Submitted:
15 May 2025
Posted:
16 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- What specific barriers impede the efficient transfer of longevity technologies from laboratory to market, and how do these differ from challenges in other fields?
- Which technology transfer models have demonstrated success in the longevity sector, and what common elements might be replicable across organizations?
- How does the academic-industry interface function in the longevity sector, and what innovations in intellectual property management and licensing strategies might accelerate commercialization?
- What market segmentation strategies enable the successful commercialization of longevity technologies across different consumer groups?
- What policy and regulatory changes could create more efficient pathways for longevity technology commercialization while maintaining appropriate safety standards?
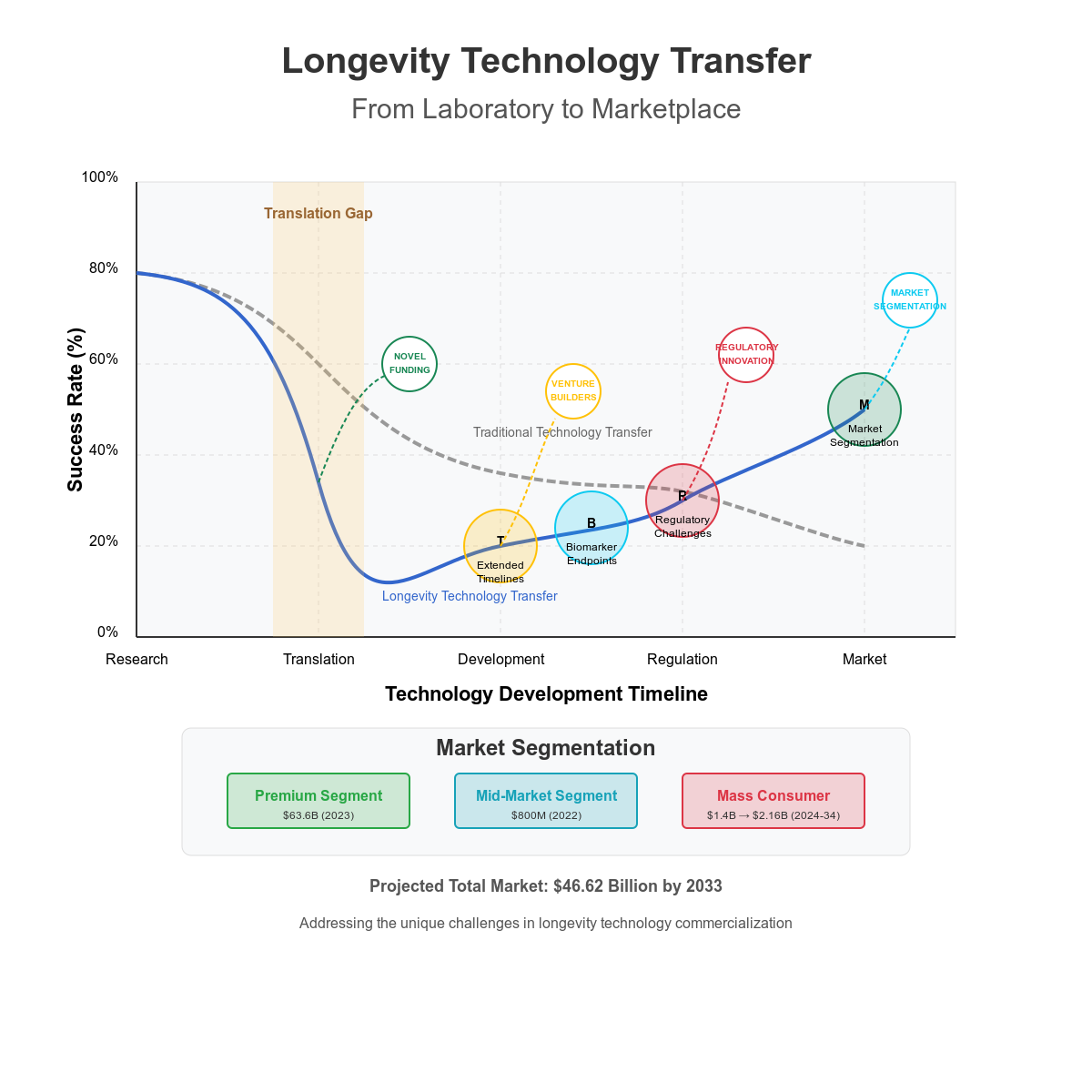
2. Unique Challenges in Longevity Technology Transfer
2.1. The Regulatory Pathway Problem
2.2. Extended Validation Timelines
2.3. Market Uncertainty and Segmentation
- Determining the optimal initial market position for a given technology
- Designing staged commercialization strategies that can move across market tiers
- Developing appropriate business models and pricing strategies for each segment
- Balancing exclusive access for early adopters with eventual broader accessibility
2.4. Appropriate Endpoints and Biomarkers
3. Case Studies in Longevity Commercialization
3.1. Success Cases
3.1.1. Alkahest: Strategic Acquisition as Technology Transfer Outcome
- Plasma Fractionation Focus: Rather than pursuing “young blood” transfusions directly, Alkahest identified specific protein fractions with therapeutic potential that could be developed as conventional biologics.
