Submitted:
19 May 2025
Posted:
20 May 2025
You are already at the latest version
Abstract
Keywords:
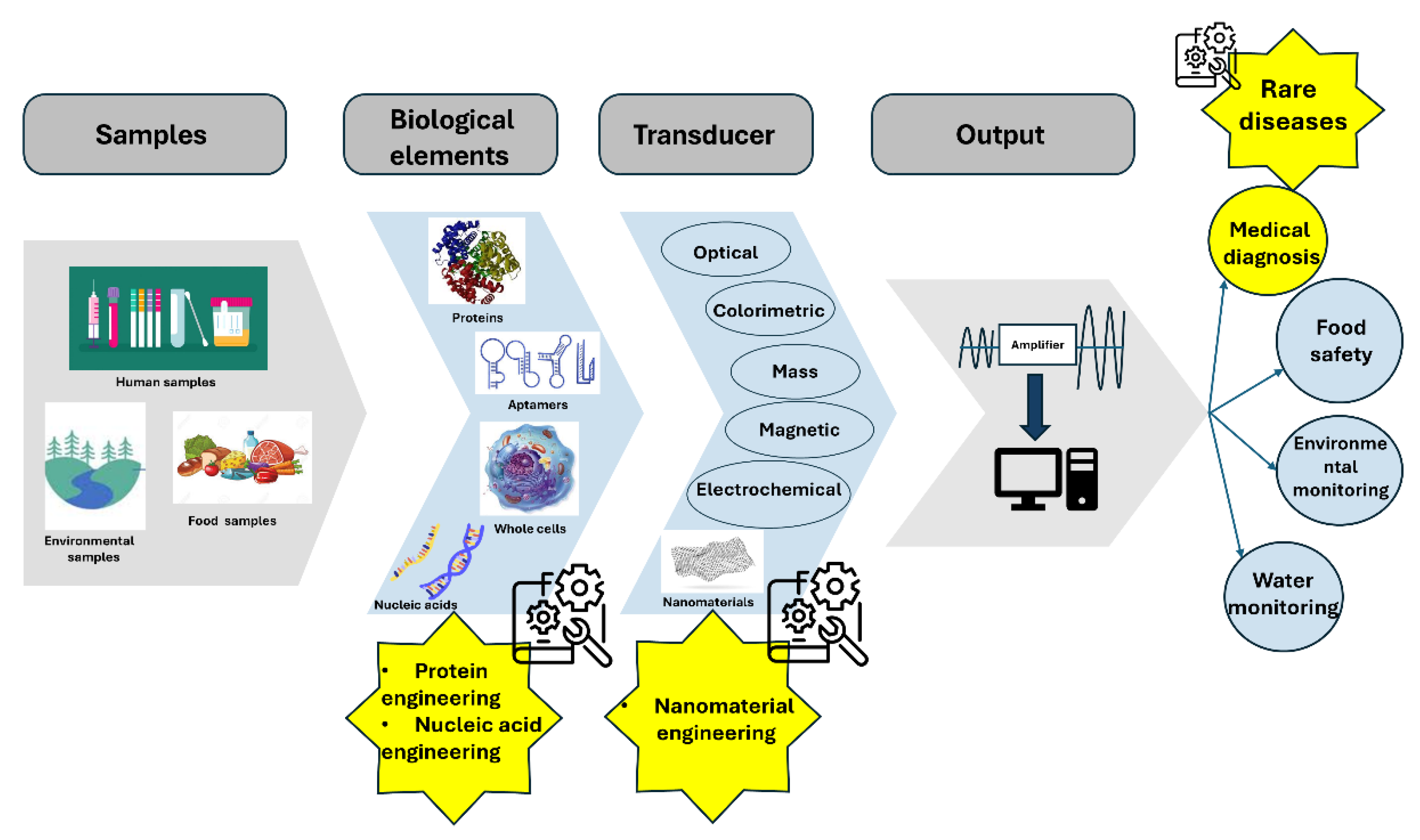
1. Introduction
2. Protein Engineering for Biosensors
2.1. Directed Evolution of Proteins for Biosensors
2.2. Semi-Rational Design of Proteins for Biosensors
2.3. Rational Design of Proteins for Biosensors
3. Nucleic Acid Engineering for Biosensors
4. Nanomaterials Engineering for Biosensors
5. Biosensors for Rare Diseases Monitoring
5.1. Biosensors for the Detection of Aβ42, a Biomarker for Alzheimer Disease Diagnosis
5.2. Biosensors for the monitoring of Hepatitis B
5.3. Biosensors for the Monitoring of Human Papillomavirus
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pellerano, M.; et al. Fluorescent Biosensor for Detection of the R248Q Aggregation-Prone Mutant of p53. Chembiochem 2019, 20, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; et al. Development of a highly sensitive PbrR-based biosensor via directed evolution and its application for lead detection. Journal of Hazardous Materials 2025, 488. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; et al. A general strategy to construct small molecule biosensors in eukaryotes. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Paulmurugan, R.; et al. A protein folding molecular imaging biosensor monitors the effects of drugs that restore mutant p53 structure and its downstream function in glioblastoma cells. Oncotarget 2018, 9, 21495–21511. [Google Scholar] [CrossRef]
- Taylor, N.D.; et al. Engineering an allosteric transcription factor to respond to new ligands. Nat Methods 2016, 13, 177–83. [Google Scholar] [CrossRef]
- Gohil, K.; et al. Biosensor Optimization Using a Forster Resonance Energy Transfer Pair Based on mScarlet Red Fluorescent Protein and an mScarlet-Derived Green Fluorescent Protein. ACS Sens 2023, 8, 587–597. [Google Scholar] [CrossRef]
- Cai, Y.; et al. Evolved Biosensor with High Sensitivity and Specificity for Measuring Cadmium in Actual Environmental Samples. Environ Sci Technol 2022, 56, 10062–10071. [Google Scholar] [CrossRef]
- Guo, Y.; et al. Anthocyanin biosynthetic pathway switched by metalloregulator PbrR to enable a biosensor for the detection of lead toxicity. Front Microbiol 2022, 13, 975421. [Google Scholar] [CrossRef]
- Lee, J.Y.; et al. C1 Compound Biosensors: Design, Functional Study, and Applications. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Ebrahimi Fana, S.; Fazaeli, A.; Aminian, M. Directed evolution of cholesterol oxidase with improved thermostability using error-prone PCR. Biotechnol Lett 2023, 45, 1159–1167. [Google Scholar] [CrossRef]
- Ding, N.; et al. Fine-tuning biosensor dynamic range based on rational design of cross-ribosome-binding sites in bacteria. bioRxiv 2020.01.27.922302. 2020. [Google Scholar]
- Moussa, S.; et al. Enhancing Electrochemical Biosensor Selectivity with Engineered D-Amino Acid Oxidase Enzymes for D-Serine and D-Alanine Quantification. Acs Applied Bio Materials 2021, 4, 5598–5604. [Google Scholar] [CrossRef]
- Nadler, D.C.; et al. Rapid construction of metabolite biosensors using domain-insertion profiling. Nature Communications 2016, 7. [Google Scholar] [CrossRef]
- Zhou, Y.K.; et al. Encoding Genetic Circuits with DNA Barcodes Paves the Way for Machine Learning-Assisted Metabolite Biosensor Response Curve Profiling in Yeast. Acs Synthetic Biology 2022, 11, 977–989. [Google Scholar] [CrossRef]
