1. Introduction
Brazil hosts an extraordinary diversity of bird species, with approximately 1,834 species currently identified, many of which are endemic to the Amazon region. Among the richest groups are the Psittaciformes [
1,
2]. Amazonian psittacids, such as macaws, parrots, and parakeets, are vital components of the Neotropical biodiversity. These birds not only contribute to the dispersal of seeds from key trees, promoting forest regeneration, but also serve as bioindicators of the overall ecosystem health. Their complex social structures and longevity (up to 70 years in some species) make the group particularly sensitive to environmental disturbances, serving as potential sentinels for diseases and contaminations present in the environment [
3]. However, psittacid populations have drastically declined due to deforestation, illegal trafficking, and more recently, chemical contamination resulting from human activities such as mining, urbanization, and port operations, which pose a serious threat due to the risk of exposure to heavy metals [
4]. Souza et al [
5] emphasize that these birds are "sentinels" of environmental degradation, reflecting habitat quality through their health and behavior.
The Amazon, despite its apparent untouchability, faces an invisible crisis: heavy metal pollution resulting from increasingly intense anthropogenic activities. Illegal gold mining is responsible for about 71% of mercury (Hg) emissions in the region, releasing tons of metal into rivers where it is transformed into methylmercury, a persistent neurotoxin that contaminates fish and invertebrates consumed by psittacids and other wildlife [
6,
7]. Simultaneously, the unregulated use of pesticides in agricultural areas introduces lead (Pb) and cadmium (Cd) into the soil, while urban and industrial waste increases arsenic (As) levels in soil and water. These non-biodegradable metals accumulate in animal tissues after contact (ingestion or inhalation) and persist for decades, even after pollution sources are curtailed [
8].
Metal bioaccumulation in psittacids occurs primarily through diet, with toxic effects amplified by the long lifespan of these birds. Feathers and eggs have been used as non-invasive matrices to monitor contamination, revealing alarming concentrations of Hg (up to 34 µg/g in
Amazona aestiva feathers) and Pb (18 µg/g in
Ara ararauna eggs) [
9]. Mercury (Hg) causes irreversible neurological damage, impairing movement, flight, and communication among individuals, while Pb induces anemia and renal failure, in addition to cardiovascular and reproductive disorders [
10]. Cadmium (Cd) when accumulated in the kidneys, is associated with reproductive dysfunction and chronic kidney failure, and as compromises immunity, increasing mortality from infectious diseases. Zinc (Zn), on the other hand, primarily affects the gastrointestinal system of birds but can also cause nervous system damage, with clinical signs including lethargy, weakness, difficulty perching, and impaired social interactions [
10].
These impacts are exacerbated by chronic metal exposure even at low doses, masking intoxication symptoms until advanced stages that can culminate in animal death, representing a multidimensional threat to the conservation of Amazonian psittacids [
11]. Populations already fragmented by deforestation face accelerated declines due to reduced fertility and chick survival. Moreover, transgenerational contamination via eggs (up to 60% of maternal Hg can be transferred to the embryo) can erode genetic diversity, limiting adaptation to climate changes and environmental variations [
12,
13]. Recent studies suggest that chronic exposure to metals like Cd and Hg can induce epigenetic changes in avian species, potentially affecting immune system genes across generations [
14].
This narrative review integrates toxicological, ecological, and socioeconomic data to propose evidence-based solutions, addressing everything from remediation techniques to public policies aimed at reducing heavy metal concentrations in the environment and consequently in animals. Given that various studies investigate only isolated aspects of environmental contamination, there are critical gaps in integrating physiological, ecological, and conservation effects on Amazonian psittacids facing heavy metal exposure. The goal is to provide a theoretical framework to guide future research and management actions, ensuring the survival of these iconic species in a biome under increasing pressure.
2. Materials and Methods
The present review is a descriptive article based on a narrative literature review, as defined by Grant and Booth [
15]. This review approach was chosen due to the interdisciplinary nature and broad scope of the topic, which encompasses toxicological, ecological, veterinary, and conservation aspects of heavy metal poisoning in Amazonian parrots. To ensure methodological rigor, the literature search was conducted across multiple electronic databases, including Periódicos Capes, PubMed, Scopus, ResearchGate, Scielo, Google Scholar, Academia.edu, BDTD, Redalyc, Science.gov, ERIC, ScienceDirect, SiBi, World Wide Science, PePSIC, and Scholarpedia. Search terms employed either independently or in combination were: heavy metal poisoning, parrots, Amazonian psittacids, psittaciformes, environmental contamination, conservation, One Health, wildlife toxicology, and Amazon Biome. Inclusion criteria comprised publications in peer-reviewed journals or credible scientific sources that addressed the specified search terms, contained relevant information on the exposure of parrots to heavy metals and discussed conservation, toxicological, or health-related implications. Exclusion criteria involved publications in non-peer-reviewed journals or unreliable scientific sources, those that did not address the specified search terms, lacked relevant information, or had insufficient focus on heavy metal toxicity in Psittaciformes. The quality and relevance of the sources were assessed based on methodological soundness, scientific credibility, and alignment with the objectives of the review. A total of 49 unique publications were identified, with an overlap rate of 94% across the consulted databases. All eligible publications were incorporated to ensure a comprehensive synthesis of the available evidence. This transparent methodology enhances reproducibility and strengthens the reliability of the conclusions drawn from the reviewed literature.
3. Sources of Contamination in the Amazon
Heavy metal contamination in the Amazon is driven by human activities that release toxins into the environment, as well as natural processes that facilitate their dispersion and accumulation. Anthropogenic activities, such as illegal artisanal mining, are one of the largest sources of Hg in the region. Mercury (Hg) is used to amalgamate gold and is released directly into rivers and soils where it deposits. It is estimated that up to 30% of the discharged Hg is transformed into methylmercury, a highly toxic form that contaminates fish and invertebrates, which are a staple in the diet of many psittacid species [
16].
Studies reveal that areas near mining sites, such as the Tapajós river, exhibit Hg concentrations in sediments up to 50 times above safe limits [
17]. Lead (Pb) and Cd are associated with intensive agricultural practices and the improper disposal of vehicle batteries. Lead (Pb), found in pesticides like lead arsenate, contaminates soils and watercourses, while Cd, derived from phosphate fertilizers, accumulates in plants that may be consumed by psittacids. In agricultural frontier areas, such as the southern Amazon, Cd levels in soils reach 3.8 mg/kg, exceeding international standards [
18].
