1. Introduction
Zirconium is widely used in the nuclear industry because of its small thermal neutron absorption cross-sectional area and good corrosion resistance [
1]. With the successful development of the new generation of molten salt nuclear reactors, high-purity zirconium tetrafluoride (ZrF
4) has also become an important part of molten salt reactors due to its small thermal neutron absorption cross-section, high heat capacity, good fluidity and thermal conductivity and stable chemical properties [
2,
3,
4,
5].
However, due to the limitations of its use environment, in order to reduce neutron losses and thermal corrosion of pipelines, the requirements for impurities in zirconium tetrafluoride are also very strict, especially for neutron absorbing materials such as hafnium and cadmium [
6,
7,
8]. Therefore, the preparation of high-purity zirconium tetrafluoride has become an inevitable trend.
In addition to being used in the nuclear industry, high-purity ZrF
4 also has great research potential in strong oxide fuel cells, surface thermal protection coatings for aerospace, and high-function optical components [
9,
10,
11]. In addition, high-purity zirconium fluoride is widely used in electrical vacuum components and instruments [
12] and can also be used as a "vitamin" in the metallurgical industry. It is an intermediate of synthetic materials and can be used for metal surface treatment [
13]. It is known that the addition of zirconium fluoride (IV) (ZrF
4) to polymeric calcium phosphate cement can alter its solidification behavior.
Existing research shows that Zr⁴⁺ and F⁻ in solution do not exist in simple combination form. Depending on the solution concentration, acidity and other factors, multiple cationic complexes (such as ZrF²⁺, ZrF₃⁺) or anionic complexes (such as ZrF₅⁻, ZrF₆²⁻) may exist in the system at the same time, and their presence will change dynamically with solution conditions [
14]
Among them, anion complexes ZrF
6²⁻ are formed under high ion concentration conditions, where zirconium acts as the central atom coordinated with six fluoride ions in an octahedral structure [
15]. The ZrF₆²⁻ anion can form grid-like structured compounds with alkali metals (Li
+, Na
+, K
+), effectively trapping these cations within the lattice interstices. As a result, these cations are particularly difficult to remove completely during ion exchange purification, making deep purification challenging to achieve. Additionally, ZrF₆²⁻ can form octahedral structures with divalent cations (e.g., Mn²⁺, Ni²⁺) [
16], where Zr and the divalent cations alternately occupy the octahedral centers. Moreover, hexafluorozirconic acid has low solubility in solution and tends to form precipitates.
ZrF2²⁺ and ZrF3⁺ were intermediate phases with incomplete fluorination reaction under low acid concentrations. When the ion exchange resin is removed from impurities, this type of substance will be adsorbed by the cationic exchange resin together with the cationic impurities. When the anion exchange resin is used to adsorb fluorozirconic acid in reverse decomposition, it will not be adsorbed, so it will reduce the overall yield and is not conducive to the ion exchange method to remove impurities. Therefore, it is necessary to control the two intermediate phases in the fluorozirconic acid solution as little as possible ZrF2²⁺ and ZrF3⁺.
Therefore, during the preparation of high purity zirconium fluoride by the hydrometallurgy (such as ion exchange and solvent extraction), and the control of solution phase composition was particularly important. Selecting the process with the highest proportion of HZrF5 phase would facilitate impurity removal via ion exchange. This experiment explored a solution preparation process that was simpler, safer, had higher purity, and enabled more precise phase control compared to the traditional process.
2. Materials and Methods
2.1. Materials
Purchasing 99.84% sponge zirconium from Beijing Xingrongyuan Technology Co., Ltd. and electronic-grade hydrofluoric acid from Shanghai Aladdin Biochemical Technology Co., Ltd. All reactions were carried out in PP (polypropylene) beakers.
2.2. Qualitative Experiments
The sponge zirconium acid solution experiment was performed by controlling the liquid-solid ratio and hydrofluoric acid concentration. However, before conducting this experiment, it was also necessary to determine the initial experimental conditions: the specific surface area of the sponge zirconium and the feeding method. Based on this, the following experiments were performed:
2.2.1. Specific Surface Area and Passivation Phenomenon Experiment
Cutting the sheet-shaped sponge zirconium and put it into 10 ml of 10% and 20% hydrofluoric acid solutions, and passivation occurred. Experimental results indicated that when the sponge zirconium was cut into approximately 3mm×3mm×4mm particles, it could be completely dissolved in a 10% and 20% concentration hydrofluoric acid solution of 10ml.
2.2.2. Feeding Method and Reaction Intensity Experiment
When 1g sponge zirconium can be put into 10 ml of 15% hydrofluoric acid solution at one time, the temperature rises, and a large number of bubbles are generated and boiled out. After experiments, within the range of 15%-45% acid concentration, as long as the sponge zirconium particles are put into five times and cooled to below 35℃ after each feeding, the maximum reaction temperature is below 65℃ and will not boil out.
2.3. Quantitative Experiments
To ensure that the main phase in the solution is zirconium pentafluoride acid (HZrF5), the following calculations were performed.
According to the standard reaction equation for HZrF
5 [
17]:
The molar concentrate ratio of the two is 1:5
.
If 1g of 99.8% purity sponge zirconium (containing 97.8% Zr) is used as the solute, and hydrofluoric acid (HF) of varying concentrations and liquid-solid ratios is used as solvent, then the molar amount of metal zirconium (Zr) can be calculated as follows:
Since the volumes of the solution in which the two are located is the same, then the corresponding molar amount of HF should be:
Then according to n=m/M, the minimum HF mass is required:
Then, under different solid-liquid ratios, the theoretical minimum concentration can be derived, and the specific values are shown in
Table 1.
Based on the preceding theoretical calculations, expanding the hydrofluoric acid concentration range, 1g of sponge zirconium sponge was reacted with hydrofluoric acid with different mass fractions (20%, 25%, 30%, 35%, 40%, 45%)and different liquid-solid ratios (ranging from 4mL·g-1 to 10mL·g-1). Experiments below the theoretical concentration threshold were excluded. According to the qualitative experimental results, the sponge zirconium was poured in five times, and the cooling was reduced to 35℃ after each feeding. In order to determine whether the solution after the reaction is stable, the samples are sealed and left to stand for 72 hours. During this period, if the solution remains in a transparent, clear and stable state, it is determined that the solution are deemed to meet the stability criteria.
2.4. XRD Experiments
In this paper, a SmartLab SE X-ray diffractometer (XRD) manufactured by Rigaku Corporation in Japan was first used to conduct phase characterizaion of the samples.
First, a part of samples were taken and subjected to evaporative crystallization at 45℃ to obtain powdered samples. Then, qualitative phase analysis was carried out. The X-ray source was operated at the voltage of 40 kV and 40 mA current density, using Cu-Kα radiation , λ = 0.154 μm. The diffraction angle was varied in the range of 5°-90° and the scanning rate was 2° per minute.
