Submitted:
18 May 2025
Posted:
19 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Investigate the role of microbiota-immune-brain crosstalk in neuroinflammatory disorders and synthesize key findings from the literature linking gut microbiota alterations to CNS pathology.
- Explore the potential of synthetic biology approaches—including engineered probiotics, microbial biosensors, and synthetic consortia—to modulate this axis and reduce neuroinflammation.
- Evaluate the current state of preclinical and translational research applying synthetic biology tools for CNS diseases.
- Propose future directions and therapeutic strategies based on existing data and knowledge gaps.
Methods
2.1. Literature Search and Selection Criteria
- Original experimental studies or systematic reviews published in peer-reviewed journals.
- Preclinical or translational studies using synthetic biology approaches to influence gut or neuroimmune responses.
- Studies published in English with full-text access.
- Exclusion criteria were:
- Non-peer-reviewed content (e.g., opinion articles without data).
- Studies focusing solely on dietary interventions without microbial engineering components.
2.2. Categories of Synthetic Biology Interventions Studied
- Engineered Probiotic Strains: These include genetically modified strains of Lactobacillus, Bifidobacterium, Escherichia coli Nissle 1917, and others, engineered to produce immunomodulatory or neuroactive molecules. For example, some strains were modified to secrete IL-10, butyrate, GABA, or kynurenic acid to influence gut-immune-brain communication [11].