Arsenic (As) is released from industrial waste, urban landfills, and fossil fuels. In Manaus and Belém (Brazil), untreated effluents raise as concentrations in rivers near feeding areas of psittacids, such as the Rio Negro [
19]. Improperly disposed batteries and contaminated plastics also contribute to metal pollution in peri-urban zones, affecting adapted species that frequent fragmented habitats, such as
Amazona ochrocephala and
Amazona amazonica [
5].
Amazonian rivers act as vectors for the dispersion of these metals. During the rainy season, floods carry contaminated sediments to floodplain areas, where psittacids feed on fruits and seeds [
19]. Mercury (Hg) particles adsorbed to sediments are transported hundreds of kilometers, contaminating regions far from primary sources, and metals like Pb and Cd bind to organic soil particles, persisting for decades. There are also hyperaccumulator plants, such as
Cecropia spp., that absorb these metals, introducing them into the food chain of numerous species [
17,
18].
Biomagnification further results in increasing metal concentrations at higher trophic levels. For instance, Hg in fish can be 10⁵ times greater than in water, affecting and increasing the risk of severe contamination in piscivorous and frugivorous birds [
16]. Invertebrates, such as ants and termites, bioaccumulate metals and are consumed by psittacid chicks, exacerbating exposure during sensitive developmental stages [
9]. The natural acidification of soils in forest areas also intensifies metal mobilization, facilitating their absorption by roots and leaves used as food and for nest building by birds [
8].
The synergy between human activities and ecological processes transforms the Amazon into a sink for heavy metals. The persistence of these contaminants in the environment, combined with biomagnification, exposes psittacids to cumulative risks, compromising their long-term survival. Given the Amazon's interconnectedness between wildlife, indigenous human populations, and ecosystem services, exposure to heavy metals in parrots reflects a broader One Health crisis, affecting food safety, zoonotic risk, and human development [
20].
4. Bioaccumulation and Toxicokinetic
The bioaccumulation of heavy metals in Amazonian psittacids is a complex process influenced by ecological, physiological, and chemical factors. Understanding toxicokinetics absorption, distribution, metabolism, and excretion is essential to assess the health risks to these birds and propose mitigation strategies [
18,
19]. The primary exposure route for psittacids to toxic metals is through the ingestion of contaminated water and food. Fruits and seeds from plants such as
Bertholletia excelsa (Brazil nut) and
Euterpe oleracea (açaí) accumulate metals from soil and water, especially in areas near mining sites or agricultural zones. Invertebrates, such as insect larvae consumed by chicks, are also critical vectors. For example,
Atta spp. ants, common in the diet of young parrots, exhibit Pb concentrations of up to 12 µg/g in mining regions [
18]. Water contamination, such as in streams near Belém (Brazil) and peri-urban regions, along with rivers associated with extractive mining, also contributes to indirect absorption via hydration and feather cleaning [
19].
The distribution of metals in tissues varies according to chemical affinity and physiological function feathers and eggs as biomarkers feathers are effective non-invasive matrices for monitoring chronic exposure. Studies with
Amazona farinosa revealed Hg concentrations ranging from 8-45 µg/g in feathers and were related to hepatic damage in animals [
9]. Eggs reflect transgenerational transfer 40-60% of the Hg accumulated in females is deposited in the yolk, compromising hatching and embryo viability [
21]. Internal organs the liver is the primary site of detoxification and can accumulate Cd (studies show up to 15 µg/g in
Ara chloropterus) and Hg, generating oxidative stress that can lead to insufficiencies. The kidneys are the primary target of Cd, binding to metallothionein and causing tubular necrosis [
22]. The nervous system has Hg as the main aggressor, crossing the blood-brain barrier and inducing neuronal degeneration, seizures, and behavioral changes [
23]. These data are summarized in
Table 1. Chicks are the most vulnerable due to the immaturity of detoxification systems and high metabolic rate at this life stage, which increases mortality rates and impacts population size and genetic diversity [
21].
The toxicity is amplified by synergistic interactions between metals and other pollutants. Co-exposure to pesticides, such as Hg combined with organophosphates (e.g., chlorpyrifos), inhibits acetylcholinesterase, exacerbating neurotoxicity [
24]. Cumulative effects like the combination of Pb and Cd in acidic soils increase their bioavailability, raising intestinal absorption of these metals by 30–50% [
17]. Regarding excretion, psittacids have a limited capacity to excrete metals. Mercury (Hg) is eliminated via bile and feathers, but its half-life in the liver can exceed 2 years. Cadmium (Cd) is retained in the kidneys for decades, while Pb is partially excreted via feces [
21]. This persistence explains why even populations in remote areas exhibit significant toxic burdens [
21].
Therefore, the bioaccumulation of metals in Amazonian psittacids is a multifactorial process, with direct consequences for individual health and population dynamics over the medium and long term. The synergy between pollutants and inefficient detoxification and excretion makes these birds particularly susceptible, reinforcing the need for continuous biomonitoring of both individuals and living areas, along with strict regulation of pollution sources [
25].
4. Physiological and Ecological Effects
Heavy metal contamination triggers a cascade of physiological dysfunctions in Amazonian psittacids, with direct repercussions on individual health and at population and ecological scales. These effects are amplified by the environmental persistence of pollutants and the vulnerability of species in already degraded habitats. It is known that long-term bioaccumulation present in animals and humans triggers a series of harmful events in the organism, such as oxygen reactions, enzyme inactivation, and oxidative stress, closely related to aging and premature cell death [
26].
Mercury (Hg) particularly in the form of methylmercury, crosses the blood-brain barrier and inhibits the activity of neurotransmitters like serotonin. In macaws (
Ara spp.), this results in spatial disorientation, reduced flight capacity, and loss of social skills, such as vocalization and interaction between individuals [
27]. In studies with
Amazona aestiva, Souza et al [
5] observed cerebellar damage associated with tremors and partial paralysis, compromising food foraging and predator evasion.
