1. Introduction
According to the Food and Agriculture Organization (FAO), total world tomato production in 2021 was 189.1 million tonnes, an increase of 4% over the previous three years (FAO1). Annual tomato production has reached or exceeded the million tonne threshold in many countries. Tomatoes are one of the most popular vegetables and are easy to grow in many parts of the world. The high yield and high quality of tomato is achieved in relatively cool and dry climates, but cultivation is adaptable to a variety of weather conditions, including humid tropical climates (Naika et al., 2005; Adenuga et al., 2013).
The way tomatoes are consumed (fresh or cooked in sauces, soups, etc.) determines the way they are harvested and stored. Preservation in the fresh form is the most delicate, particularly due to transport, diseases or pest attacks on stored foods. In fact, the production of fruit and vegetables is subject to many constraints. Good production and preservation depend to a large extent on the methods and means used or available to operators in the sector in the various countries. A high level of production is often offset by numerous losses that occur during production in the fields or in the post-production stages. For example, pest infestations in the fields account for 20-50% of losses (Khan et al., 2023; FAO2).
Post-harvest losses can be particularly important (FAO3, Goka et al., 2021). Losses and waste can be observed at different stages of the value chain: during storage, processing and packaging, transport and distribution, wholesale and retail markets. The use of low temperature and humidity control is relatively effective, but is not always accessible to industry operators and does not cover all post-production stages. There is still a significant hurdle to overcome to ensure good yields while offering simple, innovative and low-cost solutions adapted to the local production context.
A promising natural and renewable material is chitosan (CH), a food-grade polysaccharide composed of β-(1,4)-linked d-glucosamine and N-acetyl-d-glucosamine units. CH is produced by deacetylation from chitin, which is the second most abundant polysaccharide in existence and is the major component of fungal cell walls and arthropod exoskeletons (Abenaim et al., 2023). CH is a versatile and environmentally friendly biopolymer that already has numerous applications in medicine, agriculture, food preservation and the packaging industry (Abenaim et al., 2023).
CH is currently authorised in the EU as an active and non-toxic basic substance for plant protection and as an environmentally friendly and safe alternative to synthetic pesticides (Regulation EC No 1107/2009) (Farina et al., 2022). Different formulations of chitosan spray, supplemented with natural or synthetic insecticides, have been studied as pest control tools in agriculture and for the protection of food crops against pests or diseases (Abenaim et al., 2023).
The combination with essential oils (EOs) is a promising solution to protect vegetables against pests during production or post-harvest. Many EOs are considered safe for human consumption and pharmaceutical use. EOs with a repellent effect can help to protect foods from attack by several insect pests. In Ascrizzi et al. (2021), a chitosan coating film with Ferulago campestris EO applied to bean seeds showed a repellent effect against the seed pest Acanthoscelides obtectus (Coleoptera Bruchidae), without causing adverse effects on the germination and growth of bean plants. The protective effect of CH-EOs has been demonstrated in the European Union-funded FEDKITO PRIMA project (
https://fedkito.agr.unipi.it/en/home-english/), e.g. for the protection of fresh produce against Calliphoridae flies (Farina et al., 2022).
2. Materials and Methods
2.1. Essential Oils Purchase and Chemical Characterization
All essential oils used in the tests were purchased from commercial suppliers. The following essential oils were purchased from Compagnie des sens SAS (Lyon, France), certified ‘BIO’ by Ecocert (FR-BIO-01): Cinnamon (Cinnamomum cassia (L.) J.Presl), Lemon (Citrus limon (L.) Osbeck), Mandarin (Citrus reticulata Blanco), Orange (Citrus × sinensis (L.)) Osbeck) and tea tree (Melaleuca alternifolia Cheel). The chemical characterisation was carried out at the Faculty of Pharmacy, University of Pisa, Italy. The hydrodistilled EOs were diluted to 5% in HPLC grade n-hexane and then injected into a GC-MS apparatus. Gas chromatography-electron impact mass spectrometry (GC-EIMS) analyses were carried out on an Agilent 7890B gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an Agilent HP-5MS (Agilent Technologies Inc., Santa Clara, CA, USA) capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and an Agilent 5977B single quadrupole mass detector (Agilent Technologies Inc., Santa Clara, CA, USA). Analytical conditions were as follows: injector and transfer line temperatures 220 and 240 °C respectively; oven temperature programmed to rise from 60 to 240 °C at 3 °C/min; carrier gas helium at 1 ml/min; injection of 1 μL (5% HPLC grade n-hexane solution); split ratio 1:25. Acquisition parameters were as follows: full scan; scan range: 30-300 m/z; scan time: 1.0 sec. Identification of the compounds was based on comparison of their retention times with those of authentic samples (where available), comparing their linear retention indices relative to the series of n-hydrocarbons (C6-C25). Computer matching was also used against a commercial (NIST 2014) and a laboratory developed mass spectra library constructed from pure substances and components of commercial EOs of known composition and MS literature data (Adams 2007).
