers, as well as autoimmune and inflammatory disorders. Lowered quality of life, higher morbidity and mortality, sarcopenia, infections are also linked to this deficit [
3,
4,
5,
6,
7].
Acute pancreatitis (AP) is a severe inflammatory condition affecting the pancreas that can result in systemic complications and organ failure. Vitamin D might play a complex but crucial role in AP. Vitamin D may influence the severity of AP with the impact on the inflammation, immunological responses, and calcium metabolism. According to research by altering the production of pro-inflammatory cytokines vitamin D regulates both innate and adaptive immune responses [
8]. Vitamin D has an anti-inflammatory impact on macrophages with increasing interleukin (IL)-10 and decreasing inflammatory stimuli (i.e., IL-1β, IL-6, tumor necrosis factor-α (TNF-α), receptor activator of nuclear factor kappa-Β ligand, and cyclo-oxygenase-2 (COX-2)). 1,25-(OH)
2D
3 also has an anti-oxidative effect on monocytes by upregulation of glutathione reductase (GR) which results in the reduced formation of oxygen radicals [
9]. With this mechanism vitamin D might mitigate the cytokine storm in AP by controlling the expression of inflammatory cytokines that are known to be elevated in AP [
10]. Studies showed the correlation of vitamin D deficiency with the exacerbation of the inflammatory response in individuals with AP, resulting in poorer clinical outcomes [
11,
12,
13,
14].
Malheiro et al. showed correlation of cytokine levels in AP and severity of AP [
15]. AP patients showed increased levels of interleukin (IL)-6, IL-8, IL-10, vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF)-alpha, and monocyte chemoattractant protein (MCP)-1 at admission when compared with healthy controls. Pancreatic necrosis and systemic effects of AP in vitamin D-deficient circumstances may be caused by excessive inflammation and immunological dysregulation [
11,
14,
16,
17]. Additionally, vitamin D supports gut barrier integrity. A deficiency in intestinal permeability can lead to bacterial translocation, which can exacerbate the severity of AP [
18]. Moreover, vitamin D is necessary for calcium homeostasis, and dysregulated calcium signalling may increase the activation of pancreatic enzymes, so intensifying pancreatic inflammation [
19,
20].
Recent studies emphasize how crucial it is to assess vitamin D's free and bioavailable forms in addition to its overall amount. The free hormone theory states that only the unbound form of vitamin D may enter cells and have an impact on biology. Measuring free and bioavailable vitamin D offers a more accurate assessment of functional vitamin D status in conditions like AP, where VDBP and albumin concentrations may be changed [
6,
13,
14]. A study by Gallerani et al. showed the existence of circannual variation in the onset of AP, with a significantly higher frequency of events in the spring and with higher mortality from December-February [
21]. Studies have also shown that levels of both bioavailable vitamin D and free vitamin D vary seasonally, with higher levels generally observed in the summer months and lower levels observed in the winter months [
22,
23].
By assessing serum levels of total, free, and bioavailable vitamin D, our study seeks to determine the association between vitamin D status and the severity of acute pancreatitis. In order to ascertain if vitamin D levels can function as independent predictors of the course of AP, we additionally investigate their correlation with clinical outcomes, such as length of hospital stay, ICU admission, and disease severity.
2. Results
The analysis comprised eighty AP patients in all. The basic characteristics of these patients are presented in
Table 1. The sex distribution was about equal (56.3% male), and the mean age was 58.8 ± 16.1 years. The majority of patients were alcohol consumers, however, only 19 (23.8%) cases had alcoholic AP. Biliary type was present in 38 (47.5%) patients. Overall, 30 patients (37,5%) were smokers.
Vitamin D deficiency was very common: 31.3% had a deficiency and 27.5% had a severe deficiency. Vitamin D levels were optimal in just 8.8% of individuals.