2.5. LC-MS Experiments
Before analyzing the sample with LC-MS, pretreatment is first required. First, 0.5082 g of the sample was accurately weighed on an electronic balance and diluted 19.67-fold with ultrapure water. Another set of controls with water as solution was made. By comparing the LC-MS spectra of the blank and experimental groups, potential experimental variables were eliminated.
Among them, the mobile phase A was a dilute aqueous ammonia solution with a concentration of 0.05% concentration, and the B phase was acetonitrile, with a flow rate of 0.3 mL/min. The separation was performed using an ACQUITY UPLC BEH C18 (91.7 μm, 50×2.1 mm) type chromatography column, with the injection volume being 2 μL and the column temperature being 35°C. The gradient elution table is shown in
Table 2.
3. Results
3.1. Quantitative Experimental Phenomenon Analysis
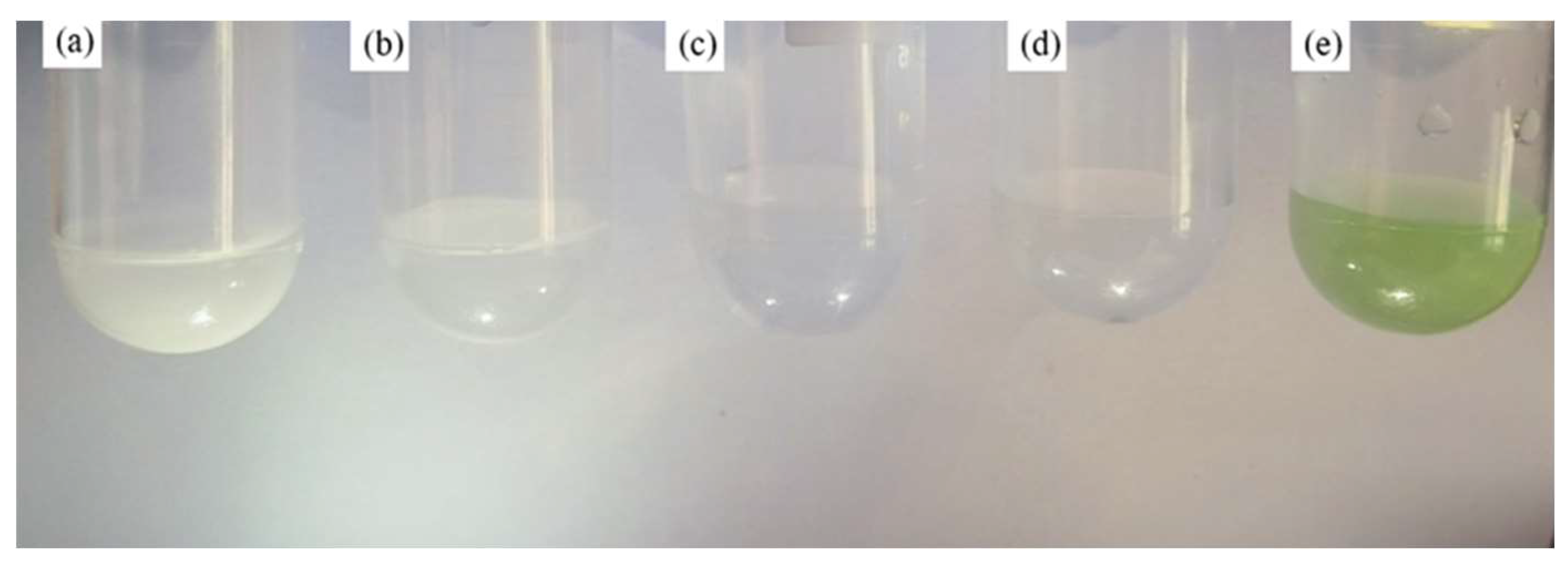
Table 3 is the status record table of the reaction solution and
Figure 1 is a reaction solution diagram under different states.
By comparing the experimental phenomena of the reaction between hydrofluoric acid and sponge zirconium at different solid-liquid ratios and different concentrations, the following conclusions are obtained:
(1) For a reaction solution with a low solid-liquid ratio, that was 4 mL·g⁻¹, a reaction was performed using different concentrations of hydrofluoric acid, and the reaction cannot be completed. According to the complex effect, it can be seen that when the fluoro-zirconium complex was stable, the reactant ions were arranged in an orderly manner due to its stable molecular structure, which hindered ion diffusion, and thus the reaction rate was greatly reduced until the reaction cannot be carried out.
At high HF concentrations (e.g., 40% and 45%), according to the activity coefficient formula αi=γi·Ci ,(where αi represents the activity of component, γi is activity coefficient, Ci is the concentration of component), it can be seen that extremely high acid ion concentration will promote the formation of fluoro-zirconium complexes, and then polymerization reaction occurs, and the complex polymerized to form colloids. The formation of colloids changed the physical properties of the solution, increased the viscosity of the system, and further reduced the reaction rate, making it impossible to complete smoothly.
(2) When the acid concentration (20%) was low, for solutions with lower liquid-solid ratio, due to the small amount of solution and more contact opportunities for sponge zirconium, they reduced the total surface area and reduced the surface energy through aggregation. During this aggregation processed, sponge zirconium was combined by van der Waals force to stabilize the reaction. Because the solution viscosity was high at this time, it was easier to generate flocculent precipitates; while solutions with lower liquid-solid ratios have relatively lower solution viscosity and better fluidity. These factors promote the increase of the reaction rate and the smooth completion of the reaction. Moreover, due to the low fluorine ion concentration, the complex polymerization situation was reduced, and only a small amount of flocculent precipitates were produced. However, for solutions with low acid concentration and smaller liquid-solid ratio, feeding was too fast, the temperature rised quickly, and the reaction rate was accelerated, which was conducive to the complexation reaction between fluorine ions and zirconium ions. Therefore, the reaction rate should not be too fast, and the solution can also be stabilized.
(3) Under high hydrofluoric acid concentrations (40%, 45%) with a moderately high liquid-solid ratio (6 mL·g⁻¹): the ion concentration was slightly reduced, which reduced the degree of fluoro-zirconium complexes aggregation and the reaction can be completed. However, when the liquid-solid ratio was 5 mL·g⁻¹ and the hydrofluoric acid concentration was higher (40%, 45%), the tendency of complex aggregation was more obvious. Moreover, the presence of excess F ions can easily form a transition state with zirconium ions, and the presence of high concentration fluorine ions will also increase its collision frequency with zirconium. All of these provided favorable conditions for the complexation reaction and accelerated the progress of the complexation reaction. From the perspective of phase change, ZrF₅⁻converted to ZrF6²⁻, discolored the solution, and finally the complex precipitates as flocculent precipitates.