Cadmium (Cd) accumulates in renal tubules, replacing Zn in essential enzymes and generating oxidative stress. In mealy parrots (
Amazona farinosa), renal necrosis was observed in 68% of individuals exposed to concentrations above 5 µg/g of Cd. Chronic renal failure leads to dehydration, electrolyte loss, and death, especially during drought periods when water availability is limited [
21].
As inhibits T lymphocyte proliferation and antibody production, leaving birds susceptible to bacterial infections like
Salmonella spp. and avian viruses. In
Ara ararauna, mortality rates from infectious diseases increased by 40% in areas where as > 2 µg/g was observed in the feathers of animals [
11]. The combination of immunosuppression and nutritional stress, due to metal-induced appetite loss, creates a vicious cycle of physiological decline often culminating in the individual's death from sepsis or even hypoglycemic shock associated with malnutrition [
28].
Metals like Hg are transferred to eggs via maternal blood plasma. In
Amazona ochrocephala, 55% of embryos exposed to Hg > 4 µg/g do not hatch, and 30% of chicks die in the first weeks due to cardiac malformations [
21,
29]. Similarly, chick survival in already fragmented populations, such as those of
Ara glaucogularis, is also reduced when exposed to high levels of metals during this life stage, rendering generational replacement unfeasible, accelerating population declines, and causing more significant genetic losses [
12,
13].
In isolated populations like those of
Ara macao in Peruvian Amazon reserves, chronic contamination by Pb and Cd is correlated with lower heterozygosity (12–15% lower than non-exposed populations). This reduces resilience to diseases and climate changes, jeopardizing individual survival in extreme conditions [
12,
13]. Additionally, selection against individuals with high toxic loads diminishes the genetic pool, favoring inbreeding and the expression of deleterious alleles [
29].
Both Pb and Zn toxicity can impair heme synthesis, shorten erythrocyte lifespan, and cause hemolysis and anemia in psittacids chronically contaminated by these metals. Hemolytic anemia and iron deficiency due to interference with intestinal absorption can also be observed, potentially leading to hypochromia, hypoxia, and, in severe cases, death. Morphological alterations in erythrocytes and greenish feces can also be observed in affected animals [
30].
Specimens of
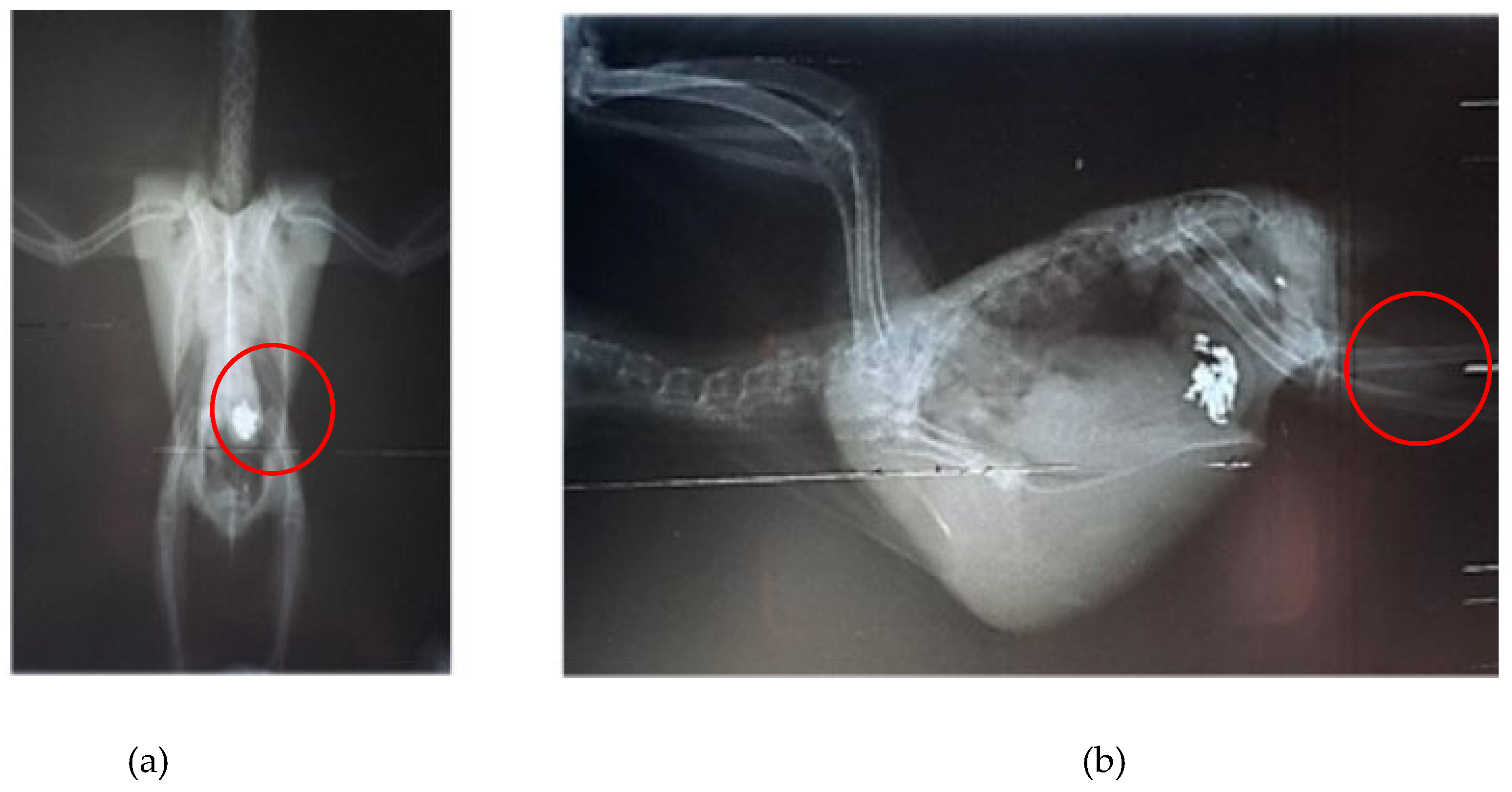
Aratinga jandaya voluntarily surrendered and treated at the Wildlife Sector of the Veterinary Hospital (HVSAS) at the Federal University of Pará (UFPA), in Castanhal/PA, exhibited symptoms (regurgitation, apathy, and disoriented behavior) of heavy metal poisoning, possibly Hg and/or Zn, and the presence of a radiopaque foreign body in the celomic cavity radiographic exam suggestive of portions of the gastrointestinal tract (
Figure 1). After symptomatic generalist treatment (Ca-EDTA 70 mg/kg PO, BID for 7 days, fluid therapy with lactated Ringer's, vitamin supplementation with complex B and omega-3, and nebulization), the animals improved, indicating the possibility of intoxication and raising awareness of the potential contact of these individuals with metals in peri-urban and urban areas where the presence of this and other psittacids species is growing (
Figure 2).
