Submitted:
22 May 2025
Posted:
26 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
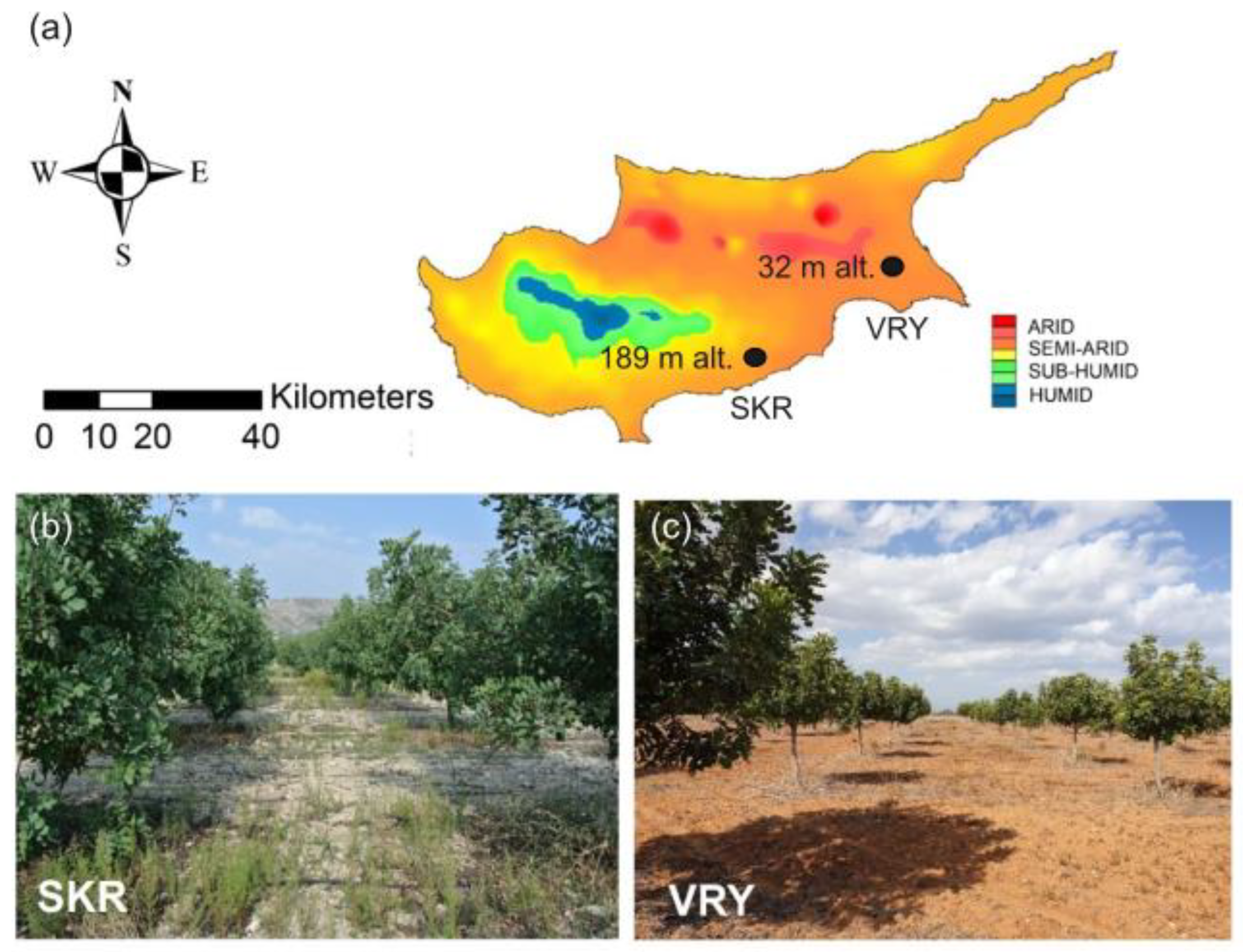
2.1. Experimental Sites
2.2. Intercropping Design
2.2.1. Carob-Thyme Intercropping System (T-System)
2.2.2. Carob-Clover Intercropping System (C-System)
2.3. Control Plots
2.3.1. Conservation Tillage Control Plots Without Irrigation (TLGdry)
2.3.2. Irrigated Conservation Tillage Carob Trees (TLGirr)
2.4. Experimental Monitoring
2.4.1. Thyme Survival Rate
2.4.2. Thyme Biomass/Thyme Soil Cover/Total Soil Cover
2.4.3. Clover Biomass and Soil Cover
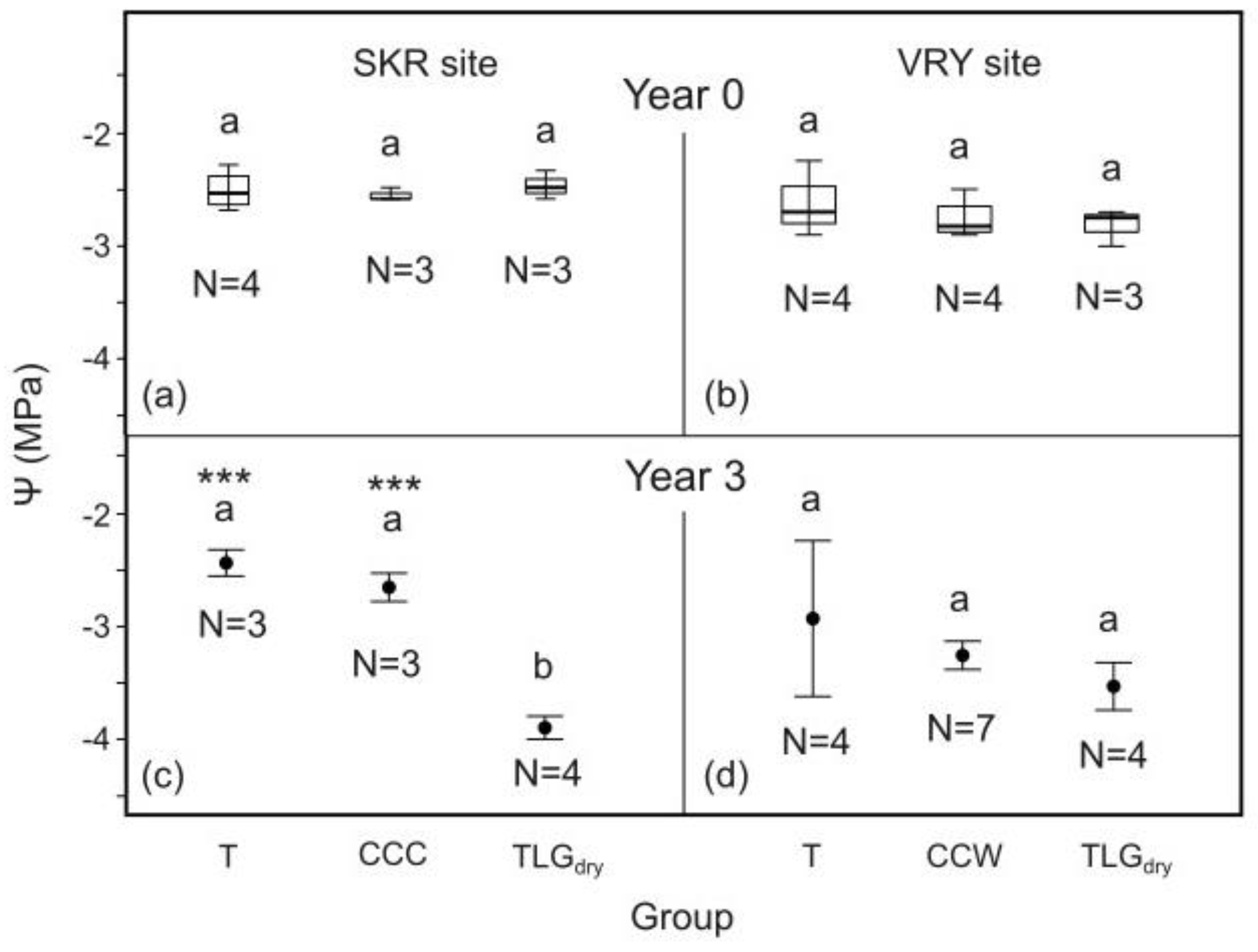
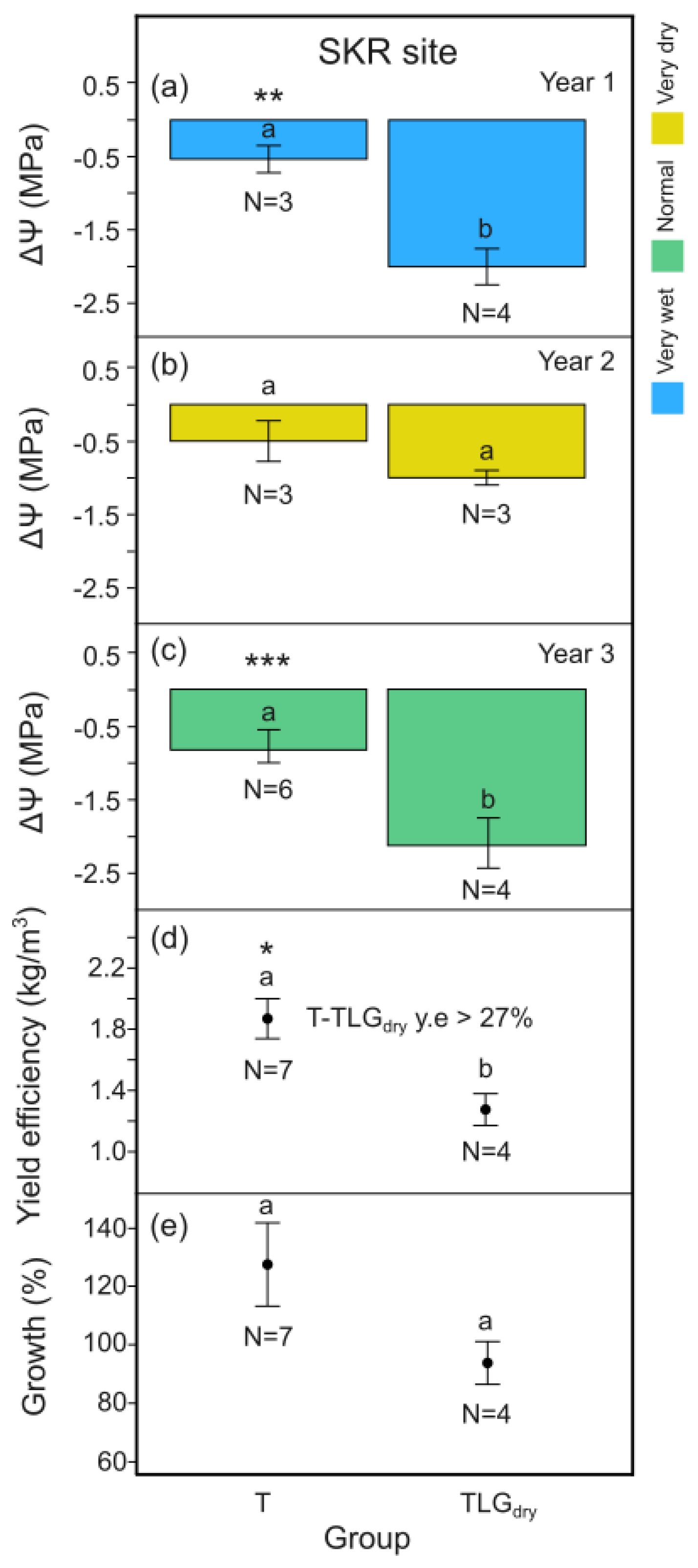
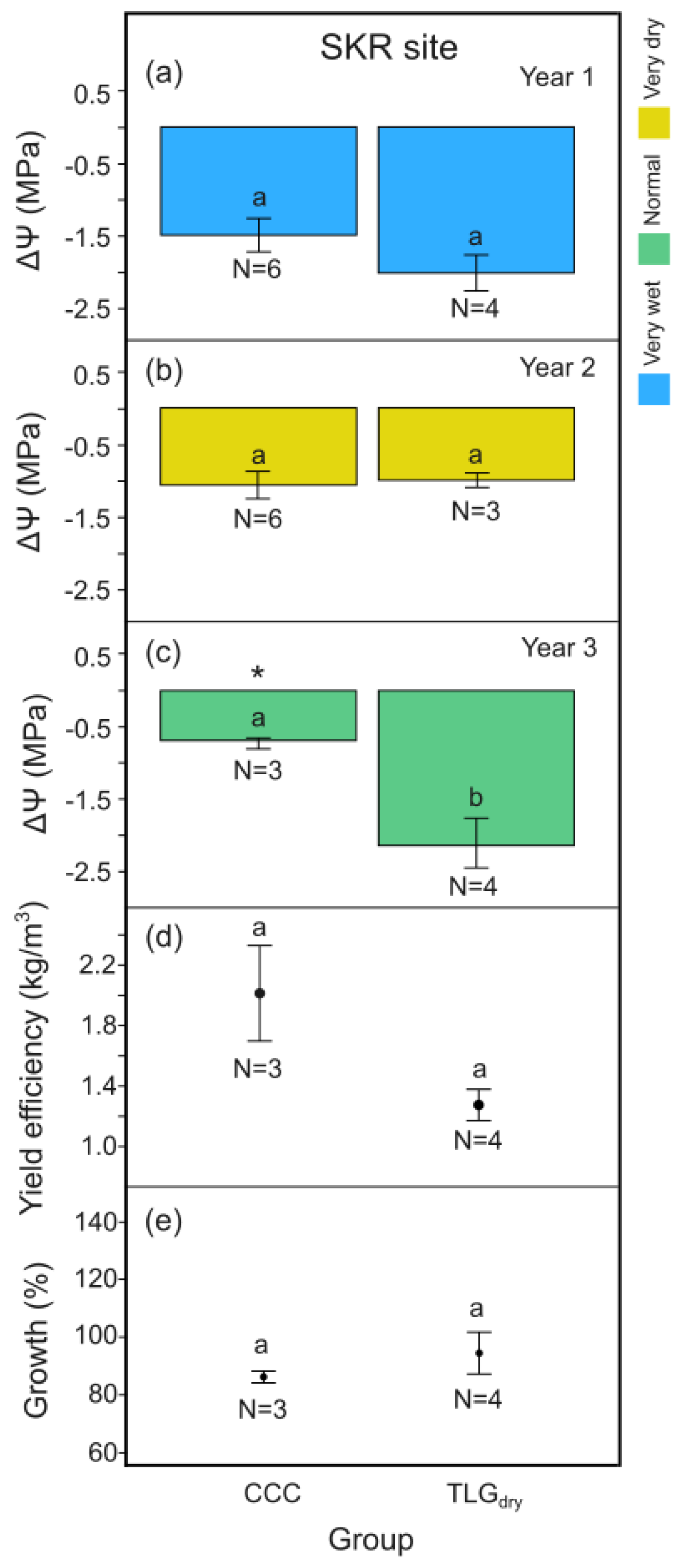
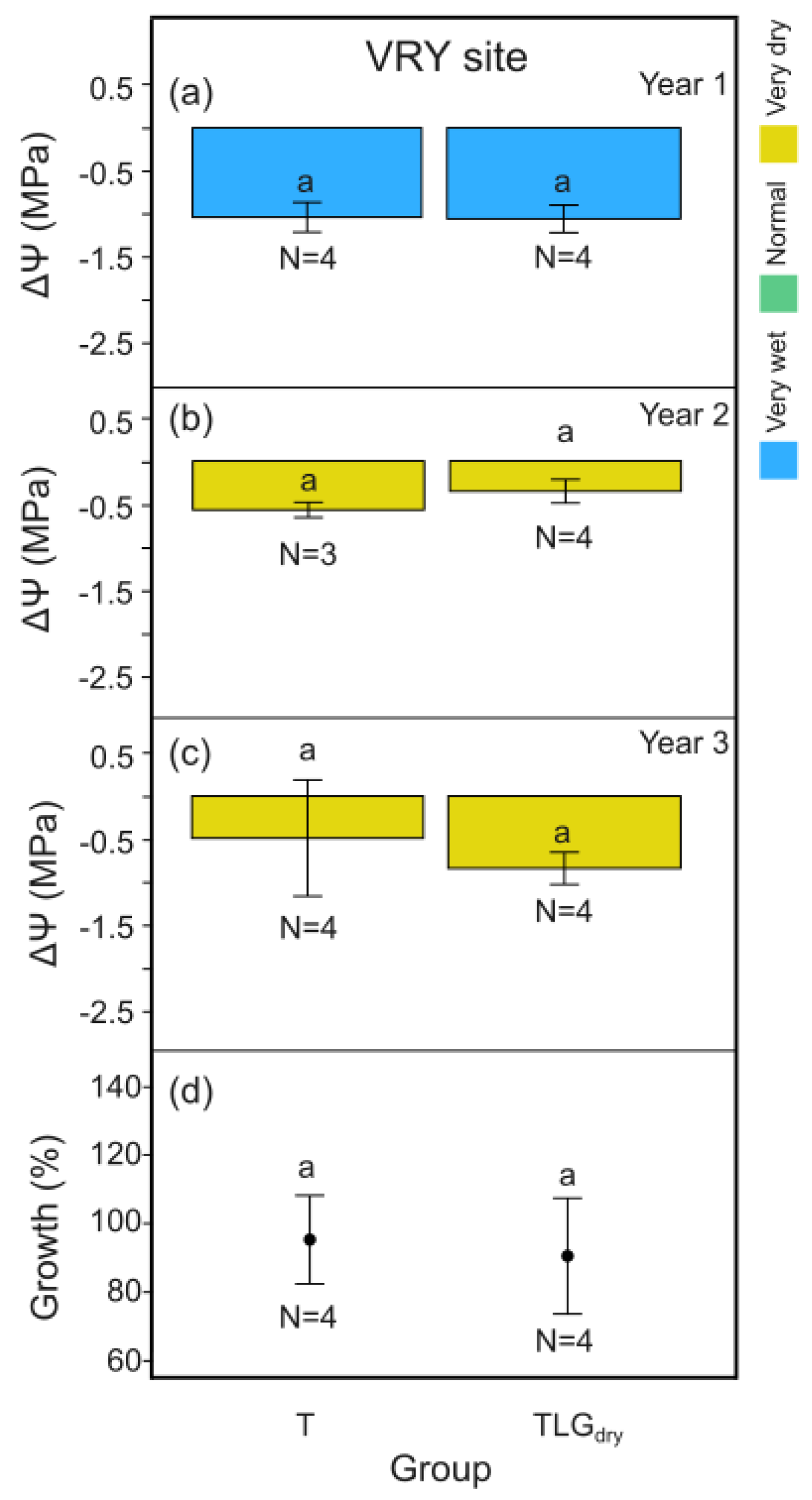
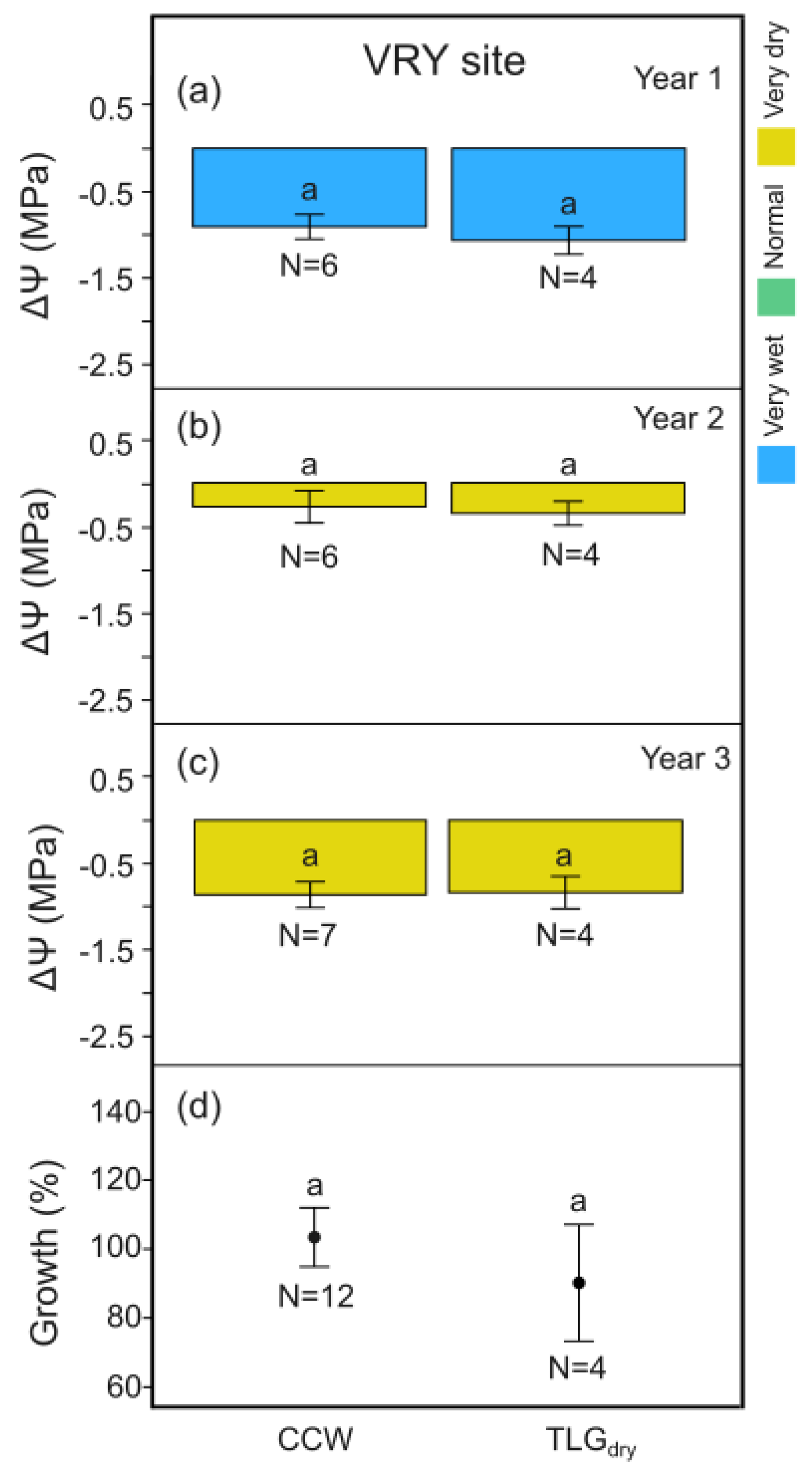
2.4.4. Water Potential