(4) When the liquid-solid ratio was increased to 10 mL·g⁻¹, clear and stable solutions can be obtained at all concentrations. However, a larger liquid-solid ratio (10 mL·g⁻¹) would lead to problems such as excessive volume of equipment during actual production, excessive feeding of hydrofluoric acid, and increased costs.
From the perspective of economics and production efficiency, the dissolution processes of 5 mL·g⁻¹-35%, 6 mL·g⁻¹-30%, 6 mL·g⁻¹-35%, and 7 mL·g⁻¹-25% are more suitable. Therefore, we only need to perform phase analysis on these four samples and select the process that is most in line with the expectations.
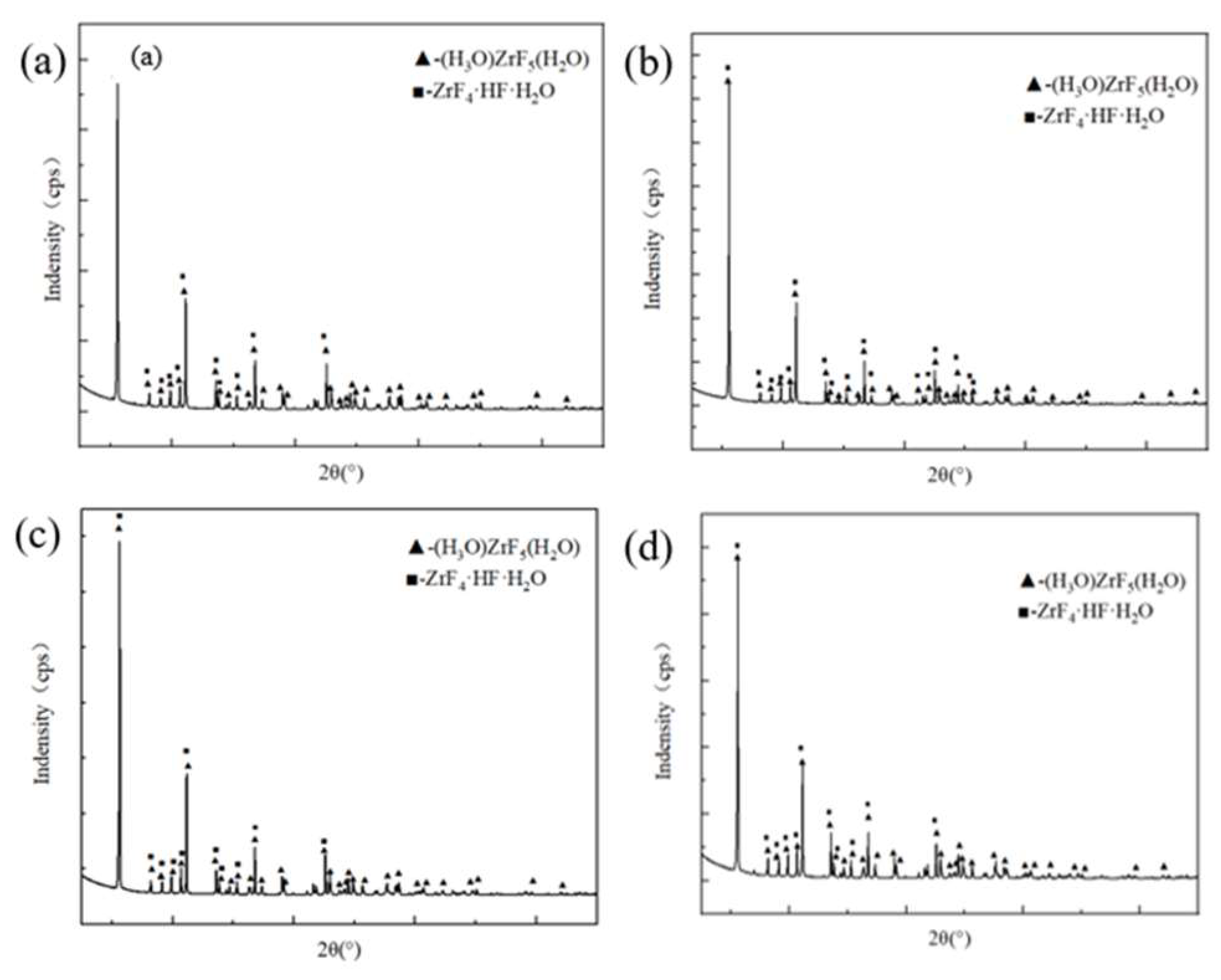
3.2. Phase Characterization by XRD
The qualitative analysis results of XRD are shown in
Figure 2.