- Early Strategic Partnership: In 2015, just one year after founding, Alkahest formed a strategic partnership with Grifols, a major plasma products company, which invested $50 million for a 45% stake and provided industry expertise.
- Multiple Clinical Programs: The company developed several clinical-stage programs for specific indications, including Alzheimer’s disease, Parkinson’s disease, and age-related macular degeneration, creating multiple potential paths to market.
3.1.2. Rejuvenate Bio: From Academia to Industry
-
Veterinary-First Approach: Rather than immediately pursuing human applications, Rejuvenate Bio first targeted companion animal applications, particularly for dogs. This strategy provided several advantages:
- o
- Faster path to market with less stringent regulatory requirements
- o
- Ability to generate revenue and efficacy data while developing human applications
- o
- Opportunity to refine delivery mechanisms and dosing in relevant mammalian models
- Incremental Financing: Unlike other longevity companies’ large early funding rounds, Rejuvenate Bio raised capital in smaller increments tied to specific milestones, maintaining greater founder control and allowing for strategic flexibility.
- Multi-Target Mechanism: The company’s gene therapy approach targets multiple aging pathways simultaneously, potentially addressing the multifactorial nature of aging more comprehensively than single-target approaches.
3.1.3. Shift Bioscience: AI-Driven Discovery and Commercialization
- AI-First Discovery Platform: The company uses computational methods to identify interventions that can target multiple hallmarks of aging simultaneously, potentially addressing the regulatory pathway problem by targeting specific age-related conditions with broader aging benefits.
- Translational Focus: The company is explicitly focused on identifying the “translatability landscape” of age-linked diseases to identify the safest and most promising paths for drug development.
- Staged Validation Approach: Shift is taking a systematic approach to validation, beginning with human fibroblasts and expanding to other cell types and animal models before proceeding to human trials.
3.2. Failure Cases
3.2.1. Unity Biotechnology: Lessons from a Pivot
- Focused Application Approach: Rather than targeting aging broadly, Unity selected specific indications, beginning with osteoarthritis, to create a clearer regulatory pathway.
- Strong IP Position: The company secured exclusive licenses to foundational patents covering senolytic approaches, creating a defensible market position.
- Scientific Credibility: The involvement of leading researchers in the aging field, including Judith Campisi and Jan van Deursen, provided scientific validation.
- The difficulty of translating promising preclinical senolytic results into clinical outcomes
- The risks of committing to a single technological approach before robust human validation
- The pressure to select disease-specific indications that may not best showcase the technology’s potential
3.2.2. Common Failure Patterns
- Premature Commercialization: Companies that rushed to market with insufficient validation data, particularly those marketing direct-to-consumer products based on preliminary science, have consistently struggled (CB Insights, 2023).
- Inflexible Development Paths: Organizations committed to specific regulatory or development strategies without contingency plans for scientific setbacks or regulatory changes have faced significant challenges (Dror, 2024).
- Misaligned Investor Expectations: Startups whose investors expected traditional drug development timelines and returns have frequently encountered pressure for premature pivots or excessive focus on short-term milestones (Deming, 2020).
- Insufficient Translational Research: Companies founded on basic research findings that had not undergone rigorous translational validation before commercialization attempts have shown high failure rates (Fishburn, 2019).
- Isolated Technology Development: Firms that developed technologies without engaging the broader ecosystem of researchers, clinicians, regulators, and patients necessary for successful implementation have struggled to gain traction (Tse et al., 2020).