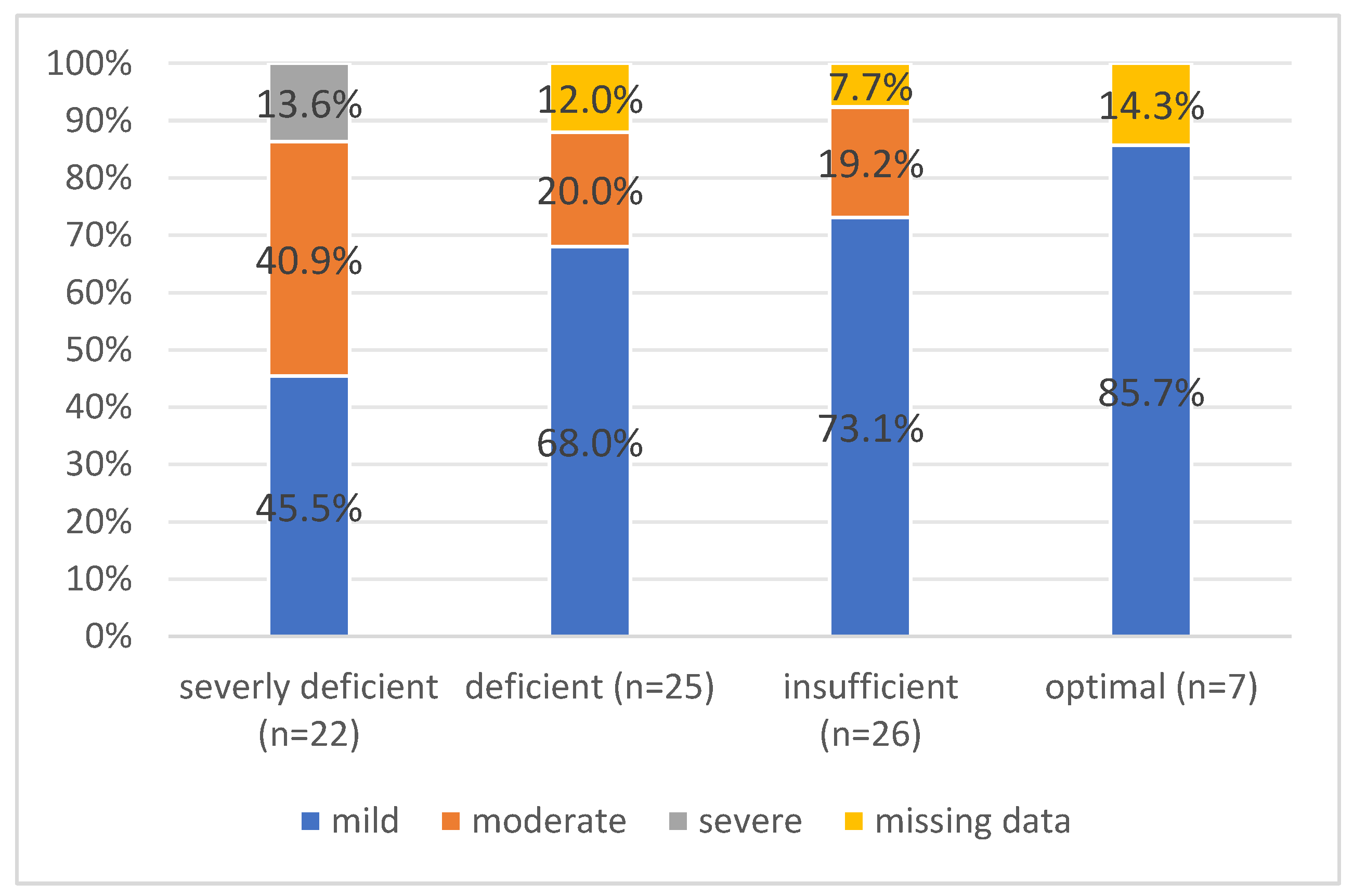
Patients were stratified into groups according to serum 25(OH)D
3 levels; severely deficient, deficient, insufficient and optimal (
Table 2). Free and bioavailable vitamin D levels were significantly higher (p<0.001) in those with optimal 25(OH)D
3 levels. Age (p=0.028), ICU admission (p=0.007), length of hospital stays (p=0.018), Ranson score at admission (p=0.045), and AP severity (p=0.029) all showed significant group differences.
(OH)D
3 levels were found in negative correlation to the severity of AP according to the resized Atlanta classification (
Figure 1).
Severe AP was present only in patients with severe deficiency of 25(OH)D3. This group also had a higher proportion of cases with moderate severity AP. Moreover, 7 patients were admitted to ICU, and out of these, 6 had severe deficiency of 25(OH)D3.
Table 2 displays the findings of the scoring system based on the degree of AP associated with vitamin D levels. 52 (65.0%) had mild AP, 19 (23.8%) had moderate AP, and 3 (3.8%) had severe AP, based on the revised Atlanta classification (
Table 2).