According to the characterization results of the crystals by XRD, the phases of the crystals are mainly HZrF
5·H
2O and HZrF
5·2H
2O. Then, the crystal was analyzed by the K value method and the results were obtained as shown in
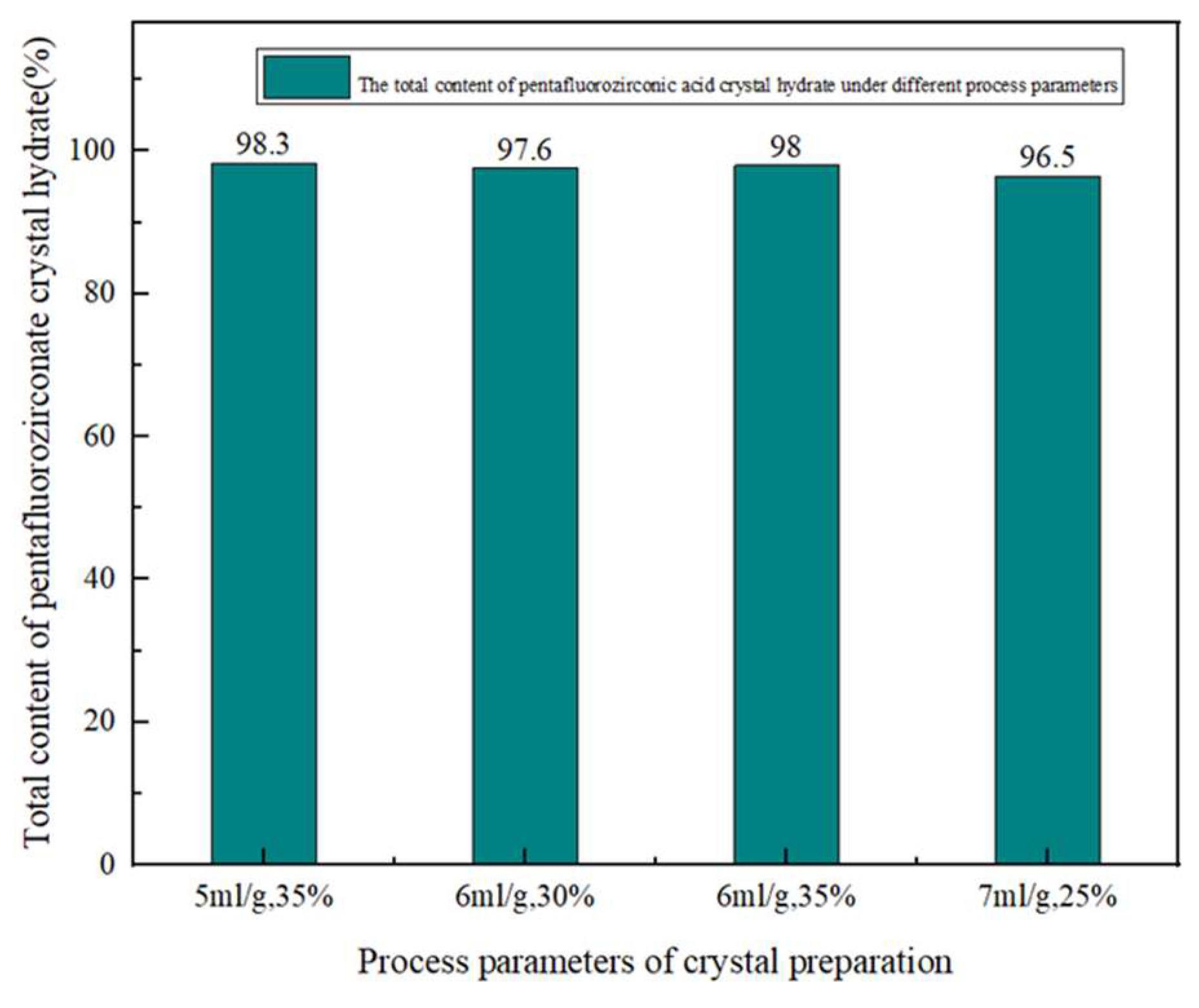
Figure 3.
When the liquid-solid ratio was 5mL·g-1 and the hydrofluoric acid concentration was 35%, the total content of pentafuorozirconate crystal hydrates in the synthesized crystals were 98.3%. When the liquid-solid ratio was 6mL·g-1 and the hydrofluoric acid mass fraction was 30%, the total content of pentafuorozirconate crystal hydrates in the synthesized crystals were 97.6%. When the liquid-solid ratio was 6mL·g-1 the hydrofluoric acid concentration was 35%, the total content of pentafuorozirconate crystal hydrates in the synthesized crystals were 98%. When the liquid-solid ratio was 7mL·g-1 and the hydrofluoric acid concentration was 25%, the total content of pentafuorozirconate crystal hydrates in the synthesized crystals were 96.5%.
Therefore, the highest content of the pentafuorozirconate crystal hydrates was achieved under the conditions of liquid-solid ratio 5 mL·g-1 and 35% HF concentration. To further refine the quantitative analysis of the solution, liquid chromatography-mass spectrometry (LC-MS) was employed for precise phase characterization of the fluorozirconic acid solution.
3.3. LC-MS Analysis
LC-MS (Liquid Chromatography-Mass Spectrometry) is widely used for the qualitative and quantitative characterization of organic solutions. Since no established method was found for the characterization of inorganic liquid phases, this method was employed to analyze the fluorozirconic acid solution.
To facilitate comparative analysis of LC-MS results across different samples, the MS spectra at RT = 5.114 min were uniformLy selected for qualitative and quantitative analysis.
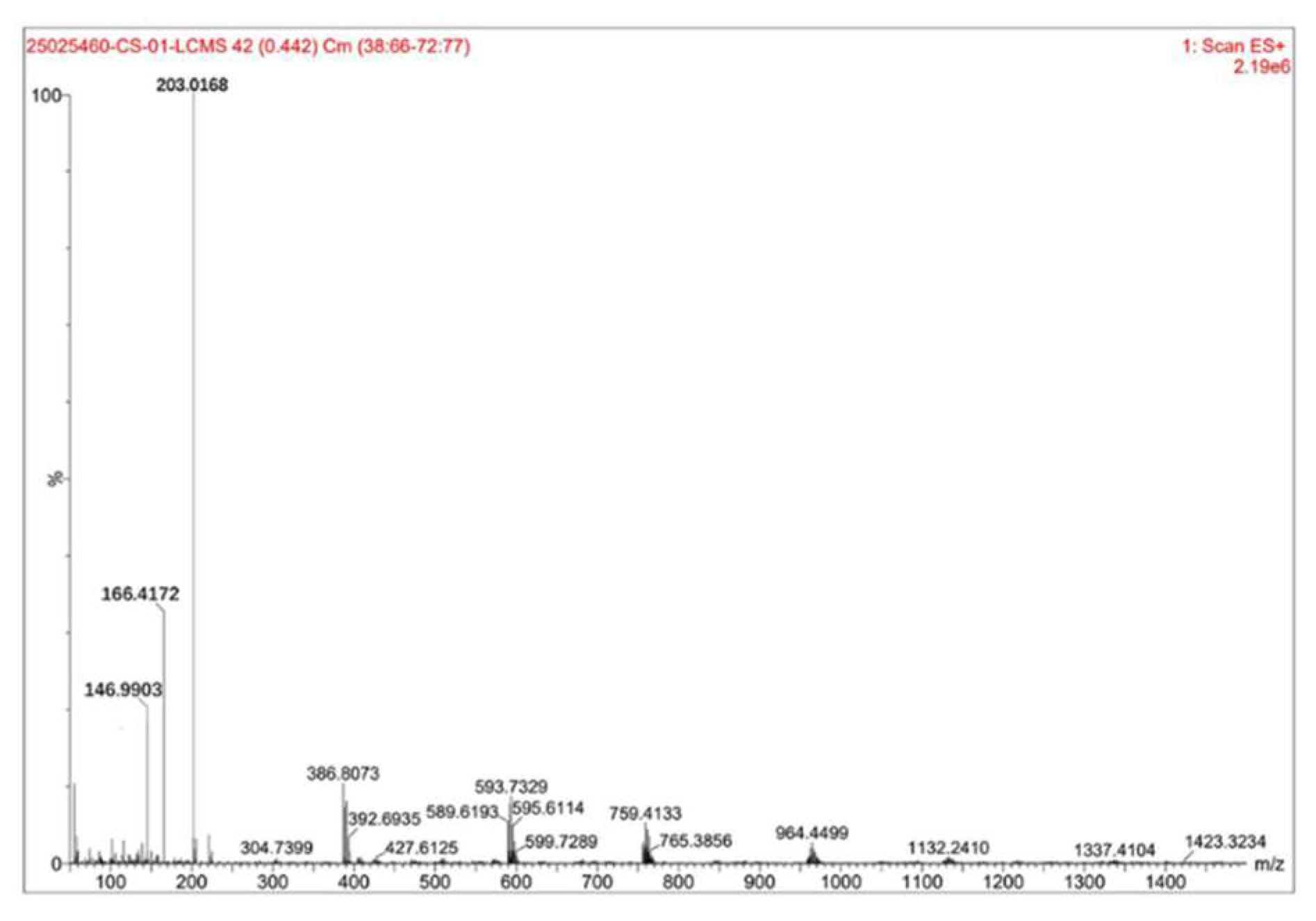
Figure 4 displays the detection results for the sample with a liquid-solid ratio of 5mL·g
-1 and 35% HF concentration at RT = 5.114 min.