4. The Academic-Industry Interface in Longevity Science
4.1. University Technology Transfer Offices and Longevity Innovation
- Valuation Challenges: Traditional approaches to valuing intellectual property rely on comparables and projections that may not exist for novel longevity interventions with uncertain regulatory paths. This uncertainty often leads to either overvaluation based on total addressable market or undervaluation based on short-term commercialization potential.
- Milestone Structure: Standard licensing agreements typically include development milestones tied to conventional drug development phases (IND filing, Phase 1/2/3 completion, marketing approval). For longevity technologies that may follow non-traditional paths or target aging processes rather than specific diseases, these milestones may be inappropriate.
- Faculty Inventor Engagement: Longevity technologies often require ongoing involvement from academic inventors due to the complex, evolving nature of the science. Traditional technology transfer models that assume clean hand-offs to industry may be insufficient.
- IP Strategy Limitations: Patents filed by universities often focus on initial discoveries rather than the translational adaptations necessary for commercial viability. For longevity technologies, where significant translation is typically required, this can leave critical developments unprotected.
4.2. Academic Spinoffs vs. Licensing to Established Companies
- Platform Potential: Technologies with applications across multiple aspects of aging benefit from dedicated organizations that can pursue several development paths simultaneously.
- Paradigm-Shifting Approaches: Truly novel approaches that don’t fit within established company research programs may require new organizations unencumbered by existing commercial commitments.
- Founder Expertise Dependence: Technologies that rely heavily on the continuing expertise of academic founders often benefit from spinoff structures that keep these individuals closely involved.
- Ecosystem Development Needs: Some longevity approaches require the simultaneous development of complementary technologies, biomarkers, and analytical tools best coordinated within a focused organization.
4.3. Alternative Technology Transfer Vehicles
- Research Collaborations with Option Rights: Long-term collaborations between academic institutions and companies that fund basic research while providing the corporate partner with options to license resulting technologies. The Novartis-Buck Institute collaboration exemplifies this approach, providing sustained funding for basic aging research while establishing predetermined licensing terms for commercial applications (Buck Institute, 2021).
- Joint Ventures Between Academia and Industry: Formally structured joint ventures that maintain academic research activities while creating dedicated commercialization capabilities. The Salk Institute’s Age Science venture with Astera Institute represents this model, combining academic research excellence with focused development resources (Salk Institute, 2022).
- Hybrid Academic-Commercial Entities: Organizations that maintain both academic research operations and commercial development activities under coordinated leadership. Human Longevity Inc.’s structure incorporated elements of this approach, though with mixed results that highlight implementation challenges.
- Technology Accelerators with Specialized Focus: Programs specifically designed to advance longevity technologies through the earliest stages of translation. The Longevity Tech Fund’s accelerator program, which provides both capital and specialized mentoring focused on aging technologies, exemplifies this approach (Dror, 2024).
5. Emerging Models for Accelerating Longevity Translation
5.1. Novel Funding Approaches
- Specialized Funds with Extended Time Horizons: Dedicated longevity investment vehicles like Apollo Health Ventures and the Longevity Fund have established extended fund lifespans (12-15 years versus the traditional 10) specifically to accommodate the longer development cycles of aging interventions (Dror, 2024).
- Non-dilutive Funding Strategies: Strategic use of government grants, foundation awards, and corporate partnerships to extend runway without dilution pressure. The National Institute on Aging’s Small Business Innovation Research (SBIR) program has become an important source of such funding for longevity startups.
- Public-Private Partnerships: Collaborative funding structures that combine public research support with private capital, often focused on specific translation challenges in aging research. The Buck Institute’s collaborations with pharmaceutical companies exemplify this approach.
5.2. Regulatory Innovation
- Biomarker Qualification Pathways: Efforts to validate aging biomarkers as acceptable surrogate endpoints for clinical trials. The FDA’s Biomarker Qualification Program provides a potential avenue for establishing such endpoints, though longevity-specific biomarkers remain in early stages of qualification (FDA, 2023).
- Adaptive Trial Designs: Clinical trial approaches that allow modification based on interim results, potentially accelerating the evaluation of aging interventions. The TAME trial design incorporates adaptive elements that could serve as a template for future aging-focused studies (Barzilai et al., 2016).