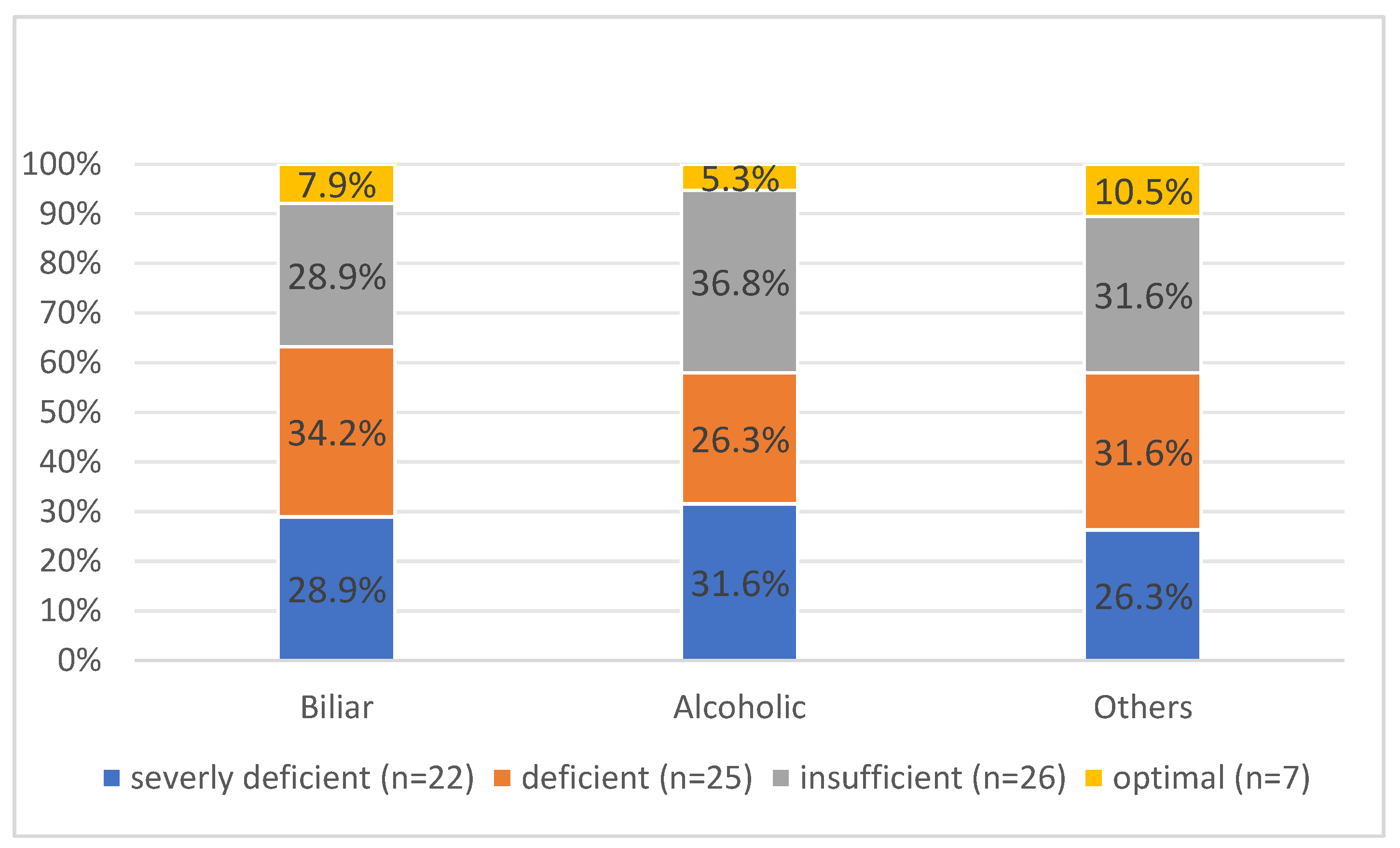
According to computer tomography, 53 (66.3%) patients with interstitial and 22 (27.5%) patients with necrotizing AP. The later has been shown as more predominant in cases with severely deficient levels of vitamin D (p=0.013).19 (23.8%) of the patients had an alcoholic aetiology, while 61 (76.2%) had another aetiology (
Figure 2).