A similar case was described by Pinheiro et al [
29] in
Nymphicus hollandicus, which also culminated in clinical improvement due to prompt symptomatic treatment, demonstrating that even psittacids known to be domestic and/or in direct contact with humans are more susceptible to heavy metal exposure. Santos et al [
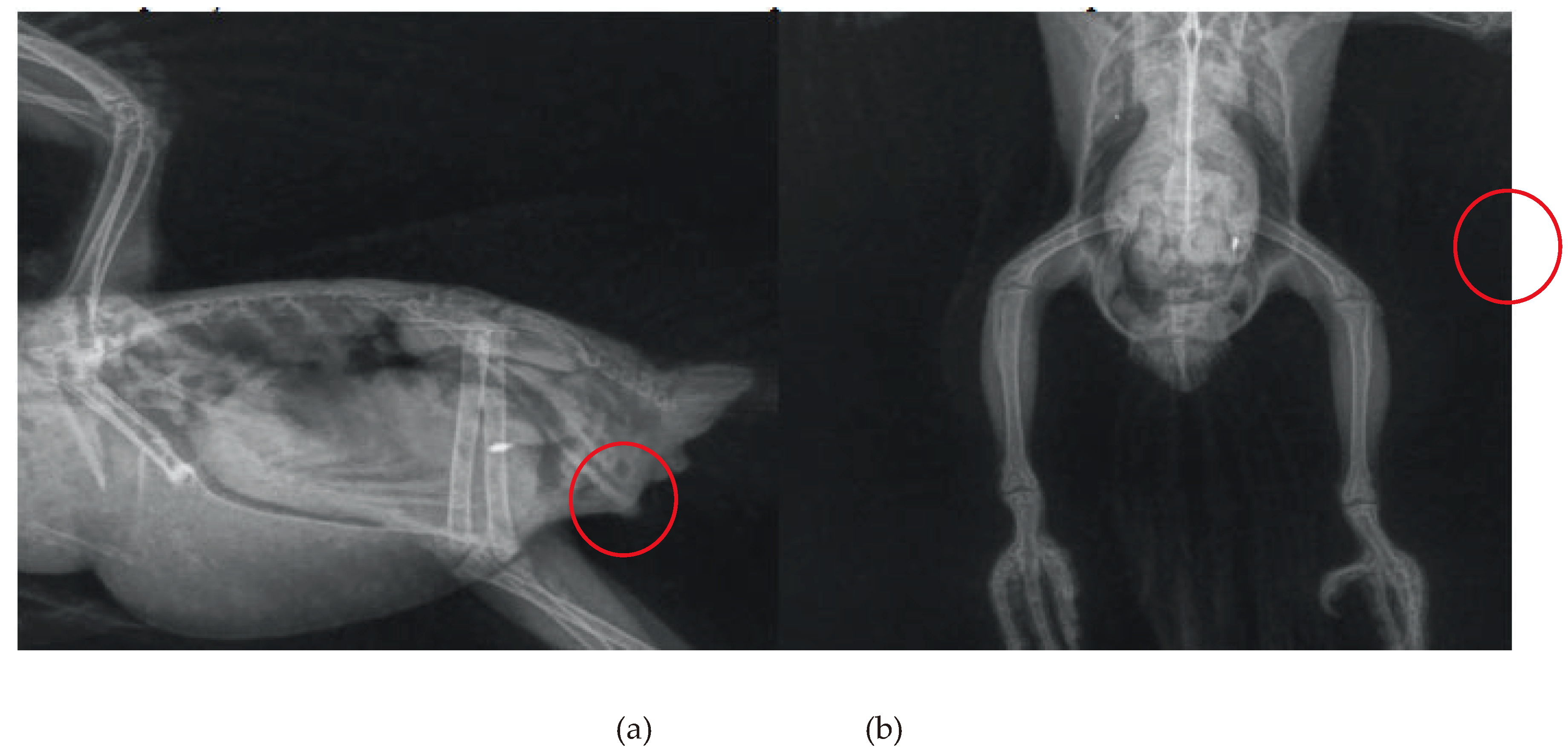
31] also detected the presence of radiopaque content (
Figure 3) and incoordination in free-living
Brotogeris chiriri that responded well to treatment against heavy metal intoxication, restoring full function to the animals. It is important to note the scarcity of reports with descriptive diagnoses regarding the type of metal found, which is a focal point of importance to be better determined in the care of these individuals despite the difficulties in collection and testing, aiming for more accurate treatment directed at the underlying cause.
The reduction in free-living psittacid populations compromises the seed dispersal of large trees (e.g.,
Dipteryx odorata), altering forest regeneration, and favors the proliferation of opportunistic generalist species (e.g., rodents), unbalancing the food chain and promoting the spread of zoonoses [
32]. Therefore, the physiological and ecological effects of heavy metal contamination represent an existential threat to Amazonian psittacids and the entire surrounding ecosystem. The interaction between individual toxicity, population collapse, and ecosystem function degradation demands urgent actions, including the decontamination of critical areas and the creation of ecological corridors to mitigate biodiversity loss [
33].
5. Conservation Challenges
The protection of Amazonian psittacids against heavy metal contamination faces multifaceted obstacles, combining environmental pressures, policy gaps, and operational limitations. These challenges interact synergistically, increasing risks to biodiversity and requiring integrated mitigation approaches. Forest fragmentation, caused by deforestation and agricultural and livestock expansion, isolates psittacid populations in smaller areas more exposed to contamination sources. For example, fragments near mining areas in Pará (Brazil) exhibit Hg concentrations in soils three times higher than in continuous forests, increasing exposure for species like
Amazona amazonica often found in fragment areas [
6].
