Submitted:
14 May 2025
Posted:
15 May 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Background
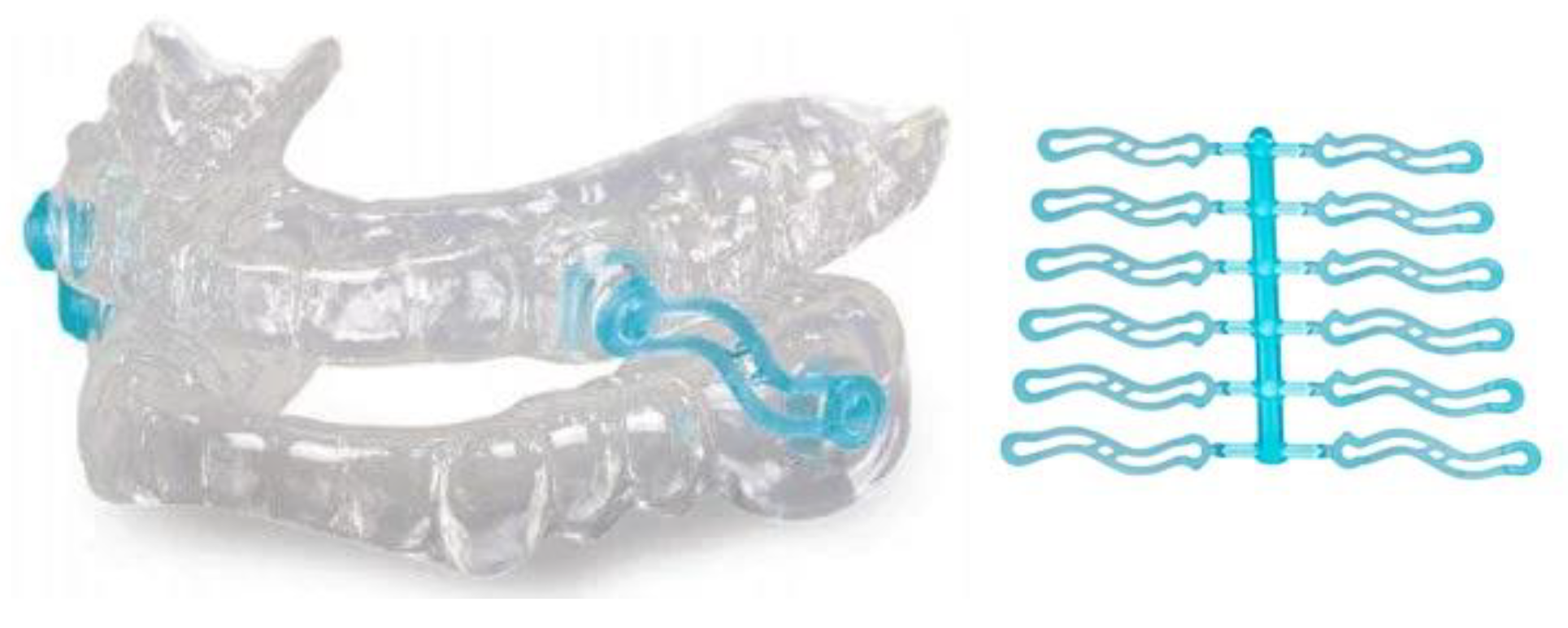
Prefabricated Displacement Screws
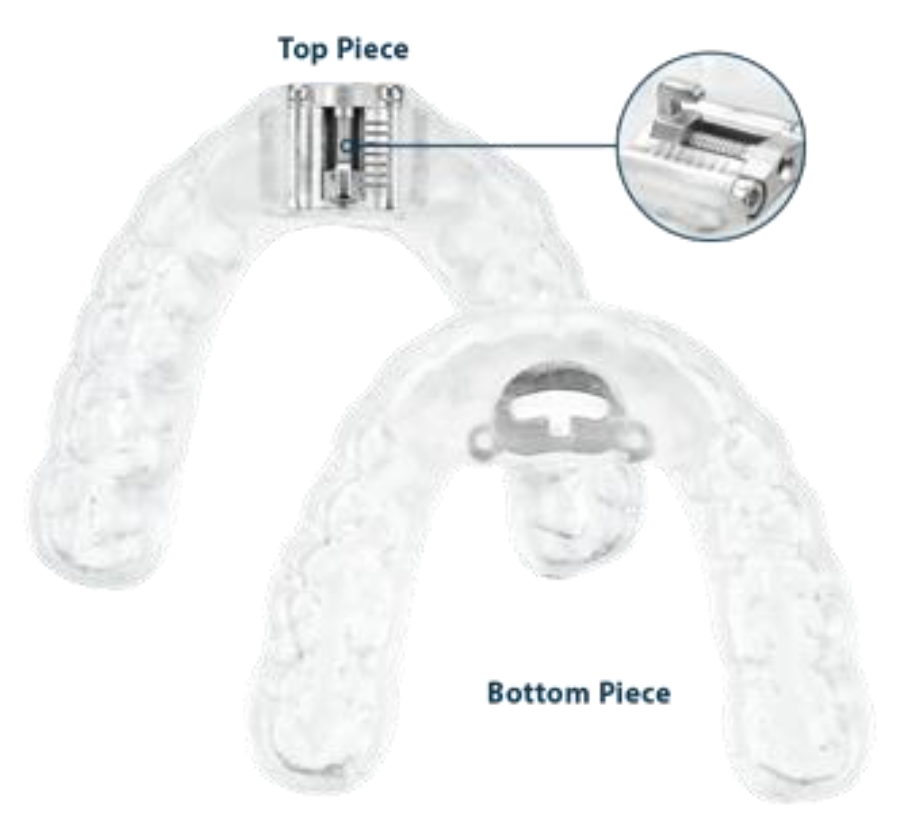
Prefabricated Interchangeable Connectors
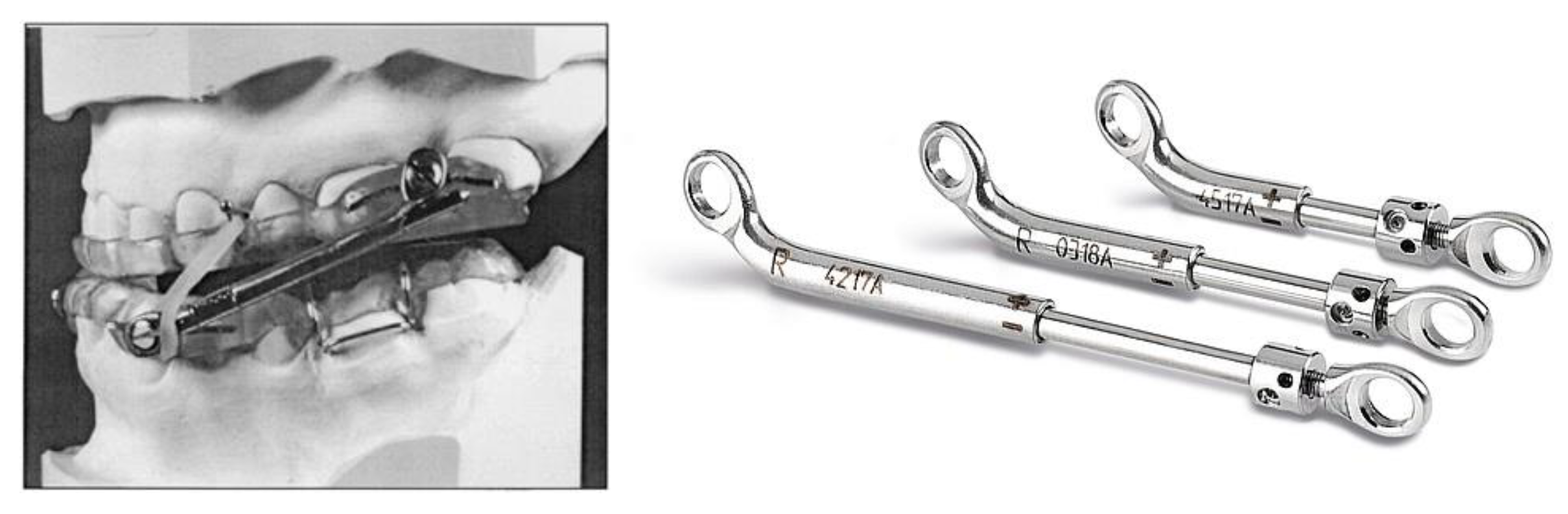
Custom Interlocking Overlays
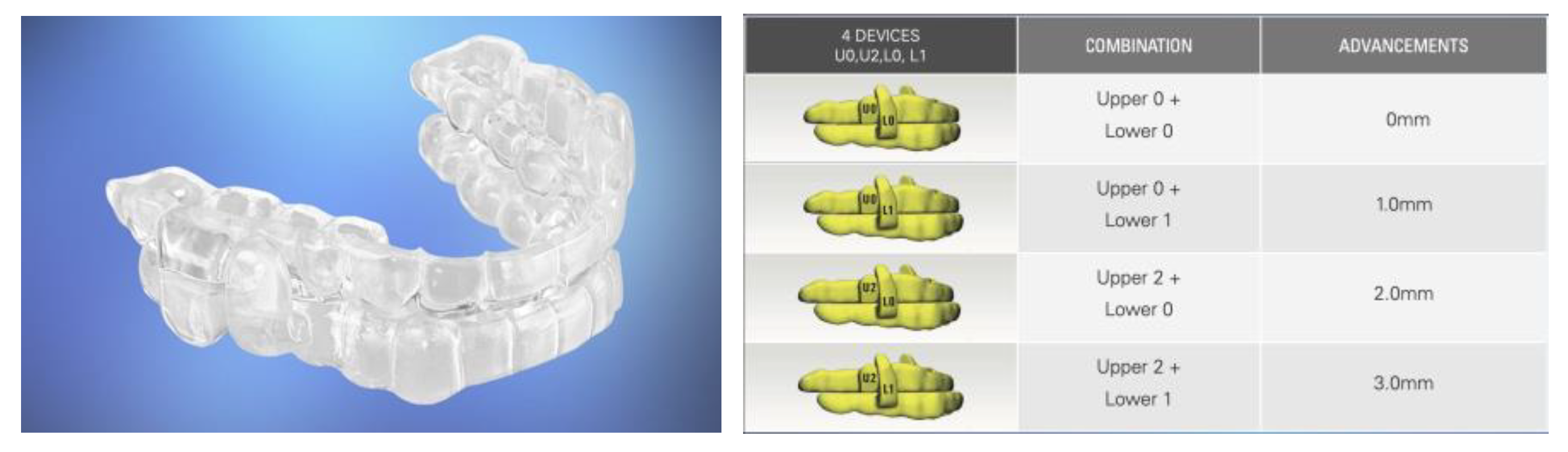
Semi-Custom Oral Appliances
Precision-Custom Oral Appliances
Review
- Is the OA made from records of an individual patient’s oral structures?