- International Regulatory Harmonization: Cross-border initiatives to standardize the evaluation of aging interventions. The European Medicines Agency’s Innovation Task Force has engaged with developers of several longevity technologies to establish consistent evaluation frameworks (EMA, 2022).
5.3. The Venture Builder Approach
- Juvenescence: Founded in 2017, Juvenescence combines venture investment with direct company creation. The organization identifies promising academic research, forms dedicated companies around specific technologies, and provides centralized resources for clinical development, regulatory affairs, and commercialization (Juvenescence, 2023).
- Life Biosciences: Established in 2017, Life Biosciences initially pursued a model of creating “daughter companies” focused on different hallmarks of aging, with shared central resources. While the company later consolidated this structure, the approach exemplified how centralized capabilities can support multiple parallel development programs targeting different aspects of aging.
- Cambrian Biopharma: Operating as a “distributed drug discovery company,” Cambrian identifies promising academic research, creates dedicated development programs around specific assets, and supports them with centralized expertise in drug development, clinical operations, and financing (Cambrian Biopharma, 2024).
- It reduces the inefficiency of each startup building its own complete infrastructure
- It provides specialized expertise in regulatory strategy specific to aging interventions
- It enables longer development runways through more efficient resource allocation
- It facilitates cross-program learning about common challenges in aging research translation
6. Market Segmentation and Commercialization Strategies
6.1. The Three-Tier Market Structure
-
Premium/Elite Segment: High-net-worth individuals accessing personalized, cutting-edge interventions often through concierge medicine models. This segment was estimated at $63.60 billion in 2023 (Grand View Research, 2024). Key characteristics include:
- o
- Limited price sensitivity
- o
- Willingness to accept experimental approaches
- o
- Demand for personalization
- o
- Preference for integrated service models over isolated products
-
Mid-Market Segment: Health-conscious consumers with disposable income accessing pharmacy-grade supplements, biomarker testing, and digital health platforms. This segment was valued at nearly $800 million in 2022 (Grand View Research, 2023). Key characteristics include:
- o
- Moderate price sensitivity
- o
- Interest in preventive health
- o
- Preference for convenience and accessibility
- o
- Desire for scientific validation but not necessarily clinical trial evidence
-
Mass Consumer Segment: Broadly available products with aging-related claims, including basic supplements, monitoring devices, and wellness programs. This segment is projected to grow from $1.4 billion in 2024 to $2.16 billion by 2034 (Future Market Insights, 2024). Key characteristics include:
- o
- High price sensitivity
- o
- Need for simple messaging and clear benefits
- o
- Distribution through established retail channels
- o
- Preference for familiar formats and minimal behavior change
6.2. Technology-Market Fit Considerations
- Regulatory Status: Technologies requiring formal approval typically enter through the premium segment before broader diffusion, while those that can be marketed as supplements or devices may target the mid-market or mass segments directly.
- Delivery Complexity: Interventions requiring specialized administration (IV delivery, medical procedures) naturally align with the premium segment, while oral supplements or digital applications can more easily reach broader markets.
- Evidence Threshold: The strength of scientific evidence influences appropriate market positioning. Clinical-grade evidence enables premium pricing and healthcare system integration, while early-stage science may be better suited to the supplement market with appropriate claim limitations.
- Cost Structure: Production costs and economies of scale determine feasible price points and, consequently, appropriate market segments. Biologics with high production costs may be constrained to premium segments, while small molecules may more readily access mass markets.
7. Global Perspectives on Longevity Commercialization
7.1. Regional Regulatory Approaches
- United States: Strong venture financing ecosystem and academic research base, but fragmented healthcare system complicates reimbursement for preventive interventions. The FDA has not formally recognized aging as a treatable indication.
- European Union: The EMA has shown greater flexibility in considering novel endpoints for age-related conditions. Several EU nations have established distinctive approaches, with France and Germany implementing mechanisms to reimburse certain preventive interventions through their healthcare systems.
- Japan: Demographic pressures have spurred regulatory accommodations for age-related technologies. The SAKIGAKE designation for accelerated approval has been applied to several aging interventions, and the country has implemented an accelerated pathway for regenerative medicines that could benefit certain longevity approaches (PMDA, 2014).
- Singapore: Strategic national investments in aging research with a centralized healthcare system have created an efficient ecosystem, attracting substantial commercialization activity.