To enable comparative analysis of LC-MS results across different samples, the MS spectra at the retention time (RT) of 5.114 minutes were consistently selected for qualitative and quantitative analysis.
Figure 4 illustrated the detection outcomes for the sample prepared with a liquid-solid ratio of 5mL·g
-1 and 35% hydrofluoric acid (HF) concentration at RT = 5.114 minutes.
The figure showed that the highest peak has a mass-to-charge ratio (m/z) of 203.0168 with a relative intensity of 100%, while the relative molecular mass of zirconium pentafluoride acid was 187.22, this peak corresponded to zirconium pentafluoride acid. The second-highest peak at m/z 166.4172, with a relative intensity of approximately 35%, aligned with ZrF₃⁺ (relative ionic mass: 148.22). The third peak at m/z 146.9903, showing a relative intensity of about 21%, matched ZrF₂²⁺ (relative molecular mass: 129.22). By calculating the proportion of zirconium pentafluoride acid among the fluoro-zirconium complex ions based on their relative intensities, it was determined that zirconium pentafluoride acid accounted for approximately 62%-67% of the composition.
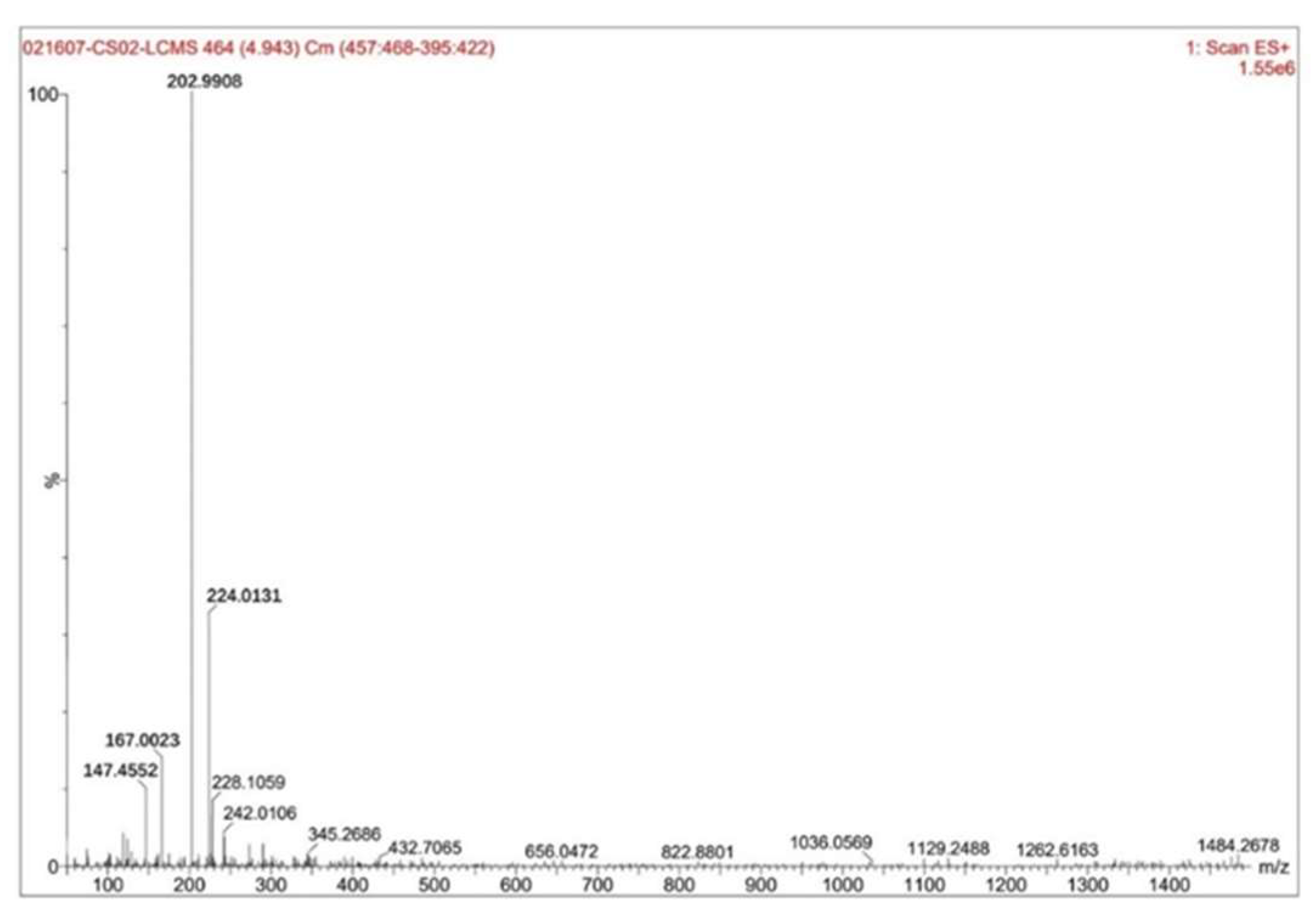
Figure 5 showed the test results of the sample liquid-solid ratio of 6 mL·g⁻¹ and the hydrofluoric acid concentration of 35%.
The figure showed that the highest peak had a mass-to-charge ratio (m/z) of 202.9908 with a relative intensity of 100%, while the relative molecular mass of zirconium pentafluoride acid was 187.22, this peak corresponded to zirconium pentafluoride acid. The second-highest peak at m/z 224.0131, with a relative intensity of approximately 34%, aligned with hexafluorozirconic acid (relative molecular mass: 207.23). The third peak at m/z 167.0023, showing a relative intensity of about 14%, matched ZrF₃⁺ (relative ionic mass: 148.22). The fourth peak at m/z 147.4552, with a relative intensity of approximately 11%, corresponded to the ZrF₂²⁺ ion (relative ionic mass: 129.22). By calculating the proportion of zirconium pentafluoride acid among the fluoro-zirconium complexes based on their relative intensities, it was determined that zirconium pentafluoride acid accounted for approximately 60%-65% of the composition.