2.4.5. Wood Production
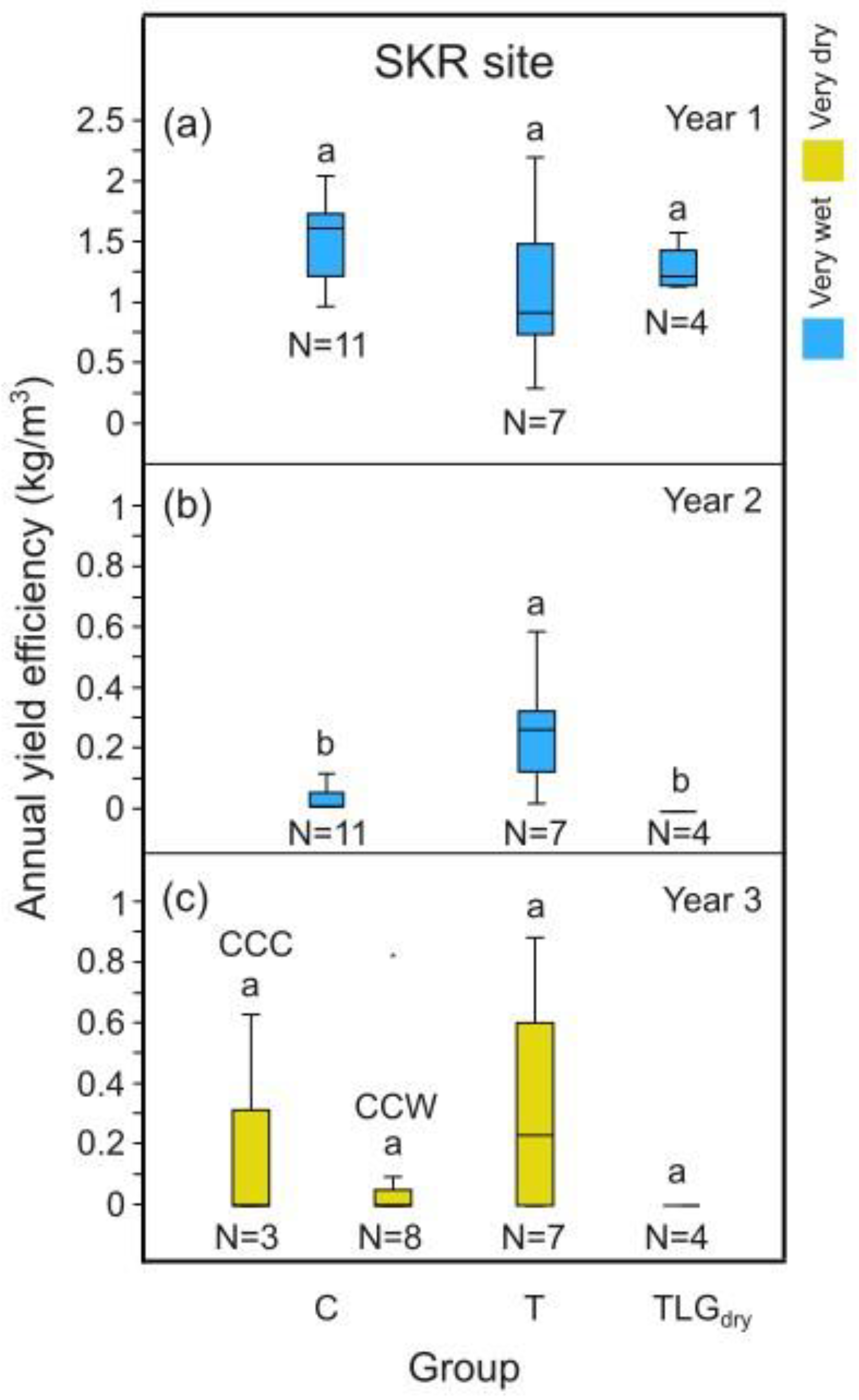
2.4.6. Carob Yield Efficiency
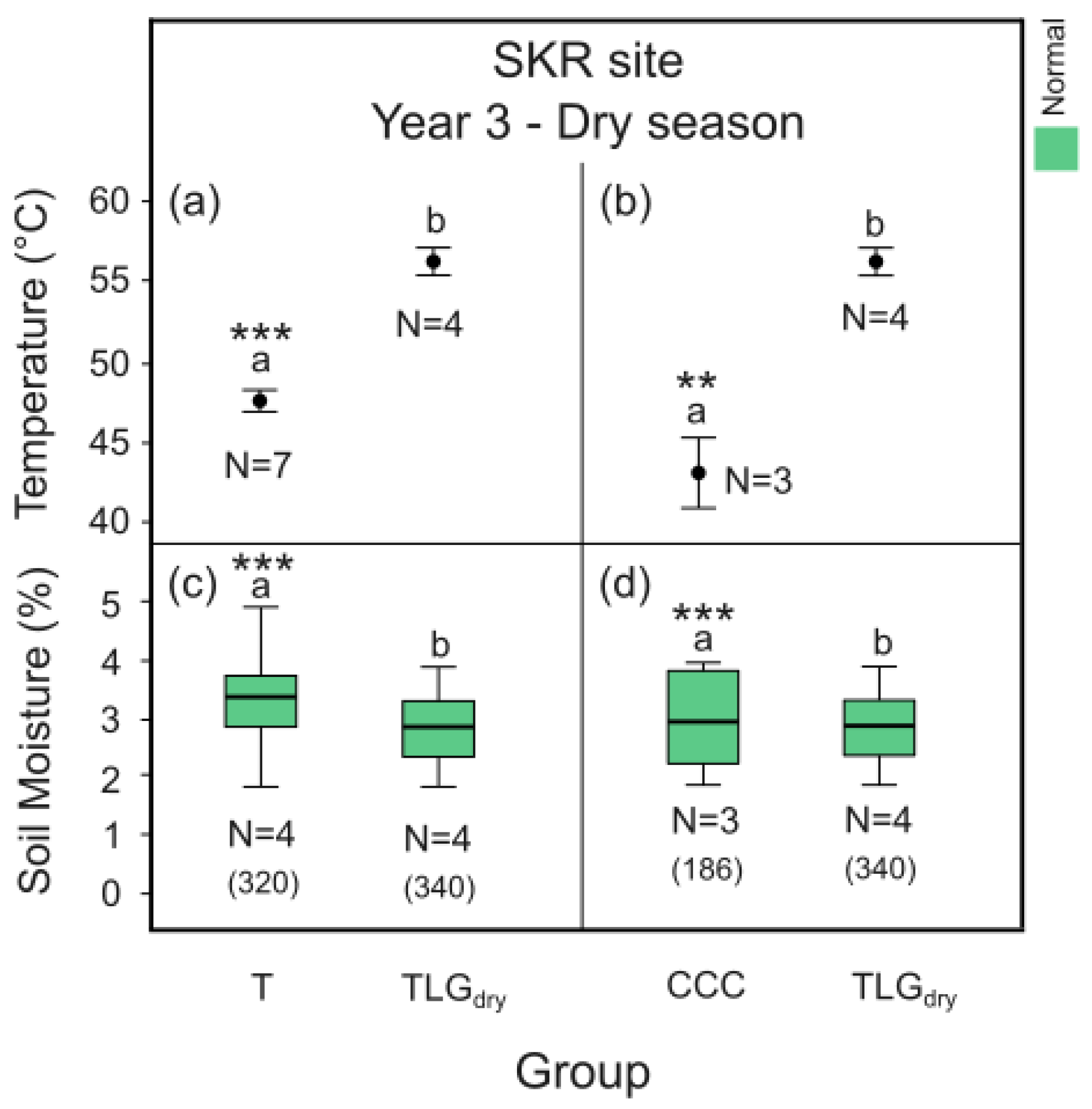
2.4.7. Soil Moisture Measurements
2.4.8. Surface Temperature Measurements
2.5. Climate Data
2.6. Environmental Indicators
2.7. Statistical Analysis
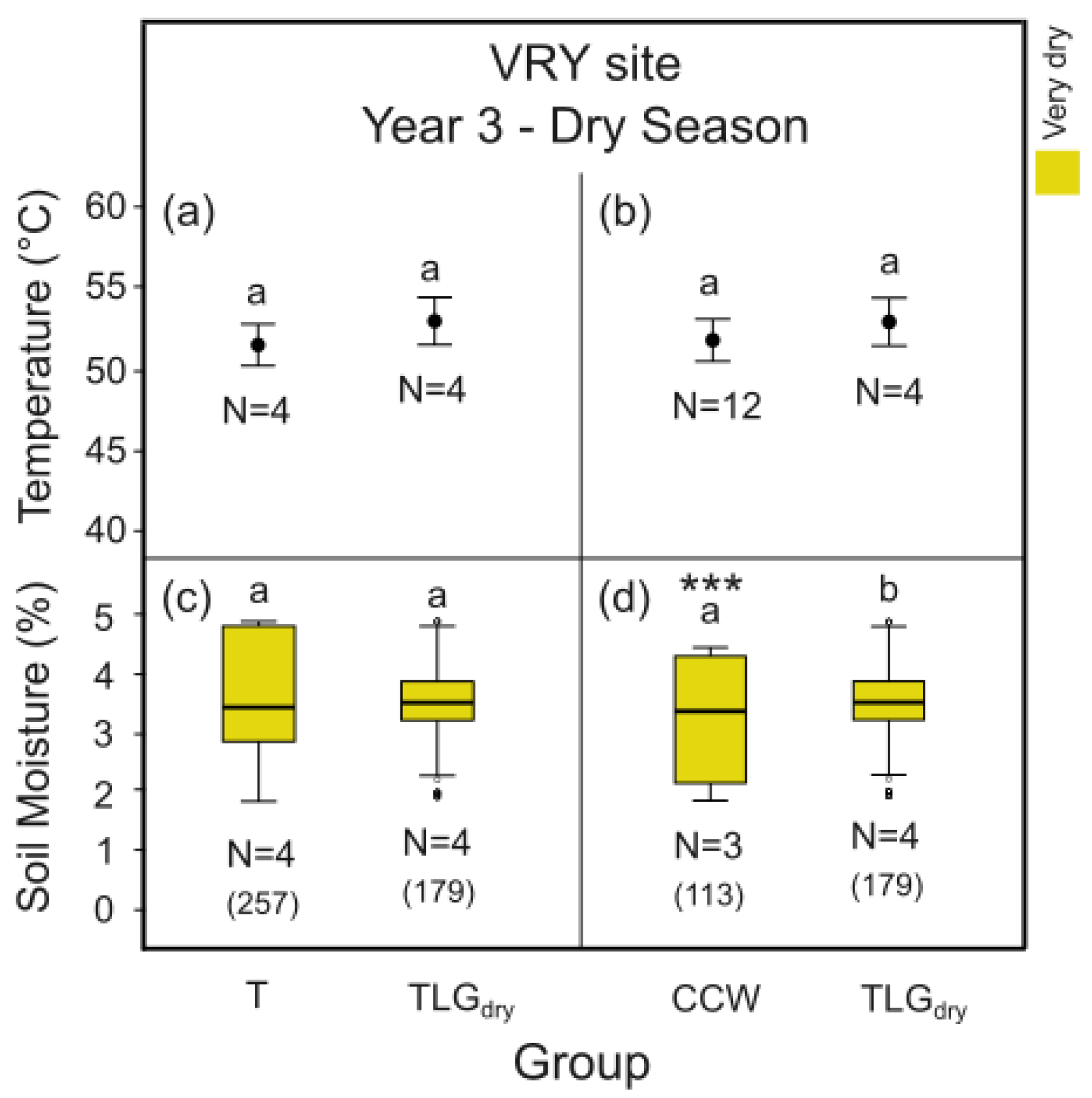
3. Results
| Exp. : Hydrological year | r (mm) | % change to previous year | % of normal r |
Ta (°C) | DMAI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SKR | VRY | SKR | VRY | SKR | VRY | SKR | VRY | SKR | VRY | |
| Y0: 2018-2019 | 678 | 469 | 160 | 142 | 20.4 | 20.5 | 22.28 | 15.35 | ||
| Y1: 2019-2020 | 655 | 513 | -<1% | +<1% | 154 | 155 | 20.8 | 20.4 | 21.29 | 16.88 |
| Y2: 2020-2021 | 311 | 289 | -53% | -44% | 73 | 87 | 21.2 | 20.8 | 9.96 | 9.40 |
