1. Introduction
Allergic contact dermatitis (ACD) is a T-cell-mediated inflammatory skin disorder triggered by exposure to specific allergens, resulting in erythema, edema, and pruritus [
1]. In recent years, its prevalence has increased, prompting the search for alternative therapeutic strategies beyond conventional corticosteroid treatments [
2]. Mast cells (MCs) play a pivotal role in allergic responses by undergoing degranulation upon exposure to allergens and releasing mediators such as histamine, prostaglandins, and cytokines. The classical pathway involves allergen-specific immunoglobulin E (IgE) bound to the high-affinity Fc epsilon receptor I (FcεRI) on the surface of MCs. Cross-linking of FcεRI-bound IgE by the allergen leads to rapid MC activation and degranulation [
3]. Moreover, recent findings highlight that MC contributes to hyperresponsiveness in allergic conditions via MRGPRX2-mediated pathways, which may play a role in ACD [
4]. Langerhans cells have been identified as key players in ACD pathogenesis, influencing MC degranulation and immune responses [
5]. These findings suggest that understanding the intricate interplay between MC and other immune cells may facilitate the development of novel therapeutic approaches.
Recently, ketone bodies, particularly β-hydroxybutyrate (BHB), have garnered attention for their potential immunomodulatory and anti-inflammatory effects beyond their role as alternative energy substrates [
6]. Ketone bodies are primarily produced in the liver during fasting, prolonged exercise, or carbohydrate restriction, serving as an essential energy source for peripheral tissues under low-glucose conditions [
7]. Emerging evidence indicates that BHB can suppress the activation of the NOD-, LRR- and Pyrin Domain-Containing Protein 3 (NLRP3) inflammasome, a key component of the innate immune response [
8], and modulate inflammation through epigenetic regulation, particularly via histone deacetylase (HDAC) inhibition [
6]. However, the precise mechanisms through which ketone bodies modulate MC-driven allergic responses remain poorly understood.
Given the immunomodulatory properties of BHB, various ketogenic compounds capable of elevating circulating BHB levels, such as medium-chain triglycerides (MCTs) and 1,3-butanediol (BD), have garnered increasing attention across the biomedical, metabolic, and exercise physiology domains [
9,
10,
11]. Although BD and MCT oil follow different metabolic pathways, their ability to elevate blood BHB levels under standard feeding conditions remains interesting [
12,
13]. Although ketogenesis from MCT oil can be influenced by hormonal factors, such as insulin regulation [
14], our study focused solely on the fed state, in which both BD and MCT oil were administered as a single oral dose. In contrast, BD is metabolized in the liver via alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), ultimately generating BHB without requiring β-oxidation [
15,
16].
Additionally, BHB functions as a ligand for GPR109A/HCAR2 (also referred to as the niacin receptor), a receptor implicated in anti-inflammatory signaling [
8]. Recent evidence suggests that GPR109A activation suppresses MC-derived cytokine secretion and function, potentially by regulating degranulation pathways [
17]. Furthermore, IgE–dependent allergic responses, including anaphylaxis, may be attenuated by GPR109A activation, as demonstrated by Nagata et al. [
18]. These findings highlight GPR109A as a promising target for modulating MC-driven allergic responses. However, whether the BD- and MCT-oil–mediated suppression of allergic responses in ACD occurs via GPR109A activation remains unclear.
To address this issue, the current study aimed to investigate the impact of dietary ketone body level elevation on ACD severity and to elucidate whether GPR109A is involved in BD–mediated suppression of allergic responses. Using a murine model of 2,4-dinitrofluorobenzene (DNFB)–induced ACD, we evaluated the effects of MCT oil and BD on blood BHB levels and disease severity to determine whether these ketogenic substrates offer a stable and effective strategy for modulating allergic inflammation. Furthermore, using mepenzolate bromide (MPN), a GPR109A antagonist [
19,
20], we assessed whether the anti-allergic effect of BD was mediated by GPR109A activation. These insights may pave the way for novel nutritional approaches to prevent and manage allergic diseases, including ACD.
2. Materials and Methods
2.1. Animals and Experimental Design
Male ICR mice (5–7 weeks old) were obtained from Japan SLC, Inc. (Shizuoka, Japan). The animals were housed under standard laboratory conditions with a 12:12 h light/dark cycle, ambient temperature maintained at 22.5 ± 0.5°C, and relative humidity at 55 ± 5%. Mice had free access to an MF diet (Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. At the start of the experiment, all animals were clinically healthy with no apparent signs of disease. The average body weight was 28.2 ± 1.3 g across all groups. Each experimental group consisted of four mice (N = 4), and all measurements were conducted independently for each animal. Mice were randomly assigned to each treatment group to minimize selection bias. All experimental procedures were approved by the Animal Experimentation Committee of Kobe Gakuin University (Approval No. 24-21) and conducted in accordance with institutional guidelines for animal care and use. No adverse events or animal deaths occurred during the course of the study.
2.2. Ketogenic Substrate Administration
MCT oil was obtained from Nisshin OilliO Group, Ltd. (Tokyo, Japan), and BD was purchased from Thermo Scientific (Waltham, MA, USA). Each compound was administered via oral gavage at a dose of either 2 g/kg or 4.5 g/kg body weight (BW) under isoflurane anesthesia in accordance with approved institutional protocols to ensure accurate and consistent delivery. Gavage was performed using a gastric tube (Fuchigami Kikai, Kyoto, Japan) to ensure uniform administration across animals. The mice were divided into the following treatment groups: the DNFB(−) group (vehicle application only), water group (DNFB challenge with water gavage), BD group (DNFB challenge with BD gavage), and MPN/BD group (DNFB challenge with MPN injection followed by BD gavage). Each group consisted of four mice (N = 4).
2.3. Blood BHB Measurement
Blood BHB concentrations were measured using a handheld ketone monitoring device (FreeStyle Optium β-Ketone, Abbott, Tokyo, Japan). Whole blood samples were collected from the tail vein or via cardiac puncture of the right ventricle, depending on the experimental stage. Measurements were performed at two time points: immediately before the DNFB application and at the time of sacrifice.
2.4. Induction of Allergic Dermatitis
ACD was induced according to the protocol described by Yoshimura [
21] and Fujii et al. [
22], ensuring reproducibility and standardization of the model. Briefly, mice were sensitized by topical application of 50 µL of 0.5% DNFB (Nacalai Tesque, Kyoto, Japan), dissolved in a 3:1 mixture of acetone and olive oil (both from Nacalai Tesque), to the shaved dorsal skin on two consecutive days. One week later, ACD was elicited by applying 20 µL of 0.2% DNFB to the dorsal surface of the right ear, whereas the left ear received 20 µL of the vehicle solution and served as a control.
MPN (Cayman Chemical, Ann Arbor, MI, USA) was dissolved in dimethyl sulfoxide at a concentration of 5 mg/mL and administered subcutaneously into the head and neck region at a dose of 1 µL/g BW (equivalent to 5 µg/kg BW).
2.5. Measurement of Ear Thickness
The ear thickness was measured as an indicator of immediate allergic inflammation mediated by MC degranulation. The procedure followed the protocols described in [
21,
22], ensuring consistency and reproducibility. Measurements were performed using a digital caliper (Mitutoyo Corporation, Kanagawa, Japan), with gentle pressure applied to avoid tissue compression. Ear thickness was recorded immediately before the DNFB application and 0.5 or 1 h after the challenge, depending on the experimental setting. Results were expressed in micrometers (μm).