- Synthetic Microbial Consortia: Multi-strain consortia designed to mimic healthy gut ecosystems or perform modular functions—such as SCFA production, immune regulation, and oxidative stress buffering—were reviewed. These consortia were designed using in silico modeling and pathway optimization strategies [12].
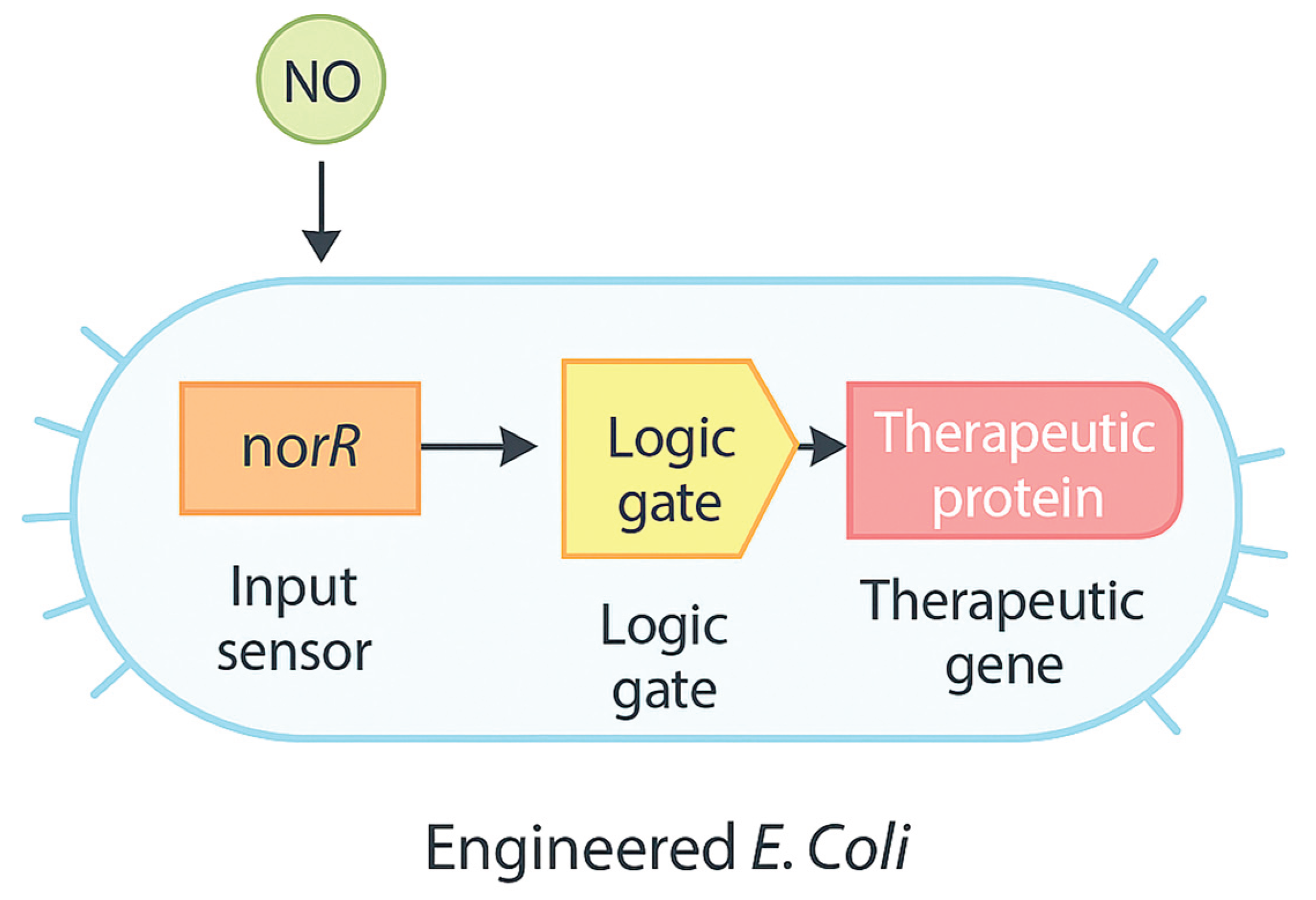
- Microbial Biosensors and Responsive Systems: Studies describing genetically encoded biosensors that detect inflammatory signals (e.g., TNF-α, nitric oxide, ROS) and trigger therapeutic gene expression in response were included. These tools allow for spatially and temporally controlled drug delivery in the gut [13], see Table 1.
- Studies focusing solely on dietary interventions without microbial engineering components.
2.3. Evaluation Metrics
- Immune modulation markers: TNF-α, IL-6, IL-10, Treg/Th17 ratio.
- Neuroinflammation indicators: Microglial activation (Iba1), CNS cytokine levels, astrocyte reactivity.
- Neurobehavioral endpoints: Anxiety- and depression-like behavior in murine models (e.g., open field test, forced swim test).
- Metabolite quantification: Butyrate, propionate, GABA levels via LC-MS/MS or NMR.
- Barrier integrity: Tight junction protein expression (occludin, claudin-5) and blood-brain barrier permeability assays.
2.4. Data Integration and Review Strategy
3. Results
3.1. Engineered Probiotics Produce Neuroactive and Anti-inflammatory Molecules
3.2. Synthetic Microbial Consortia Restore Gut-CNS Homeostasis
3.3. Biosensor-Equipped Bacteria Enable Inflammation-Responsive Therapy
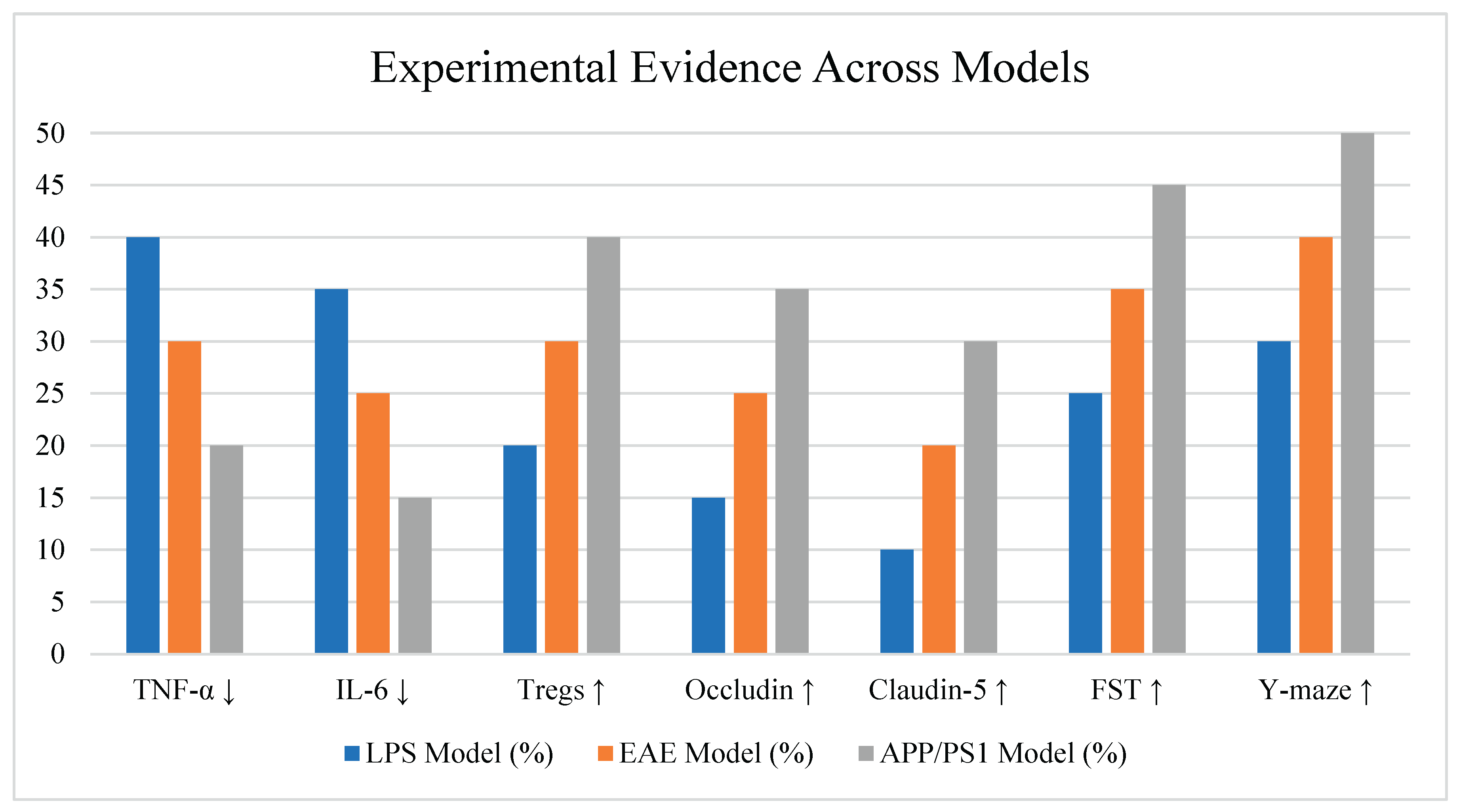
3.4. Behavioral and Cognitive Improvements Correlate with Inflammatory Reduction