2.2. Chitosan and Essential Oils-Enriched Chitosan Solutions
High viscosity chitosan (CH) from crab shells, molecular weight ~50,000, CAS number: 9012-76-4, was purchased from Sigma-Aldrich (St. Louis, MO, USA). For all solutions, the protocol of Peng and Li (2014) was followed with minor modifications. For the 0.5, 1.0, and 2.0% (w/v) pure CH solution, 0.5, 1.0, and 2.0 g CH were dispersed in 100 mL of demineralised water containing 1.0% (v/v) glacial acetic acid (Carlo Erba Reagents s.r.l., Cornaredo, Italy), respectively. The solution was then stirred on a hot-plate stirrer (new type, VELP Scientifica, Usmate, Italy) at 25 °C and 7× g for 2 h. For the EOs-enriched CH solutions, 0.5% (v/v) vegetable glycerol (A.C.E.F. s.p.a, Fiorenzuola d’Arda, Italy), 0.6% (v/v) Tween® 80 (Sigma-Aldrich) and 0.1 or 1.0% (v/v) of the five selected EOs were added to the previously dissolved CH. Three EO concentrations were used in this study, corresponding to 5%, 3% and 1% of the corresponding EO diluted in chitosan solution prior to application to tomatoes. The EOs-enriched CH solutions were homogenised on a hot plate stirrer at 18 °C and 28× g for 4 min. Glycerol is a plasticiser that improves the mechanical properties of CH and Tween® 80 is a surfactant used to ensure wettability (Casariego et al. 2008). The solutions were prepared on the same day as the experiment. The tomatoes were completely soaked in the corresponding chitosan solution for 1 min and then left to dry for 30 min on an inert support in the open air at room temperature (i.e. 19-21 °C) before being used in the behavioural tests.
1.3. Insects Rearing
The cotton leafworm S. littoralis is an African noctuid pest that has spread to southern Europe and Asia, with larvae attacking a wide range of crops (CABI 2020). Test animals were obtained from a laboratory strain reared on a semi-artificial diet (Hinks 1976) at 23°C, 70% relative humidity and a 16:8 light/dark cycle. Animals were observed daily to allow accurate dating of larval, pupal and adult stages. The moult of the larvae was identified by the presence of the head capsule of the previous stage. Observation of this head capsule indicated that the larvae had molted within 24 hours, allowing us to perform tests on larvae of known age (comparison between one, two and three day old larvae).
1.4. Two-Choices Behavioral Test
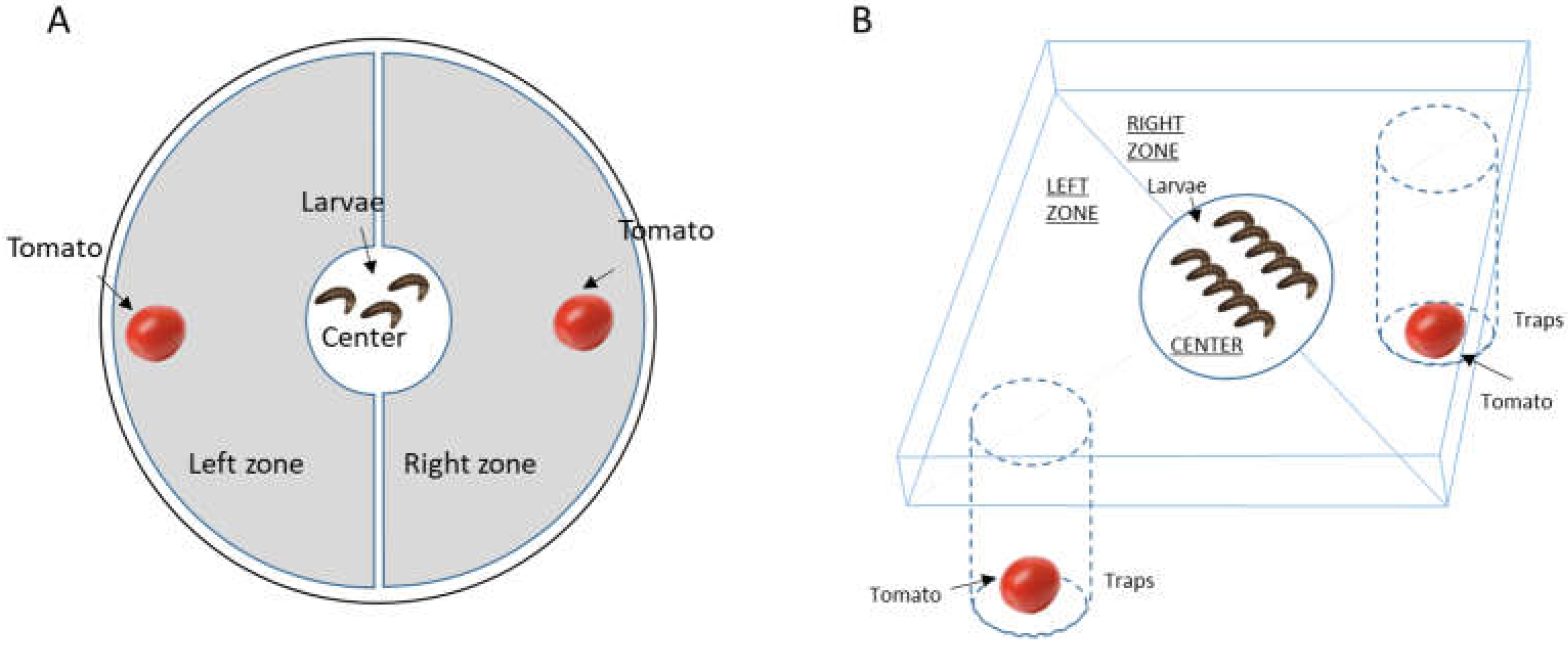