The reduction of food availability in degraded areas forces birds to consume resources in contaminated zones, such as
Spondias mombin fruits at mining edges, aggravating the possibility of intoxications and bioaccumulation [
29]. Another issue is related to the increase in average environmental temperature (≥1.5°C in eastern Amazon) which intensifies the release of metals retained in soils, like Cd, which becomes more bioavailable under acidic and hot conditions [
34]. Thermal stress from high temperatures also reduces the hepatic detoxification efficiency of psittacids, increasing mortality mainly due to Hg during prolonged drought periods or in areas with limited water availability. Species as
Ara ararauna, for example, show 20% lower metabolic rates at temperatures above 35°C, compromising toxin excretion [
34].
Another challenge is related to poor enforcement and the growing illegal mining operating under ambiguous legislation, such as Law 13.575/2017, which does not clearly set limits for Hg use in operations. It is estimated that only 12% of mines in the Legal Amazon have environmental licenses, facilitating the discharge of 30 to 50 tons of Hg/year into rivers [
6]. Proposals such as replacing Hg with less toxic alloys (e.g., sodium thiosulfate) are still poorly implemented due to lack of oversight and economic incentives, as well as the absence of continuous monitoring stations in regions like the Javari Valley (Amazonas, Brazil) and Yanomami lands (Rondônia, Brazil)), critical focal points associated with illegal mining, which prevent detection of contamination peaks, hindering actions that could significantly reduce river contamination in these regions [
7]. It is known that less than 5% of protected Amazonian areas have real-time water analysis systems, and the implementation of mining and exploration areas in various parts of the forest is increasing, sometimes remote and difficult to access, further complicating the control and oversight of these sites [
5].
Part of the issues associated with enforcement could be better addressed with the use of promising new technologies, such as spectral sensors attached to drones, however, these are still underutilized due to high costs and lack of technical training. Other techniques that could help address the challenge of managing large inaccessible areas, such as phytoremediation with Heliconia spp. (effective in absorbing Pb), are tested on a small scale and also lack funding for expansion [
5]. Considering the deficiencies in oversight and the absence of focal control points, it is demonstrated that governmental efforts towards the rational use and control of heavy metals are still discreet and insufficient. The Amazon Basin is vast and encompasses nine countries, but only 15% of bilateral agreements include concrete targets for heavy metal control [
12,
13]. This shows that without the articulation and development of joint actions between entities and governments for the cause, the implementation of mitigation actions against massive forest contamination and psittacid contact with toxic agents is impaired [
12,
13].
The challenges for the conservation of Amazonian psittacids require interdisciplinary solutions, combining technological innovation, institutional strengthening, and community participation. Without strict regulation of polluting activities and the integration of climate policies, preservation efforts will remain fragmented and insufficient to reverse the population decline of these species. The proliferation and contamination of the Amazon forest with heavy metals is a silent issue that encompasses various nations housing diverse portions of this biome in their territories and is only perceived by the surrounding populations, making it crucial to conduct studies and actions with this focus to expose this issue and pressure institutions and countries to promote mitigation and control measures in their territories [
35].
6. Mitigation Strategies
The conservation of Amazonian psittacids requires integrated approaches, combining technological innovation, ecosystem restoration, and robust public policies. These strategies aim to reduce the use and exposure to heavy metals, restore degraded habitats, and engage local communities in preservation efforts. Monitoring and diagnosis through advanced technologies such as remote sensors, where platforms like drones equipped with mass spectrometers identify contamination sources in real-time, are promising strategies to enable a better understanding of the issue even considering large territorial extents. In the Tapajós River (Pará, Brazil), sensors detected Hg peaks of 8 µg/L during periods of intense mining activity [
19].
Environmental restoration to conserve living and feeding areas for psittacids, as well as planting trees and plants capable of absorbing metals present in the soil, are also interesting and more easily executed mitigation strategies by local communities inhabiting the forest and its surroundings [
4]. The Amazon shrub
Baccharis spp. absorbs up to 150 mg/kg of Pb in contaminated soils through phytoremediation, reducing the bioavailability of toxic metals in the psittacids' food chain; another tree contributing to soil health maintenance is the buriti (
Mauritia flexuosa), whose roots filter 40 to 60% of water from streams, according to tests in the Rio Negro State Park (Brazil) [
29].
The protection of ecological corridors between conservation units, such as the Mamirauá Sustainable Development Reserve (Brazil) and the Jaú National Park (Brazil), is also essential for the conservation of remaining fragments and allows gene flow of species like
Ara macao, increasing the genetic diversity of populations by up to 18% [
12,
13]. In addition to these practices, the restoration of riparian forests, like the "Amazônia Viva" project that replanted 200 ha/year of rubber trees (
Hevea brasiliensis), a tree that also influences metal absorption in the soil, serve as an interesting strategy to block contaminated sediments and reduce psittacids' contact with heavy metals [
12,
13].
Other strategies relate to the biomonitoring of the psittacids inhabiting the forest. One such strategy involves isotopic analyses through techniques like δ²⁰²Hg in psittacids from critical points. This technique reveals sources of environmental contamination, allowing the tracing of the metal path and proving the existence of toxic compounds present in the animals, facilitating diagnosis and directing actions to the main occurrence sites according to their home ranges. About 70% of Hg was detected in
Amazona farinosa from areas that may contain illegal mining, compared to 30% from those with access to non-contaminated natural sources, which tested negative, proving the existence of environmental contamination in specific points [
21].
Another technology that can be associated with animal monitoring is the use of portable spectrometry, such as portable X-ray fluorescence (XRF), which can be used to quantify the concentration of Cd, Hg, Pb, and other heavy metals directly in body organs and tissues [
36]. The analysis of feces, in addition to other potentially collected tissues from deceased animals, constitutes a non-invasive strategy and allows the quantification of metal accumulation such as As, Pb, Cd, and Hg in birds with prolonged contact with such agents, serving as another environmental monitoring tool that does not require direct contact with the animals [
37,
38].