- Is the OA modified (trimmed, bent, relined) or primarily a prefabricated item?
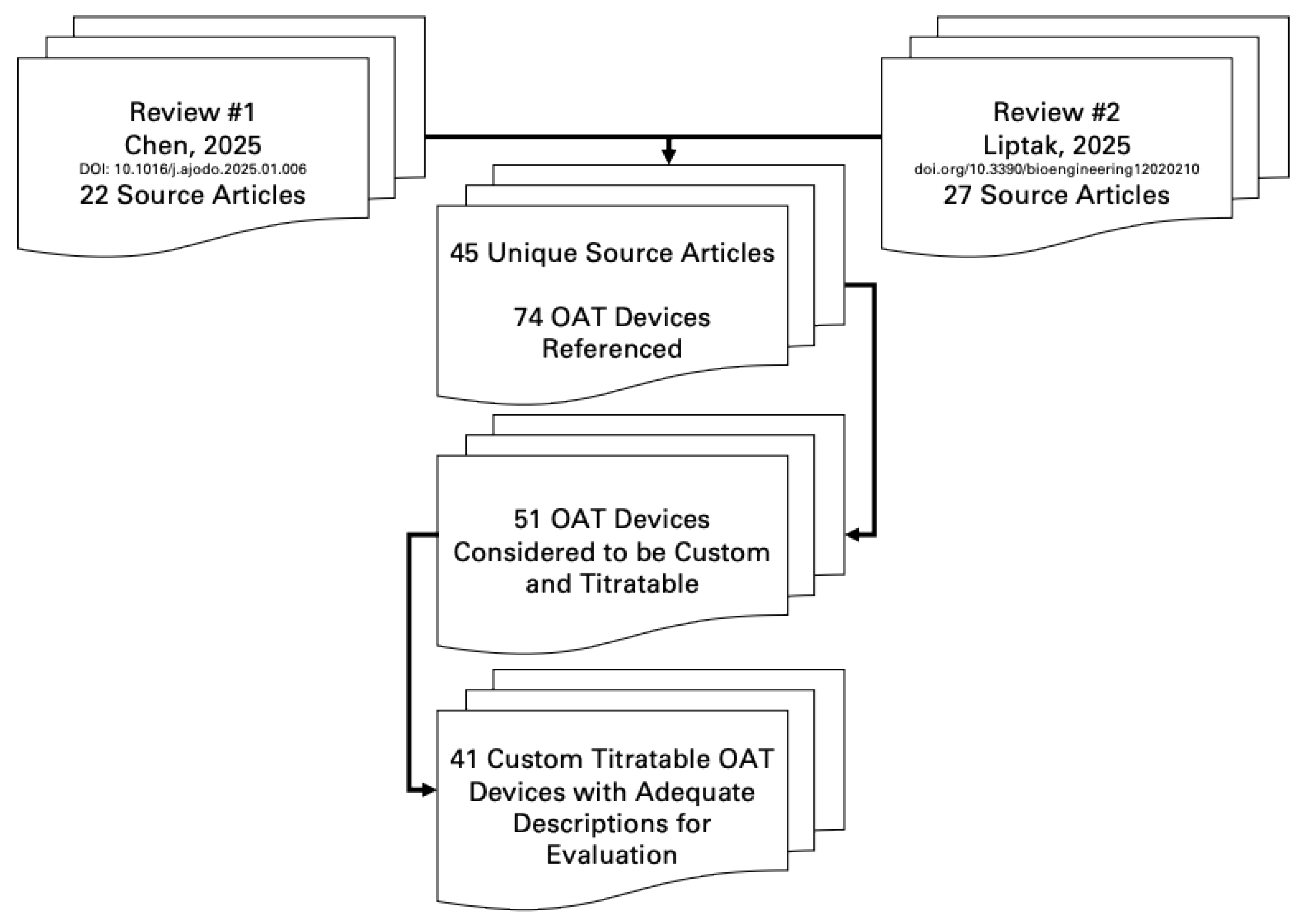
Results
Discussion
Implications for Efficacy?
Signs of Different Efficacy
Signs of Different Patient Preferences
Signs of Different Symptom Alleviation
Signs of Different Side Effects
Conclusions
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA. 2020 Apr 14;323(14):1389-1400. [CrossRef] [PubMed]
- Anandam, A.; Patil, M.; Akinnusi, M.; Jaoude, P.; El-Solh, A.A. Cardiovascular mortality in obstructive sleep apnoea treated with continuous positive airway pressure or oral appliance: An observational study. Respirology 2013, 18, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.L.; Woehrle, H.; Liu, D.; Shao, S.; Armitstead, J.P.; Cistulli, P.A.; Benjafield, A.V.; Malhotra, A. Adherence to Positive Airway Therapy After Switching From CPAP to ASV: A Big Data Analysis. J. Clin. Sleep Med. 2018, 15, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Bakker, J.P.; Stitt, C.J.; Aloia, M.S.; Nouraie, S.M. Age and Sex Disparities in Adherence to CPAP. Chest 2021, 159, 382–389. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 15, 773–827. [Google Scholar] [CrossRef]
- VA/DOD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF CHRONIC INSOMNIA DISORDER AND OBSTRUCTIVE SLEEP APNEA 2025. Available online: https://www.healthquality.va.gov/guidelines/CD/insomnia/I-OSA-CPG_2025-Guildeline_final_20250422.pdf (accessed on day month year).