| Y3: 2021-2022 | 496 | 283 | 59% | -2% | 117 | 85 | 20 | 20.3 | 15.77 | 9.33 |
| SKR site | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T system | C-System: CCC | ||||||||||||
| Exp. year | Climate year | Weed comp. | TB (g/m2) mean |
TSC (%) mean |
TWSC (%) mean |
TSR (%) median |
CB (g/m2) mean |
TB (g/m2) mean |
TSC (%) mean |
CWB (g/m2) mean |
CR (%) mean |
CWSC (%) mean |
TSR (%) median |
| Y1 | Very wet | Low | 43 | 15 | >40 | 97 | 157 | 1 | <0.3 | 215 | 70 | >90 | 2 |
| Y2 | Very dry | Low | 335 | 35 | 55 | 97 | 329* | 97* | |||||
| Y3 | Normal | Low | 420 | 83 | 85 | 94 | >180 | >320 | 55 | 100 | |||
| VRY site | |||||||||||||
| T system | C-System: CCW | ||||||||||||
| Y1 | Very wet | High | 28 | 11 | >55 | 72 | 171 | 0 | 0 | 305 | 50 | 97 | 0 |
| Y2 | Very dry | High | 39 | 9 | >80 | 40 | >400* | >95* | |||||
| Y3 | Very dry | High | 45 | 25 | >97 | 40 | 22 | 316 | 7 | 100 | |||
4. Discussion
4.1. The Drivers for the Greater Carob Tree Productivity in T-System Plots
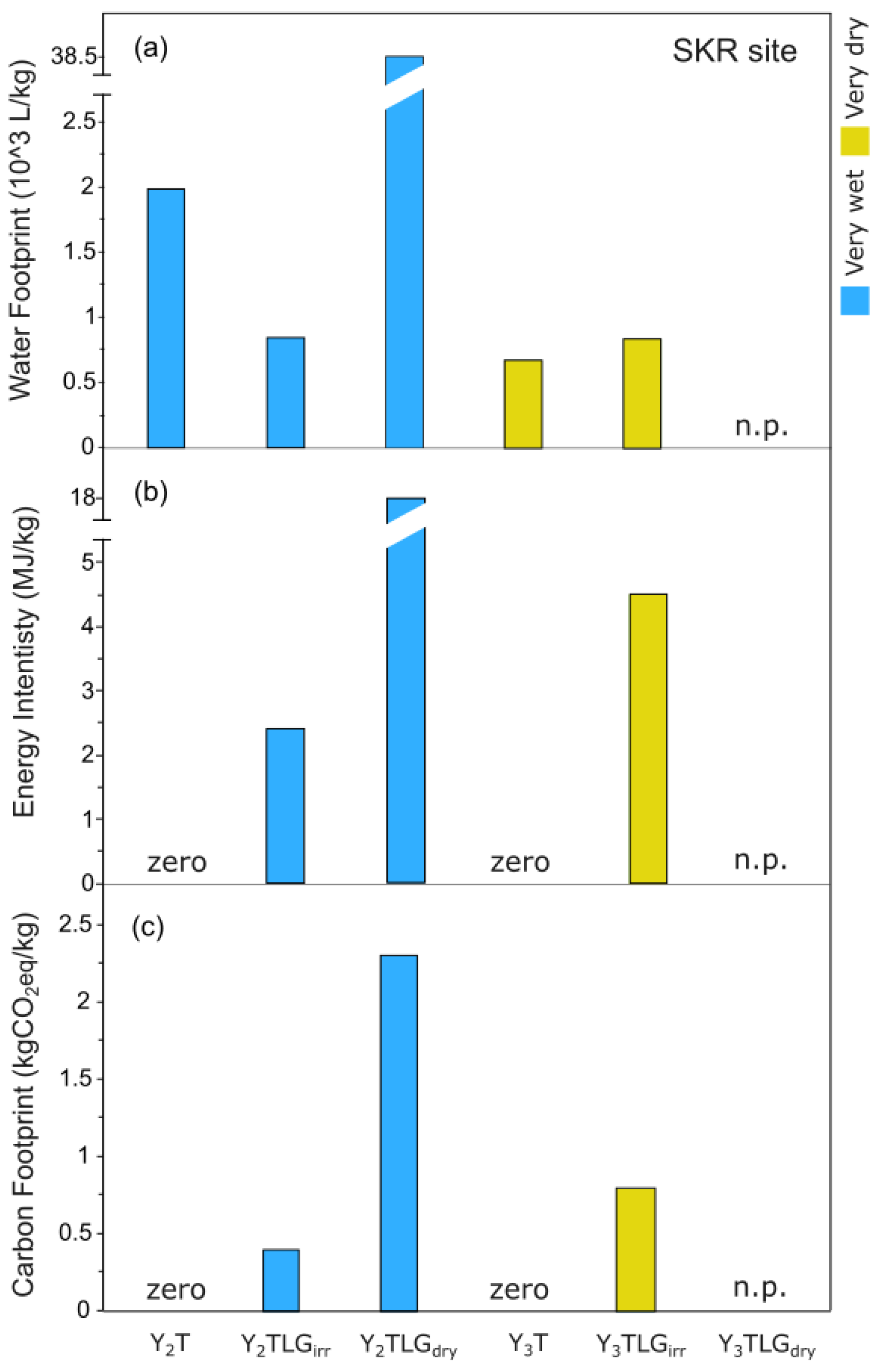
4.2. Overall Yield and Environmental Performance
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CB | Clover biomass |
| CCC | C-system with clover-based living mulch in 3 out of 3 years of monitoring |
| CCW | C-system with clover-based living mulch in 2 out of 3 years of monitoring |
| CF | Carbon Footprint |
| CR | Clover to clover plus weeds ratio |
| C-system | Clover (Trifolium squarossum)-based intercropping system with carob trees |
| CWB | Clover and weed biomass |
| CWSC | Soil cover by clover & wild vegetation |
| EI | Energy Intensity |
| SES | South (sun) exposed soil side of carob trees |
| SKR | Skarinou site |
| TB | Thyme biomass |
| TLGdry | Conservation tillage carob trees without irrigation (control) |
| TLGirr | Conservation tillage carob trees with irrigation |
| TSC | Soil cover only by thyme |
| TSR | Thyme survival rate |
| T-system | Thyme (Thymbra capitata)-based intercropping system with carob trees |
| TWSC | Soil cover by thyme & wild vegetation |
| VRY | Vrysoulles site |
| WF | Water Footprint |
References
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2015, 6, 166–171. [Google Scholar] [CrossRef]
- IUCN. Drylands and climate change. Issues Brief. International Union for Conservation of Nature and Natural Resources. 2019. Available online: https://www.iucn.org/resources/issues-brief/drylands-and-climate-change (accessed on 18 February 2023).