The National Action Plan for Psittacids (PAN Psittacids), which monitors 15 species of this family, notably
Ara chloropterus, includes population health programs including the assessment of metals in feathers, considered a non-invasive and essential method for identifying bioaccumulated toxins in these individuals [
9]. Additionally, tissue biobanks, such as liver and kidney samples from deceased birds, can be stored for retrospective studies (e.g., Cd trends from 2000–2020) and can predict the real situation and vulnerability of the affected population. This strategy needs to be a priority in the coming years for more robust longitudinal studies that allow better delimitation of populations most vulnerable to contaminants [
39].
The adoption of public policies and implementation of the Minamata Convention, which mandates the prohibition of Hg in mining by 2030, is fundamental for the conservation of populations and habitats and one of the main governmental strategies in combating heavy metal contamination [
40]. Brazil has already eliminated 80% of its use by 2023, but enforcement is flawed (only 3% of mines are shut down annually) [
41]. Therefore, technological alternatives are necessary, such as artisanal mining support centers promoting the use of gravimetric concentrators (e.g., shaking tables) and reducing Hg dependence by up to 90%, and the use of magnetic nanoparticles, which adsorb metals in rivers (e.g., 95% of Cd in Xingu waters - Brazil) and are recoverable via magnetic fields; and social support through community environmental education, with the training of riverine populations through active and pedagogical methodologies, such as workshops in Marajó Island (Pará Brazil), which teach non-burning agroforestry techniques, for instance, which have already reduced Cd and Pb release by up to 50% in the region [
5,
34,
42,
43,
44]. Another example is awareness campaigns about different educational projects, such as the "Healthy Parrot" project, which distributes rapid test kits for metals in water and has already benefited 120 riverine communities in Pará (Brazil) [
45].
Thus, mitigation strategies will only be effective with the synergy between science, public policies, and social participation [
46]. While phytoremediation and ecological corridors combat existing damage, Hg regulation and environmental education prevent new contaminations [
47]. The survival of Amazonian psittacids thus depends on a management model that unites innovation with socio-environmental justice. Wetland restoration and sediment dredging in the Danube and Mississippi rivers have successfully reduced heavy metal levels in birds and fish, demonstrating the efficacy of integrated watershed approaches [
48,
49,
50].
7. Conclusions
Heavy metal contamination poses a silent, multidimensional threat to Amazonian psittacids, jeopardizing not only individual birds but entire populations and the ecosystems that sustain them. The persistence of these pollutants combined with habitat fragmentation and climate change creates a cumulative risk scenario where synergistic effects accelerate population declines and ecological degradation. Despite recent scientific advances, critical knowledge gaps and the slow implementation of effective policies keep these species and their habitats trapped in a cycle of vulnerability. This threat is often underestimated, as contamination acts insidiously, with effects manifesting over prolonged time scales such as transgenerational mercury bioaccumulation and across vast spatial scales, such as the fluvial transport of cadmium throughout watersheds. These processes result in cascading impacts, including neurotoxicity, immunosuppression, and genetic diversity loss, ultimately undermining species resilience and destabilizing ecological mutualisms like seed dispersal.
To reverse this scenario, it is essential to prioritize actions that close existing knowledge gaps especially concerning chronic, low-dose exposure and the mechanisms by which metals like arsenic modulate pathogen virulence and immune suppression. Multidisciplinary research efforts should integrate ecological, toxicological, and genomic data to develop predictive models of contamination risks and species vulnerability. Initiatives such as the proposed Amazon BioMetal Atlas modeled on platforms like GBIF and Movebank could provide centralized, open-access data on contamination and species movements. International cooperation is also critical. Organizations such as the Amazon Cooperation Treaty Organization and the Regional Amazon Initiative must strengthen monitoring standards (e.g., under the Minamata Convention) and promote cross-border funding for phytoremediation and technological monitoring. Moreover, community engagement through “citizen scientist” programs, conservation partnerships (e.g., with the Arara Azul Institute), and the deployment of portable diagnostic kits will be vital. Emerging tools such as CRISPR-Cas9 and artificial intelligence-driven contamination prediction models must be developed within ethical frameworks. Ultimately, Amazonian parrots are sentinel species living indicators of environmental health. Their decline reflects systemic governance failures, and safeguarding them will require embracing the One Health approach to integrate animal, human, and ecosystem well-being.
Author Contributions
Conceptualization, M.S.C.B.; K.A.C.S.; B.J.S.d.S. and F.M.S.; methodology, M.S.C.B.; K.A.C.S.; B.J.S.d.S. and F.M.S.; formal analysis, M.S.C.B.; K.A.C.S.; B.J.S.d.S. and F.M.S.; data curation, M.S.C.B.; K.A.C.S.; B.J.S.d.S. and F.M.S.; writing—original draft preparation, M.S.C.B.; K.A.C.S.; B.J.S.d.S. and F.M.S.; writing—review and editing, F.M.S.; supervision, F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Moraes, K.F.; Santos, M.P.D.; Gonçalves, G.S.R.; De Oliveira, G.L.; Gomes, L.B.; Lima, M.G.M. Climate change and bird extinctions in the Amazon. PLoS ONE 2020, 15, e0236103. [CrossRef]
- Silveira, L.F.; De Lima, F.C.T.; Hofling, E. A new species of Aratinga parakeet (psittaciformes: psittacidae) from Brazil, with taxonomic remarks on the Aratinga solstitialis complex. Ornithology 2005, 122, 292–305. [CrossRef]
- Piacentini, V.D.Q.; Aleixo, A.; Agne, C.E.; Maurício, G.N.; Pacheco, J.F.; Bravo, G.A. Lista comentada das aves do Brasil pelo Comitê Brasileiro de Registros Ornitológicos. Rev. Bras. Ornitol. 2015, 23, 91–298. https://doi.org/urn:lsid:zoobank.org:pub:30856542‐FFD1‐44CA‐B249‐9F321CD4CF4C.