- Jiang, S.Q.; et al. Semi-rational design for enhancing thermostability of acetylcholinesterase and sensitivity analysis of acephate. Science of the Total Environment 2024, 934. [Google Scholar] [CrossRef] [PubMed]
- Balaz, A.M.; et al. Semi-rational design of cellobiose dehydrogenase for increased stability in the presence of peroxide. Molecular Diversity 2020, 24, 593–601. [Google Scholar] [CrossRef]
- Qiu, C.X.; Zhai, H.T.; Hou, J. Biosensors design in yeast and applications in metabolic engineering. Fems Yeast Research 2019, 19. [Google Scholar] [CrossRef]
- Pedotti, M.; et al. Rationally Modified Estrogen Receptor Protein as a Bio-Recognition Element for the Detection of EDC Pollutants: Strategies and Opportunities. International Journal of Environmental Research and Public Health 2015, 12, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.K.; et al. Synthetic biosensors for precise gene control and real-time monitoring of metabolites. Nucleic Acids Research 2015, 43, 7648–7660. [Google Scholar] [CrossRef]
- Hiraka, K.; et al. Rational engineering of L-lactate oxidase for the mediator modification to achieve quasi-direct electron transfer type lactate sensor. Biosensors & Bioelectronics 2020, 151. [Google Scholar]
- Golynskiy, M.V.; et al. Engineering Protein Switches: Sensors, Regulators, and Spare Parts for Biology and Biotechnology. Chembiochem 2011, 12, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, L.; et al. A general method for the development of multicolor biosensors with large dynamic ranges. Nature Chemical Biology 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; et al. Directed Evolution of the Stringency of the LuxR Quorum Sensor without OFF-State Selection. Acs Synthetic Biology 2020, 9, 567–575. [Google Scholar] [CrossRef]
- Du, H.X.; et al. Directed Evolution of 4-Hydroxyphenylpyruvate Biosensors Based on a Dual Selection System. International Journal of Molecular Sciences 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Q.; Ching, C.B. Development of Colorimetric-Based Whole-Cell Biosensor for Organophosphorus Compounds by Engineering Transcription Regulator DmpR. Acs Synthetic Biology 2016, 5, 1290–1298. [Google Scholar] [CrossRef]
- Dong, Z.Z.; et al. Recent Progress in DNA Biosensors for Detecting Biomarkers in Living Cells. Acs Biomaterials Science & Engineering 2024, 10, 5595–5608. [Google Scholar]
- Stephen, B.J.; et al. DNA biosensor based detection for neglected tropical disease: moving towards smart diagnosis. Sensor Review 2022, 42, 517–525. [Google Scholar] [CrossRef]
- Machado, L.F.M.; Currin, A.; Dixon, N. Directed evolution of the PcaV allosteric transcription factor to generate a biosensor for aromatic aldehydes. Journal of Biological Engineering 2019, 13. [Google Scholar] [CrossRef]
- Huo, Y.Y.; et al. Development of a specific biosensor for sesquiterpene based on SELEX and directed evolution platforms. Talanta 2025, 283. [Google Scholar] [CrossRef]
- Townshend, B.; Kaplan, M.; Smolke, C.D. Highly multiplexed selection of RNA aptamers against a small molecule library. Plos One 2022, 17. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Nikolaus, N.; Strehlitz, B. Capture-SELEX: Selection of DNA Aptamers for Aminoglycoside Antibiotics. Journal of Analytical Methods in Chemistry 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.Q.; et al. Integration of an Expression Platform in the SELEX Cycle to Select DNA Aptamer Binding to a Disease Biomarker. Acs Omega 2022, 7, 10804–10811. [Google Scholar] [CrossRef] [PubMed]
- Kramat, J.; et al. Sensing Levofloxacin with an RNA Aptamer as a Bioreceptor. Biosensors-Basel 2024, 14. [Google Scholar] [CrossRef]