- Open field and elevated plus maze tests (less anxiety-like behavior)
- Y-maze and Morris water maze tests (better spatial memory)
- Forced swim and tail suspension tests (reduced depressive-like behavior)
4. Discussion
Conflict Of Interest Statement
Acknowledgments
Funding Statement
Ethical Approval Statement
Data Availability Statement
References
- Bober, J. R., Beisel, C. L., & Nair, N. U. (2018). Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. Annual Review of Biomedical Engineering, 20, 277–300. [CrossRef]
- Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Toth, M., & Pettersson, S. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine, 6(263), 263ra158. [CrossRef]
- Cameron, D. E., Bashor, C. J., & Collins, J. J. (2014). A brief history of synthetic biology. Nature Reviews Microbiology, 12(5), 381–390. [CrossRef]
- Charbonneau, M. R., Isabella, V. M., Li, N., & Kurtz, C. B. (2020). Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nature Communications, 11, 1738. [CrossRef]
- Chowdhury, S., Castro, S., Coker, C., et al. (2019). Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nature Medicine, 25(7), 1057–1063. [CrossRef]
- Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., & Dinan, T. G. (2019). The microbiota-gut-brain axis. Physiological Reviews, 99(4), 1877–2013. [CrossRef]
- Dalile, B., Van Oudenhove, L., Vervliet, B., & Verbeke, K. (2019). The role of short-chain fatty acids in microbiota–gut–brain communication. Nature Reviews Gastroenterology & Hepatology, 16(8), 461–478. [CrossRef]
- Dempsey, J. L., & Cui, J. Y. (2019). Interspecies differences in gut microbiota and host drug metabolism. Toxicological Research, 35(2), 75–84.