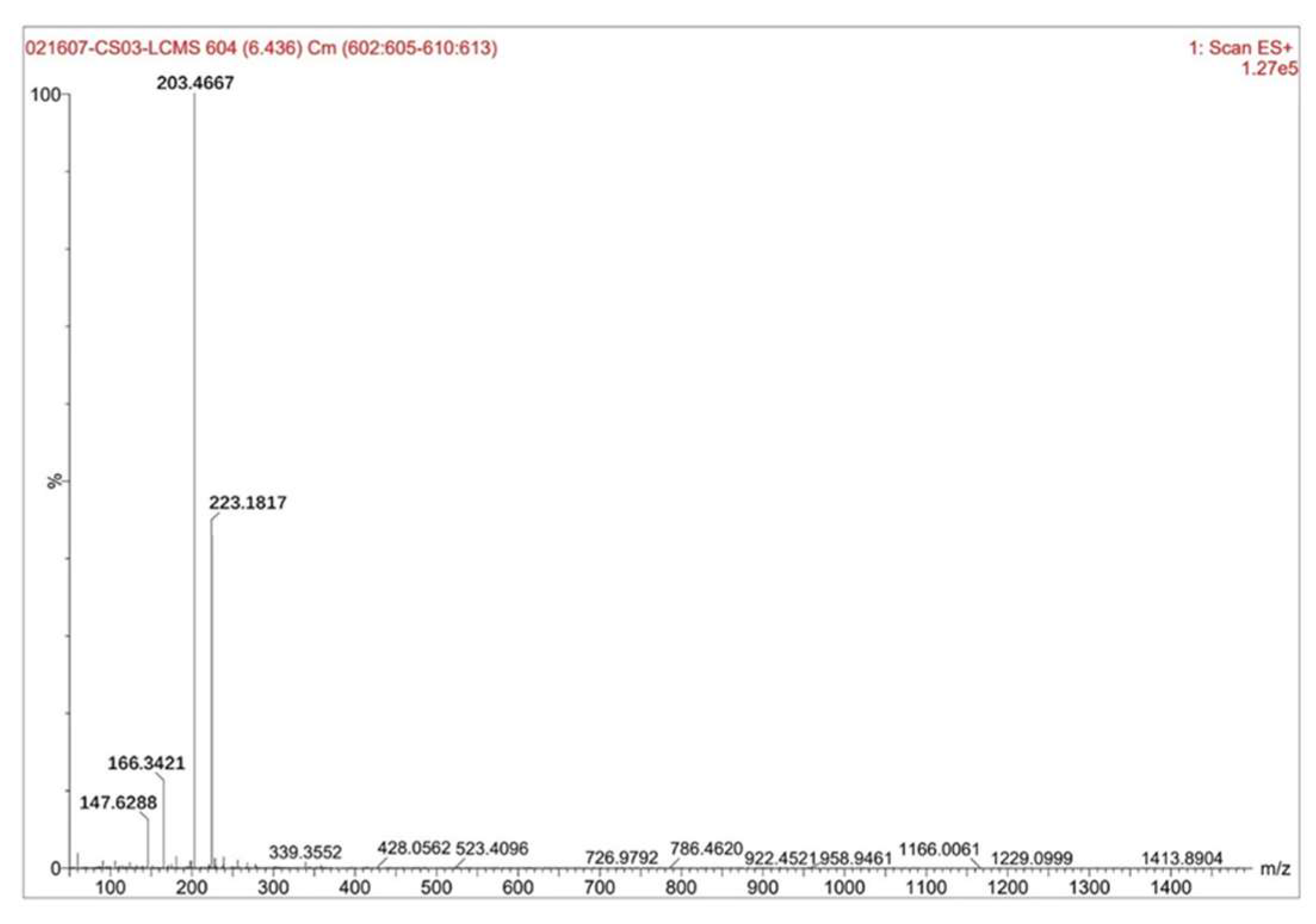
Figure 6 showed the test results of the sample liquid-solid ratio of 6 mL·g⁻¹ and the hydrofluoric acid concentration of 30%.
The figure revealed the following: The highest peak had a mass-to-charge ratio (m/z) of 203.4667 with a relative intensity of 100%, while the relative molecular mass of zirconium pentafluoride acid was 187.22, this peak corresponded to zirconium pentafluoride acid. The second-highest peak at m/z 223.1817, with a relative intensity of ~46%, aligned with hexafluorozirconic acid (relative molecular mass: 207.23). The third peak at m/z 166.3421, showing a relative intensity of ~12%, matched ZrF₃⁺ (relative ionic mass: 148.22). The fourth peak at m/z 147.6288, with a relative intensity of ~7%, corresponded to the ZrF₂²⁺ ion (relative ionic mass: 129.22). By calculating the proportion of zirconium pentafluoride acid among the fluoro-zirconium complexes based on relative intensities, it was determined that zirconium pentafluoride acid accounted for approximately 58%-63% of the composition.