- Jaeger, J.; et al. Characterization and Inkjet Printing of an RNA Aptamer for Paper-Based Biosensing of Ciprofloxacin. Biosensors-Basel 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Synergetic Effects of Combined Nanomaterials for Biosensing Applications. Sensors 2017, 17. [Google Scholar] [CrossRef]
- El-Kady, M.M.; et al. Nanomaterials: A comprehensive review of applications, toxicity, impact, and fate to environment. Journal of Molecular Liquids 2023, 370. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Hwang, H.S.; et al. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; et al. Applications of carbon-based conductive nanomaterials in biosensors. Chemical Engineering Journal 2022, 442. [Google Scholar] [CrossRef]
- Ansari, M.A.; Mohd-Naim, N.F.; Ahmed, M.U. Electrochemical Nanoaptasensor Based on Graphitic Carbon Nitride/Zirconium Dioxide/Multiwalled Carbon Nanotubes for Matrix Metalloproteinase-9 in Human Serum and Saliva. Acs Applied Bio Materials 2024, 7, 1579–1587. [Google Scholar] [CrossRef]
- Shi, Z.H.; et al. Sensitive Electrochemical Biosensor Using Gold and Manganese (IV) Oxide Nanomaterials on Multiwalled Carbon Nanotubes for the Determination of Homocysteine With a Portable Potentiostat. Analytical Letters 2025, 58, 492–505. [Google Scholar] [CrossRef]
- du Plooy, J.; et al. Carbon Nanostructured Immunosensing of Anti-SARS-CoV-2 S-Protein Antibodies. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.R.; et al. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Frontiers in Chemistry 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; et al. Graphene quantum dots: Synthesis, optical properties and navigational applications against cancer. Materials Today Communications 2022, 31. [Google Scholar] [CrossRef]
- El-Said, W.A.; et al. Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Mirzaei, S.S.; et al. Novel detection of H.pylori using ultrasensitive electrochemical aptasensor based on surface modified graphene oxide doped gold nanoparticles conjugated polythiophene( vol 200, 110279, 2024). Microchemical Journal 2024, 204. [Google Scholar]
- Jaradat, H.; et al. Detection of H. pylori outer membrane protein (HopQ) biomarker using electrochemical impedimetric immunosensor with polypyrrole nanotubes and carbon nanotubes nanocomposite on screen-printed carbon electrode. Biosensors & Bioelectronics 2024, 249. [Google Scholar]
- Yu, T.T.; et al. Noble metal-free high-entropy oxide/Co-N-C bifunctional electrocatalyst enables highly reversible and durable Zn-air batteries. Applied Surface Science 2023, 610. [Google Scholar] [CrossRef]
- Lv, C.L.; et al. Self-supported PtPdMnCoFe high-entropy alloy with nanochain-like internetworks for ultrasensitive electrochemical immunoassay of biomarker. Sensors and Actuators B-Chemical 2024, 401. [Google Scholar] [CrossRef]
- Tang, C.; et al. Dendritic quinary PtRhMoCoFe high-entropy alloy as a robust immunosensing nanoplatform for ultrasensitive detection of biomarker. Bioelectrochemistry 2024, 157. [Google Scholar] [CrossRef]
- Kurup, C.P. and M.U. Ahmed, Nanozymes towards Personalized Diagnostics: A Recent Progress in Biosensing. Biosensors-Basel 2023, 13. [Google Scholar]
- Keum, C.; et al. Modular conductive MOF-gated field-effect biosensor for sensitive discrimination on the small molecular scale. Chemical Engineering Journal 2023, 456. [Google Scholar] [CrossRef]
- Li, X.Y.; et al. Electrochemical biosensor for ultrasensitive exosomal miRNA analysis by cascade primer exchange reaction and MOF@Pt@MOF nanozyme. Biosensors & Bioelectronics 2020, 168. [Google Scholar]