- Fragata, M.M.; Baccaro, F.; Gonçalves, A.L.S.; Borges, S.H. Living in a tropical concrete jungle: diversity and abundance variation in a parrot assemblage (Aves, Psittacidae) of a major Amazonian city. Urban Ecosyst. 2022, 25, 977–987. [CrossRef]
- Souza, N.D.; Sant’Anna Neto, A.; Santos Junior, A.J.; Oliveira, A.C.L.; Sampaio, D.A.; Cupertino, G.F.M.; Gonçalves, A.N.; Dias Júnior, A.F. A Brazilian Amazon Species with High Potential to Phytoextract Potential Toxic Elements. Floresta Ambient. 2022, 29, 1. [CrossRef]
- Sousa, E.P.; Martins, M.B.; Freitas Junior, J.A.B.; Pinheiro, M.C.N. Contaminação por mercúrio em peixes na Amazônia brasileira. Rev. Electrónica Ambient. Sust. 2024, 24, 8. [CrossRef]
- Rodrigues, A.A.; Robeiro, S.L.; Costa, B.S.M.; Albuquerque, A.H.S.; Oliveira Júnior, Z.; Silva, L.C. Contaminação de corpos hídricos por mercúrio - Impactos do garimpo de ouro nos direitos e cultura do povo Yanomami em Roraima. Ambiente Gest. Desenv. 2024, 17, 3. [CrossRef]
- Alloway, B.; Ayres, D.C. Chemical Principles of Environmental Pollution, 2nd ed.; CRC Press: Florida, USA, 1997.
- Martinez, J.; Prestes, N.P. Biologia da conservação: Programa Nacional para Conservação do Papagaio-de-peito-roxo e outras iniciativas; LEW, 2021.
- Guthrie, A.L.; Jayson, S.L.; Strike, T.B.; Sparrow, S.J.; Flach, E.J. Diagnosis and Treatment of Heavy Metal Toxicosis in Six Waldrapp Ibis (Geronticus eremita). J. Avian Med. Surg. 2020, 34, 371–380. [CrossRef]
- Beck, K.K.; Mariani, M.; Fletcher, M.S.; Schneider, L.; Aquino-López, M.A.; Gadd, P.S.; Heijnis, H.; Saunders, K.M.; Zawadzki, A. The impacts of intensive mining on terrestrial and aquatic ecosystems: A case of sediment pollution and calcium decline in cool temperate Tasmania, Australia. Environ. Pollut. 2020, 265, 114695. [CrossRef]
- Sheewagen, C.L. Threats of environmental mercury to birds: Knowledge gaps and priorities for future research. Bird Conserv. Int. 2010, 20, 112–123. [CrossRef]
- Whitney, M.C.; Cristol, D. Impacts of Sublethal Mercury Exposure on Birds: A Detailed Review. Rev. Environ. Contam. Toxicol. 2017, 244. [CrossRef]
- Rainio, M. J., Eeva, T. Metal-related oxidative stress in birds. Environ. Pollut. 2010, 158, 2359–2370. [CrossRef]
- Grant, M.J.; Booth, A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [CrossRef]
- Nunes, M.S.B.; Silva, S.S.; Silveira, R.N.P.; Almeida, R.S.; Almeida, A.C.G. Impacto do consumo de peixes contaminados por mercúrio na saúde humana em habitantes da Amazônia brasileira: revisão sistemática. Arch. Health Invest. 2023, 6, 4. [CrossRef]
- Costa, A.G.; Borges, A.M.; Soto-Blanco, B. Metais tóxicos e seus efeitos sobre a reprodução dos animais. Rev. Bras. Hig. Sanit. Anim. 2020, 14, 108. [CrossRef]
- Silva, G.N.; Nazaré, M.L. Impactos das ações antrópicas na história ambiental da bacia hidrogáfica do rio Apeú. Rev. Cien. Multid. 2024, 5, 4117. [CrossRef]
- Santos, C.C.M.; Nauar, A.R.; Ferreira, J.A.; Montes, C.S.; Adolfo, F.R.; Leal, G.; Reis, G.M.; Lapinsky, J.; Carvalho, L.M.; Amado, L.L. Multiple anthropogenic influences in the Pará River (Amazônia, Brazil): A spatial-temporal ecotoxicological monitoring in abiotic and biotic compartments. Chemosphere 2023, 323, 138090. [CrossRef]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; Paillard, C.; Pontier, D.; Sueur, C.; Voituron, Y. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018 12, 14. [CrossRef]
- Barbosa, P.S.; Soares, R.V.; Peres, J.A. Mensuração de metais pesados em ovos de aves comerciais. Ed. Atena, Paraná, Brazil, 2021, p.131–135. [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [CrossRef]
- Dos Santos, A.A.; Hort, M.A.; Culbreth, M.; López-Granero, C.; Farina, M.; Rocha, J.B.; Aschner, M. Methylmercury and brain development: A review of recent literature. J. Trace Elem. Med. Biol. 2016, 38, 99-107. [CrossRef]
- Lemos, M.; Ribeiro, R.O.R.; Targino, F.J.; Almeida, A.L.; Fedullo, L.P.L.; Pires, J.R.; Balthazar, C.F.; Mano, S.B.; Mársico, E.T. Mercury in liver and feathers of some birds of prey in Rio de Janeiro State, Brazil: Preliminary results. Res. Soc. Dev. 2024, 13, 7. [CrossRef]
- Souza, D.C.D.d.; Viana, L.F.; Kummrow, F.; Cardoso, C.A.L.; Lima, N.A.d.; Lacerda, I.A.R.; Crispim, B.d.A.; Barufatti, A.; Dias, L.A.V.; Florentino, A.C. Bioaccumulation of Metals in Fish Collected from Macapá Urban Aquatic Environments (Brazilian Amazon) and the Risks to Human Health. Toxics 2025, 13, 67. [CrossRef]
- Elnabi, M.K.A.; Elkaliny, E.N.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Elaty, A.E.A.; Elgendy, I.M.; Etman, A.E.; Saad, K.E.; Tsigkou, K.; Ali, S.S.; Kornaros, M.; Mahmoud, Y.A.G. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [CrossRef]
- Almeida, M.; Nascimento, D.V.; Mafalda Júnior, P.O.; Patire, V.; Barbosa, A.C.R. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of a Tropical Bay influenced by anthropogenic activities (Todos os Santos Bay, BA, Brazil). Mar. Pollut. Bull. 2018, 137, 399–407. [CrossRef]
