Submitted:
09 May 2025
Posted:
12 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
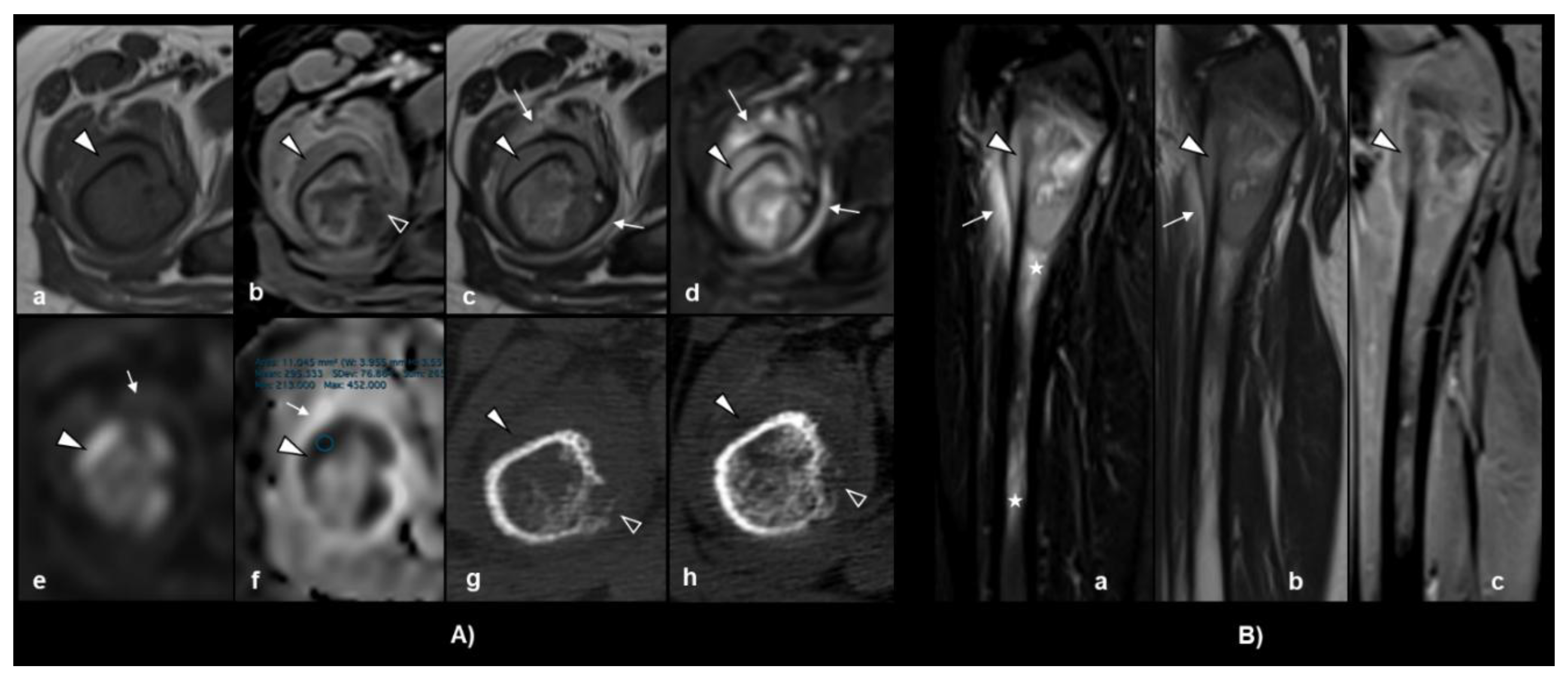
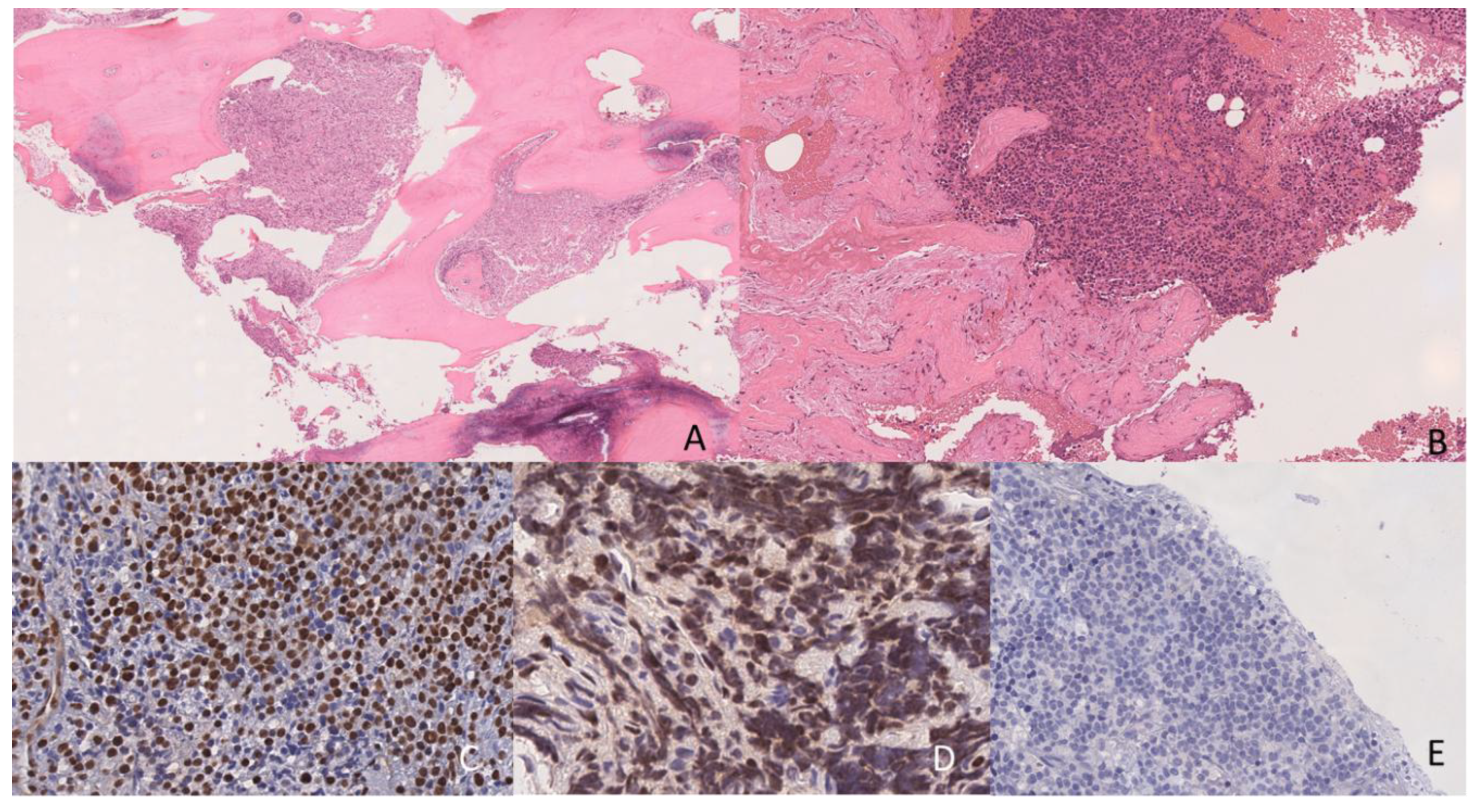
2. Case Presentation
3. Laboratory Studies at the Time of Relapse Diagnosis
4. Discussion
5. Methods
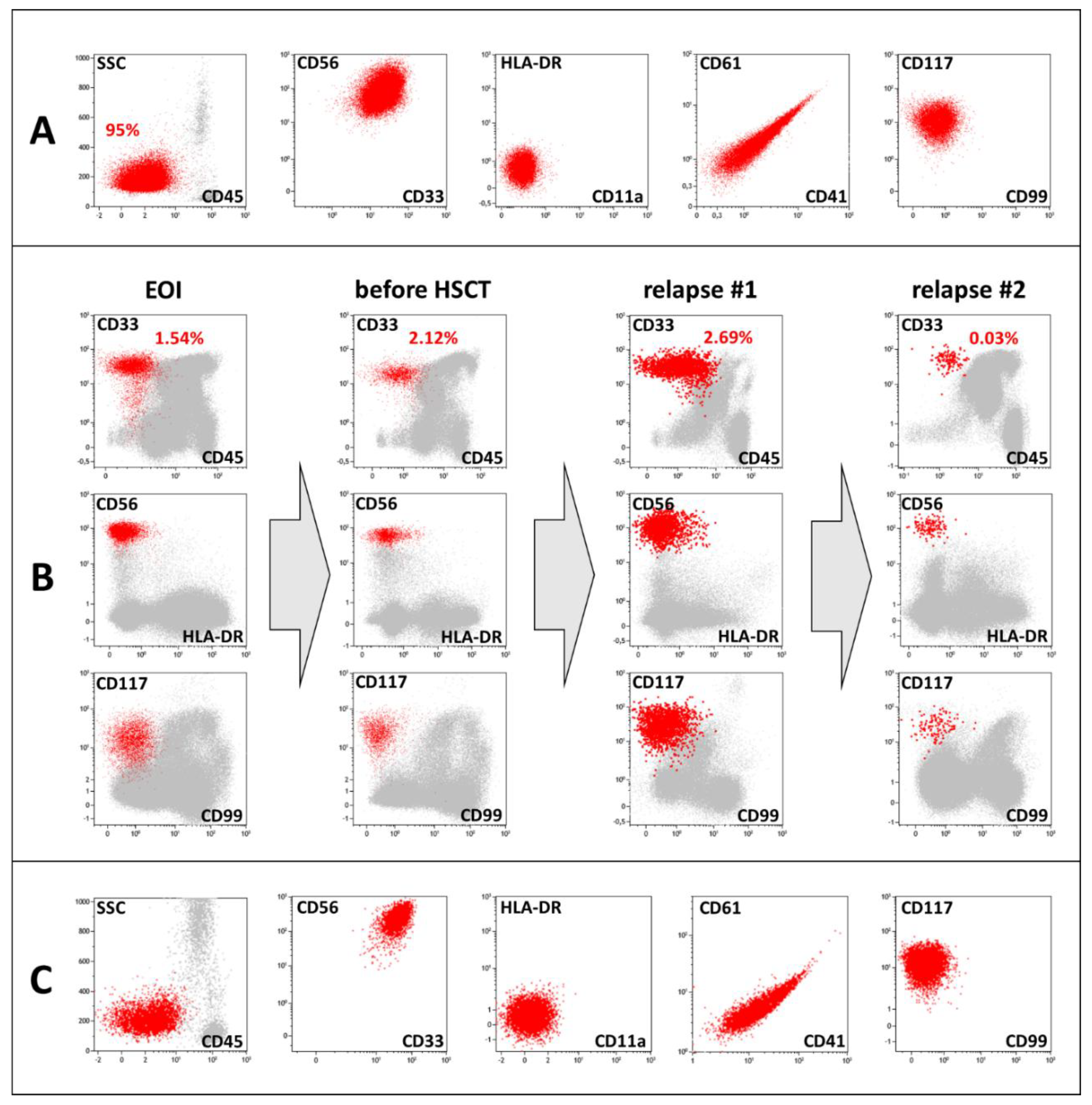
Immunophenotyping
Cytogenetic and Molecular Studies
Author Contributions
Funding
Patient Consent Statement
Data Sharing Statement
Conflicts of Interests
References
- de Rooij, J.D.; Branstetter, C.; Ma, J.; Li, Y.; Walsh, M.P.; Cheng, J.; Obulkasim, A.; Dang, J.; Easton, J.; Verboon, L.J.; et al. Pediatric non-Down syndrome acute megakaryoblastic leukemia is characterized by distinct genomic subsets with varying outcomes. Nat Genet 2017, 49, 451-456. [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Criteria for the diagnosis of acute leukemia of megakaryocyte lineage (M7). A report of the French-American-British Cooperative Group. Ann Intern Med 1985, 103, 460-462. [CrossRef]