2.6. Histological Preparation and Toluidine Blue (TB) Staining
At the time of sacrifice, a 2- to 3-mm central portion of the lateral half of the auricular tissue (external ear) was excised and fixed in 4% paraformaldehyde (Nacalai Tesque, Kyoto, Japan). Frozen sections (8- to 10-µm thick) were prepared from this tissue using a cryostat. TB staining was performed using a 0.5% TB solution (Nacalai Tesque), and the stained sections were examined under a light microscope at ×400 magnification (Carl Zeiss, Oberkochen, Germany). MC degranulation was assessed across the entire tissue section to avoid sampling bias and to capture the overall inflammatory response.
2.7. Statistical Analysis
Statistical analyses were performed using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test or Dunnett’s test, as appropriate. All analyses were conducted using the R software (version 4.4.2; R Foundation for Statistical Computing, Vienna, Austria). A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Fasting Increases BHB Levels and Suppresses Immediate-Type Allergic Response
To examine the effects of fasting-induced ketosis on ACD, we measured blood BHB levels and assessed allergic responses following the DNFB challenge. Based on a previous report demonstrating that 20 h of fasting reliably induced a physiological increase in circulating BHB levels in mice [
23], we adopted the same fasting duration in our model. As expected, the BHB levels were significantly higher in the fasted group than in the fed group (
Figure 1a), confirming the successful induction of a ketotic state. Macroscopic observation of the ears 0.5 h after the DNFB challenge revealed clear differences between the groups (
Figure 1b, upper panel). In the fed group, the right ear treated with DNFB exhibited visible redness compared with the left ear treated with vehicle alone. In contrast, the fasted group showed markedly less redness in the DNFB-treated right ear than the fed group, suggesting that fasting attenuated the immediate inflammatory response. Histological analysis using TB staining revealed fewer degranulated MCs in the fasted group, whereas extensive degranulation was observed in the fed group (
Figure 1b, lower panel). Quantitative measurements further supported these observations: ear thickness at 0.5 h post-challenge was significantly lower in fasted mice than in fed mice (
Figure 1c), and the MC degranulation ratio was significantly reduced in the fasted group (
Figure 1d). These values were comparable with those observed in the non-challenged control group. These findings indicated that fasting for 20 h, which is sufficient to induce nutritional ketosis, increases circulating BHB levels and attenuates immediate-type allergic responses by suppressing MC degranulation.
3.2. Nutritional Ketosis via MCT Oil and BD Attenuates Immediate-Type Allergic Response
To assess whether nutritional interventions that elevate ketone body levels can replicate the effects of fasting, we administered MCT oil and BD—two known ketogenic substrates—before the DNFB challenge. As illustrated in the experimental timeline (
Figure 2a), the mice received a single oral gavage (2 g/kg BW) of MCT oil or BD 1 h before DNFB exposure. Visual inspection of the right ear challenged with DNFB revealed that mice treated with MCT oil or BD displayed markedly less redness than those in the water group (
Figure 2b). Quantitative analysis confirmed that ear thickness was significantly reduced in both the MCT and BD groups compared with that in the water-treated group (
Figure 2c). Although swelling in the MCT and BD groups appeared similar to that in the non-sensitized control group, statistical analysis revealed that the measurements remained significantly higher than those in the DNFB(−) group. Blood BHB levels measured 1 h after the DNFB challenge were significantly elevated in the MCT and BD groups relative to those in the water group (
Figure 2d), indicating the successful induction of nutritional ketosis. No significant difference exists in the BHB levels between the MCT and BD groups. Furthermore, a significant negative correlation was observed between blood BHB concentrations and ear swelling across all groups (R² = 0.5935,
p < 0.004), suggesting that elevated BHB levels are associated with suppression of the immediate-type allergic response (
Figure 2e). These results demonstrated that oral administration of MCT oil or BD at a dose of 2 g/kg BW effectively increased circulating BHB levels and reduced MC-mediated acute allergic inflammation, similar to the effects observed under fasted conditions.
3.3. MCT Oil and BD Suppress MC Degranulation in Association with Elevated BHB Levels
To investigate whether the suppressive effects of MCT oil and BD on immediate-type allergic responses involved inhibition of MC degranulation, we evaluated MC activation in ear tissues 1 h after the DNFB challenge using TB staining. As shown in the histological images (
Figure 3a), degranulated MCs were more frequently observed in the water-treated group than in the MCT and BD groups. Quantitative analysis revealed that the MC degranulation rate was significantly higher in the water group than in the MCT and BD groups, both of which showed comparable and significantly reduced levels of degranulation (
Figure 3b). These results indicated that MCT oil and BD suppressed MC activation in this model. Furthermore, a strong negative correlation was observed between blood BHB concentrations and MC degranulation rates across all groups (R² = 0.7868,
p < 0.0002), suggesting that elevated BHB levels were associated with reduced MC degranulation (
Figure 3c).
3.4. GPR109A Antagonist Mepenzolate Abolishes the Inhibitory Effects of BD on Allergic Inflammation
To investigate whether the anti-allergic effects of BD were mediated by GPR109A, mice were pretreated with the GPR109A antagonist MPN before BD administration (MPN/BD group). In this experiment, BD was administered at a dose of 4.5 g/kg BW, which was higher than that used in previous experiments (
Figure 2). This higher dose was selected because the co-administration of MPN at 2 g/kg previously resulted in insufficient elevation of blood BHB levels, potentially making it difficult to assess whether the anti-allergic effect of BD is mediated by GPR109A. Therefore, a higher dose was used to ensure adequate BHB level elevation and appropriately assess whether the anti-allergic effects of BD were mediated by GPR109A. The experimental timeline (
Figure 4a) shows that MPN was administered subcutaneously 1 h before BD gavage. Visual inspection revealed that ear redness, which was markedly reduced in the BD-treated mice, reappeared in the MPN/BD group (
Figure 4b). Quantitative analysis confirmed that ear swelling was significantly suppressed in the BD group compared with the water group, whereas co-administration of MPN completely abolished this suppressive effect (
Figure 4c). Blood BHB levels were significantly elevated following BD treatment; however, this increase was markedly reduced by MPN co-administration (
Figure 4d). This attenuation of BHB levels is likely attributable to the influence of MPN on systemic metabolism rather than a direct effect of GPR109A signaling. Degranulated MCs were observed more frequently in the water and MPN/BD groups than in the DNFB(−) and BD groups, indicating that BD administration suppressed MC degranulation unless co-treated with MPN (
Figure 4e). The quantitative assessment showed that the MC degranulation rates were significantly lower in the BD group than in the water group, and this suppression was abolished by MPN co-treatment (
Figure 4f). These results indicate that the anti-allergic effects of BD, including the suppression of MC degranulation and ear swelling, were at least partially mediated through GPR109A signaling.