AP did not exhibit a statistically significant difference between the groups based on stratified etiologic variables (p=0.988). In order to predict moderate-to-severe AP, an independent logistic regression was conducted. The results indicated the relationships between 25(OH)D
3, free 25(OH)D
3, bioavailable 25(OH)D
3, necrotizing AP, alcoholic type of AP, and CTSI score (
Table 3). Moderate-to-severe AP was linked to lower levels of all three vitamin D variants. Conversely, more severe AP is associated with higher CT score values.
To independently predict ICU admission, a comparable study has been carried out (
Table 4). There were statistically significant associations between ICU admission and lower levels of 25(OH)D
3, free 25(OH)D
3, and bioavailable 25(OH)D
3. Higher CTSI scores have shown the similar trend. Admission to the intensive care unit was not linked to the type or cause of AP.
The impact of independent variables on Ranson score and hospital stay duration was also examined using linear regression analysis.
Table 5 and
Table 6 present the findings. Patients who were older (p<0.001) and had lower albumin levels (p=0.018) were predicted to have higher Ranson scores.
There was no correlation between vitamin D levels and the Ranson score at admission. However, there was a negative correlation between the length of hospital stay and the levels of 25(OH)D
3, free 25(OH)D
3 and bioavailable 25(OH)D
3 (
Table 6).
The patients in the most significantly vitamin D inadequate group spent the longest time in the hospital (14.6±15.2 days) (
Table 2). Longer hospital stays were also associated with lower albumin levels. Longer hospital stays were also associated with necrotizing form of AP and higher CTSI scores.
3. Discussion
In our study, the relationship of total, free, and bioavailable 25(OH)D3 with the severity of acute pancreatitis was evaluated. According to our findings, there is a direct correlation between patients who had lower levels of all three types of vitamin D, especially those who had a severe deficiency and more severe disease courses, such as necrotizing pancreatitis, longer hospital stays, and higher ICU admission rates.
Acute pancreatitis is still challenging, and potentially lethal disorder. At first it originates locally in the pancreas and soon becomes systemic disease, the systemic inflammatory response and risk of organ failure depend on many factors. Previous studies showed low vitamin D levels in acute pancreatitis [
24,
25]. Two studies also showed the relationship of 25(OH)D3 with severity of AP [
26,
27]. Our research shows a significant correlation between more severe outcomes in acute pancreatitis and vitamin D deficiency, particularly in its severe form. Our study expands on earlier findings associating vitamin D insufficiency to poor outcomes in inflammatory disorders and is, to our knowledge, the first to objectively demonstrate the predictive usefulness of free and bioavailable 25(OH)D3 in AP severity. These results lend credence to the idea that vitamin D may influence the immunological and inflammatory reactions that contribute to the development of AP.
Comparing Healthy Individuals
According to the research vitamin D levels were much lower in AP patients than in the general healthy population [
14,
28]. Studies in healthy populations and recognized criteria indicate that blood concentrations of serum 25(OH)D3 should be between 75 and 125 nmol/L to be deemed optimal. Levels below 50 nmol/L indicate insufficiency, while concentrations below 30 nmol/L suggest severe deficiency [
14,
29]. Concentrations between 50 and 75 nmol/L are frequently categorized as insufficient. The average 25(OH)D3 concentration in our group of AP patients was 45.7±25.3 nmol/L, which is significantly below the sufficiency level. As a result, most patients fall into the category of deficient or severely deficient.
In contrast to large-scale population-based studies, where severe deficiency is typically assumed to impact fewer than 10% of individuals, our AP patients showed a prevalence of severe deficiency (<30 nmol/L) of 27.5%, which is approximately three times higher [
1]. Numerous disease-related factors, such as decreased levels of vitamin D-binding protein and albumin, altered vitamin D metabolism brought on by inflammation, and poor intestinal absorption, could be responsible for this disparity. These results confirm the idea that acute inflammatory conditions may worsen underlying insufficiency or quickly exhaust accessible vitamin D pools, and they highlight the increased susceptibility of AP patients to functional vitamin D deficiency.
Additionally, healthy individuals typically have higher levels of free and bioavailable vitamin D due to stable VDBP and albumin concentrations. However, AP patients often experience metabolic and inflammatory changes that may impact vitamin D absorption [
11]. Because the levels of free and bioavailable vitamin D in our sample are significantly lower, it is possible that traditional measures of total vitamin D may underestimate functional insufficiency in AP. These findings support the hypothesis that, rather than being the sole outcome of population-wide trends, vitamin D deficiency in AP patients may be exacerbated by disease-specific factors such as malabsorption, systemic inflammation, and altered protein synthesis [
8,
14].