- Durand, H., Bernatchez, M., & Langlois, M. R. (2022). GABA-producing probiotics for stress and sleep: Mechanistic insights and translational potential. Current Opinion in Clinical Nutrition and Metabolic Care, 25(4), 328–334.
- Erny, D., Hrabe de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., & Prinz, M. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience, 18(7), 965–977. [CrossRef]
- Evrensel, A., & Ceylan, M. E. (2015). The gut-brain axis: The missing link in depression. Clinical Psychopharmacology and Neuroscience, 13(3), 239–244. [CrossRef]
- Isabella, V. M., Ha, B. N., Castillo, M. J., et al. (2018). Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nature Biotechnology, 36(9), 857–864. [CrossRef]
- Mimee, M., Citorik, R. J., & Lu, T. K. (2015). Programming a human commensal bacterium to sense and respond to stimuli in the murine gut. Cell Systems, 1(1), 62–71. [CrossRef]
- Odah, M.A.A. Exploring the Role of DNA Methylation in Regulating Gene Expression and Adaptation in Plants: A Case Study on the Impact of Environmental Stress on Gene Regulation. African Research Journal of Biosciences 2024, 1, 117–123. [CrossRef]
- Odah, M.A.A. From the Double Helix to Precision Genomics: A Comprehensive Review of DNA and Its Transformative Role in Biomedical Sciences. African Research Journal of Biosciences 2024, 1, 72–88. [CrossRef]
- Odah, M. (2025). Photosynthetic Reprogramming Enhancing Carbon Fixation in Crops through Synthetic Biology. Preprints. [CrossRef]
- Odah, M. (2025). Ultra-Short DNA Satellites as Environmental Sensing Elements in Soil Microbiomes: A Frontier Review. Preprints. [CrossRef]
- Odah, M. A. A. (2025). Mitochondrial Epitranscriptomics: The Role of RNA Modifications in Cellular Energy Regulation and Aging. Preprints. [CrossRef]
- Odah, M. (2025). Artificial Intelligence Meets Drug Discovery: A Systematic Review on AI-Powered Target Identification and Molecular Design. Preprints. [CrossRef]
- Riglar, D. T., & Silver, P. A. (2018). Engineering bacteria for diagnostic and therapeutic applications. Nature Reviews Microbiology, 16(4), 214–225. [CrossRef]
- Steidler, L., Hans, W., Schotte, L., et al. (2000). Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science, 289(5483), 1352–1355. [CrossRef]
- Sun, M., He, C., Cong, Y., & Liu, Z. (2020). Regulatory role of commensal microbiota in neurodegenerative diseases. Frontiers in Immunology, 11, 2828.
- Thursby, E., & Juge, N. (2017). Introduction to the human gut microbiota. Biochemical Journal, 474(11), 1823–1836. [CrossRef]
- Tremlett, H., Bauer, K. C., Appel-Cresswell, S., Finlay, B. B., & Waubant, E. (2017). The gut microbiome in human neurological disease: A review. Annals of Neurology, 81(3), 369–382. [CrossRef]
- Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Scientific Reports, 7(1), 13537. [CrossRef]
- Wang, H., Xu, X., Nguyen, C. M., Liu, Y., Gao, Y., Lin, X., & Yang, L. (2020). CRISPR-mediated microbial therapeutics: Challenges and future prospects. Trends in Biotechnology, 38(9), 918–933. [CrossRef]
- Zhou, Y., Li, Y., Chen, C., et al. (2023). Synthetic microbial consortia for gut-brain axis modulation. ACS Synthetic Biology, 12(2), 217–230.