4. Discussion
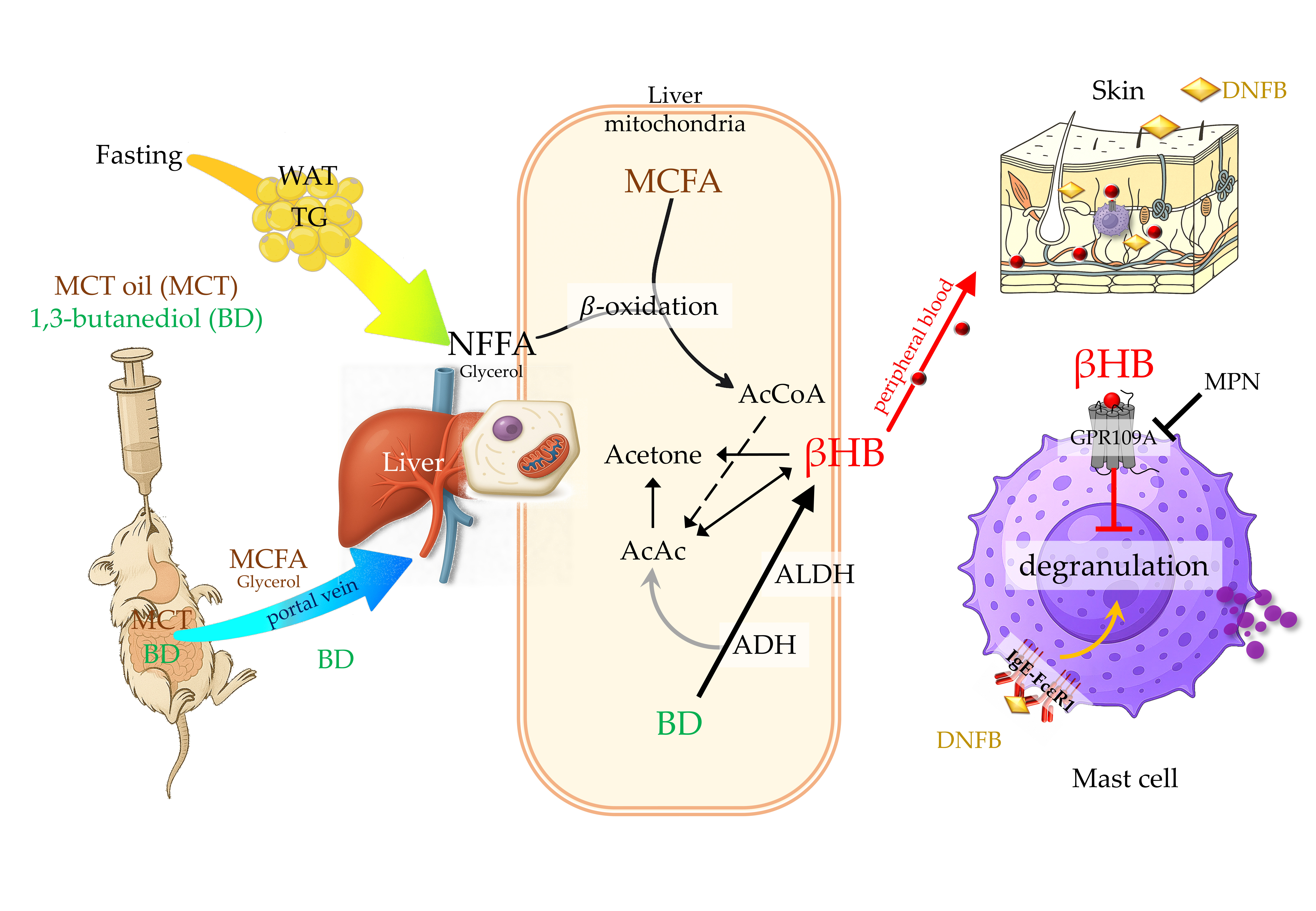
To our knowledge, this is the first study to demonstrate that BHB suppresses MC–mediated allergic responses in a murine model of ACD, thereby providing a novel link between ketone metabolism and contact hypersensitivity. Our findings further show that nutritional ketosis, achieved through either fasting or oral administration of ketogenic substrates such as MCT oil and BD, attenuates immediate-type allergic inflammation in DNFB-induced ACD. As illustrated in
Figure 5, elevated circulating BHB levels were associated with reduced ear swelling and mast cell degranulation, suggesting a mechanistic connection between ketone body metabolism and allergic inflammation. Moreover, pharmacological blockade of the BHB receptor GPR109A using MPN abolished the anti-allergic effects of BD, indicating that these effects are mediated through GPR109A-dependent signaling.
Fasting-induced ketosis significantly increased blood BHB levels and attenuated immediate allergic responses following the DNFB challenge, as evidenced by reductions in ear swelling and MC degranulation (
Figure 1). These results align with previous findings indicating that BHB possesses anti-inflammatory properties through the inhibition of the NLRP3 inflammasome and HDACs [
8,
24] and a previous report showing that fasting-induced elevation of endogenous BHB suppresses immediate hypersensitivity reactions [
23]. However, our study provides new evidence that BHB modulates acute allergic responses by inhibiting MC degranulation.
To assess the translational potential of this mechanism, we examined whether nutritional supplementation with MCT oil or BD could replicate the fasting-induced effects. Both compounds effectively increased the circulating BHB levels and significantly suppressed DNFB-induced inflammation and MC degranulation (
Figure 2 and
Figure 3). BD remained effective even in the fed state, indicating its potential as a more robust and dietary-independent ketogenic substrate. These findings support the hypothesis that BHB is a bioactive metabolite capable of modulating immune responses.
Given the growing interest in steroid-sparing alternatives due to concerns over the adverse effects of topical corticosteroids (TCS) [
25,
26], our findings provide a rationale for developing nutritional strategies to manage allergic skin conditions. The BHB-induced suppression of MC activation may offer a novel therapeutic pathway, particularly for patients who are reluctant to use TCS or for whom long-term steroid use is contraindicated.
The involvement of GPR109A is supported by experiments using MPN, a pharmacological antagonist of this receptor [
19,
20]. MPN co-administration abolished BD-induced suppression of ear swelling and MC degranulation (
Figure 4), supporting a GPR109A-dependent mechanism. Although GPR109A was the intended target, MPN has anti-muscarinic properties [
27], which may confound our interpretation. MPN partially reduced the BHB level elevation induced by BD (
Figure 4d), raising the possibility that it interferes with BD metabolism. BD is converted into BHB in the liver by ADH and ALDH [
15,
16]. Although direct inhibition of these enzymes by MPN is unproven, muscarinic blockade can alter hepatic autonomic tone and affect enzyme activity or cofactor availability, such as nicotinamide adenine dinucleotide and its reduced form balance [
28,
29]. Muscarinic signaling, particularly through hepatic M3 receptors, influences mitochondrial function and metabolic regulation [
30,
31], suggesting that MPN suppresses hepatic BHB production by impairing BD metabolism. Alternatively, MPN might reduce intestinal BD absorption by inhibiting gastrointestinal motility and secretion. These off-target actions may limit systemic availability of BD, contributing to an attenuated BHB response. Although these mechanisms remain hypothetical, they underscore the importance of using more selective GPR109A inhibitors or genetic models in future studies. Although MPN only partially reduced the BD-induced elevation in circulating BHB levels (
Figure 4d), its anti-allergic effects—suppression of MC degranulation and ear swelling—were completely abolished (
Figure 4c, f). This dissociation highlights the essential role of GPR109A signaling in mediating the effects of BHB, even at physiologically relevant BHB levels. Further validation, using more selective antagonists or GPR109A-deficient models, is warranted.
Although our study focused primarily on MCs as effector cells, other immune or structural cells within the skin, such as keratinocytes, dendritic cells, and T lymphocytes, may respond to BHB. Keratinocytes have been shown to express GPR109A [
32], suggesting that BHB directly modulates their functions. Keratinocytes are known to influence skin barrier integrity and local immune responses, and they can produce immunoregulatory cytokines such as interleukin-10 and transforming growth factor–β, which may, in turn, affect MC activation [
33,
34]. Therefore, BHB may exert its anti-allergic effects by acting directly on MCs and indirectly via keratinocyte-derived signals. Further investigation is warranted to clarify the contribution of these GPR109A-expressing structural cells to the overall anti-inflammatory response.