- Aguilera, E.; Díaz-Gaona, C.; García-Laureano, R.; Reyes-Palomo, C.; Guzmán, G.I.; Ortolani, L.; Sánchez-Rodríguez, M.; Rodríguez-Estévez, V. Agroecology for adaptation to climate change and resource depletion in the Mediterranean region. A review. Agric. Syst. 2020, 181. [Google Scholar] [CrossRef]
- MedECC. Climate and Environmental Change in the Mediterranean Basin–Current Situation and Risks for the Future; First Mediter-ranean Assessment Report; Cramer, W., Guiot, J., Marini, K., Eds.; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; 632p, ISBN 978-2-9577416-0-1. [Google Scholar] [CrossRef]
- Merino, A.; et al. Best Practices in Evaluation and Restoration of Degraded Mediterranean Environments. Library (Lond) 2019, 327. [Google Scholar]
- Palm, C.; Blanco-Canqui, H.; DeClerck, F.; Gatere, L.; Grace, P. Conservation agriculture and ecosystem services: An overview. Agric. Ecosyst. Environ. 2014, 187, 87–105. [Google Scholar] [CrossRef]
- Wittwer, R.A.; Dorn, B.; Jossi, W.; van der Heijden, M.G.A. Cover crops support ecological intensification of arable cropping systems. Sci. Rep. 2017, 7, srep41911. [Google Scholar] [CrossRef] [PubMed]
- Iseman, T.; Miralles-Wilhelm, F. Nature-based solutions in agriculture: The case and pathway for adoption; Food & Agriculture Organization, 2021. [Google Scholar]
- Palm, C.; Blanco-Canqui, H.; DeClerck, F.; Gatere, L.; Grace, P. Conservation agriculture and ecosystem services: An overview. Agric. Ecosyst. Environ. 2014, 187, 87–105. [Google Scholar] [CrossRef]
- Almagro, M.; de Vente, J.; Boix-Fayos, C.; García-Franco, N.; Melgares de Aguilar, J.; González, D.; Solé-Benet, A.; Martínez-Mena, M. Sustainable land management practices as providers of several ecosystem services under rainfed Mediterranean agroecosystems. Mitig. Adapt. Strat. Glob. Change 2016, 21, 1029–1043. [Google Scholar] [CrossRef]
- Lee, H.; Lautenbach, S.; Nieto, A.P.G.; Bondeau, A.; Cramer, W.; Geijzendorffer, I.R. The impact of conservation farming practices on Mediterranean agro-ecosystem services provisioning—a meta-analysis. Reg. Environ. Chang. 2019, 19, 2187–2202. [Google Scholar] [CrossRef]
- McLennon, E.; Dari, B.; Jha, G.; Sihi, D.; Kankarla, V. Regenerative agriculture and integrative permaculture for sustainable and technology driven global food production and security. Agron. J. 2021, 113, 4541–4559. [Google Scholar] [CrossRef]
- Mrunalini, K.; Behera, B.; Jayaraman, S.; Abhilash, P.C.; Dubey, P.K.; Swamy, G.N.; Prasad, J.V.N.S.; Rao, K.V.; Krishnan, P.; Pratibha, G.; et al. Nature-based solutions in soil restoration for improving agricultural productivity. Land Degrad. Dev. 2022, 33, 1269–1289. [Google Scholar] [CrossRef]
- Conti, J.; et al. Strategies for Operationalizing Nature-Based Solutions in the Private Sector – Environmental News Bits. Nat. Conserv. 2019, 27. [Google Scholar]
- Lv, W.; Zhao, X.; Wu, P.; Lv, J.; He, H. A Scientometric Analysis of Worldwide Intercropping Research Based on Web of Science Database between 1992 and 2020. Sustainability 2021, 13, 2430. [Google Scholar] [CrossRef]
- Marotti, I.; Whittaker, A.; Bağdat, R.B.; Akin, P.A.; Ergün, N.; Dinelli, G. Intercropping Perennial Fruit Trees and Annual Field Crops with Aromatic and Medicinal Plants (MAPs) in the Mediterranean Basin. Sustainability 2023, 15, 12054. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Brahmachari, K.; Shankar, T.; Bhadra, P.; Palai, J.B.; et al. Intercropping—A Low Input Agricultural Strategy for Food and Environmental Security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Gu, C.; Bastiaans, L.; Anten, N.P.; Makowski, D.; van der Werf, W. Annual intercropping suppresses weeds: A meta-analysis. Agric. Ecosyst. Environ. 2021, 322. [Google Scholar] [CrossRef]
- Restuccia, A.; Scavo, A.; Lombardo, S.; Pandino, G.; Fontanazza, S.; Anastasi, U.; Abbate, C.; Mauromicale, G. Long-Term Effect of Cover Crops on Species Abundance and Diversity of Weed Flora. Plants 2020, 9, 1506. [Google Scholar] [CrossRef]
- Tziolas, E.; Ispikoudis, S.; Mantzanas, K.; Koutsoulis, D.; Pantera, A. Economic and Environmental Assessment of Olive Agroforestry Practices in Northern Greece. Agriculture 2022, 12, 851. [Google Scholar] [CrossRef]
- Casas, G.L.; Ciaccia, C.; Iovino, V.; Ferlito, F.; Torrisi, B.; Lodolini, E.M.; Giuffrida, A.; Catania, R.; Nicolosi, E.; Bella, S. Effects of Different Inter-Row Soil Management and Intra-Row Living Mulch on Spontaneous Flora, Beneficial Insects, and Growth of Young Olive Trees in Southern Italy. Plants 2022, 11, 545. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; van der Werf, W. Syndromes of production in intercropping impact yield gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Tamburini, G.; Bommarco, R.; Wanger, T.C.; Kremen, C.; van der Heijden, M.G.A.; Liebman, M.; Hallin, S. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.I.; Nguyen, T.; Wing, C.; Lozano-Rojas, F.; Ahn, Y.; Simon, K. Evidence from internet search data shows information-seeking responses to news of local COVID-19 cases. Proc Natl Acad Sci. USA 2020, 117, 11220–11222. [Google Scholar] [CrossRef] [PubMed]
- Kassam, A.; Friedrich, T.; Derpsch, R.; Lahmar, R.; Mrabet, R.; Basch, G.; González-Sánchez, E.J.; Serraj, R. Conservation agriculture in the dry Mediterranean climate. Field Crop. Res. 2012, 132, 7–17. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Linares, C.; Gómez-López, M.D.; Faz, Á.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2020, 178, 102736. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.; Song, M.; Tian, J.; Song, B.; Hu, Y.; Zhang, J.; Yao, Y. Intercropping With Aromatic Plants Increased the Soil Organic Matter Content and Changed the Microbial Community in a Pear Orchard. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Sánchez-Navarro, V.; Shahrokh, V.; Martínez-Martínez, S.; Acosta, J.A.; Almagro, M.; Martínez-Mena, M.; Boix-Fayos, C.; Díaz-Pereira, E.; Zornoza, R. Perennial alley cropping contributes to decrease soil CO2 and N2O emissions and increase soil carbon sequestration in a Mediterranean almond orchard. Sci. Total. Environ. 2022, 845, 157225. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Steenwerth, K. Influence of Floor Management Technique on Grapevine Growth, Disease Pressure, and Juice and Wine Composition: A Review. Am. J. Enol. Vitic. 2012, 63, 149–164. [Google Scholar] [CrossRef]
- Belal, B.E.A.; El-Kenawy, M.A.; Ismail, S.I.I.; El-Hameed, A.M.A. Effect of Intercropping of Thompson Seedless Grapevines with some Medicinal Plants on Vine Nutritional Status, Yield, Berry Quality and the Microbiological Activity of the Soil. J. Plant Prod. 2017, 8, 495–501. [Google Scholar] [CrossRef]
- Rao, M.R.; Palada, M.C.; Becker, B.N.