- Bloomfield, C.D.; Brunning, R.D. FAB M7: acute megakaryoblastic leukemia--beyond morphology. Ann Intern Med 1985, 103, 450-452. [CrossRef]
- Athale, U.H.; Razzouk, B.I.; Raimondi, S.C.; Long, X.; Behm, F.G.; Head, D.R.; Srivastava, D.K.; Rubnitz, J.E.; Bowman, L.; Pui, C.H.; et al. Biology and outcome of childhood acute megakaryoblastic leukemia: a single institution's experience. Blood 2001, 97, 3727-3732. [CrossRef]
- Barnard, D.R.; Alonzo, T.A.; Gerbing, R.B.; Lange, B.; Woods, W.G. Comparison of childhood myelodysplastic syndrome, AML FAB M6 or M7, CCG 2891: Report from the Children's Oncology Group. Pediatric Blood and Cancer 2007, 49, 17-22. [CrossRef]
- Pagano, L.; Pulsoni, A.; Vignetti, M.; Mele, L.; Fianchi, L.; Petti, M.C.; Mirto, S.; Falcucci, P.; Fazi, P.; Broccia, G.; et al. Acute megakaryoblastic leukemia: experience of GIMEMA trials. Leukemia 2002 16:9 2002, 16, 1622-1626. [CrossRef]
- Ribeiro, R.C.; Oliveira, M.S.P.; Fairclough, D.; Hurwitz, C.; Mirro, J.; Behm, F.G.; Head, D.; Silva, M.L.M.; Raimondi, S.C.; Crist, W.M.; et al. Acute Megakaryoblastic Leukemia in Children and Adolescents: A Retrospective Analysis of 24 Cases. Leukemia & Lymphoma 1993, 10, 299-306. [CrossRef]
- Tallman, M.S.; Neuberg, D.; Bennett, J.M.; Francois, C.J.; Paietta, E.; Wiernik, P.H.; Dewald, G.; Cassileth, P.A.; Oken, M.M.; Rowe, J.M. Acute megakaryocytic leukemia: the Eastern Cooperative Oncology Group experience. Blood 2000, 96, 2405-2411.
- Du, Y.; Yang, L.; Qi, S.; Chen, Z.; Sun, M.; Wu, M.; Wu, B.; Tao, F.; Xiong, H. Clinical Analysis of Pediatric Acute Megakaryocytic Leukemia With CBFA2T3-GLIS2 Fusion Gene. J Pediatr Hematol Oncol 2024, 46, 96-103. [CrossRef]
- Gruber, T.A.; Larson Gedman, A.; Zhang, J.; Koss, C.S.; Marada, S.; Ta, H.Q.; Chen, S.C.; Su, X.; Ogden, S.K.; Dang, J.; et al. An inv(16)(p13.3q24.3)-encoded CBFA2T3-GLIS2 fusion protein defines an aggressive subtype of pediatric acute megakaryoblastic leukemia. Cancer cell 2012, 22, 683-683. [CrossRef]
- Rørvik, S.D.; Torkildsen, S.; Bruserud, Ø.; Tvedt, T.H.A. Acute myeloid leukemia with rare recurring translocations—an overview of the entities included in the international consensus classification. Annals of Hematology 2024, 103, 1103-1119. [CrossRef]
- Wu, K.; Liu, H.; Xie, Y.; LiuCui, Y.; Cai, J.; Wang, R.; Wang, Y.; Wang, X.; Chen, X.; Zhao, S.; et al. Genomic Landscape of Pediatric Non-Down's Syndrome Acute Megakaryoblastic Leukemia in China. In Proceedings of the 65th ASH Annual Meeting, San Diego, California, 29 November 2023, 2023; p. 4331.