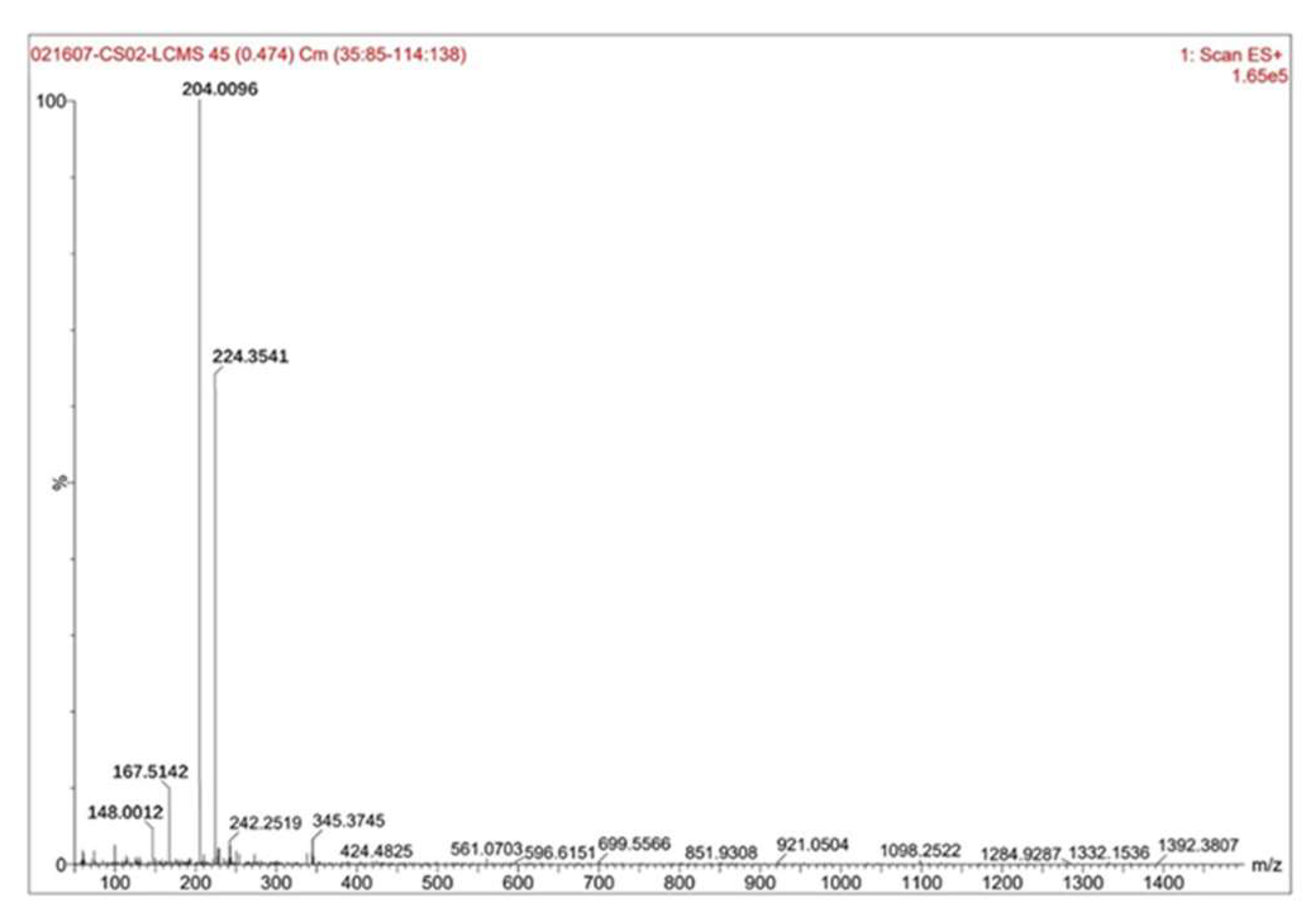
Figure 7 showed the test results of the sample liquid-solid ratio of 7 mL·g⁻¹ and the hydrofluoric acid concentration of 25%.
Figure 7 showed the MS spectrum of Sample 1(liquid-solid ratio: 7 mL·g⁻¹, hydrofluoric acid concentration: 25%) at RT=5.114 min. The figure revealed the following: The highest peak had a mass-to-charge ratio (m/z) of 204.0096 with a relative intensity of 100%, while the relative molecular mass of zirconium pentafluoride acid was 187.22, this peak corresponded to zirconium pentafluoride acid. The second-highest peak at m/z 224.3541, with a relative intensity of approximately 65%, aligned with hexafluorozirconic acid (relative molecular mass: 207.23).The third peak at m/z 167.5142, showing a relative intensity of ~12%, matched ZrF₃⁺ (relative ionic mass: 148.22).The fourth peak at m/z 148.0012, with a relative intensity of approximately 5%, corresponded to the ZrF₂²⁺ ion (relative ionic mass: 129.22). By calculating the proportion of zirconium pentafluoride acid among the fluoro-zirconium complex ions based on relative intensities, it was determined that zirconium pentafluoride acid accounted for approximately 53%-58% of the composition.
Based on the above MS spectral results, it was found that the fluoro-zirconium complexes in the sample may exist in two forms, namely cationic fluoro-zirconium complexes and anion fluoro-zirconium complexes. And there may be two types of anionic fluoro-zirconium complexes in the solution in form: ZrF5⁻ , ZrF62, and there may be two types of fluoro-zirconium complex ions in the solution in the solution in cationic form: ZrF22+, ZrF3+.
A rough calculation of the proportion of zirconium pentafluoride acid (HZrF5) among the fluoro-zirconium complexes, based on relative intensities, revealed that under the process parameters of a liquid-solid ratio of 5 mL·g⁻¹ and 35% hydrofluoric acid concentration, the proportion of zirconium pentafluoride acid reached its highest value, approximately 62%-67%. Therefore, we concluded that it is meaningful to use LC-MS to characterize the solution.
4. Discussion
By analyzing the mass spectra of LC-MS, it was found that the mass-to-charge ratio (m/z) of each peak in the mass spectra was higher than the theoretical value. This may be because the solution is in a dynamic state, and the molecular structure composed of fluorozirconic acid solution is in a metastable state. During the electrospray ionization (ESI) process of LC-MS, the original ionic groups in the solution are affected by the electric field force, which is prone to dissociation or rearrangement, which affects the mass-to-charge ratio of each peak, and thus causes a deviation from the theoretical value.
Based on the relative peak intensities, the content of zirconium pentafluoride acid (HZrF5) in the samples was quantified as follows: Sample 5 mL·g-1, 35%: HZrF5 content ranged between 62% and 67%.Sample 6 mL·g-1, 35%: HZrF5 content ranged between 60% and 65%.Sample 6mL·g-1, 30%: HZrF5 content ranged between 58% and 63%.Sample 7 mL·g-1, 20%: HZrF5 content ranged between 53% and 58%.
Among these four samples, ZrF5- is the highest in the phase. This result is highly consistent with the theoretical model constructed based on chemical reaction formula derivation in the early stage of the experiment. In the reaction system in this paper, a series of reactions between zirconium ions and fluoride ions will occur, generating a variety of anionic/cationic complexes, but experiments have confirmed that the content of phase is largely affected by nZr/nF ratio (molar ratio of Zr to F).
As mentioned earlier, the phase distribution of the sample is related to acidity and acid ions (fluorine ions) concentration. Next, this article will discuss these two aspects. Through chemical reaction kinetics, it can be seen that the higher the concentration of fluorine ions, the greater the tendency of the phase ZrF62--generated phase, and the results of the MS map are contrary to this theory. The trend of ZrF62- generated by samples sorted by fluoride ion concentrations of 5 mL·g-1, 35% > 6mL·g-1, 35% > 6 mL·g-1, 30% > 7mL·g-1, 25%, 5 mL·g-1, 35% should be greater, but the facts are exactly the opposite.
In these four samples, 5mL·g-1, 35% had a pH of 2.13, sample 6 mL·g-1, 35% had a pH of 2.43, sample 6 mL·g-1, 30% had a pH of 2.86, sample 7mL·g-1, 25% had a pH of 3.17. And the sample with the highest acidity of this product is 5 mL·g-1, 35%, and its ZrF5- content is also the highest. It shows that the solution phase is greatly affected by acidity, and the higher the acidity, the more conducive it is to generate cationic complexes.