- Su, Q.; Ying, H.; Pan, H.L.; Khalid, M.; Zhaoxin, T.; Lianmei, H. Toxicity of inorganic arsenic to animals and its treatment strategies. Comp. Biochem. Physiol. 2023, 271, 109654. [CrossRef]
- Pinheiro, E.C.; Melo, R.C.; Grespan, A.; Peixoto, T.M.B.; dos Santos, M.H.; Cabral, L.A.R.; Costa, P.P.C. Heavy Metal Poisoning in a Cockatiel (Nymphicus hollandicus). Acta Sci. Vet. 2018, 46, 251. [CrossRef]
- Cristopher, M.M.; Shooshtari, M.P.; Levengood, J.M. Assessment of erythrocyte morphologic abnormalities in mallards with experimentally induced zinc toxicosis. Am. J. Vet. Res. 2004, 65, 440–446. [CrossRef]
- Santos, C.B.; Canavessi, L.; Silva, A.H.; Telles, P.H.F.; Cubas, Z.S. Heavy metal poisoning in periquito (Brotogeris Chiriri): case report. Arch. Health Invest. 2021, 7, 11. [CrossRef]
- Triay-Limonta, O.; Hechavarría-García, G.G.; Valdivia, C.E.; Napolitano, C. Knowledge Gaps on the Effectiveness of Seed Dispersal by Mammals and the Effect of Human Disturbances: A Review. Diversity 2024, 16, 780. [CrossRef]
- Loera, Y.; Gruppi, C.; Swing, K.; Campbell-Staton, S.C.; Milá, B.; Smith, T.B. Heavy Metal Contamination in Birds from Protected Regions in the Amazon. Environ. Toxicol. Chem. 2024, 43, 2601–2607. [CrossRef]
- Nascimento, S.P.G.; Santos, E.O.; Santos, J.R.U.; Silva, J.M. Influência do clima a partir da análise da precipitação pluviométrica no Município de Pão de Açúcar, Semiárido Alagoano, Nordeste do Brasil. Rev. Bras. Gest. Amb. Sustent. 2022, 9, 22. [CrossRef]
- Fearnside, P.M. Strategic Challenges for Science and Technology in the Amazon. In: A Floresta Amazônica nas Mudanças Globais, 2nd ed.; Instituto Nacional de Pesquisas da Amazônia—INPA: Manaus, Brazil, 2009; pp. 115–124. Available online: http://philip.inpa.gov.br (accessed on 04/04/2025).
- Estevam, M.; Appoloni, C.R. Uso da fluorescência de raios X portátil (XRF) in vivo como técnica alternativa para acompanhamento dos níveis de ferro em pacientes com sobrecarga de ferro. Rev. Bras. Hematol. Hemoter. 2009, 31, 153–159. [CrossRef]
- Hurtado, T.C.; Brum, B.R.; Batista, M.S.; D’Ávila, R.S.; Ignácio, A.R.A. Estudo quantitativo temporal sobre a contaminação de aves aquáticas por metais. Res. Soc. Dev. 2020, 9, 8. [CrossRef]
- Pinheiro, L.V.S.; Leite, G.; Martinez, C.; Gonsioroski, G.; Pereira, S.; Melo, H.; Arantes, F.; Cerqueira, P.V.; Melinski, R.D.; Oliveira, A.; Bovo, A.A.A.; Las-Casas, F.M.G.; Carvalho, L.G.; Sousa, A.E.B.A.; Ubaid, F.K. Aves da Reserva Biológica do Gurupi: implementação de protocolo avançado de monitoramento e atualizações. Biodivers. Bras. 2024, 14, 3. [CrossRef]
- Ackerman, J.T.; Peterson, S.H.; Herzog, M.P.; Yee, J.L. Methylmercury Effects on Birds: A Review, Meta-Analysis, and Development of Toxicity Reference Values for Injury Assessment Based on Tissue Residues and Diet. Environ. Toxicol. Chem. 2024, 43, 1195–1241. [CrossRef]
- Biswas, S. Birds as Intrinsic Bio-Indicators for Probing Heavy Metal Contamination Signatures in Polluted Environmental Matrices. In Birds - Evolution, Behavior and Habitat Conservation. IntechOpen 2023. [CrossRef]
- Khwankitrittikul, P.; Poapolathep, A.; Poapolathep, S.; Prasanwong, C.; Kulprasertsri, S.; Khidkhan, K. Species Differences and Tissue Distribution of Heavy Metal Residues in Wild Birds. Animals 2024, 14, 308. [CrossRef]
- Scheuhammer, A.M. The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: A review. Environ. Pollut. 1987, 46, 263. [CrossRef]
- Khan, B.N.; Ullah, H.; Ashfaq, Y.; Hussain, N.; Atique, U.; Aziz, T.; Alharbi, M.; Albekairi, T.H.; Alasmari, A.F. Elucidating the effects of heavy metals contamination on vital organ of fish and migratory birds found at fresh water ecosystem. Heliyon 2023, 9, e20968. [CrossRef]
- Pandiyan, J.; Poiyamozhi, A.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Manzoor, I.; Govindarajan, M. Assessment of the Toxic Effects of Heavy Metals on Waterbirds and Their Prey Species in Freshwater Habitats. Toxics 2022, 10, 641. [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [CrossRef]
- Berglund, Å.M.M.; Rainio, M.J.; Eeva, T. Temporal Trends in Metal Pollution: Using Bird Excrement as Indicator. PLoS ONE 2015, 10, e0117071. [CrossRef]
- Seewagen, C.L. Threats of environmental mercury to birds: knowledge gaps and priorities for future research. Bird Conserv. Int. 2010, 20, 112–123. [CrossRef]
- Ilyas, A.; Zhahbaz, S.; Zahra, R. Detection and Comparative Analysis of Heavy Metal Toxicity in Birds. J. Phys. Biomed. Biol. Sci. 2024, 1, 25. https://jpbab.com/index.php/home/article/view/25.
- Pain, D.J.; Mateo, R.; Green, R.E. Effects of lead from ammunition on birds and other wildlife: A review and update. Ambio 2019, 48, 935–953. [CrossRef]
- Abdullah, M.; Fasola, M.; Muhammad, A.; Malik, S.A.; Bostan, N.; Bokhari, H.; Kamran, M.A.; Shafqat, M.N.; Alamdar, A.; Khan, M.; Ali, N.; Eqani, S.A.M.A.S. Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: a case study from severely contaminated areas. Chemosphere 2015, 119, 553–561. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).