Low levels of vitamin D have been shown to affect oxidative stress responses and autophagy, two processes that are essential for regulating inflammation in AP [
30,
31].
Numerous inflammatory conditions, such as sepsis, rheumatoid arthritis, and inflammatory bowel disease (IBD), have been linked to vitamin D deficiency [
16,
32,
33,
34,
35,
36,
37]. Vitamin D-deficient patients with these illnesses have higher disease severity, more inflammation, and more extended hospital stays, just like those with AP. Similar to studies in AP, vitamin D deficiency is linked to increased intestinal permeability and bacterial translocation in IBD. Low vitamin D levels in sepsis are associated with immunological dysfunction and unfavourable outcomes, which supports vitamin D's function as a vital regulator of inflammatory processes [
37]. These similarities imply that vitamin D might modulate inflammatory illnesses in ways other than AP, which calls for more research.
Previous studies showed the correlation of serum vitamin D level and severity of AP and suggested, that serum 25(OH)D3 level at the admission could be a useful marker for predicting the severity of AP. As the bioavailibility of vitamin D in different conditions could vary, our study showed, that the serum level of vitamin D in patients with AP correlates with free 25(OH)D3, and bioavailable 25(OH)D3. This finding suggest that the detection of 25(OH)D3 at the admission for predicting the severity of AP could be enough, without the additional detecting of free 25(OH)D3, and bioavailable 25(OH)D3.
Our research has several advantages. First of all, it is among the few studies that assesses both free and bioavailable vitamin D in addition to total serum vitamin D levels, which could provide a more accurate evaluation of vitamin D status in AP patients. Second, our findings are more reliable since we used a well-defined patient group with standardized diagnostic criteria, such as the Atlanta categorization. Third, there is substantial statistical support for the predictive utility of vitamin D status in AP severity thanks to the use of logistic regression analysis. However, our study has limitations as well. Our findings may be less generalizable due to the small sample size (N=80). Furthermore, as this is an observational study, we cannot prove a link between the severity of AP and vitamin D deficiency. The absence of information on seasonal fluctuations in vitamin D levels, which may impact the prevalence of deficiency, is another drawback. Finally, even though our research points to a link between vitamin D level and AP outcomes, further research is required to ascertain whether vitamin D treatment can enhance clinical results for AP patients. Despite these drawbacks, our results highlight the need for more studies in this field and add to the increasing body of data connecting vitamin D to inflammatory disorders.
4. Materials and Methods
4.1. Patients
We included adult patients older than 18 who were hospitalized for a first episode of AP. Patients with previous episodes of AP were excluded. The diagnosis of AP was confirmed if two of the three criteria were present: Upper abdominal pain typical for AP, serum amylase and/or lipase elevated by at least 3x above the upper limit of normal, and radiological signs of AP. Patients were hospitalized in the gastroenterology department of internal medicine. We obtained a detailed patient history, determined etiological factors, and recorded co-pathology and regular therapy. Body mass index (BMI) was calculated as the weight in kilograms divided by square by square of height in meters. All patients received parenteral hydration from admission onwards. And received a fluid bolus when there were signs of hypovolemia. All patients enrolled in the study were started on 1.5 ml/kg/h of 0,9% saline solution with a prior bolus of 10 ml/kg if the patient was hypovolemic (moderate fluid resuscitation). Patients were monitored for fluid overload status, urine output, blood urea nitrogen levels, and blood pressure, and fluid resuscitation was adjusted accordingly. The fluid resuscitation approach was standard for all patients.
Peripheral blood was collected on admission for laboratory analysis: hemogram, electrolytes, calcium, albumin, urea, creatinine, glucose, lactate dehydrogenase, hepatogram, CRP, and vitamin D level. Vitamin D level was measured within 24 hours of admission as serum 25-hydroxyvitamin D3 (25(OH)D3). Vitamin D deficiency was defined as a vitamin D level of 30 to 50 nmol/L, severe deficiency as below 30 nmol/L, and insufficient as 50 to 75. Normal values were defined as a level above 75 nmol/L.