- de Ruiter, M.H.T.; Aarab, G.; de Vries, N.; Lobbezoo, F.; de Lange, J. A Stepwise Titration Protocol for Oral Appliance Therapy in Positional Obstructive Sleep Apnea Patients: Proof of Concept. Sleep Breath. Schlaf Atm. 2020, 24, 1229–1236. [Google Scholar] [CrossRef]
- Kato J, Isono S, Tanaka A, Watanabe T, Araki D, Tanzawa H, Nishino T. Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest. 2000 Apr;117(4):1065-72. [CrossRef] [PubMed]
- Liptak, L.A.; Sall, E.; Kim, S.; Mosca, E.; Charkhandeh, S.; Remmers, J.E. Different Oral Appliance Designs Demonstrate Different Rates of Efficacy for the Treatment of Obstructive Sleep Apnea: A Review Article. Bioengineering 2025, 12, 210. [Google Scholar] [CrossRef]
- Chen Y, Zhang J, Gao X, Almeida FR. Efficacy and adherence of different mandibular advancement devices designs in treatment of obstructive sleep apnea: A systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2025 Mar 7:S0889-5406(25)00019-8. [CrossRef] [PubMed]
- Bloch, K.E.; Iseli, A.; Zhang, J.N.; Xie, X.; Kaplan, V.; Stoeckli, P.W.; Russi, E.W. A randomized, controlled crossover trial of two oral appliances for sleep apnea treatment. Am. J. Respir. Crit. Care Med. 2000, 162, 246–251. [Google Scholar] [CrossRef]
- Pépin JL, Raymond N, Lacaze O, Aisenberg N, Forcioli J, Bonte E, Bourdin A, Launois S, Tamisier R, Molinari N. Heat-moulded versus custom-made mandibular advancement devices for obstructive sleep apnoea: a randomised non-inferiority trial. Thorax. 2019 Jul;74(7):667-674. [CrossRef] [PubMed]
- Randerath, W.J.; Heise, M.; Hinz, R.; Ruehle, K.H. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest. 2002, 122, 569–575. [Google Scholar] [CrossRef]
- Ghazal, A.; Sorichter, S.; Jonas, I.; Rose, E.C. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J. Sleep Res. 2009, 18, 321–328. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Fleury, B.; Vielle, B.; Pételle, B.; Meslier, N.; N'Guyen, X.L.; Trzepizur, W.; Racineux, J.L. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur. Respir. J. 2009, 34, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Yanamoto S, Harata S, Miyoshi T, Nakamura N, Sakamoto Y, Murata M, Soutome S, Umeda M. Semi-fixed versus fixed oral appliance therapy for obstructive sleep apnea: A randomized crossover pilot study. J Dent Sci. 2021 Jan;16(1):404-409. PMCID: PMC7770364. [CrossRef] [PubMed]
- Zhou J, Liu YH. A randomised titrated crossover study comparing two oral appliances in the treatment for mild to moderate obstructive sleep apnoea/hypopnoea syndrome. J Oral Rehabil. 2012 Dec;39(12):914-22. [CrossRef] [PubMed]
- Isacsson G, Fodor C, Sturebrand M. Obstructive sleep apnea treated with custom-made bibloc and monobloc oral appliances: a retrospective comparative study. Sleep Breath. 2017 Mar;21(1):93-100. PMCID: PMC5343082. [CrossRef] [PubMed]
- Vecchierini, M.F.; Attali, V.; Collet, J.M.; d'Ortho, M.P.; El Chater, P.; Kerbrat, J.B.; Leger, D.; Monaca, C.; Monteyrol, P.J.; Morin, L.; et al. A custom-made mandibular repositioning device for obstructive sleep apnoea-hypopnoea syndrome: The ORCADES study. Sleep Med. 2016, 19, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tegelberg, Å.; Nohlert, E.; Bornefalk-Hermansson, A.; Fransson, A.; Isacsson, G. Respiratory outcomes after a 1-year treatment of obstructive sleep apnoea with bibloc versus monobloc oral appliances: A multicentre, randomized equivalence trial. Acta Odontol. Scand. 2020, 78, 401–408. [Google Scholar] [CrossRef]
- Kuna, S.T.; Giarraputo, P.C.; Stanton, D.C.; Levin, L.M.; Frantz, D. Evaluation of an oral mandibular advancement titration appliance. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2006, 101, 593–603. [Google Scholar] [CrossRef]