Behavioural tests were conducted to determine the effects of the addition of EOs to the chitosan film on larval behaviour (
Figure 1A). Batches of five L4 stage larvae collected from our breeding stock were placed in individual boxes on the day of the experiment and placed in the dark for four hours to reduce stress until the experiment. The five larvae from the same batch were then placed in the centre of 150 mm diameter Petri dishes containing a control tomato treated with chitosan alone and a tomato treated with chitosan + EO (
Figure 1A). After one hour, the number of larvae present in the centre (initial zone) and in the two zones (right or left) with the treated tomatoes was recorded. A control experiment with tomatoes treated only with chitosan was systematically carried out in parallel with these series. All tests were carried out under red light conditions (invisible spectrum for insects).
1.5. Tomato Consumption by Larvae
Tomatoes treated with chitosan alone or with chitosan + EO were individually placed in a Petri dish. The tomatoes were weighed before the experiment to know their initial fresh weight. Ten L4 larvae were placed in a Petri dish. The weight of the tomatoes was recorded 48 hours after the start of the experiment. We also carried out the same study on tomatoes that had not been exposed to larval attack, in order to take into account the weight loss of the tomatoes due to dehydration.
1.6. Behavioral Tests on Larvae Aged and Weighted
After a 4-hour fasting period, ten L4 larvae were placed at the start of the experiment in a pitfall-type arena with a central area 2 cm in diameter (
Figure 1B). At the start of the experiment, the plastic lid holding the larvae was removed, allowing the larvae to move freely within the box. Two holes were drilled in the bottom of the arena, each opening onto a closed tube, called a pitfall, in which treated or untreated tomatoes were placed. Individuals, attracted by the odours, approached the hole and had to fall into it to reach the tomato. Larvae were unable to escape from the pitfall. The control devices, which contained only tomatoes with chitosan in the branches, were also monitored in parallel to ensure random distribution between the two branches during the experiment. The experiment was carried out over 18 hours (from 14.00 hrs on day D to 08.00 hrs on day D+1). The masses of the two tomatoes and the ten L4 larvae placed in each trap were measured.