- Huang, W.; et al. Dual nanozyme based on ultrathin 2D conductive MOF nanosheets intergraded with gold nanoparticles for electrochemical biosensing of HO in cancer cells. Talanta 2022, 249. [Google Scholar] [CrossRef] [PubMed]
- Koukouviti, E.; et al. 3D-printed electrochemical glucose device with integrated Fe(II)-MOF nanozyme. Microchimica Acta 2023, 190. [Google Scholar] [CrossRef]
- Yu, Y.Y.; et al. Gelsolin bound β-amyloid peptides: Electrochemical evaluation of levels of soluble peptide associated with Alzheimer's disease. Biosensors & Bioelectronics 2015, 68, 115–121. [Google Scholar]
- Diba, F.S., S. Kim, and H.J. Lee, Electrochemical immunoassay for amyloid-beta 1-42 peptide in biological fluids interfacing with a gold nanoparticle modified carbon surface. Catalysis Today 2017, 295, 41–47. [Google Scholar] [CrossRef]
- Ding, M.L.; et al. Electrochemical Immunosensor for the Sensitive Detection of Alzheimer's Biomarker Amyloid-β (1-42) Using the Heme-amyloid-β (1-42) Complex as the Signal Source. Electroanalysis 2022, 34, 263–274. [Google Scholar] [CrossRef]
- Hsu, C.H.; et al. Sensing Alzheimer's Disease Utilizing Au Electrode by Controlling Nanorestructuring. Chemosensors 2022, 10. [Google Scholar] [CrossRef]
- Le, H.T.N.; Park, J.; Cho, S. A Probeless Capacitive Biosensor for Direct Detection of Amyloid Beta 1-42 in Human Serum Based on an Interdigitated Chain-Shaped Electrode. Micromachines 2020, 11. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; et al. Potentiometric Biosensor Based on Artificial Antibodies for an Alzheimer Biomarker Detection. Applied Sciences-Basel 2022, 12. [Google Scholar] [CrossRef]
- Chen, J.Y.; et al. Development of an Electrochemical Sensing Technique for Rapid Genotyping of Hepatitis B Virus. Sensors 2014, 14, 5611–5621. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; et al. An innovative wireless electrochemical card sensor for field-deployable diagnostics of Hepatitis B surface antigen. Scientific Reports 2023, 13. [Google Scholar] [CrossRef]
- Fan, P.Y.; et al. A new electrochemical DNA biosensor based on the density control strategy of TiCNH MXene@Au nanocomposites for the detection of hepatitis B virus-DNA. Ionics 2024, 30, 541–552. [Google Scholar] [CrossRef]
- Li, X.; Scida, K.; Crooks, R.M. Detection of Hepatitis B Virus DNA with a Paper Electrochemical Sensor. Analytical Chemistry 2015, 87, 9009–9015. [Google Scholar] [CrossRef]
- Pareek, S.; et al. An ultrasensitive electrochemical DNA biosensor for monitoring (HPV-16) using graphene oxide/Ag/Au nano-biohybrids. Analytical Biochemistry 2023, 663. [Google Scholar] [CrossRef]
- Yang, Y.X.; et al. APTES-Modified Remote Self-Assembled DNA-Based Electrochemical Biosensor for Human Papillomavirus DNA Detection. Biosensors-Basel 2022, 12. [Google Scholar] [CrossRef]
- Yang, Y.X.; et al. Electrochemical DNA Biosensors with Dual-Signal Amplification Strategy for Highly Sensitive HPV 16 Detection. Sensors 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, E.; et al. Electrochemical DNA-nano biosensor for the detection of cervical cancer-causing HPV-16 using ultrasmall Fe3O4-Au core-shell nanoparticles. Sensing and Bio-Sensing Research 2023, 40. [Google Scholar] [CrossRef]
- Chaibun, T.; et al. A Multianalyte Electrochemical Genosensor for the Detection of High-Risk HPV Genotypes in Oral and Cervical Cancers. Biosensors-Basel 2022, 12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