- Henke, K.G.; Frantz, D.E.; Kuna, S.T. An oral elastic mandibular advancement device for obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2000, 161, 420–425. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Van Daele, M.; Verbraecken, J.; Braem, M.J.; Dieltjens, M. Comparative analysis of two custom-made mandibular advancement devices with varied designs for treating moderate to severe obstructive sleep apnea. Sleep Med. 2024, 117, 95–98. [Google Scholar] [CrossRef]
- Schneiderman, E.D.; Schramm, P.J.; Hui, J.; Wilson, P.D.; Marques, P.; German, Z.; McCann, A.L.; Newton, M.N. Randomized Trial of 2 Self-Titrated Oral Appliances for Airway Management. J. Dent. Res. 2020, 100, 155–162. [Google Scholar] [CrossRef]
- Pancer, J.; Al-Faifi, S.; Al-Faifi, M.; Hoffstein, V. Evaluation of variable mandibular advancement appliance for treatment of snoring and sleep apnea. Chest. 1999, 116, 1511–1518. [Google Scholar] [CrossRef]
- Van Haesendonck, G.; Dieltjens, M.; Hamans, E.; Braem, M.J.; Vanderveken, O.M. Treatment efficacy of a titratable oral appliance in obstructive sleep apnea patients: A prospective clinical trial. B-ENT. 2016, 12, 1–8. [Google Scholar]
- Remmers, J.; Charkhandeh, S.; Grosse, J.; Topor, Z.; Brant, R.; Santosham, P.; Bruehlmann, S. Remotely Controlled Mandibular Protrusion during Sleep Predicts Therapeutic Success with Oral Appliances in Patients with Obstructive Sleep Apnea. Sleep 2013, 36, 1517–1525. [Google Scholar] [CrossRef]
- Mehta, A.; Qian, J.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. A Randomized, Controlled Study of a Mandibular Advancement Splint for Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2001, 163, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellah ME, Mohamed FS, Khamis MM, Abdel Wahab NH. Modified biblock versus monoblock mandibular advancement appliances for treatment of obstructive sleep apnea: A randomized controlled trial. J Prosthet Dent. 2024 Apr;131(4):633-642. [CrossRef] [PubMed]
- Isacsson G, Nohlert E, Fransson AMC, Bornefalk-Hermansson A, Wiman Eriksson E, Ortlieb E, Trepp L, Avdelius A, Sturebrand M, Fodor C, List T, Schumann M, Tegelberg Å. Use of bibloc and monobloc oral appliances in obstructive sleep apnoea: a multicentre, randomized, blinded, parallel-group equivalence trial. Eur J Orthod. 2019 Jan 23;41(1):80-88. PMCID: PMC6343726. [CrossRef] [PubMed]
- Bosschieter PFN, Uniken Venema JAM, Vonk PE, Ravesloot MJL, Hoekema A, Plooij JM, Lobbezoo F, de Vries N. Equal effect of a noncustom vs a custom mandibular advancement device in treatment of obstructive sleep apnea. J Clin Sleep Med. 2022 Sep 1;18(9):2155-2165. PMCID: PMC9435323. [CrossRef] [PubMed]
- Johal A, Haria P, Manek S, Joury E, Riha R. Ready-Made Versus Custom-Made Mandibular Repositioning Devices in Sleep Apnea: A Randomized Clinical Trial. J Clin Sleep Med. 2017 Feb 15;13(2):175-182. PMCID: PMC5263072. [CrossRef] [PubMed]
- Friedman M, Hamilton C, Samuelson CG, Kelley K, Pearson-Chauhan K, Taylor D, Taylor R, Maley A, Hirsch MA. Compliance and efficacy of titratable thermoplastic versus custom mandibular advancement devices. Otolaryngol Head Neck Surg. 2012 Aug;147(2):379-86. [CrossRef] [PubMed]
- Lettieri CJ, Paolino N, Eliasson AH, Shah AA, Holley AB. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2011 Oct 15;7(5):439-45. PMCID: PMC3190841. [CrossRef] [PubMed]
- Sari E, Menillo S. Comparison of titratable oral appliance and mandibular advancement splint in the treatment of patients with obstructive sleep apnea. ISRN Dent. 2011;2011:581692. PMCID: PMC3169918. [CrossRef] [PubMed]
- Stern, J.; Lee, K.; Kuhns, D.; Martinez-Kratz, J.F. Efficacy and Effectiveness of the ProSomnus® [IA] Sleep Device for the Treatment of Obstructive Sleep Apnea: EFFECTS Study. Cureus 2021, 13, e15391. [Google Scholar] [CrossRef] [PubMed]
- Silva R, Pires L, Belchior I, Moniri A, New generation oral appliances for treatment of obstructive sleep apnea. Sleep Med. 2024, 115 (Suppl. 1).