5. Conclusions
This study demonstrates that BHB suppresses immediate-type allergic inflammation by inhibiting MC degranulation. BHB was elevated either endogenously by fasting or exogenously by ketogenic substrates, such as MCT oil or BD, and its effects were abolished by GPR109A antagonism. The observed effects were strongly correlated with elevated blood BHB levels and were abolished by GPR109A antagonism, indicating that the anti-allergic actions of BHB are at least partially mediated through GPR109A signaling. These findings highlight the therapeutic potential of targeting the ketone body–GPR109A axis to prevent or mitigate allergic conditions such as ACD. Nutritional modulation of ketone metabolism may represent a novel non-pharmacological strategy for managing allergic skin diseases, particularly in patients seeking alternatives to TCS. However, this study has certain limitations. The causal role of GPR109A was inferred pharmacologically and awaits confirmation using genetic approaches. The specific cellular targets of BHB within skin tissue remain to be fully elucidated. Additionally, the GPR109A antagonist mepenzolate may have off-target effects, such as anti-muscarinic activity, which could influence systemic metabolism and confound interpretation. Further studies using selective tools and chronic models are required to validate the therapeutic potential of this pathway.
Author Contributions
Conceptualization, Y.Y.; Methodology, Y.Y.; Validation, Y.Y.; Formal Analysis, Y.Y., A.F. and K.N.; Investigation, Y.Y. and A.F.; Data Curation, Y.Y. and A.F.; Writing—Original Draft Preparation, A.F. and Y.Y.; Writing—Review and Editing, Y.Y.; Visualization, Y.Y.; Supervision, Y.Y.; Project Administration, Y.Y.; Funding Acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI [grant numbers 22K11738 and 25K14927].
Institutional Review Board Statement
All animal experiments were approved by the Kobe Gakuin University Animal Experimentation Committee (Animal Experimentation Certificate No. 24-21).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Abbreviations
The following abbreviations are used in this manuscript:
| ACD |
Allergic Contact Dermatitis |
| ADH |
Alcohol Dehydrogenase |
| ALDH |
Aldehyde Dehydrogenase |
| ANOVA |
Analysis of Variance |
| BHB |
β-Hydroxybutyrate |
| BD |
1,3-Butanediol |
| COX-2 |
Cyclooxygenase-2 |
| DNFB |
2,4-Dinitrofluorobenzene |
| FcεRI |
Fc epsilon receptor I |
| GPR109A |
G Protein-Coupled Receptor 109A |
| HDAC |
Histone Deacetylase |
| IgE |
Immunoglobulin E |
| IL |
Interleukin |
| MC |
Mast Cell |
| MCT |
Medium-Chain Triglyceride |
| MPN |
Mepenzolate Bromide |
| NLRP3 |
NOD-, LRR- and Pyrin Domain-Containing Protein 3 |
| NFFA |
Non-esterified Fatty Acids |
| SEM |
Standard Error of the Mean |
| TB |
Toluidine Blue |
| TCS |
Topical Corticosteroids |
References
- Mraz, V.; Geisler, C.; Bonefeld, C.M. Dendritic Epidermal T Cells in Allergic Contact Dermatitis. Front Immunol 2020, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Brites, G.S.; Ferreira, I.; Sebastiao, A.I.; Silva, A.; Carrascal, M.; Neves, B.M.; Cruz, M.T. Allergic contact dermatitis: From pathophysiology to development of new preventive strategies. Pharmacol Res 2020, 162, 105282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kurashima, Y. Two Sides of the Coin: Mast Cells as a Key Regulator of Allergy and Acute/Chronic Inflammation. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Aitella, E.; De Martinis, M.; Romano, C.; Azzellino, G.; Ginaldi, L. Neurogenic Inflammation in Allergic Contact Dermatitis. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Numata, T.; Harada, K.; Nakae, S. Roles of Mast Cells in Cutaneous Diseases. Front Immunol 2022, 13, 923495. [Google Scholar] [CrossRef]
- Qi, J.; Gan, L.; Fang, J.; Zhang, J.; Yu, X.; Guo, H.; Cai, D.; Cui, H.; Gou, L.; Deng, J.; et al. Beta-Hydroxybutyrate: A Dual Function Molecular and Immunological Barrier Function Regulator. Front Immunol 2022, 13, 805881. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Tapia, E.; Pedraza-Chaverri, J. beta-Hydroxybutyrate: A signaling metabolite in starvation response? Cell Signal 2016, 28, 917–923. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Boleslawska, I.; Kowalowka, M.; Boleslawska-Krol, N.; Przyslawski, J. Ketogenic Diet and Ketone Bodies as Clinical Support for the Treatment of SARS-CoV-2-Review of the Evidence. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Koutnik, A.P.; Goldberg, E.L.; Upadhyay, V.; Turnbaugh, P.J.; Verdin, E.; Newman, J.C. Investigating Ketone Bodies as Immunometabolic Countermeasures against Respiratory Viral Infections. Med 2020, 1, 43–65. [Google Scholar] [CrossRef]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Plews, D.; Laursen, P.; Dulson, D.K. The Effect of 1,3-Butanediol on Cycling Time-Trial Performance. Int J Sport Nutr Exerc Metab 2019, 29, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Blonquist, T.M.; Mah, E.; Sanoshy, K.; Beckman, D.; Nieman, K.M.; Winters, B.L.; Anthony, J.C.; Verdin, E.; Newman, J.C.; et al. Tolerability and Safety of a Novel Ketogenic Ester, Bis-Hexanoyl (R)-1,3-Butanediol: A Randomized Controlled Trial in Healthy Adults. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.M.; Rho, J.M.; D’Agostino, D.P. Ketone Administration for Seizure Disorders: History and Rationale for Ketone Esters and Metabolic Alternatives. Front Neurosci 2019, 13, 1041. [Google Scholar] [CrossRef] [PubMed]
- Heidt, C.; Fobker, M.; Newport, M.; Feldmann, R.; Fischer, T.; Marquardt, T. Beta-Hydroxybutyrate (BHB), Glucose, Insulin, Octanoate (C8), and Decanoate (C10) Responses to a Medium-Chain Triglyceride (MCT) Oil with and without Glucose: A Single-Center Study in Healthy Adults. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Tate, R.L.; Mehlman, M.A.; Tobin, R.B. Metabolic fate of 1,3-butanediol in the rat: conversion to -hydroxybutyrate. J Nutr 1971, 101, 1719–1726. [Google Scholar] [CrossRef]