- Masetti, R.; Pigazzi, M.; Togni, M.; Astolfi, A.; Indio, V.; Manara, E.; Casadio, R.; Pession, A.; Basso, G.; Locatelli, F. CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood 2013, 121, 3469-3472. [CrossRef]
- Smith, J.L.; Ries, R.E.; Hylkema, T.; Alonzo, T.A.; Gerbing, R.B.; Santaguida, M.T.; Brodersen, L.E.; Pardo, L.; Cummings, C.L.; Loeb, K.R.; et al. Comprehensive Transcriptome Profiling of Cryptic CBFA2T3-GLIS2 Fusion-positive AML Defines Novel Therapeutic Options – A COG and TARGET Pediatric AML Study. Clinical cancer research : an official journal of the American Association for Cancer Research 2020, 26, 726-726. [CrossRef]
- Eidenschink Brodersen, L.; Alonzo, T.A.; Menssen, A.J.; Gerbing, R.B.; Pardo, L.; Voigt, A.P.; Kahwash, S.B.; Hirsch, B.; Raimondi, S.; Gamis, A.S.; et al. A recurrent immunophenotype at diagnosis independently identifies high-risk pediatric acute myeloid leukemia: a report from Children's Oncology Group. Leukemia 2016, 30, 2077-2080. [CrossRef]
- Zangrando, A.; Cavagnero, F.; Scarparo, P.; Varotto, E.; Francescato, S.; Tregnago, C.; Cuccurullo, R.; Fagioli, F.; Nigro, L.L.; Masetti, R.; et al. CD56, HLA-DR, and CD45 recognize a subtype of childhood AML harboring CBFA2T3-GLIS2 fusion transcript. Cytometry A 2021, 99, 844-850. [CrossRef]
- Liu, H.; Wu, K.; Hu, W.; Chen, X.; Tang, Y.; Ma, Y.; Chen, C.; Xie, Y.; Yu, L.; Huang, J.; et al. Immunophenotypic clustering in paediatric acute myeloid leukaemia. Br J Haematol 2024, 204, 2275-2286. [CrossRef]
- Pardo, L.M.; Voigt, A.P.; Alonzo, T.A.; Wilson, E.R.; Gerbing, R.B.; Paine, D.J.; Dai, F.; Menssen, A.J.; Raimondi, S.C.; Hirsch, B.A.; et al. Deciphering the Significance of CD56 Expression in Pediatric Acute Myeloid Leukemia: A Report from the Children's Oncology Group. Cytometry B Clin Cytom 2020, 98, 52-56. [CrossRef]
- Ferreira-Facio, C.S.; Botafogo, V.; Ferrao, P.M.; Canellas, M.C.; Milito, C.B.; Romano, S.; Lopes, D.V.; Teixeira, L.C.; Oliveira, E.; Bruno-Riscarolli, E.; et al. Flow Cytometry Immunophenotyping for Diagnostic Orientation and Classification of Pediatric Cancer Based on the EuroFlow Solid Tumor Orientation Tube (STOT). Cancers (Basel) 2021, 13. [CrossRef]
- Gajendra, S.; Anupurba, S.; Gupta, R.; Mallick, S.; Panda, D.; Thakral, D.; Gupta, S.K.; Bakhshi, S.; Seth, R.; Rai, S.; et al. Acute myeloid leukemia with RAM immunophenotype: A new underdiagnosed entity. Int J Lab Hematol 2023, 45, 541-552. [CrossRef]
- Panda, D.; Chatterjee, G.; Sardana, R.; Khanka, T.; Ghogale, S.; Deshpande, N.; Badrinath, Y.; Shetty, D.; Narula, G.; Banavali, S.; et al. Utility of CD36 as a novel addition to the immunophenotypic signature of RAM-phenotype acute myeloid leukemia and study of its clinicopathological characteristics. Cytometry B Clin Cytom 2021, 100, 206-217. [CrossRef]
- Chisholm, K.M.; Smith, J.; Heerema-McKenney, A.E.; Choi, J.K.; Ries, R.E.; Hirsch, B.A.; Raimondi, S.C.; Wang, Y.C.; Dang, A.; Alonzo, T.A.; et al. Pathologic, cytogenetic, and molecular features of acute myeloid leukemia with megakaryocytic differentiation: A report from the Children's Oncology Group. Pediatr Blood Cancer 2023, 70, e30251. [CrossRef]
- Brouwer, N.; Matarraz, S.; Nierkens, S.; Hofmans, M.; Novakova, M.; da Costa, E.S.; Fernandez, P.; Bras, A.E.; de Mello, F.V.; Mejstrikova, E.; et al. Immunophenotypic Analysis of Acute Megakaryoblastic Leukemia: A EuroFlow Study. Cancers (Basel) 2022, 14. [CrossRef]
- Bisschop, M.M.; Revesz, T.; Bierings, M.; van Weerden, J.F.; van Wering, E.R.; Hahlen, K.; van der Does-van den Berg, A. Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukaemia. Leukemia 2001, 15, 46-49. [CrossRef]