- Odah, M. (2024). Epigenetic Reprogramming of Aging: Reversing the Clock for Regenerative Medicine. Preprints. [CrossRef]
- Odah, M. A. A. (2024). The Vital Role of Non-coding RNA Regions in Hemoglobin Gene Regulation. Preprints. [CrossRef]
- Odah, M. (2024). Barcoding of Atropa Belladonna’s DNA: A Systemic Review. Preprints. [CrossRef]

| Microbial Strain | Engineered Function | Target Disease Model | Delivery Mode | Key Outcome |
|---|---|---|---|---|
| L. plantarum | GABA production | LPS-induced anxiety | Oral gavage | Reduced inflammation, anxiety |
| E. coli Nissle 1917 | IL-10 secretion | EAE (MS model) | Oral capsule | Decreased microglial activation |
| Synthetic consortia | SCFA production | APP/PS1 (AD model) | Oral mix | Improved cognition, BBB repair |
| Study | Model | Inflammatory Markers | Neurobehavioral Effects | Intervention |
|---|---|---|---|---|
| Durand et al., 2022 | LPS model | ↓ IL-6, TNF-α | ↓ Anxiety behavior | GABA-probiotic |
| Steidler et al., 2000 | EAE mice | ↓ Microglial Iba1 | ↑ Motor recovery | IL-10 probiotic |
| Zhou et al., 2023 | APP/PS1 mice | ↓ Astrocyte GFAP | ↑ Memory retention | Synthetic SCFA mix |
| Challenge | Impact | Potential Synthetic Biology Solution | References |
|---|---|---|---|
| Colonization inefficiency | Reduced therapeutic persistence and efficacy | Engineer strains with adhesion factors and niche-specificity | Riglar & Silver, 2018 |
| Host-to-host microbiome variability | Variable outcomes and lack of standardization | Design personalized microbial consortia using metagenomic data | Dempsey & Cui, 2019 |
| Safety concerns (e.g., horizontal gene transfer) | Potential ecological risks and off-target effects | Integrate kill switches and biocontainment circuits | Wang et al., 2020 |
| Immune rejection or dysregulation | Inflammatory response or probiotic clearance | Use immune-modulatory gene circuits or tolerogenic strains | Mimee et al., 2016 |
| Limited regulatory frameworks | Barriers to clinical approval and scalability | Develop standard biosafety frameworks and genetic part registries | Bober et al., 2018 |
| Category | Biomarker | Function / Significance |
|---|---|---|
| Microbial Metabolites | Butyrate | SCFA; HDAC inhibitor; anti-inflammatory; improves BBB integrity |
| Propionate | SCFA; modulates immune tolerance; affects neurotransmitter balance | |
| Acetate | SCFA; enhances mucosal immunity and brain energy metabolism | |
| GABA | Inhibitory neurotransmitter; modulates vagal nerve and immune response | |
| Indole derivatives | Tryptophan catabolites; AHR ligands; immune modulators and tight-junction regulators | |
| Immune Markers | IL-10 | Anti-inflammatory cytokine; downregulates Th1/Th17 responses |
| IL-6 | Pro-inflammatory cytokine; elevated in CNS and gut inflammation | |
| TNF-α | Major pro-inflammatory cytokine; stimulates microglial activation | |
| IFN-γ | Th1 cytokine; increases blood-brain barrier permeability and inflammation | |
| Barrier Proteins | Claudin-5 | Tight junction protein; crucial for BBB integrity |
| Occludin | Maintains epithelial and BBB tight junctions | |
| ZO-1 | Zonula occludens-1; scaffolds tight junction assembly in epithelial and endothelial tissues | |
| CNS Inflammation Indicators | Iba1 | Marker of microglial activation; increased in neuroinflammation |
| GFAP | Marker of astrocyte reactivity; elevated in neurodegenerative diseases | |
| Amyloid-β (Aβ) | Protein aggregates implicated in Alzheimer’s pathology; inflammation promotes aggregation |
| Challenge | Impact | Synthetic Biology Solution | Reference |
|---|---|---|---|
| Colonization inefficiency | Reduced therapeutic effect | Use of colonization factors or adhesins | [Riglar & Silver, 2018] |
| Safety concerns | Regulatory and patient risk | Kill switches, biocontainment systems | [Wang et al., 2020] |
| Host variability | Response inconsistency | Personalized microbiota-based designs | [Dempsey & Cui, 2019] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