The severity of AP was assessed according to the revised Atlanta 2012 criteria and classified as mild, moderate, or severe. Mild AP is defined by the absence of organ failure (OF) and local or systemic complications. Moderate AP is described as transient OF lasting less than 48 hours and accompanied by local or systemic complications. Severe AP is defined as one or more organ failures with a duration of more than 48 hours of OF. Endoscopic retrograde cholangiopancreatography was performed when biliary pancreatitis with signs of biliary obstruction was diagnosed. Due to the need for scoring (Ranson score, CTSI, Revised Atlanta classification) and monitoring of the clinical picture, peripheral blood was collected several times.
All patients underwent abdominal ultrasound, and some patients (especially those with suspected severe or necrotizing pancreatitis) underwent contrast-enhanced CT of the abdominal organs.
The Ethics Committee approved the study protocol for Human Research of the Medical Ethics Commission of the Republic of Slovenia. All patients gave written informed consent for the study before inclusion.
4.2. Blood Analyses
Blood was taken into a biochemical blood tube (4 ml). Albumin and total 25(OH)D3 were analysed from fresh serum after blood collection, while the aliquot for vitamin D binding protein (DBP) was stored at minus 80 degrees Celsius until analysis. All samples were analysed simultaneously. Measurements were performed at the Clinical Institute of Clinical Chemistry and Biochemistry (University Medical Centre, Ljubljana).
25(OH)D
3, S-albumin and S-DBP in serum, were measured in all participants using the following methods: The concentration of 25(OH)D
3 vitamin was measured using competitive luminescent immunoassay with intra-laboratory CV <6% and the limit of quantification 6 nmol/L (Architect analyser, Abbott Diagnostics, Lake Forest, USA), ADVIA® 1650 Chemistry Albumin BCP Assay (Siemens, New York, USA), Human Vitamin D Binding Protein was measured with ELISA (MyBioSource, Inc., San Diego, CA, USA), the limit of quantification was 31 mg/L. Free and bioavailable 25(OH)D
3 was calculated using an online calculator and based on a modified Vermeulen [
38].
4.3. Statistical Analysis
Statistical analyses were performed using SPSS 22.0 software (SPSS Inc, Chicago, IL, USA). Continuous variables were presented as mean values with standard deviation, and categorical variables as frequencies and percentages. Normality of distribution was confirmed using the Shapiro-Wilk test. Severity of AP, namely the prediction of moderate-to-severe AP according to Atlanta classification was considered as dependent variable. The remaining variables were categorized as independent. The main investigated independent variables in the statistical analysis were levels of total 25(OH)D
3, free 25(OH)D
3 and bioavailable 25(OH)D
3, if they can predict the severity of AP. The Endocrine Society cut-off values were used to assess total serum 25(OH)D
3 levels target concentration for the optimal vitamin D effect: 75–125 nmol/L, insufficiency: 50–75 nmol/L and deficiency: <50 nmol/L [
14].
The main differences of the dependent variables were analysed using an One-way ANOVA test for those variables that were normally distributed. In the case of non-parametric non-normally distributed variables, we used the Kruskal-Wallis test for comparisons. Pearson’s chi-squared test was used to compare categorical variables. The correlations between dependant and independent variables were measured by Spearman’s correlation test. Logistic and multivariate logistic regressions with odds ratios were used to predict moderate-to-severe AP. All tests were considered as statistically significant with p<0.05.
5. Conclusions
Acute pancreatitis (AP) patients are very likely to have deficiency or severe deficiency of vitamin D. According to the revised Atlanta classification, higher severity, longer hospital stays, and higher ICU admission rates are all indicative of more severe disease, which is substantially correlated with lower concentrations of total 25(OH)D3 and its free and bioavailable forms. Specifically, moderate-to-severe AP and necrotizing pancreatitis are highly associated with severe vitamin D deficiency and are both connected to worse clinical outcomes.
Low levels of each of the three types of vitamin D are independent predictors of disease severity, as confirmed by multivariate logistic regression analysis. In order to further stratify risk in AP patients, these results demonstrate the therapeutic significance of measuring not only total vitamin D but also free and bioavailable vitamin D. Mechanistically, immunological dysregulation, increased intestinal permeability, and disruptions in calcium homeostasis are among ways that vitamin D deficiency may advance the course of disease.