- Sall, E.; Smith, K.; Desai, A.; Carollo, J.A.; Murphy, M.T.; Kim, S.; Liptak, L.A. Evaluating the Clinical Performance of a Novel, Precision Oral Appliance Therapy Medical Device Made Wholly from a Medical Grade Class VI Material for the Treatment of Obstructive Sleep Apnea. Cureus 2023, 15, e50107. [Google Scholar] [CrossRef]
- Sall, E. 434 Precision Oral Appliance Therapy: The Prime-Time Treatment for OSA. Sleep 2021, 44 (Suppl. 2). [Google Scholar] [CrossRef]
- Remmers, J.E.; Topor, Z.L.; Grosse, J.; Vranjes, N.; Mosca, E.V.; Brant, R.; Bruehlmann, S.; Charkhandeh, S.; Jenabali, A. A Feedback-Controlled Mandibular Positioner Identifies Individuals with Sleep Apnea Who Will Respond to Oral Appliance Therapy. J. Clin. Sleep Med. 2017, 13, 871–880. [Google Scholar] [CrossRef]
- Murphy, M.; Munro, K. Dose Management in DSM; Analysis of Efficacy, Starting Position, Advancement and side effects in consecutive Patient Series Treated with Precision Platform. J. Dent. Sleep Med. 2021, 8, No.3. [Google Scholar]
- Mosca, E.V.; Bruehlmann, S.; Zouboules, S.M.; Chiew, A.E.; Westersund, C.; Hambrook, D.A.; Jahromi, S.A.Z.; Grosse, J.; Topor, Z.L.; Charkhandeh, S.; et al. In-Home Mandibular Repositioning during Sleep Using MATRx plus Predicts Outcome and Efficacious Positioning for Oral Appliance Treatment of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2022, 18, 911–919. [Google Scholar] [CrossRef]
- Kang, R.S.; Knowles, S.; Dekow, M. The Success of Oral Appliance Therapy Based on Symptom-Driven Titration. Mil. Med. 2022, 189, 620–626. [Google Scholar] [CrossRef]
- Knowles, S.; Dekow, M.; Williamson, M.L. Oral Appliances for OSA Treatment: Meeting the Quadruple Aim. Mil. Med. 2021, 188, e718–e724. [Google Scholar] [CrossRef]
- https://doi.org/10.1016/j.cirpj.2019.02.001. [CrossRef]
- https://sleepreviewmag.com/sleep-treatments/therapy-devices/oral-appliances/barriers-sleep-physicians-oat/ (Accessed May 13, 2025).
- Pack, A. Developing a Personalized Approach to Obstructive Sleep Apnea. Sleep Med Clin. 2025 Mar;20(1):127-134. [CrossRef] [PubMed]
- Xu Z, Zhang SY, Huang M, Hu R, Li JL, Cen HJ, Wang ZP, Ou JS, Yin SL, Xu YQ, Wu ZK, Zhang X. Genotype-Guided Warfarin Dosing in Patients With Mechanical Valves: A Randomized Controlled Trial. Ann Thorac Surg. 2018 Dec;106(6):1774-1781. [CrossRef] [PubMed]
- Mancinelli L, Cronin M, Sadée W. Pharmacogenomics: the promise of personalized medicine. AAPS PharmSci. 2000;2(1):E4. PMCID: PMC2750999. [CrossRef] [PubMed]
- Su JH, Zhu YH, Ren TY, Guo L, Yang GY, Jiao LG, Wang JF. Distribution and Antimicrobial Resistance of SalmonellaIsolated from Pigs with Diarrhea in China. Microorganisms. 2018 Nov 26;6(4):117. PMCID: PMC6313467. [CrossRef] [PubMed]
- Yalameha B, Birjandi M, Nouryazdan N, Nasri H, Shahsavari G. Association between the FABP2 Ala54Thr and CRP+1059C/G polymorphisms and small dense LDL level in patients with atherosclerosis: a case-control study. Arch Physiol Biochem. 2023 Feb;129(1):246-252. [CrossRef] [PubMed]
- Ryser AS, Sabol J, Handel S, Walworth P, Dimalanta W, et al. (2024) Soldier Preference in Mandibular Advancement Devices in Patients Who Brux. J Dent Oral Epidemiol 4(2). d. [CrossRef]
- Ioerger P, Afshari A, Hentati F, Strober W, Kallogjeri D, Ju YE, Piccirillo JF. Mandibular Advancement vs Combined Airway and Positional Therapy for Snoring: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2024 Jul 1;150(7):572-579. PMCID: PMC11117146. [CrossRef] [PubMed]
- Vranjes N, Santucci G, Schulze K, Kuhns D, Khai A. Assessment of potential tooth movement and bite changes with a hardacrylic sleep appliance: A 2-year clinical study. J Dent Sleep Med. 2019;6(2). https://www.aadsm.org/docs/jdsm.4.10.19.o2.