- Kirsch, J.R.; D’Alecy, L.G.; Mongroo, P.B. Butanediol induced ketosis increases tolerance to hypoxia in the mouse. Stroke 1980, 11, 506–513. [Google Scholar] [CrossRef]
- Thio, C.L.; Lai, A.C.; Ting, Y.T.; Chi, P.Y.; Chang, Y.J. The ketone body beta-hydroxybutyrate mitigates ILC2-driven airway inflammation by regulating mast cell function. Cell Rep 2022, 40, 111437. [Google Scholar] [CrossRef]
- Nagata, K.; Ando, D.; Ashikari, T.; Ito, K.; Miura, R.; Fujigaki, I.; Goto, Y.; Ando, M.; Ito, N.; Kawazoe, H.; et al. Butyrate, Valerate, and Niacin Ameliorate Anaphylaxis by Suppressing IgE-Dependent Mast Cell Activation: Roles of GPR109A, PGE2, and Epigenetic Regulation. J Immunol 2024, 212, 771–784. [Google Scholar] [CrossRef]
- Singh, V.; Jamwal, S.; Jain, R.; Verma, P.; Gokhale, R.; Rao, K.V. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe 2012, 12, 669–681. [Google Scholar] [CrossRef]
- Tran, M.T.; Zsengeller, Z.K.; Berg, A.H.; Khankin, E.V.; Bhasin, M.K.; Kim, W.; Clish, C.B.; Stillman, I.E.; Karumanchi, S.A.; Rhee, E.P.; et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016, 531, 528–532. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Takahashi, M. Effects of Triacetin on AMPK Activation and Immune Responses in Allergic Contact Dermatitis. Allergies 2024, 4, 254–267. [Google Scholar] [CrossRef]
- Fujii, A.; Kimura, R.; Mori, A.; Yoshimura, Y. Sucrose Solution Ingestion Exacerbates Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Rats. Nutrients 2024, 16, 1962. [Google Scholar] [CrossRef]
- Nakamura, S.; Hisamura, R.; Shimoda, S.; Shibuya, I.; Tsubota, K. Fasting mitigates immediate hypersensitivity: a pivotal role of endogenous D-beta-hydroxybutyrate. Nutr Metab (Lond) 2014, 11, 40. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Li, A.W.; Yin, E.S.; Antaya, R.J. Topical Corticosteroid Phobia in Atopic Dermatitis: A Systematic Review. JAMA Dermatol 2017, 153, 1036–1042. [Google Scholar] [CrossRef]
- Rathi, S.K.; D’Souza, P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol 2012, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Tanaka, K.; Asano, T.; Yamakawa, N.; Kobayashi, D.; Ishihara, T.; Hanaya, K.; Shoji, M.; Sugai, T.; Wada, M.; et al. Synthesis and biological comparison of enantiomers of mepenzolate bromide, a muscarinic receptor antagonist with bronchodilatory and anti-inflammatory activities. Bioorg Med Chem 2014, 22, 3488–3497. [Google Scholar] [CrossRef]
- Walker, M.A.; Tian, R. NAD(H) in mitochondrial energy transduction: implications for health and disease. Curr Opin Physiol 2018, 3, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 2007, 30, 5–13. [Google Scholar]
- Vatamaniuk, M.Z.; Horyn, O.V.; Vatamaniuk, O.K.; Doliba, N.M. Acetylcholine affects rat liver metabolism via type 3 muscarinic receptors in hepatocytes. Life Sci 2003, 72, 1871–1882. [Google Scholar] [CrossRef]
- Li, J.H.; Gautam, D.; Han, S.J.; Guettier, J.M.; Cui, Y.; Lu, H.; Deng, C.; O’Hare, J.; Jou, W.; Gavrilova, O.; et al. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes 2009, 58, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Gille, A.; Zwykiel, S.; Lukasova, M.; Clausen, B.E.; Ahmed, K.; Tunaru, S.; Wirth, A.; Offermanns, S. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest 2010, 120, 2910–2919. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat Rev Immunol 2009, 9, 679–691. [Google Scholar] [CrossRef]
Figure 1.
Effect of fasting on blood β-hydroxybutyrate levels, ear swelling, and mast cell degranulation in DNFB-induced allergic contact dermatitis (a) Blood BHB concentrations under fed and fasted conditions. Blood samples were collected after 20 h of fasting, and BHB levels were measured. Data are expressed as mean ± SEM. *p < 0.05 vs. Fed (Student’s t-test). (b) Representative dorsal view images of mouse ears after DNFB challenge under fed and fasted conditions (top). TB-stained histological sections of ear tissue showing degranulated MC (arrowheads) in each group (bottom). Scale bars: 50 μm. (c) Ear swelling measured at 0.5 h after the DNFB challenge in control (DNFB(−)), fed, and fasted mice. Different letters indicate statistically significant differences between groups (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (d) MC degranulation rates at 0.5 h after the DNFB challenge assessed using TB staining. The percentage of degranulated MC was calculated relative to the total number of MC. Different letters indicate statistically significant differences between groups (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05).
Figure 1.
Effect of fasting on blood β-hydroxybutyrate levels, ear swelling, and mast cell degranulation in DNFB-induced allergic contact dermatitis (a) Blood BHB concentrations under fed and fasted conditions. Blood samples were collected after 20 h of fasting, and BHB levels were measured. Data are expressed as mean ± SEM. *p < 0.05 vs. Fed (Student’s t-test). (b) Representative dorsal view images of mouse ears after DNFB challenge under fed and fasted conditions (top). TB-stained histological sections of ear tissue showing degranulated MC (arrowheads) in each group (bottom). Scale bars: 50 μm. (c) Ear swelling measured at 0.5 h after the DNFB challenge in control (DNFB(−)), fed, and fasted mice. Different letters indicate statistically significant differences between groups (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (d) MC degranulation rates at 0.5 h after the DNFB challenge assessed using TB staining. The percentage of degranulated MC was calculated relative to the total number of MC. Different letters indicate statistically significant differences between groups (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05).
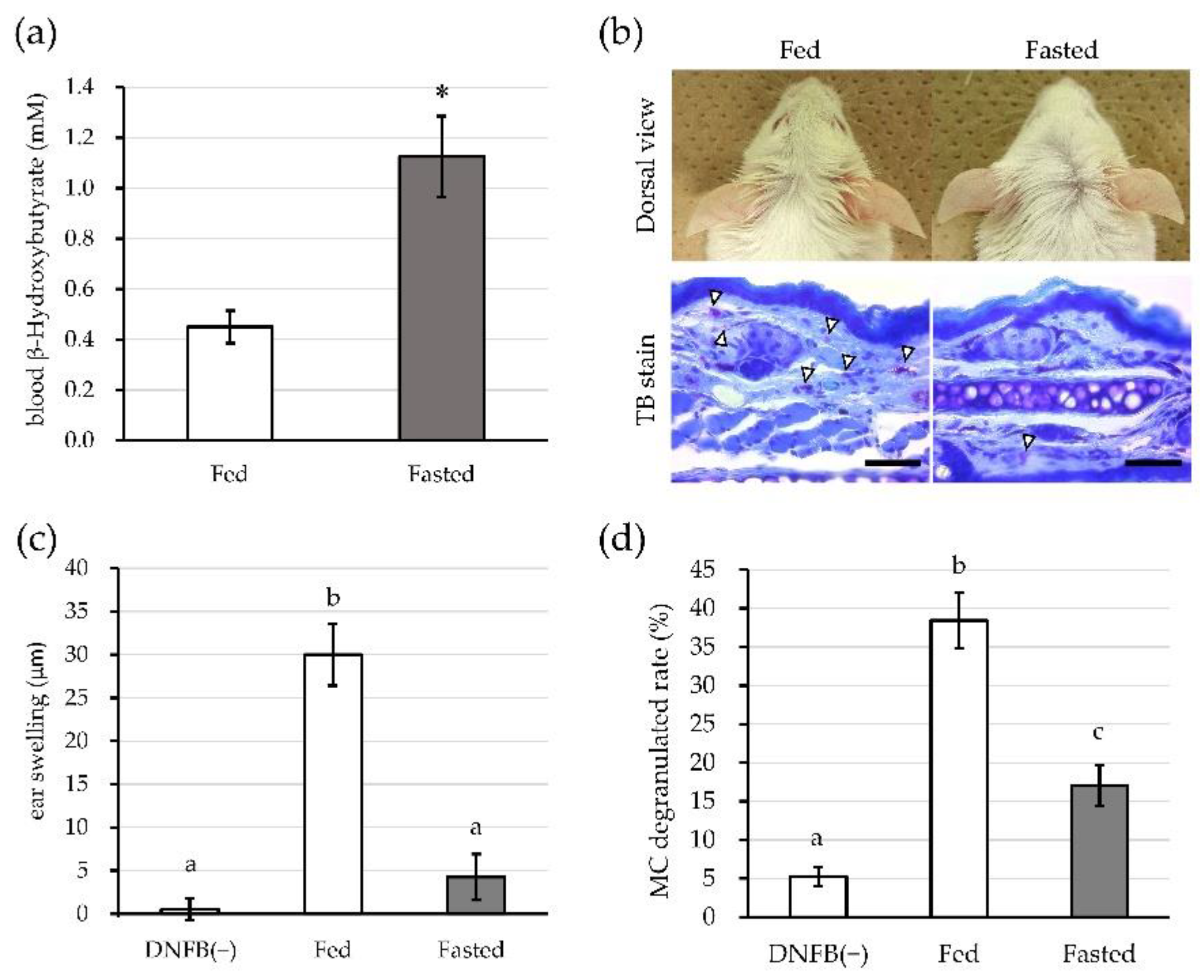
Figure 2.