- Dinikina, Y.V.; Maschan, A.A. Extramedullary involvement in pediatric myeloid leukemia: challenges of diagnosis and treatment. Clinical cases and a literature review (In Russ.). Pediatric Hematology/Oncology and Immunopathology 2023, 22, 123-141. [CrossRef]
- Samborska, M.; Derwich, K.; Skalska-Sadowska, J.; Kurzawa, P.; Wachowiak, J. Myeloid sarcoma in children – diagnostic and therapeutic difficulties. Contemporary Oncology 2016, 20, 444-444. [CrossRef]
- Støve, H.K.; Sandahl, J.D.; Abrahamsson, J.; Asdahl, P.H.; Forestier, E.; Ha, S.Y.; Jahnukainen, K.; Jónsson, Ó.G.; Lausen, B.; Palle, J.; et al. Extramedullary leukemia in children with acute myeloid leukemia: A population-based cohort study from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Pediatric blood & cancer 2017, 64. [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703-1719. [CrossRef]
- Meyer, H.J.; Beimler, M.; Borte, G.; Pönisch, W.; Surov, A. Radiological and Clinical Patterns of Myeloid Sarcoma. Radiology and Oncology 2019, 53, 213-213. [CrossRef]
- Roberts, A.S.; Shetty, A.S.; Mellnick, V.M.; Pickhardt, P.J.; Bhalla, S.; Menias, C.O. Extramedullary haematopoiesis: radiological imaging features. Clin Radiol 2016, 71, 807-814. [CrossRef]
- Vasilyeva, M.S.; Kalinina, I.I.; Venyov, D.A.; Lebedeva, S.A.; Bankole, V.A.; Abashidze, Z.A.; Aleinikova, O.V.; Zerkalenkova, E.A.; Gaskova, M.V.; Itov, A.B.; et al. Preliminary results of treatment of intermediate-risk patients according to the AML-MRD-2018 protocol. Pediatric Hematology/Oncology and Immunopathology 2025, 24, 14-25.
- Lebedeva, S.A.; Kalinina, I.I.; Kazakova, A.N.; Bankole, V.A.; Vasileva, M.S.; Venyov, D.A.; Baydildina, D.D.; Aleinikova, O.V.; Popa, A.V.; Maschan, A.A.; et al. The prognostic significance of partner genes and breakpoint locations in children with KMT2A-rearranged acute myeloid leukemia. Pediatric Hematology/Oncology and Immunopathology 2025, 24, 58-65.
- Alexenko, M.; Illarionova, O.; Verzhbitskaya, T.; Zerkalenkova, E.; Novikova, I.; Panferova, A.; Fechina, L.; Tsaur, G.; Olshanskaya, J.; Popov, A. Immunophenotypic characterization of acute megakaryoblastic leukaemia in children. Pediatric Hematology/Oncology and Immunopathology 2019, 18, 35-40. [CrossRef]
- Schweitzer, J.; Zimmermann, M.; Rasche, M.; von Neuhoff, C.; Creutzig, U.; Dworzak, M.; Reinhardt, D.; Klusmann, J.H. Improved outcome of pediatric patients with acute megakaryoblastic leukemia in the AML-BFM 04 trial. Ann Hematol 2015, 94, 1327-1336. [CrossRef]
- Hara, Y.; Shiba, N.; Ohki, K.; Tabuchi, K.; Yamato, G.; Park, M.J.; Tomizawa, D.; Kinoshita, A.; Shimada, A.; Arakawa, H.; et al. Prognostic impact of specific molecular profiles in pediatric acute megakaryoblastic leukemia in non-Down syndrome. Genes Chromosomes Cancer 2017, 56, 394-404. [CrossRef]
- Thiollier, C.; Lopez, C.K.; Gerby, B.; Ignacimouttou, C.; Poglio, S.; Duffourd, Y.; Guegan, J.; Rivera-Munoz, P.; Bluteau, O.; Mabialah, V.; et al. Characterization of novel genomic alterations and therapeutic approaches using acute megakaryoblastic leukemia xenograft models. J Exp Med 2012, 209, 2017-2031. [CrossRef]
- Theodorakos, I.; Paterakis, G.; Papadakis, V.; Vicha, A.; Topakas, G.; Jencova, P.; Karchilaki, E.; Taparkou, A.; Tsagarakis, N.J.; Polychronopoulou, S. Interference of bone marrow CD56(+) mesenchymal stromal cells in minimal residual disease investigation of neuroblastoma and other CD45(-)/CD56(+) pediatric malignancies using flow cytometry. Pediatr Blood Cancer 2019, 66, e27799. [CrossRef]