We conjecture that this is because at low hydrofluoric acid concentration, the acidity has a greater impact on the phase of the solution than the fluorine ion concentration on the phase of the solution. Based on the above analysis, we speculate that the main factor affecting the phase of fluorozirconic acid solution is nZr/nF, followed by acidity and finally the acid ion concentration. And the liquid-solid ratio is determined to be 5 mL·g-1 and the mass fraction is 35% as the optimal process parameter.
5. Conclusions
In order to improve the yield of zirconium, this study is conducive to the subsequent cationic removal of fluorozirconic acid solution by using the ion exchange method to prepare high-purity zirconium tetrafluoride. We need to promote the high content of ZrF5- phase in the solution as high as possible. Then, through a series of experiments and analysis, the optimal preparation process of fluorozirconic acid solution was determined.
First, the initial experimental conditions for the reaction between sponge zirconium and hydrofluoric acid were determined: the size of sponge zirconium should be 3mm × 3mm × 4mm, and the sponge zirconium particles were put into several times, and the temperature was lowered below 35℃ after each feeding. On this basis, quantitative experiments were conducted on the reaction of hydrofluoric acid and sponge zirconium at different liquid-solid ratios and different concentrations, and the experimental phenomena were recorded and analyzed. Finally, the phase characteristics of four samples with a Liquid-solid ratio of 5 mL·g-1 and a hydrofluoric acid concentration of 35%; a Liquid-solid ratio of 6 mL·g-1 and a hydrofluoric acid concentration of 35%; a Liquid-solid ratio of 6 mL·g-1and a hydrofluoric acid concentration of 30%; a Liquid-solid ratio of 7 mL·g-1 and a hydrofluoric acid concentration of 20%.
After taking some samples to evaporate and crystallize and performing XRD qualitative quantitative analysis, in order to conduct more accurate quantitative analysis, it was determined that the remaining liquid samples were detected using LC-MS, and the optimal preparation process was determined to be 5 mL·g-1 liquid-solid ratio and 35% hydrofluoric acid concentration. At the same time, the factors affecting the phase were also discussed, and the following speculation was obtained: the main factor affecting the phase of fluorozirconic acid solution is nZr/nF, followed by acidity, and finally the acid ion concentration.
Author Contributions
Conceptualization, F.Y.; methodology Y.Y. and J.R.; validation, J.R.; formal analysis, J.R. and Y.Y. ; investigation, J.R.; data curation, J.R.; writing—original draft preparation, J.R.; writing—review and editing, F.Y. ,Y.Y. and J. R..; supervision, F.Y.; project administration, F.Y.; funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Foundation of China, grant number 52171017.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We acknowledge Prof. Danmin Liu and Dr. Kezhu Ren for their assistance in the qualitative and quantitative XRD characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nielsen R, H. Schlewitz J, Nielsen H, et al. Zirconium and Zirconium Compounds[J]. Kirk-Othmer Encyclopedia of Chemical Technology, 2000: 1-46. [CrossRef]
- Ali Rezvani S, Ogawa K, Fuji T. Highly coherent multi-octave polarization-maintained supercontinuum generation solely based on ZBLAN fibers[J]. Optics Express, 2020, 28(20): 18-26. [CrossRef]
- Santos F, Delben J, Delben A, et al. Thermal stability and crystallization behavior of TiO2 doped ZBLAN glasses [J]. Journal of non-crystalline solids, 2011, 357(15): 07-18. [CrossRef]
- Kavun V Y, Voit E I, Yaroshenko R M , et al. Structure and ion mobility in glasses in the BiF3-PbF2-ZrF4 systems studied by Raman and NMR spectroscopy[J]. Journal of Non-crystalline Solids, 2014, 401: 224-231. [CrossRef]
- Rezvani S A, Ogawa K, Fuji T. Highly coherent multi-octave polarization-maintained supercontinuum generation solely based on ZBLAN fibers[J]. Optics Express, 2020, 28(20): 29918-29926. [CrossRef]
- Miyazaki K, et al. Polymeric calcium phosphate cements: setting reaction modifiers. Dental Materials: official Publication of the Academy of Dental Materials (1993). [CrossRef]
- Guo Y, Li J, Chai S, et al. Nanomaterials for the optical detection of fluoride[J]. Nanoscale, 2017, 9(45): 17667-17680. [CrossRef]
- Zhu X, Peyghambarian N. High-power ZBLAN glass fiberlasers: Review and prospect[J]. Advances in OptoElectronics 2010, 2010(1): 501956. [CrossRef]
- Miyagawa A, Komatsu H, Nagatomo S, et al. Thermodynamic complexation mechanism of zinc ion with 8-hydroxyquinoline-5-sulfonic acid in molecular crowding environment [J]. Journal of Molecular Liquids, 2023, 372: 121-181. [CrossRef]
- Bedü S, Pollnau M, Lithy W, et al. Saturation of the 2.71 um laser output in erbium-doped ZBLAN fibers[J]. Optics Communications, 19. [CrossRef]
- Kotsar' M L, Bateev V B, Baskov P B, et al. Preparation of High-Purity ZrF4 and HfF4 for Optical Fibers and Radiation Resistant Glasses[J]. lnorganic materials, 2001,37: 1085-1091. [CrossRef]
- Mitachi S, Terunuma Y, Ohishi Y, et al. Reduction of impurities in fluoride glass fibers[J]. Journal of lightwave technology, 1984, 2(5): 587-592. [CrossRef]
- Hall G., Sutcliffe, H. Carboxylato complexes of zirconium (IV) Part VI Potential new synthetic routes to ZrF4[J]. Journal of materials science letters, 1991, 10(19): 1156-1157. [CrossRef]
- Lowalekar V, Raghavan S. Etching of zirconium oxide, hafnium oxide, and hafnium silicates in dilute hydrofluoric acidsolutions[J]. Journal of materials research, 2004, 19(4): 1149-1156. [CrossRef]
- Davidovich R L, Marinin D V, Stavila V, et al. Stereochemistry of fluoride and mixed-igand fluoride complexes of zirconium and hafnium[J]. Coordination Chemistry Reviews, 2013,257(21-22): 3074-3088. [CrossRef]
- Hu, L., Chen, J., Xu, J., et al. Atomic linkage flexibility tuned isotropic negative, zero, and positive thermal expansion in MZrF6 (M= Ca, Mn, Fe, Co, Ni, and Zn). Journal of the American Chemical Society, 2016, 138(44): 14530-14533. [CrossRef]
- Xu X, Ling L, Ding X, et al. Synthesis and characterization of a novel, fluoride-releasing dimethacrylate monomer and its dental composite[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2004, 42(4): 985-998. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).