Nutritional ketosis using MCT oil and 1,3-butanediol suppresses DNFB-induced immediate-type allergic response (a) Experimental timeline. Mice were sensitized with DNFB on days −7 and −6, followed by oral gavage (2 g/kg body weight) of MCT oil, BD, or water 1 h before the DNFB challenge. Blood samples were collected for BHB measurement at 1 h after the DNFB challenge. (b) Representative dorsal views of mouse ears at 1 h after DNFB challenge. The right ear challenged with DNFB revealed that mice treated with MCT oil or BD displayed markedly less redness than those in the water group. (c) Ear swelling at 1 h after the DNFB challenge in each group. MCT and BD groups exhibited significantly reduced swelling compared with the water group. Although ear swelling appeared similar to that in the DNFB(−) group, it remained significantly higher. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (d) Blood BHB concentrations measured 1 h after the DNFB challenge. Both the MCT and BD groups demonstrated significantly increased BHB levels compared with the water group. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). (e) Correlation between blood BHB concentrations and ear swelling. A significant negative correlation was observed between BHB levels and ear thickness (R² = 0.5935, p < 0.004).
Figure 2.
Nutritional ketosis using MCT oil and 1,3-butanediol suppresses DNFB-induced immediate-type allergic response (a) Experimental timeline. Mice were sensitized with DNFB on days −7 and −6, followed by oral gavage (2 g/kg body weight) of MCT oil, BD, or water 1 h before the DNFB challenge. Blood samples were collected for BHB measurement at 1 h after the DNFB challenge. (b) Representative dorsal views of mouse ears at 1 h after DNFB challenge. The right ear challenged with DNFB revealed that mice treated with MCT oil or BD displayed markedly less redness than those in the water group. (c) Ear swelling at 1 h after the DNFB challenge in each group. MCT and BD groups exhibited significantly reduced swelling compared with the water group. Although ear swelling appeared similar to that in the DNFB(−) group, it remained significantly higher. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (d) Blood BHB concentrations measured 1 h after the DNFB challenge. Both the MCT and BD groups demonstrated significantly increased BHB levels compared with the water group. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). (e) Correlation between blood BHB concentrations and ear swelling. A significant negative correlation was observed between BHB levels and ear thickness (R² = 0.5935, p < 0.004).
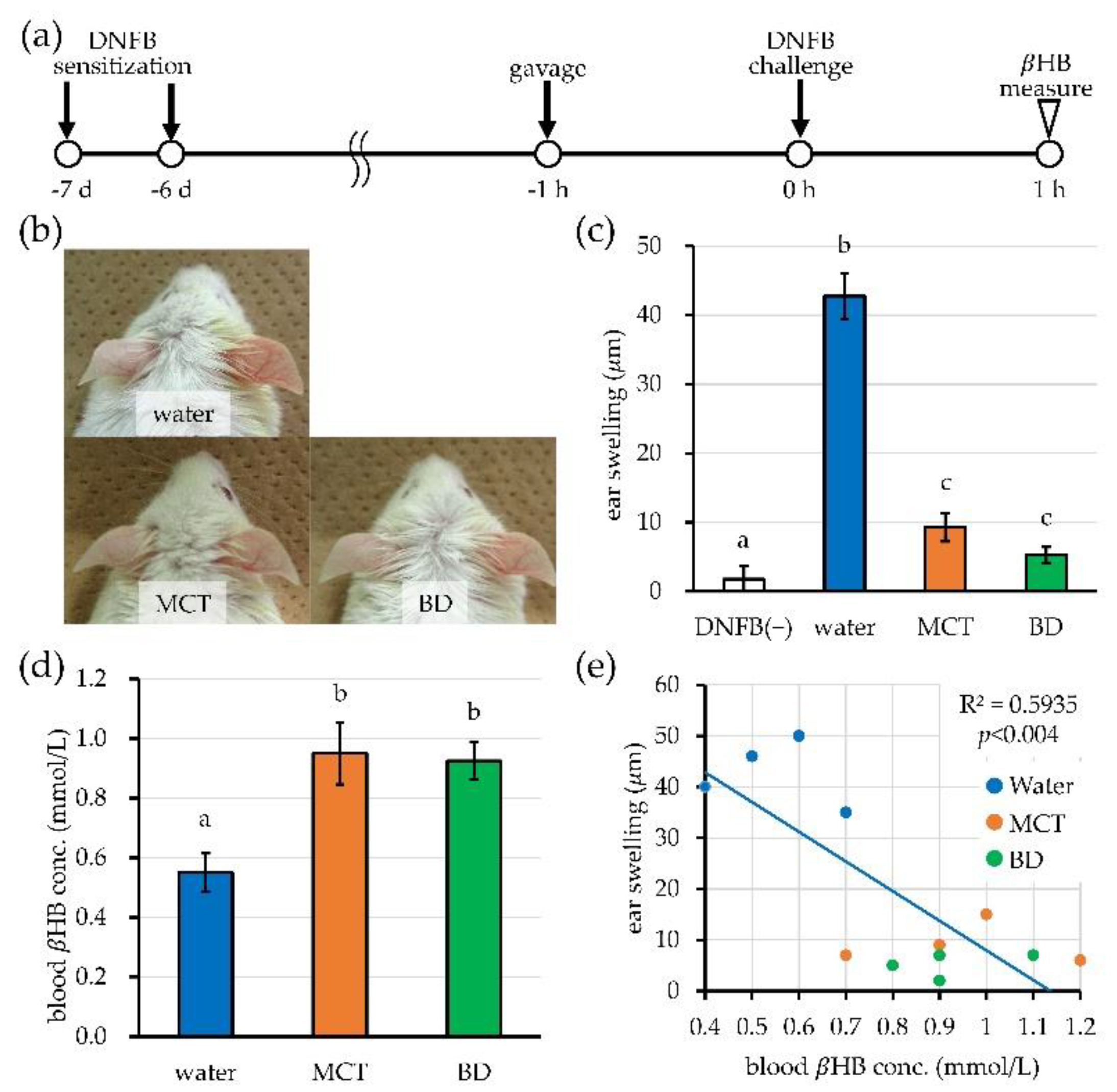
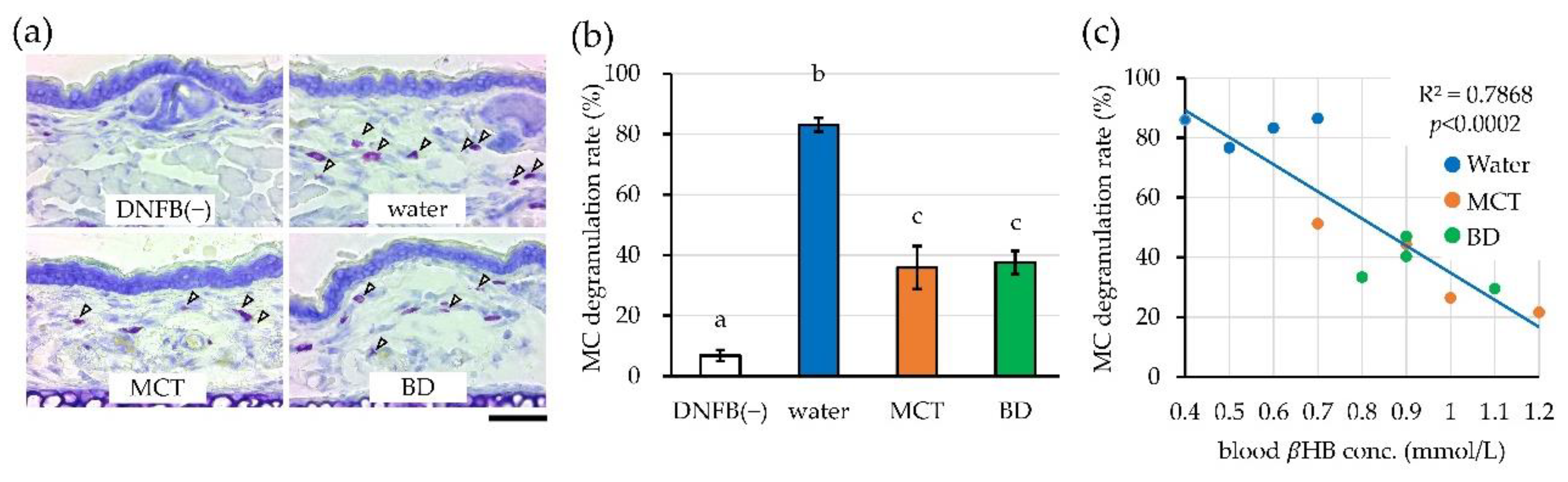
Figure 3.
MCT oil and 1,3-butanediol suppress MC degranulation following the DNFB challenge. (a) Representative images of toluidine blue–stained ear tissue sections 1 h after the DNFB challenge in each group. Degranulated MCs (arrowheads) are more abundant in the water group than in the MCT and BD groups. Scale bar: 50 μm. (b) Quantification of MC degranulation rates. Both MCT and BD significantly reduced MC degranulation compared with the water group. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (c) Correlation between blood BHB concentrations and MC degranulation rates. A significant negative correlation was observed (R² = 0.7868, p < 0.0002), suggesting that elevated BHB levels were associated with reduced MC activation.
Figure 3.