- Aziz R, Somaiah S, Kalha AS, Reddy G, Muddaiah S, Shetty B. Comparative assessment of changes in pharyngeal airway space in cases of obstructive sleep apnoea with a customized mandibular repositioning appliance - a clinical study. Sleep Sci. 2021 Jan-Mar;14(Spec 1):16-24. PMCID: PMC8663729. [CrossRef] [PubMed]


| Custom OA Definition Criteria (Summarized) | |||
|---|---|---|---|
| Proposed Classifications | Made from Oral Records of an Individual Patient? | Modified (Trimmed, Bent, Relined)? | Includes Prefabricated Items? |
| Semi-Custom | Yes | Yes | |
| Precision-Custom | Yes | No | |
| Review Article | Author | Reference | Basic Description | 1. Made from Records of Oral Structures? | 2. Is the Device Modified or Primarily Prefabricated? |
|---|---|---|---|---|---|
| Chen 2025 | Bloch 2000 | [12] | Push | Yes | Yes |
| Chen 2025 | Pepin 2019 | [13] | Push | Yes | Yes |
| Chen 2025 | Pepin 2019 | [13] | Push | Yes | Yes |
| Liptak 2025 | Randerath, 2022 | [14] | Push | Yes | Yes |
| Liptak 2025 | Ghazal, 2008 | [15] | Push | Yes | Yes |
| Liptak 2025 | Gagnadoux, 2009 | [16] | Push | Yes | Yes |
| Chen 2025 | Yanamoto 2021 | [17] | Pull | Yes | Yes |
| Chen 2025 | Zhou 2012 | [18] | Pull | Yes | Yes |
| Chen 2025 | Isacsson 2017 | [19] | Pull | Yes | Yes |
| Liptak 2025 | Vecchierini, 2016 | [20] | Pull | Yes | Yes |
| Liptak 2025 | Vecchierini, 2016 | [14] | Pull | Yes | Yes |
| Liptak 2025 | Tegelberg, 2020 | [21] | Pull | Yes | Yes |
| Liptak 2025 | Kuna, 2005 | [22] | Pull | Yes | Yes |
| Liptak 2025 | Henke, 1999 | [23] | Pull | Yes | Yes |
| Liptak 2025 | Vanderveken, 2024 | [24] | Pull | Yes | Yes |
| Liptak 2025 | Schneiderman, 2020 | [25] | Pull | Yes | Yes |
| Liptak 2025 | Pancer, 1999 | [26] | Pull | Yes | Yes |
| Liptak 2025 | Ghazal, 2008 | [12] | Pull | Yes | Yes |
| Chen 2025 | Gagnadoux 2012 | [13] | Interlocking | Yes | Yes |
| Liptak 2025 | Vanderveken, 2024 | [18] | Interlocking | Yes | Yes |
| Liptak 2025 | Van Haesendonck, 2016 | [27] | Interlocking | Yes | Yes |
| Liptak 2025 | Schneiderman, 2020 | [19] | Interlocking | Yes | Yes |
| Liptak 2025 | Remmers, 2013 | [28] | Interlocking | Yes | Yes |
| Liptak 2025 | Mehta, 2001 | [29] | Interlocking | Yes | Yes |
| Liptak 2025 | de Ruiter, 2020 | [7] | Interlocking | Yes | Yes |
| Chen 2025 | Abd-Ellah 2022 | [30] | Bi Block | Yes | Yes |
| Chen 2025 | Isacsson 2019 | [31] | Bi Block | Yes | Yes |
| Chen 2025 | Bosschieter 2022 | [32] | Anterior | Yes | Yes |
| Chen 2025 | Johal 2015 | [33] | Anterior | Yes | Yes |
| Chen 2025 | Friedman 2012 | [34] | Anterior | Yes | Yes |
| Chen 2025 | Lettieri 2011 | [35] | Anterior | Yes | Yes |
| Chen 2025 | Sari 2011 | [36] | Anterior | Yes | Yes |
| Liptak 2025 | Stern, 2021 | [37] | Dual Post | Yes | No |
| Liptak 2025 | Silva, 2023 | [38] | Dual Post | Yes | No |
| Liptak 2025 | Sall, 2023 | [39] | Dual Post | Yes | No |
| Liptak 2025 | Sall, 2021 | [40] | Dual Post | Yes | No |
| Liptak 2025 | Remmers, 2017 | [41] | Dual Post | Yes | No |
| Liptak 2025 | Murphy, 2021 | [42] | Dual Post | Yes | No |
| Liptak 2025 | Mosca, 2022 | [43] | Dual Post | Yes | No |
| Liptak 2025 | Kang, 2024 | [44] | Dual Post | Yes | No |
| Liptak 2025 | Knowles, 2023 | [45] | Dual Post | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