- Masetti, R.; Bertuccio, S.N.; Pession, A.; Locatelli, F. CBFA2T3-GLIS2-positive acute myeloid leukaemia. A peculiar paediatric entity. Br J Haematol 2019, 184, 337-347. [CrossRef]
- Pession, A.; Masetti, R.; Rizzari, C.; Putti, M.C.; Casale, F.; Fagioli, F.; Luciani, M.; Lo Nigro, L.; Menna, G.; Micalizzi, C.; et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood 2013, 122, 170-178. [CrossRef]
- Masetti, R.; Pigazzi, M.; Togni, M.; Astolfi, A.; Indio, V.; Manara, E.; Casadio, R.; Pession, A.; Basso, G.; Locatelli, F. CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood 2013, 121, 3469-3472. [CrossRef]
- Zollner, S.K.; Kauertz, K.L.; Kaiser, I.; Kerkhoff, M.; Schaefer, C.; Tassius, M.; Jabar, S.; Jurgens, H.; Ladenstein, R.; Kuhne, T.; et al. Ewing Sarcoma as Secondary Malignant Neoplasm-Epidemiological and Clinical Analysis of an International Trial Registry. Cancers (Basel) 2022, 14. [CrossRef]
- Loning, L.; Zimmermann, M.; Reiter, A.; Kaatsch, P.; Henze, G.; Riehm, H.; Schrappe, M. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood 2000, 95, 2770-2775.
- Applebaum, M.A.; Goldsby, R.; Neuhaus, J.; DuBois, S.G. Clinical features and outcomes in patients with secondary Ewing sarcoma. Pediatr Blood Cancer 2013, 60, 611-615. [CrossRef]
- Kim, G.E.; Beach, B.; Gastier-Foster, J.M.; Murata-Collins, J.L.; Rowland, J.M.; O'Donnell, R.J.; Goldsby, R.E. Ewing sarcoma as a second malignant neoplasm after acute lymphoblastic leukemia. Pediatr Blood Cancer 2005, 45, 57-59. [CrossRef]
- Zhu, N.; Ni, H.; Guo, S.; Shen, Y.Q.; Chen, Q. Bone complications of cancer treatment. Cancer Treat Rev 2024, 130, 102828. [CrossRef]
- Almond, L.M.; Charalampakis, M.; Ford, S.J.; Gourevitch, D.; Desai, A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk 2017, 17, 263-267. [CrossRef]
- Semchenkova, A.; Mikhailova, E.; Komkov, A.; Gaskova, M.; Abasov, R.; Matveev, E.; Kazanov, M.; Mamedov, I.; Shmitko, A.; Belova, V.; et al. Lineage Conversion in Pediatric B-Cell Precursor Acute Leukemia under Blinatumomab Therapy. Int J Mol Sci 2022, 23. [CrossRef]
- Dorantes-Acosta, E.; Pelayo, R. Lineage switching in acute leukemias: a consequence of stem cell plasticity? Bone Marrow Res 2012, 2012, 406796. [CrossRef]
- Silbert, S.K.; Rankin, A.W.; Hoang, C.N.; Semchenkova, A.; Myers, R.M.; Zerkalenkova PhD, E.; Wang, H.-W.; Kovach, A.E.; Yuan, C.M.; Delgado Colon, D.; et al. Project EVOLVE: An international analysis of postimmunotherapy lineage switch, an emergent form of relapse in leukemia. Blood 2025, blood.2024026655-blood.2024026655. [CrossRef]
- Permikin, Z.; Popov, A.; Verzhbitskaya, T.; Riger, T.; Plekhanova, O.; Makarova, O.; Fronkova, E.; Trka, J.; Meyer, C.; Marschalek, R.; et al. Lineage switch to acute myeloid leukemia during induction chemotherapy for early T-cell precursor acute lymphoblastic leukemia with the translocation t(6;11)(q27;q23)/KMT2A-AFDN: A case report. Leuk Res 2022, 112, 106758. [CrossRef]
- Demina, I.; Dagestani, A.; Borkovskaia, A.; Semchenkova, A.; Soldatkina, O.; Kashpor, S.; Olshanskaya, Y.; Roumiantseva, J.; Karachunskiy, A.; Novichkova, G.; et al. Immunophenotypic but Not Genetic Changes Reclassify the Majority of Relapsed/Refractory Pediatric Cases of Early T-Cell Precursor Acute Lymphoblastic Leukemia. International journal of molecular sciences 2024, 25. [CrossRef]
- Popov A.; Verzhbitskaya T.; Movchan L.; Demina I.; Mikhailova E.; Semchenkova A.; Permikin Zh.; Shman T.; Karachunskiy A.; G., N. Flow cytometry in acute leukemia diagnostics. Guidelines of Russian-Belarusian multicenter group for pediatric leukemia studies. Pediatric Hematology/Oncology and Immunopathology 2023, 22, 165-177.