- Montemurro, F.; Persiani, A.; Diacono, M. Cover Crop as Living Mulch: Effects on Energy Flows in Mediterranean Organic Cropping Systems. Agronomy 2020, 10, 667. [Google Scholar] [CrossRef]
- Angon, P.B.; Anjum, N.; Akter, M.M.; Kc, S.; Suma, R.P.; Jannat, S. An Overview of the Impact of Tillage and Cropping Systems on Soil Health in Agricultural Practices. Adv. Agric. 2023, 2023, 1–14. [Google Scholar] [CrossRef]
- Rusu, T. Energy efficiency and soil conservation in conventional, minimum tillage and no-tillage. Int. Soil Water Conserv. Res. 2014, 2, 42–49. [Google Scholar] [CrossRef]
- Reicosky, D.C. Conservation tillage is not conservation agriculture. J. Soil Water Conserv. 2015, 70, 103A–108A. [Google Scholar] [CrossRef]
- Battle, I.; Tous, J. Carob tree Ceratonia siliqua L.. Promoting the Conservation and Use of Underutilized and Neglected Crops. Leibniz Institute of Plant Genetics and Crop Plant Research, 1997.
- Palaiogianni, A.; Stylianou, M.; Sarris, D.; Agapiou, A. Carob-agro-industrial waste and potential uses in the circular economy. In Mediterranean Fruits Bio-wastes: Chemistry, Functionality and Technological Applications; Springer International Publishing, 2022, pp. 765–797.
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Flintan, F. Participatory rangeland management-an enabling process for improving silvopastoral management and governance. In Grazing with trees; Haddad, F.F., Herrera, P.M., Besbes, B., Eds.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar]
- Valdecantos, A.; et al. Report on the restoration potential for preventing and reversing regime shifts (No. 13). Cascade Project. 2016.
- Diamond, N.K. An agroforestry system for semi-arid Mediterranean areas and its potential for technology transfer, California. 1988, 32.
- Martins-Loução, M.A.; Correia, P.J.; Romano, A. Carob: A Mediterranean Resource for the Future. Plants 2024, 13, 1188. [Google Scholar] [CrossRef]
- Qin, J.; Duan, W.; Zou, S.; Chen, Y.; Huang, W.; Rosa, L. Global energy use and carbon emissions from irrigated agriculture. Nat. Commun. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Karimi, F.; Ghahderijani, M.; Bakhoda, H. Optimizing cropping patterns and resource allocation for sustainable agricultural development: A case study of Ilam province, Iran. Environ. Sustain. Indic. 2024, 23. [Google Scholar] [CrossRef]
- Litskas, V.D.; Chrysargyris, A.; Tzortzakis, N.; Stavrinides, M.C.; Petropoulos, S.A. Can the commercial cultivation of wild edible species contribute to sustainable food production? A case study of golden thistle (Scolymus hispanicus L.). Int. J. Life Cycle Assess. 2025, 30, 446–461. [Google Scholar] [CrossRef]
- Moradi, P.; Ford-Lloyd, B.; Pritchard, J. Plant-water responses of different medicinal plant thyme (Thymus spp.) species to drought stress condition. Aust. J. Crop Sci. 2014, 8, 666–673. [Google Scholar]
- Cordovilla, M.P.; Bueno, M.; Aparicio, C.; Urrestarazu, M. EFFECTS OF SALINITY AND THE INTERACTION BETWEENTHYMUS VULGARISANDLAVANDULA ANGUSTIFOLIAON GROWTH, ETHYLENE PRODUCTION AND ESSENTIAL OIL CONTENTS. J. Plant Nutr. 2014, 37, 875–888. [Google Scholar] [CrossRef]
- Martínez-Mena, M.; Boix-Fayos, C.; Carrillo-López, E.; Díaz-Pereira, E.; Zornoza, R.; Sánchez-Navarro, V.; Acosta, J.A.; Martínez-Martínez, S.; Almagro, M. Short-term impact of crop diversification on soil carbon fluxes and balance in rainfed and irrigated woody cropping systems under semiarid Mediterranean conditions. Plant Soil 2021, 467, 499–514. [Google Scholar] [CrossRef]
- Almagro, M.; Díaz-Pereira, E.; Boix-Fayos, C.; Zornoza, R.; Sánchez-Navarro, V.; Re, P.; Fernández, C.; Martínez-Mena, M. The combination of crop diversification and no tillage enhances key soil quality parameters related to soil functioning without compromising crop yields in a low-input rainfed almond orchard under semiarid Mediterranean conditions. Agric. Ecosyst. Environ. 2022, 345. [Google Scholar] [CrossRef]
- Zuazo, V.H.D.; Pleguezuelo, C.R.R.; Martínez, J.R.F.; Raya, A.M.; Panadero, L.A.; Rodríguez, B.C.; Moll, M.C.N. Benefits of plant strips for sustainable mountain agriculture. Agron. Sustain. Dev. 2008, 28, 497–505. [Google Scholar] [CrossRef]
- IACO. Consultation Services for the Production of a National Action Plan to Combat Desertification in Cyprus - Contract No. 3/2007 – Environment Service Executive Summary – Final Report. 2007. [Google Scholar]
- Matsi, S.; Sarris, D.; Konstantinou, M. Effects of Climate and Weeds on the Establishment of Thyme-Based Living Mulch Systems in Drylands of Cyprus. Int. J. Environ. Clim. Chang. 2024, 14, 791–803. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Hermoso, J.F.; Ninot, A.; Plana, J.; Batlle, I. Agronomic and commercial performance of four Spanish carob cultivars. Horttechnology 2009, 19, 465–470. [Google Scholar] [CrossRef]
- Pawar, A.D. Orchard Tree Canopy Detection. 2019; pp. 7642–7644.
- Liang, X.; Rehman, S.U.; Zhiqi, W.; Raza, M.A.; Haider, I.; Bin Khalid, M.H.; Saeed, A.; Iqbal, Z.; Fatima, S.; Siddiqa, A.; et al. Impacts of Conservation Tillage on Agricultural Land Development: A Review. J. Soil Sci. Plant Nutr. 2024, 25, 428–449. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Eldridge, D.J.; Berdugo, M.; Trivedi, P.; Sokoya, B.; Cano-Díaz, C.; Abades, S.; Alfaro, F.; Bamigboye, A.R.; Bastida, F.; et al. Unearthing the soil-borne microbiome of land plants. Glob. Chang. Biol. 2024, 30, e17295. [Google Scholar] [CrossRef]
- Yu, R.; Li, X.; Xiao, Z.; Lambers, H.; Li, L. Phosphorus facilitation and covariation of root traits in steppe species. New Phytol. 2020, 226, 1285–1298. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dordas, C.A.; Damalas, C.A.; Vlachostergios, D.N. Annual intercrops: an alternative pathway for sustainable agriculture. Aust. J. Crop Sci. 2011, 5, 396–410. [Google Scholar]
- Constantinou, E.; Sarris, D.; Vogiatzakis, I.N. The possible role of Ziziphus lotus as an ecosystem engineer in semiarid landscapes. J. Arid. Environ. 2021, 195. [Google Scholar] [CrossRef]
- Constantinou, E.; Sarris, D.; Psichoudaki, M.; Cabello, J.; Vogiatzakis, I.N. How can ecosystem engineer plants boost productivity in east Mediterranean drylands. Ecol. Process. 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Constantinou, E.; Montesinos-Navarro, A.; Sarris, D.; Vogiatzakis, I.N. Facilitation network in phryganic plant communities: evidence from a Mediterranean island. Plant Biosyst. - Int. J. Deal. all Asp. Plant Biol. 2025, 1–10. [Google Scholar] [CrossRef]
- Gerowitt, B.; Bàrberi, P.; Darmency, H.; Petit, S.; Storkey, J.; Westerman, P. Weeds and biodiversity. In Weed research: expanding horizons, 2017, pp. 115–147.