1.7. Statistical Analyses
We analysed the two-choice tests in the arena using chi-squared tests with a Marascuilo procedure to compare the number of larvae between the arena zones. We analysed behavioural tests on aged and weighed larvae in two steps. First, we performed a logistic analysis to test whether the frequency of larvae that stayed in the experimental area (compared to larvae that were caught) depended on the age of the larvae, on the presence of chitosan, (E)-cinnamaldehyde or cinnamon EO, and on the interaction between these two factors. Secondly, a logistic analysis was performed on the frequency of larvae choosing the trap with the treated tomato (compared to the control tomato), with the age and body mass of the larvae as factors. For the experiment examining the persistence of the effects of the tomato coating treatments over ten days, the frequency of larvae choosing the trap with the treated tomato (compared to the control tomato) was tested using a logistic analysis with age of the larvae and time after treatment as factors. Statistical analyses were performed using JMP software (JMP Pro 16, SAS Institute Inc., Cary, NC) or XLSTAT (2023 3.0 Addinsoft).
3. Results
3.1. Essential Oil (EO) Compositions
The full EO compositions are given in
Table 1. A total of 84 compounds were identified among all the EOs analysed. Cinnamon (Cinnamomum cassia (L.) J.Presl) EO was mainly composed of phenylpropanoids (over 85%), of which (E)-cinnamaldehyde was detected as the most abundant compound (70.1%) in the total composition, followed by (E)-o-methoxycinnamaldehyde (15.2%) and (E)-cinnamyl acetate (2.1%).