- Novello, M.; Coli, A.; Della Pepa, G.M.; Martini, M.; Doglietto, F.; De Stefano, V.; Bellesi, S.; Pescarmona, E.; Lauriola, L. Myeloid sarcoma with megakaryoblastic differentiation mimicking a sellar tumor. Neuropathology : official journal of the Japanese Society of Neuropathology 2014, 34, 179-184. [CrossRef]
- Cannatella, J.; Ganapathi, K.; Horvai, A. Hematolymphoid Neoplasms Rarely Mimic Undifferentiated Pleomorphic Sarcoma of Soft Tissue. Archives of pathology & laboratory medicine 2020, 144, 1547-1552. [CrossRef]
- Kalina, T.; Flores-Montero, J.; Lecrevisse, Q.; Pedreira, C.E.; van der Velden, V.H.; Novakova, M.; Mejstrikova, E.; Hrusak, O.; Bottcher, S.; Karsch, D.; et al. Quality assessment program for EuroFlow protocols: summary results of four-year (2010-2013) quality assurance rounds. Cytometry A 2015, 87, 145-156. [CrossRef]
- Buldini, B.; Maurer-Granofszky, M.; Varotto, E.; Dworzak, M.N. Flow-Cytometric Monitoring of Minimal Residual Disease in Pediatric Patients With Acute Myeloid Leukemia: Recent Advances and Future Strategies. Front Pediatr 2019, 7, 412. [CrossRef]
- Boztug, H.; Schumich, A.; Potschger, U.; Muhlegger, N.; Kolenova, A.; Reinhardt, K.; Dworzak, M. Blast cell deficiency of CD11a as a marker of acute megakaryoblastic leukemia and transient myeloproliferative disease in children with and without Down syndrome. Cytometry B Clin Cytom 2013, 84, 370-378. [CrossRef]
- Semchenkova, A.; Zerkalenkova, E.; Demina, I.; Kashpor, S.; Volchkov, E.; Zakharova, E.; Larin, S.; Olshanskaya, Y.; Novichkova, G.; Maschan, A.; et al. Recognizing Minor Leukemic Populations with Monocytic Features in Mixed-Phenotype Acute Leukemia by Flow Cell Sorting Followed by Cytogenetic and Molecular Studies: Report of Five Exemplary Cases. Int J Mol Sci 2023, 24. [CrossRef]
- den Nijs, J.I.; Gonggrijp, H.S.; Augustinus, E.; Leeksma, C.H. Hot bands: a simple G-banding method for leukemic metaphases. Cancer Genet Cytogenet 1985, 15, 373-374. [CrossRef]
- ISCN 2020: An International System for Human Cytogenomic Nomenclature (2020). ISCN 2020 2020. [CrossRef]
- Uhrig, S.; Ellermann, J.; Walther, T.; Burkhardt, P.; Frohlich, M.; Hutter, B.; Toprak, U.H.; Neumann, O.; Stenzinger, A.; Scholl, C.; et al. Accurate and efficient detection of gene fusions from RNA sequencing data. Genome Res 2021, 31, 448-460. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