7.2. Regulatory Arbitrage in Longevity Development
- Cell Therapies for Aging: Companies developing cell-based interventions for age-related conditions have increasingly conducted initial trials in Japan, which implemented an accelerated approval pathway for regenerative medicines in 2014.
- Combination Approaches to Aging: Interventions that combine multiple compounds or modalities have found more receptive regulatory environments in Singapore and parts of the EU, where regulators have shown greater willingness to consider holistic approaches to age-related syndromes.
- Consumer Applications of Aging Biomarkers: Direct-to-consumer applications of aging biomarkers have seen accelerated commercialization in regulatory environments with less restrictive oversight of laboratory-developed tests, notably including certain EU countries and Australia.
8. Recommendations and Conclusions
8.1. For Researchers and Entrepreneurs
- Develop Multi-Path Commercialization Strategies: Plan for multiple potential development pathways rather than committing to a single approach, recognizing the regulatory uncertainty and potential for scientific surprises in aging research.
- Establish Biomarker Validation Early: Integrate aging biomarker development and validation into research programs from the beginning, creating potential surrogate endpoints for later clinical studies.
- Consider Staged Market Entry: Evaluate whether your technology has applications that could reach market more quickly (consumer products, veterinary applications) while pursuing longer-term medical applications in parallel.
- Build Interdisciplinary Teams: Ensure your research or founding team includes expertise not only in aging biology but also in translational medicine, regulatory affairs, and commercial development.
- Engage Regulators Early: Initiate discussion with regulatory authorities about potential pathways for your technology well before clinical development, potentially shaping how your intervention will be evaluated.
8.2. For Technology Transfer Professionals
- Develop Specialized Expertise: The unique aspects of longevity technology commercialization require dedicated knowledge of aging biology, relevant regulatory frameworks, and specialized development pathways.
- Implement Flexible Licensing Structures: Develop adaptable frameworks with milestone structures that reflect the realities of aging intervention development rather than conventional drug timelines.
- Facilitate Connections to Specialized Investors: Cultivate relationships with investors specifically focused on the longevity sector and familiar with its unique characteristics.
- Consider Alternative Commercialization Vehicles: Beyond traditional licensing and spinoffs, explore joint development programs, phased option agreements, and public-private consortia that better accommodate longevity technologies.
- Adopt Global Perspective from Inception: Incorporate international considerations from the earliest stages of licensing strategy rather than treating international markets as secondary opportunities.
8.3. For Policymakers
- Establish Regulatory Pathways for Aging Interventions: Create clearly defined regulatory frameworks that recognize aging processes as appropriate targets for intervention.
- Develop Standardized Aging Biomarker Validation: Coordinate international efforts to validate and standardize biomarkers of aging as acceptable endpoints for clinical development.
- Create Specialized Funding Mechanisms: Establish public funding programs specifically designed to bridge the gap between basic aging research and commercial investment.
- Implement Longevity-Specific Technology Transfer Training: Support educational programs focused on the unique aspects of commercializing aging research.
- Establish International Harmonization Initiatives: Coordinate international approaches to regulation, data sharing, and intellectual property protection for aging interventions.
8.4. Conclusion and Future Outlook (Continued)
- The integration of artificial intelligence across the longevity technology value chain, from target discovery to clinical trial optimization
- The convergence of digital health with biological aging interventions, creating hybrid approaches to monitoring and modifying aging processes
- The increasing engagement of major healthcare systems in preventive aging strategies as demographic pressures intensify
- The evolution of reimbursement models incorporating healthspan metrics as economic arguments for prevention strengthen
- The development of personalized aging intervention approaches based on individual aging trajectories and genetic profiles
References
- Amaglobeli, D., Chai, H., Dabla-Norris, E., Dybczak, K., Soto, M., & Tieman, A. (2019). The future of saving: The role of pension system design in an aging world. IMF Staff Discussion Note.
- Aging Analytics Agency. (2022). Longevity Industry 2022: Financial Industry Report. Aging Analytics Agency Ltd.
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Cuervo, A.M.; Austad, S. Aging as a Biological Target for Prevention and Therapy. JAMA 2018, 320, 1321–1322. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S. R., Hayter, C. S., & Link, A. N. (2013). Models and methods of university technology transfer. Foundations and Trends in Entrepreneurship, 9(6), 571-650.