MCT oil and 1,3-butanediol suppress MC degranulation following the DNFB challenge. (a) Representative images of toluidine blue–stained ear tissue sections 1 h after the DNFB challenge in each group. Degranulated MCs (arrowheads) are more abundant in the water group than in the MCT and BD groups. Scale bar: 50 μm. (b) Quantification of MC degranulation rates. Both MCT and BD significantly reduced MC degranulation compared with the water group. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (c) Correlation between blood BHB concentrations and MC degranulation rates. A significant negative correlation was observed (R² = 0.7868, p < 0.0002), suggesting that elevated BHB levels were associated with reduced MC activation.
Figure 4.
GPR109A antagonist mepenzolate abolishes the inhibitory effects of 1,3-butanediol on DNFB-induced allergic inflammation (a) Experimental timeline. Mice were sensitized with DNFB on days −7 and −6, followed by subcutaneous injection of the GPR109A antagonist mepenzolate bromide (MPN, 5 mg/kg) 1 h before oral gavage of 1,3-butanediol (BD, 4.5 g/kg body weight) or water. The DNFB challenge was performed 1 h later, and blood samples were collected 1 h after the challenge for BHB measurement. (b) Representative dorsal views of mouse ears at 1 h after the DNFB challenge. BD-treated mice showed reduced redness compared with the water group, whereas this suppressive effect was diminished in the MPN/BD group. (c) Ear swelling at 1 h after DNFB challenge. BD significantly suppressed ear swelling; however, this effect was abolished in the MPN/BD group. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (d) Blood BHB concentrations measured 1 h after the DNFB challenge. BD increased BHB levels, whereas MPN co-administration significantly reduced this elevation. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). (e) Representative toluidine blue–stained sections of ear tissue showing mast cell morphology. Degranulated MCs (arrowheads) were more frequently observed in the water and MPN/BD groups than in the BD group. Scale bars: 50 μm. (f) Quantification of MC degranulation rates. BD significantly suppressed MC degranulation, whereas this effect was abolished by MPN administration. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05).
Figure 4.
GPR109A antagonist mepenzolate abolishes the inhibitory effects of 1,3-butanediol on DNFB-induced allergic inflammation (a) Experimental timeline. Mice were sensitized with DNFB on days −7 and −6, followed by subcutaneous injection of the GPR109A antagonist mepenzolate bromide (MPN, 5 mg/kg) 1 h before oral gavage of 1,3-butanediol (BD, 4.5 g/kg body weight) or water. The DNFB challenge was performed 1 h later, and blood samples were collected 1 h after the challenge for BHB measurement. (b) Representative dorsal views of mouse ears at 1 h after the DNFB challenge. BD-treated mice showed reduced redness compared with the water group, whereas this suppressive effect was diminished in the MPN/BD group. (c) Ear swelling at 1 h after DNFB challenge. BD significantly suppressed ear swelling; however, this effect was abolished in the MPN/BD group. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). Each group consisted of four mice (N = 4 per group). (d) Blood BHB concentrations measured 1 h after the DNFB challenge. BD increased BHB levels, whereas MPN co-administration significantly reduced this elevation. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05). (e) Representative toluidine blue–stained sections of ear tissue showing mast cell morphology. Degranulated MCs (arrowheads) were more frequently observed in the water and MPN/BD groups than in the BD group. Scale bars: 50 μm. (f) Quantification of MC degranulation rates. BD significantly suppressed MC degranulation, whereas this effect was abolished by MPN administration. Different letters indicate statistically significant differences (one-way ANOVA followed by Tukey’s post hoc test, p < 0.05).