Similar immunopathological pathways are seen when compared to other inflammatory illnesses, such as sepsis and inflammatory bowel disease, which supports the findings' biological plausibility. All of these data points to the necessity of additional interventional research to ascertain whether vitamin D administration could enhance the prognosis of patients suffering from acute pancreatitis.
Author Contributions
Conceptualization, D.S., and J.O.; Methodology, D.S., M.R., A.V., L.V., B.Š., D.Š., D.D., A.J., H.M. and J.O.; Software, D.S., D.Š., and D.D.; Validation, J.O.; Formal Analysis, D.S., D.Š., and J.O.; Investigation, D.S., M.R., A.V., L.V., B.Š., and D.D.; Data Curation, D.S., M.R., A.V., L.V., B.Š., D.Š., D.D., A.J., H.M. and J.O.; Writing – Original Draft Preparation, D.S.; M.R., D.Š., and J.O.; Writing – Review & Editing, B.Š., D.Š., D.D., and J.O.; Visualization, J.O.; Supervision, B.Š. and J.O.; Project Administration, D.S., and J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethics Committee approved the study protocol for Human Research of the Medical Ethics Commission of the Republic of Slovenia (Protocol ID: 0120-60/2021/5).
Informed Consent Statement
All patients gave written informed consent for the study before inclusion.
Acknowledgments
Funding was not provided for the project or its implementation.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Hribar, M.; Hristov, H.; Gregorič, M.; Blaznik, U.; Zaletel, K.; Oblak, A.; Osredkar, J.; Kušar, A.; Žmitek, K.; Rogelj, I.; Pravst, I. Nutrihealth Study: Seasonal Variation in Vitamin D Status Among the Slovenian Adult and Elderly Population. Nutrients 2020, 12, 1838. [Google Scholar] [CrossRef]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr J 2010, 9, 65. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. Vitamin D and Vitamin D-Binding Protein in Health and Disease. Int J Mol Sci 2023, 24, 4642. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Holleczek, B.; Schöttker, B. Vitamin D Insufficiency and Deficiency and Mortality from Respiratory Diseases in a Cohort of Older Adults: Potential for Limiting the Death Toll during and beyond the COVID-19 Pandemic? Int J Environ Res Public Health 2023, 20, 4642. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front Endocrinol 2020, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front Endocrinol (Lausanne) 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Kuznia, S.; Boakye, D.; Schöttker, B.; Brenner, H. Vitamin D-Binding Protein, Bioavailable, and Free 25(OH)D, and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2834-8. [Google Scholar] [CrossRef]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front Physiol 2014, 5, 244. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Garg, S.; Makhija, N. A Study on Effect of Vitamin D Supplementation in Vitamin D Deficient Females With Polycystic Ovarian Syndrome. Int J Reprod Contracept Obstet Gynecol 2022, 11, 2398–2405. [Google Scholar] [CrossRef]
- Kolosovych, I.; Hanol, I.; Bystrytska, M.; Uzun, H. Changes in Vitamin D and Calcium-Phosphorus Metabolism in Patients with Severe Acute Pancreatitis. Turk J Surg 2022, 38, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ocal, S.; Cerci, K.; Buldukoglu, O.C.; Atar, G.E.; Harmandar, F.A.; Cekin, A.H. Effect of Serum Vitamin D Levels on the Severity of Acute Pancreatitis: A Prospective Study. Pancreatology 2024, 24, 206–210. [Google Scholar] [CrossRef]
- Huh, J.H.; Kim, J.W.; Lee, K.J. Vitamin D Deficiency Predicts Severe Acute Pancreatitis. United Eur Gastroenterol J 2019, 7, 90–95. [Google Scholar] [CrossRef]
- Vieth, R.; Holick, M.F. Chapter 57B - The IOM—Endocrine Society Controversy on Recommended Vitamin D Targets in Support of the Endocrine Society Position. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1091–1107. [Google Scholar]
- Malheiro, A.P.G.; Gianfrancesco, L.; Nogueira, R.J.N.; Grotta, M.B.; Morcillo, A.M.; Ribeiro, J.D.; Toro, A.A.D.C. Association between serum Vitamin D levels and asthma severity and control in children and adolescents. Lung 2023, 201, 181–187. [Google Scholar] [CrossRef]