Monoterpene hydrocarbons dominated the composition of all the EOs analysed from Citrus species. Within this chemical class, limonene was identified as the most abundant compound, ranging from 64.2% in lemon (Citrus limon (L.) Osbeck) to 93.7% in sweet orange EO (Citrus × sinensis (L.) Osbeck). Lemon and red mandarin EOs also contained relevant relative amounts of γ-terpinene (10.5% and 19.1%, respectively), β-pinene (10.1% and 1.4%, respectively) and α-pinene (1.4% and 1.5%, respectively). Among the Citrus species analysed, the highest relative abundance of oxygenated monoterpenes was found in lemon EO (7.5%), with trans-citral, neral and geranyl acetate being the most abundant.
More than 50% of tea tree (Melaleuca alternifolia Cheel) EO was composed of oxygenated monoterpenes, the most abundant of which was 4-terpineol (42.7%). Within this chemical group, α-terpineol is typically found in tea tree EO composition, as well as p-cymene and γ-terpinene, which were detected as the two most abundant monoterpene hydrocarbons (9.6% and 3.5%, respectively) (Burdock 2009).
3.2. Two-Choices Behavioral Test
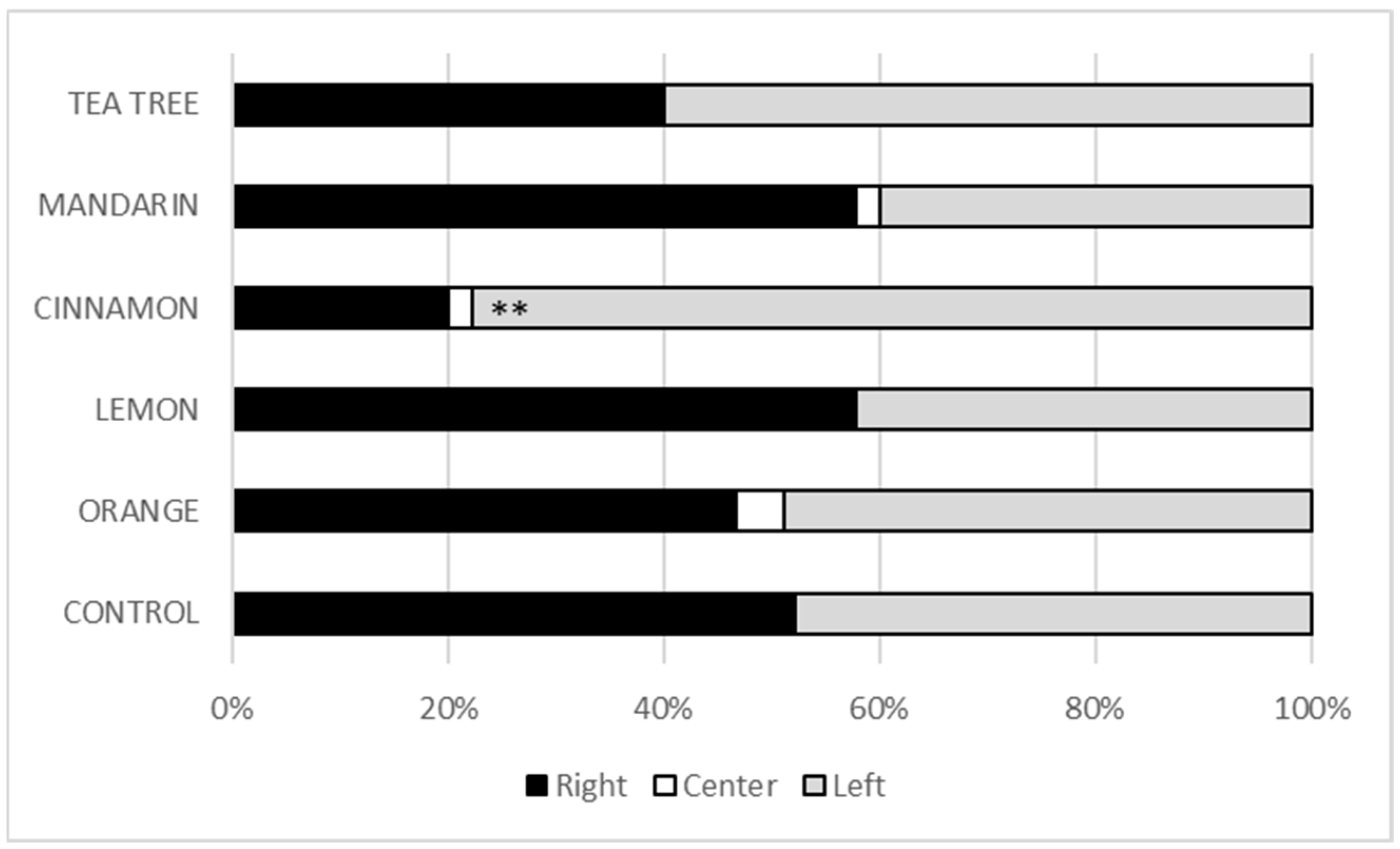
The relevance of our two-choice experiments was supported by the observation of very few larvae remaining in the starting zone (centre zone) of the arena and the balanced proportion of larvae in the control condition, which faced two tomatoes treated with chitosan alone (