- Valdes, Y.B. The role of weeds as a component of biodiversity in agroecosystems. Cultiv. Trop. 2016, 37, 34–56. [Google Scholar] [CrossRef]
- Correia, P.J.; Cota, T.; Pestana, M. Evaluation of Carob Tree Productivity during a 30-Year Period, in Relation to Precipitation and Air Temperature. Environ. Process. 2020, 7, 1221–1233. [Google Scholar] [CrossRef]
- Correia, P.J.; Anastácio, I.; Candeias, M.d.F.; Martins-Loução, M.A. Nutritional Diagnosis in Carob-Tree: Relationships between yield and leaf mineral concentration. Crop. Sci. 2002, 42, 1577–1583. [Google Scholar] [CrossRef]
- Bonanomi, G.; Stinca, A.; Chirico, G.B.; Ciaschetti, G.; Saracino, A.; Incerti, G. Cushion plant morphology controls biogenic capability and facilitation effects of Silene acaulis along an elevation gradient. Funct. Ecol. 2015, 30, 1216–1226. [Google Scholar] [CrossRef]
- Bonanomi, G.; Idbella, M.; Stinca, A.; Maisto, G.; De Marco, A.; Del Galdo, G.P.G.; Guarino, R.; Zotti, M. Nitrogen-fixing cushion Astragalus siculus modulates soil fertility, microclimate, plant facilitation, bacterial and fungal microbiota along an elevation gradient. J. Veg. Sci. 2023, 34. [Google Scholar] [CrossRef]
- Momberg, M.; le Roux, P.C. Testing for consistency in ecosystem engineering: Do cushion plants always turn up the heat? Acta Oecologica 2020, 104. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Losapio, G.; Schöb, C. Species interactions involving cushion plants in high-elevation environments under a changing climate. Ecosistemas 2021, 30, 2186. [Google Scholar] [CrossRef]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress inThymus citriodorus. Int. J. Agron. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Karanja, N.N.; Gachene, C.K.K.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M.L. Intercropping Optimizes Soil Temperature and Increases Crop Water Productivity and Radiation Use Efficiency of Rainfed Potato. Am. J. Potato Res. 2019, 96, 457–471. [Google Scholar] [CrossRef]
- Safari, N.; Kazemi, F.; Tehrani, A. Examining temperature and soil moisture contents of mulches in the urban landscaping of an arid region. Desert 2021, 26, 139–156. [Google Scholar] [CrossRef]
- Correia, P.J.; Martins-Loução, M.A. The use of macronutrients and water in marginal Mediterranean areas: the case of carob-tree. Field Crop. Res. 2005, 91, 1–6. [Google Scholar] [CrossRef]
- Kolyva, F.; Stratakis, E.; Rhizopoulou, S.; Chimona, C.; Fotakis, C. Leaf surface characteristics and wetting in Ceratonia siliqua L. Flora 2012, 207, 551–556. [Google Scholar] [CrossRef]
- Sarris, D.; Mazza, G. Mediterranean pine root systems under drought. In Pines and Their Mixed Forest Ecosystems in the Mediterranean Basin, 2021, pp. 129–140.
- Sarris, D.; Christodoulakis, D. Topographic and climatic effects on Pinus halepensis s.l. growth at its drought tolerance margins under climatic change. J. For. Res. 2024, 35, 1–20. [Google Scholar] [CrossRef]
- Markou, L. Effects of Intercropping Systems on Carob Productivity Under Rainfed Conditions. University of Cyprus. 2021. [Google Scholar]
- Dorman, M.; Perevolotsky, A.; Sarris, D.; Svoray, T. Amount vs. temporal pattern: On the importance of intra-annual climatic conditions on tree growth in a dry environment. J. Arid. Environ. 2015, 118, 65–68. [Google Scholar] [CrossRef]
- Mazza, G.; Sarris, D. Identifying the full spectrum of climatic signals controlling a tree species' growth and adaptation to climate change. Ecol. Indic. 2021, 130. [Google Scholar] [CrossRef]
- Mazza, G.; Markou, L.; Sarris, D. Species-specific growth dynamics and vulnerability to drought at the single tree level in a Mediterranean reforestation. Trees 2021, 35, 1697–1710. [Google Scholar] [CrossRef]
- von Hasalberg, C.D. Vegetative Growth and Flower and Fruit Development in Carob Tree (Ceratonia siliqua L.) with Special Emphasis on Environmental Conditions at Marginal Production Sites in South Portugal. 2000.
- Tous, I.; Romero, J.; Batlle, A. Editorial Board, Volume 44 Horticultural Reviews is sponsored by. Horticultural Reviews 2013, 41, 385–456. [Google Scholar]
- Haselberg, V.C. Factors influencing flower and fruit development in carob (Ceratonia siliqua L.). 1996.
- Moore, E.A.; Norton, U. Improving semi-arid agroecosystem services with cover crop mixes. PLOS ONE 2024, 19, e0306567. [Google Scholar] [CrossRef] [PubMed]
- Opoku, A.; Ogunleye, A.M.; Solomon, J.K.; Payne, W.A. Cover crop systems impact on biomass production, carbon-to-nitrogen ratio, forage quality, and soil health in a semi-arid environment. Heliyon 2024, 10, e39600. [Google Scholar] [CrossRef] [PubMed]
- Everwand, G.; Cass, S.; Dauber, J.; Williams, M.; Stout, J. Legume crops and biodiversity. Legum. J. Crop. Syst. 2017, 55–69. [Google Scholar] [CrossRef]
- Simoes, M.P.; Belo, A.F.; Pinto-Cruz, C.; Pinheiro, A.C. Natural vegetation management to conserve biodiversity and soil water in olive orchards. Span. J. Agric. Res. 2014, 12, 633–643. [Google Scholar] [CrossRef]
- Zhu, S.-G.; Zhu, H.; Zhou, R.; Zhang, W.; Wang, W.; Zhou, Y.-N.; Wang, B.-Z.; Yang, Y.-M.; Wang, J.; Tao, H.-Y.; et al. Intercrop overyielding weakened by high inputs: Global meta-analysis with experimental validation. Agric. Ecosyst. Environ. 2022, 342. [Google Scholar] [CrossRef]
- Brooker, R.; Kikvidze, Z.; Pugnaire, F.I.; Callaway, R.M.; Choler, P.; Lortie, C.J.; Michalet, R. The importance of importance. Oikos 2005, 109, 63–70. [Google Scholar] [CrossRef]
- Echarte, L.; Della Maggiora, A.; Cerrudo, D.; Gonzalez, V.; Abbate, P.; Cerrudo, A.; Sadras, V.; Calviño, P. Yield response to plant density of maize and sunflower intercropped with soybean. Field Crop. Res. 2011, 121, 423–429. [Google Scholar] [CrossRef]
- Willey, R. W. Resource use in intercropping systems. Agric. Water Manag. 1990, 1–3, 215–231. [Google Scholar] [CrossRef]
- Rossi, L.; Regni, L.; Rinaldi, S.; Sdringola, P.; Calisti, R.; Brunori, A.; Dini, F.; Proietti, P. Long-Term Water Footprint Assessment in a Rainfed Olive Tree Grove in the Umbria Region, Italy. Agriculture 2019, 10, 8. [Google Scholar] [CrossRef]
- Litskas, V.; Mandoulaki, A.; Vogiatzakis, I.N.; Tzortzakis, N.; Stavrinides, M. Sustainable Viticulture: First Determination of the Environmental Footprint of Grapes. Sustainability 2020, 12, 8812. [Google Scholar] [CrossRef]
- Caruso, M.; Distefano, G.; Ye, X.; La Malfa, S.; Gentile, A.; Tribulato, E.; Roose, M.L. Generation of expressed sequence tags from carob (Ceratonia siliqua L.) flowers for gene identification and marker development. Tree Genet. Genomes 2008, 4, 869–879. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