- Buck Institute for Research on Aging. (2021). Translational Research Partnerships. Retrieved from https://www.buckinstitute.org/research/partnerships/.
- Business Research Insights. (2024). Longevity and Anti-senescence Market Report, 2024-2033.
- Cambrian Biopharma. (2024). Company Overview. https://www.cambrianbio.com.
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- CB Insights. (2023). Digital Health 150: The Digital Health Companies Transforming Healthcare.
- Deloitte. (2024). The future of health: How innovation will blur traditional healthcare boundaries.
- Deming, T. J. (2020). Biotech’s long road from innovation to impact. Nature Biotechnology, 38, 1280-1283.
- DePinho, R., et al. (2024). Advancing therapeutic strategies for age-related diseases. Cell, 187(3), 565-580.
- Dror, D. M. (2024). Investors in Longevity: Big Capital and the Future of Extending Life. Literary Letters.
- European Medicines Agency (EMA). (2022). Innovation Task Force Annual Report.
- FDA. (2023). Biomarker Qualification Program: Qualified Biomarkers.
- Fishburn, C. S. (2019). Why senescence is a hard sell. SciBX: Science-Business eXchange, 12(9), 254-255.
- Future Market Insights. (2024). Longevity and Anti-Aging Market Outlook 2024-2034.
- Grand View Research. (2023). Longevity Technology Market Size, Share & Trends Analysis Report.
- Grand View Research. (2024). Longevity and Anti-Aging Products Market Size Report, 2024-2032.
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Juvenescence Ltd. (2023). Company Overview. https://www.juvlabs.com.
- Kennedy, B.K.; Pennypacker, J.K. Drugs that modulate aging: the promising yet difficult path ahead. Transl. Res. 2014, 163, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- LabBiotech. (2024). Shift Bioscience secures $16M seed funding for AI cell simulation platform. Retrieved from https://labiotech.
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C., et al. (2023). The hallmarks of aging: An expanded framework. Cell, 186(2), 243-278.
- Marks, P.; Gottlieb, S. Balancing Safety and Innovation for Cell-Based Regenerative Medicine. New Engl. J. Med. 2018, 378, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Mowery, D. C., Nelson, R. R., Sampat, B. N., & Ziedonis, A. A. (2015). Ivory tower and industrial innovation: University-industry technology transfer before and after the Bayh-Dole Act. Stanford University Press.
- Newman, J.C.; Milman, S.; Hashmi, S.K.; Austad, S.N.; Kirkland, J.L.; Halter, J.B.; Barzilai, N. Strategies and Challenges in Clinical Trials Targeting Human Aging. Journals Gerontol. Ser. A Boil. Sci. Med Sci. 2016, 71, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, S. J. (2018). From lifespan to healthspan. JAMA, 320(13), 1323-1324.
- Papapetrou, E., & Tsalaporta, P. (2020). Macroeconomic implications of demographic aging: A global perspective. International Monetary Fund Working Paper.
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532. [Google Scholar] [CrossRef] [PubMed]
- PMDA (Pharmaceuticals and Medical Devices Agency). (2014). SAKIGAKE designation system. PMDA, Japan.
- Rothaermel, F.T.; Agung, S.D.; Jiang, L. University entrepreneurship: a taxonomy of the literature. Ind. Corp. Chang. 2007, 16, 691–791. [Google Scholar] [CrossRef]
- Salk Institute for Biological Studies. (2022). Age Science initiative launched with the Astera Institute.
- Sierra, F. (2020). Moving geroscience from bench to bedside: New targets, biomarkers, and clinical trials. Geroscience, 42(1), 1-3.
- Tse, H. F., Ho, J. C., Choi, S. W., & Lee, Y. K. (2020). Patient-specific induced-pluripotent stem cells-derived cardiomyocytes recapitulate the pathogenic phenotypes of dilated cardiomyopathy. Human Molecular Genetics, 29(18), 3456-3464.
- Unity Biotechnology. (2020). Unity Biotechnology announces 12-week data from UBX0101 Phase 2 clinical study in patients with painful osteoarthritis of the knee. Retrieved from https://ir.unitybiotechnology.com/news-releases/news-release-details/unity-biotechnology-announces-12-week-data-ubx0101-phase-2.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