Figure 2). Comparison of the different treatment conditions with tomatoes treated with chitosan alone showed no significant attraction or repellent effect of the tea tree, mandarin, lemon and orange EOs. Only cinnamon EO showed a repellent effect (P = 0.003,
Figure 2). Twenty percent of the larvae were found in the arena zone with cinnamon EO compared to 78% in the arena zone with chitosan alone.
1.3. Tomato Consumption by Larvae
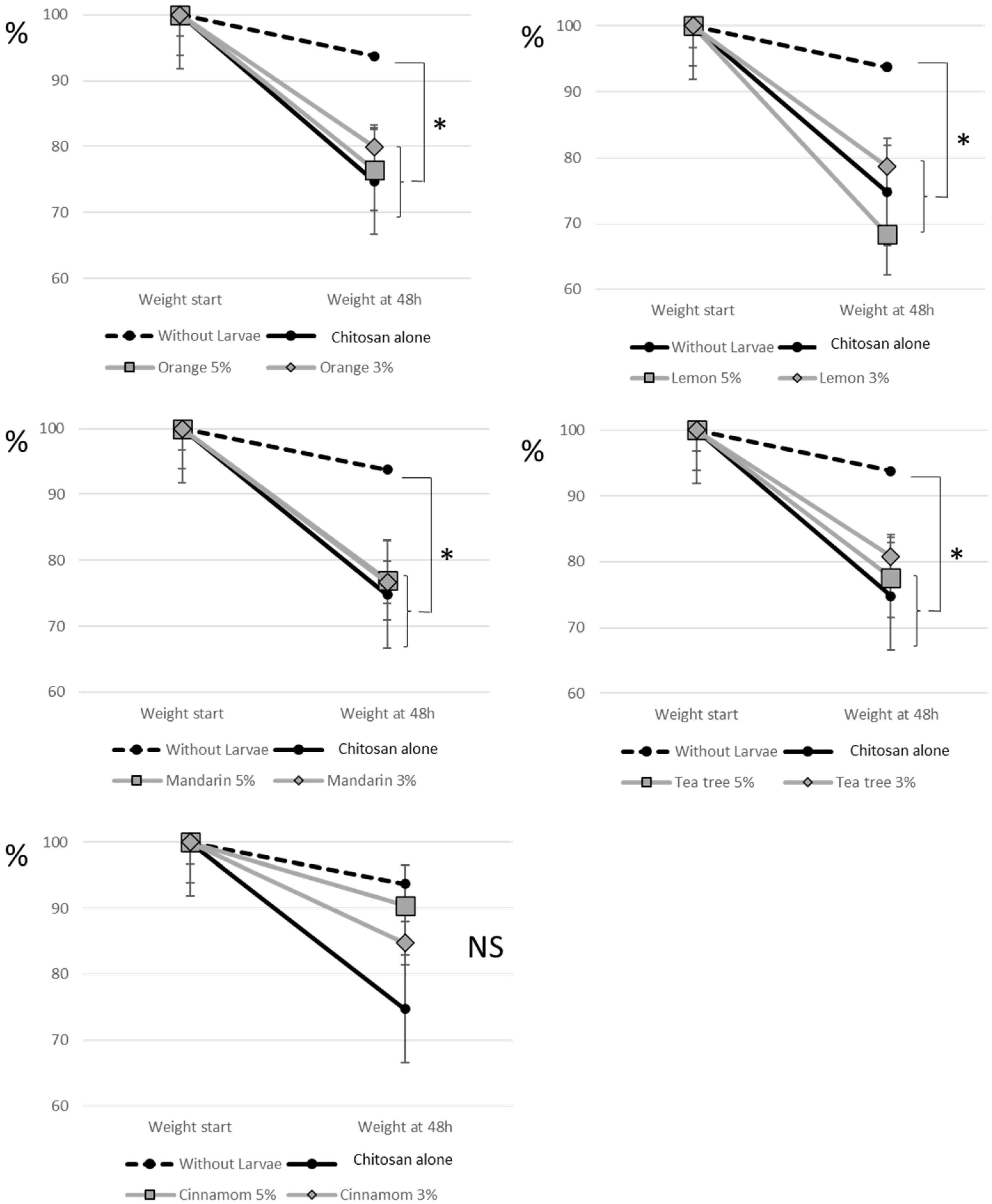
To complement the previous one-hour behavioural tests, we carried out 48-hour tests to investigate the effects of treatments on larval feeding behaviour. Consumption of tomatoes resulted in loss of tomato weight and the presence of numerous bites and larval attacks. Tomato weight loss was 25.2% in the control condition with chitosan alone. Tomatoes with chitosan alone and without larvae lost 6.2% of their weight in 48 hours, mainly due to dehydration. The difference between tomatoes with and without larvae was significant (P = 0.029) and showed the consumption of tomatoes by larvae. In comparison with the chitosan control without larvae, we also found a significant consumption of tomatoes by larvae in the conditions combining chitosan with orange, lemon, mandarin and tea tree, both for the percentages of EO of 5 and 3% (