- Cai, F.; Hu, C.; Chen, C.J.; Han, Y.P.; Lin, Z.Q.; Deng, L.H.; et al. Vitamin D and Pancreatitis: A Narrative Review of Current Evidence. Nutrients 2022, 14, 2113. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; et al. Immunomodulatory Actions of Vitamin D in Various Immune-Related Disorders: A Comprehensive Review. Front Immunol 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; He, C.; Zhu, Y.; Lu, N.H. Role of Gut Microbiota on Intestinal Barrier Function in Acute Pancreatitis. World J Gastroenterol 2020, 26, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, R.; Zhang, J.; Li, Z.F. Calcium Signaling of Pancreatic Acinar Cells in the Pathogenesis of Pancreatitis. World J Gastroenterol 2014, 20, 16146–16152. [Google Scholar] [CrossRef]
- Gerasimenko, J.V.; Gerasimenko, O.V.; Petersen, O.H. The Role of Ca2+ in the Pathophysiology of Pancreatitis. J Physiol 2014, 592, 269–280. [Google Scholar] [CrossRef]
- Gallerani, M.; Boari, B.; Salmi, R.; Manfredini, R. Seasonal variation in the onset of acute pancreatitis. World J Gastroenterol 2004, 10, 3328–3331. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am J Clin Nutr 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Hirche, F.; Stangl, G.I.; Hinz, K.; Westphal, S.; Dierkes, J. Bioavailability of Vitamin D₂ and D₃ in healthy volunteers: a randomized placebo-controlled trial. J Clin Endocrinol Metab 2013, 98, 4339–4345. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, Assessment, and Management of Vitamin D Inadequacy: Suggestions, Recommendations, and Warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R.; DiBaise, J.K. Vitamin D deficiency in patients with pancreatitis: Is vitamin D replacement required? J Nutr Disord Ther 2015, 5, 3. [Google Scholar]
- Huh, J.H.; Kim, J.W.; Lee, K.J. Vitamin D deficiency predicts severe acute pancreatitis. United Eur Gastroenterol J 2019, 7, 90–95. [Google Scholar] [CrossRef]
- Ocal, S.; Cerci, K.; Buldukoglu, O.C.; Atar, G.E.; Harmandar, F.A.; Cekin, A.H. Effect of serum vitamin D levels on the severity of acute pancreatitis: A prospective study. Pancreatology 2024, 24, 206–210. [Google Scholar] [CrossRef]
- Margulies, S.L.; Kurian, D.; Elliott, M.S.; Han, Z. Vitamin D deficiency in patients with intestinal malabsorption syndromes—think in and outside the gut. J Dig Dis 2015, 16, 617–633. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Suh, H.W.; Kim, J.K.; Kim, T.S.; Jo, E.K. New insights into vitamin D and autophagy in inflammatory bowel diseases. Curr Med Chem 2017, 24, 898–910. [Google Scholar] [CrossRef]
- Bhutia, S.K. Vitamin D in autophagy signaling for health and diseases: Insights on potential mechanisms and future perspectives. J Nutr Biochem 2022, 99, 108841. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J Inflamm Res 2014, 7, 69–87. [Google Scholar] [PubMed]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between inflammatory bowel disease and vitamin D deficiency: A systematic review and meta-analysis. Inflamm Bowel Dis 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Milestone, A.N.; Walters, J.R.; Hart, A.L.; Ghosh, S. Vitamin D and gastrointestinal diseases: inflammatory bowel disease and colorectal cancer. Therap Adv Gastroenterol 2011, 4, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The role of vitamin D in inflammatory bowel disease: Mechanism to management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef]
- Upala, S.; Sanguankeo, A.; Permpalung, N. Significant association between vitamin D deficiency and sepsis: A systematic review and meta-analysis. BMC Anesthesiol 2015, 15, 84. [Google Scholar] [CrossRef]
- Kempker, J.A.; Han, J.E.; Tangpricha, V.; Ziegler, T.R.; Martin, G.S. Vitamin D and sepsis: An emerging relationship. Dermatoendocrinol 2012, 4, 101–108. [Google Scholar] [CrossRef]
- Vičič, V.; Kukec, A.; Kugler, S.; Geršak, K.; Osredkar, J.; Pandel Mikuš, R. Correction: Vičič et al. Assessment of Vitamin D status in Slovenian premenopausal and postmenopausal women, using total, free, and bioavailable 25-hydroxyvitamin D (25(OH)D). Nutrients 2023, 15, 2103. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).