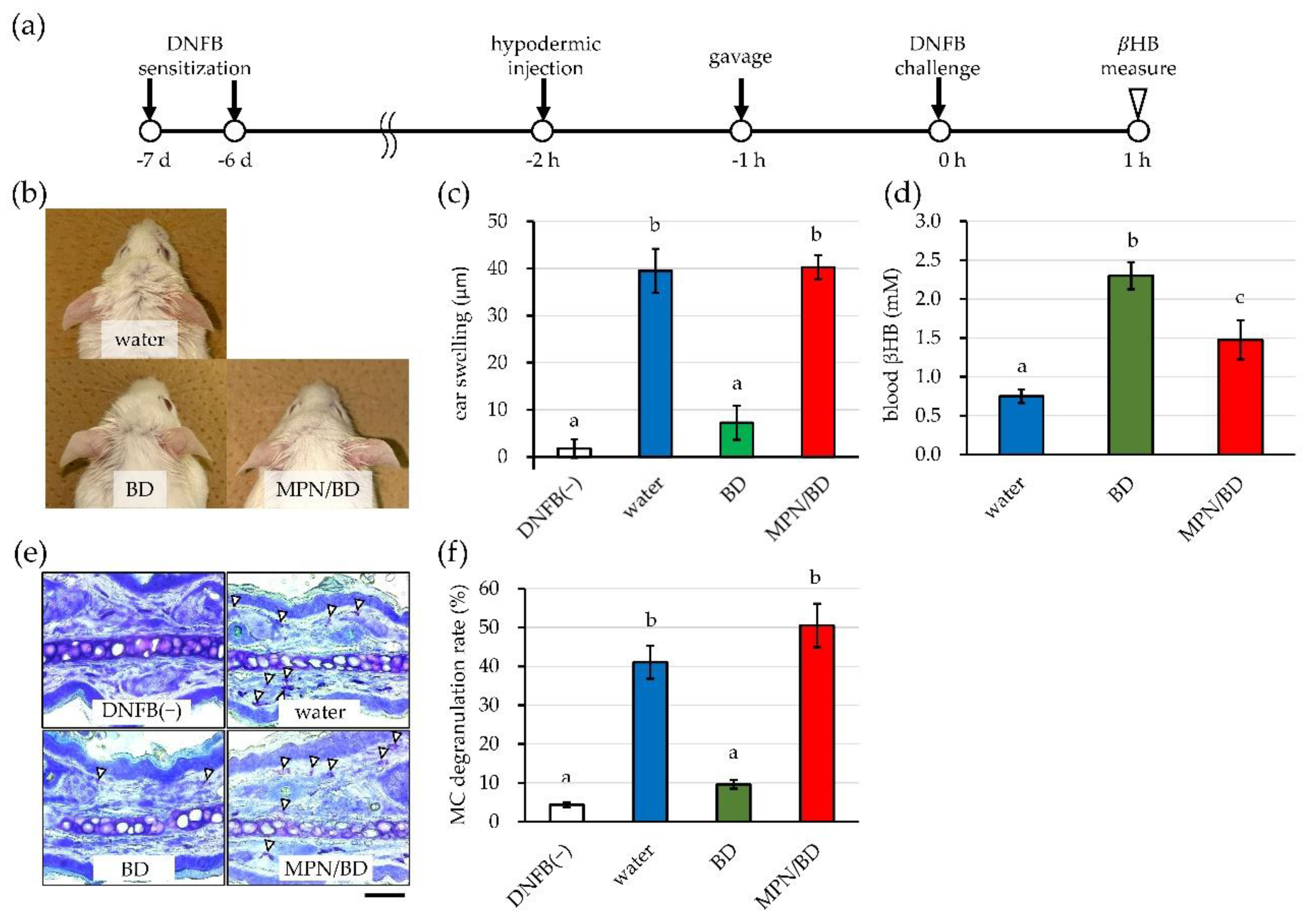
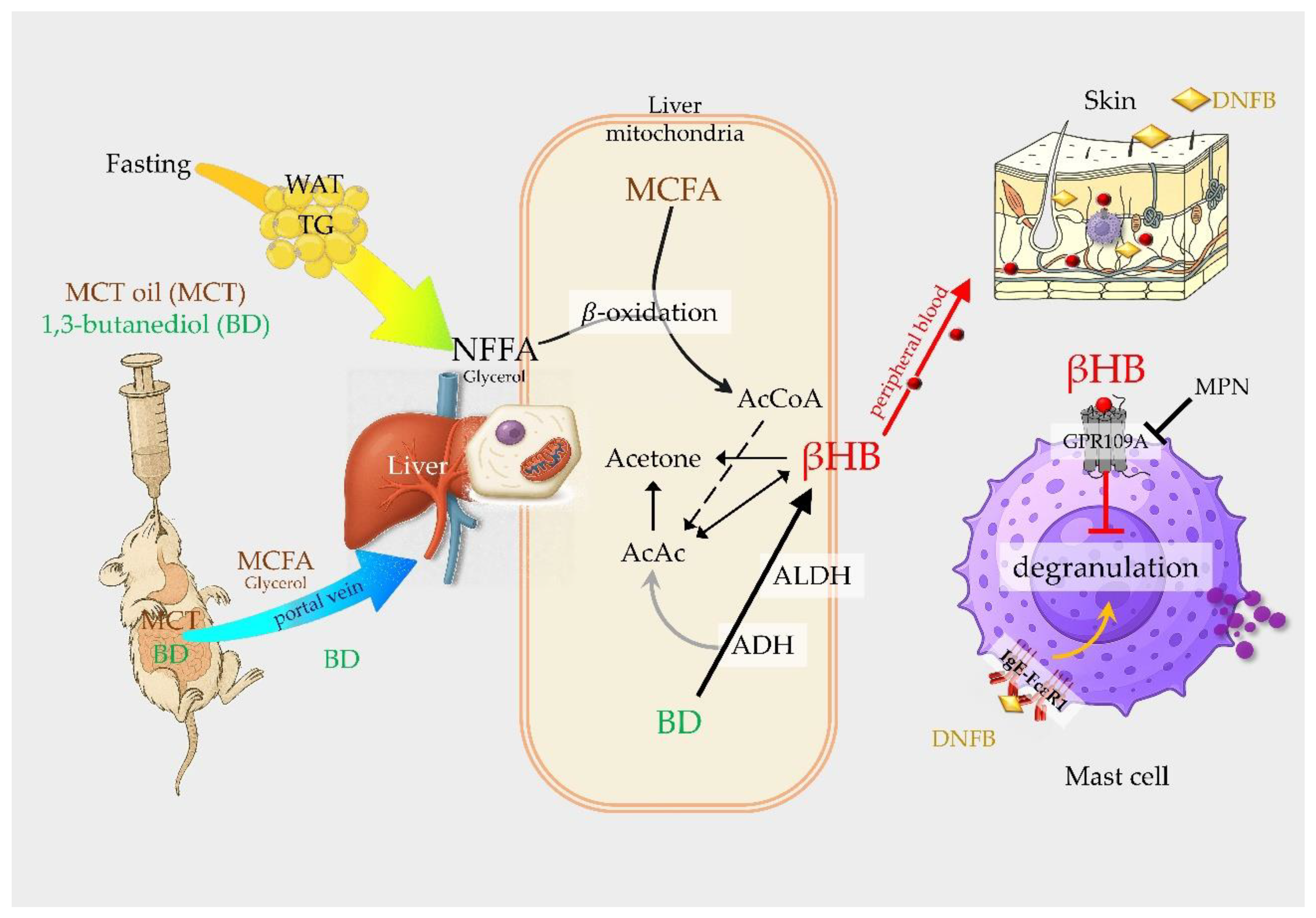
Figure 5.
Proposed mechanism by which BHB suppresses immediate-type allergic inflammation via GPR109A-mediated inhibition of mast cell degranulation.
Figure 5.
Proposed mechanism by which BHB suppresses immediate-type allergic inflammation via GPR109A-mediated inhibition of mast cell degranulation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).