Figure 3). For cinnamon, no difference was observed with the same control without larvae, whatever the percentage of essential oil. We measured a loss of 9.6 and 15.2% of the tomato weight for 5 and 3% cinnamon EO, respectively, whereas for the other conditions (chitosan alone or chitosan with EO) we reported losses between 20.2 and 31.7%. This means that tomato consumption by larvae was particularly reduced by cinnamon EO (
Figure 3).
2.4. Behavioral Tests on Larvae Aged and Weighted
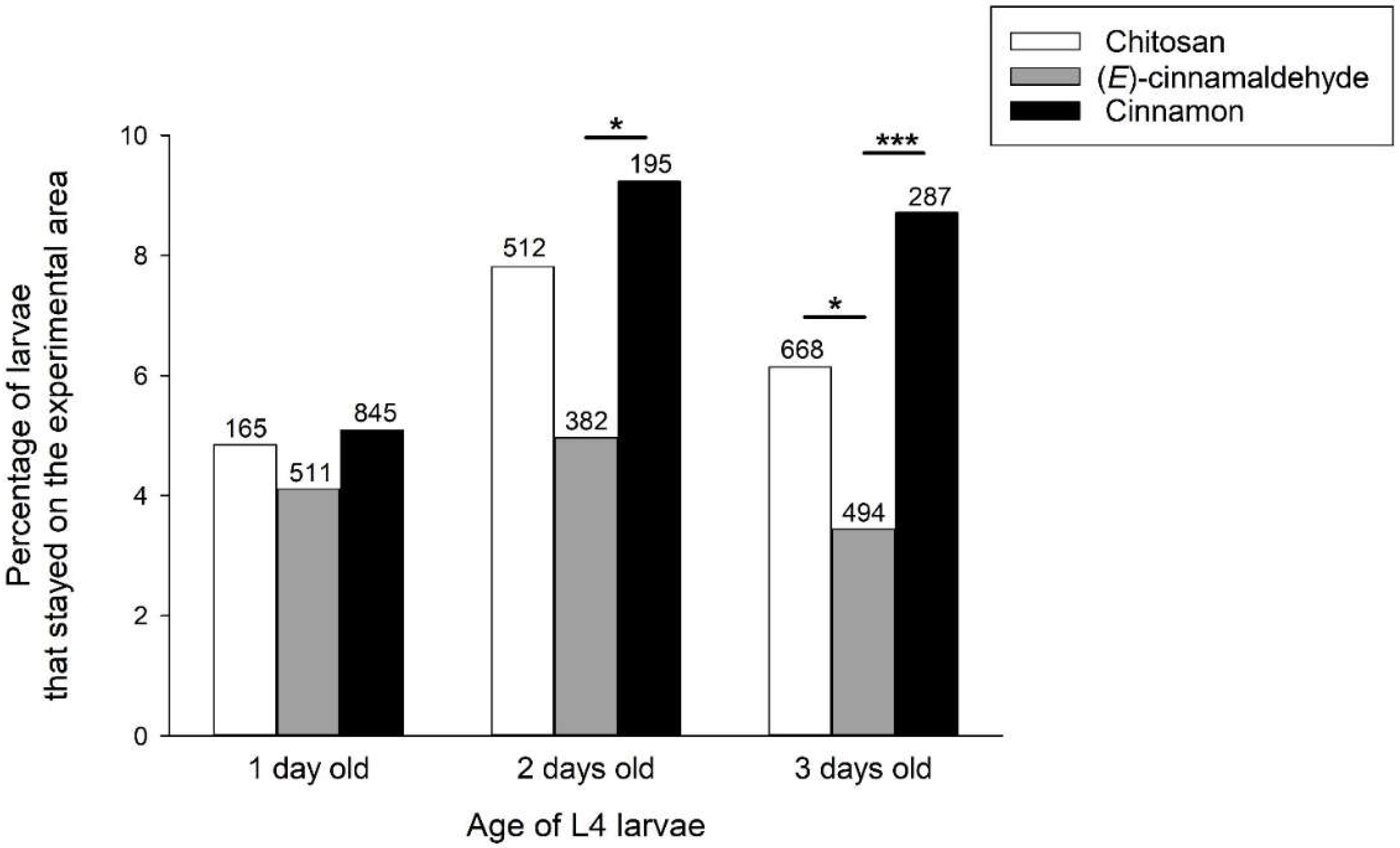
Additional analyses were performed with cinnamon EO and its main compound (E)-cinnamaldehyde to investigate the possible influence of age and body mass of L4 larvae. We first analysed the number of larvae that remained in the experimental area, i.e. larvae that were inactive or did not move into a trap with control or treated tomatoes. The number of larvae that remained in the experimental area depended on the age of the larvae (X22 = 7.5 P = 0.024) and the treatment of the tomatoes with chitosan, cinnamal or cinnamon (X22 = 11.8 P = 0.003). These effects were additive (X24 = 3.3 P = 0.506 for the interaction age × treatment). For the age effect, two- and three-day-old L4 larvae were captured less frequently than one-day-old L4 larvae (
Figure 4). For the effect of tomato treatment, larvae were on average less frequently captured in the presence of cinnamon EO (with significant post hoc tests in two- and three-day L4 larvae) and more frequently captured in the presence of (E)-cinnamaldehyde (with a significant post hoc test in three-day L4 larvae) (
Figure 4).
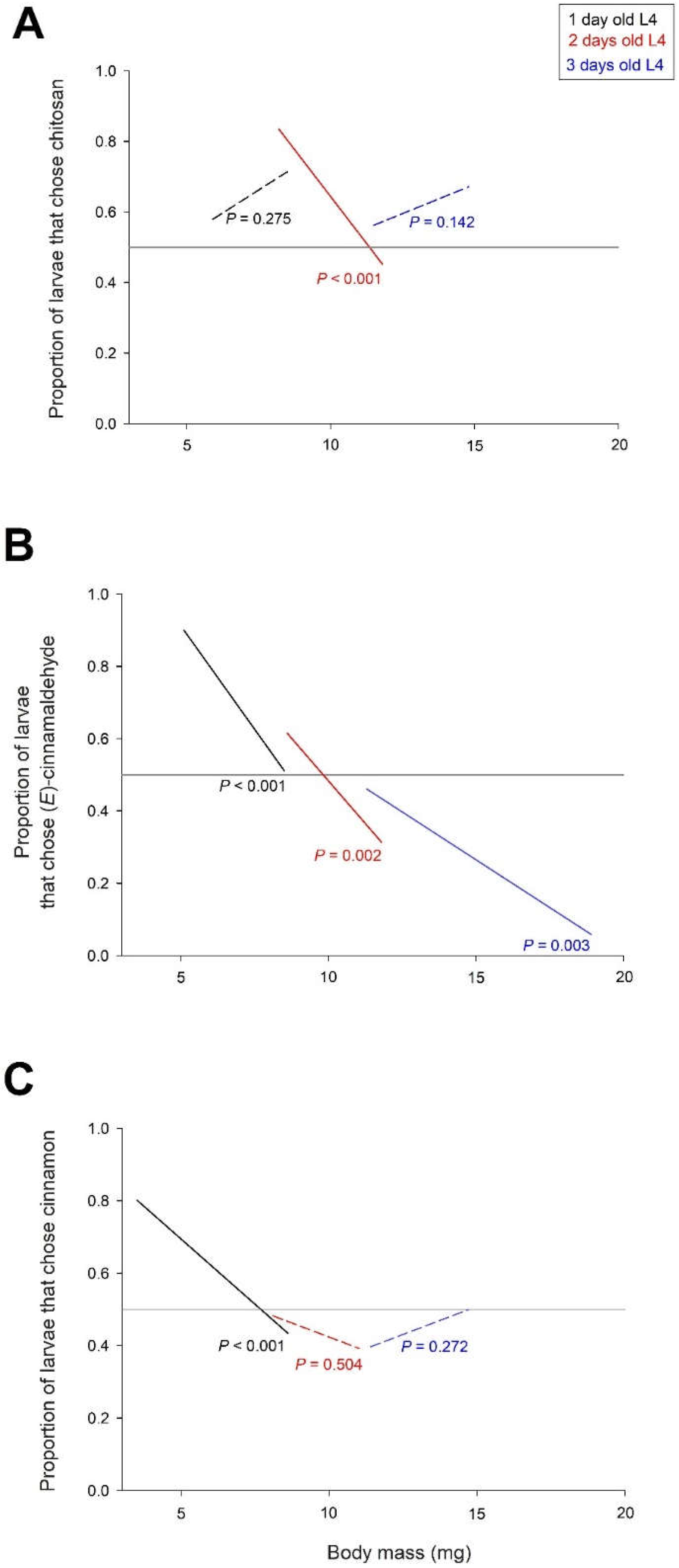
Looking at the captured larvae, we found that their choice between the treated and control tomatoes depended on the age and body mass of the L4 larvae (
Figure 5). In the chitosan/control choice experiment (
Figure 5A), L4 larvae were more likely to choose the chitosan-treated tomato trap than the untreated control tomato. However, the frequency of larvae choosing the chitosan trap was dependent on the interaction between body mass and age of the larvae (X22 = 20.0 P < 0.001). This interaction was caused by a significant decrease in the attractiveness of chitosan with larval body mass, observed only in the two-day-old L4 larvae (
Figure 5A).
In the (E)-cinnamaldehyde/control experiment (
Figure 5B), the frequency of larvae choosing the trap with the tomato treated with (E)-cinnamaldehyde was dependent on body mass (X21 = 38.8 P < 0.001) and age of the larvae (X22 = 10.2 P = 0.006). The interaction between body mass and larval age was not significant (X22 = 3.8 P = 0.147).
Figure 5B shows that the frequency of larvae choosing the (E)-cinnamaldehyde trap decreased with both body mass and age of the larvae. Overall, (E)-cinnamaldehyde was attractive in 1-day-old L4 larvae, repulsive in 3-day-old L4 larvae and with an intermediate pattern in 2-day-old L4 larvae.
In the cinnamon EO/control experiment (
Figure 5C), the frequency of larvae choosing the cinnamon EO-treated tomato trap over the control tomato was dependent on the interaction between larval body mass and age (X22 = 11.6 P = 0.003). As shown in
Figure 5C, this interaction was caused by a significant decrease in attractiveness of cinnamon EO with larval body mass, which was only observed for 1-day-old L4 larvae. Cinnamon EO was only attractive to the smallest larvae, i.e. the smallest one-day-old L4 larvae.
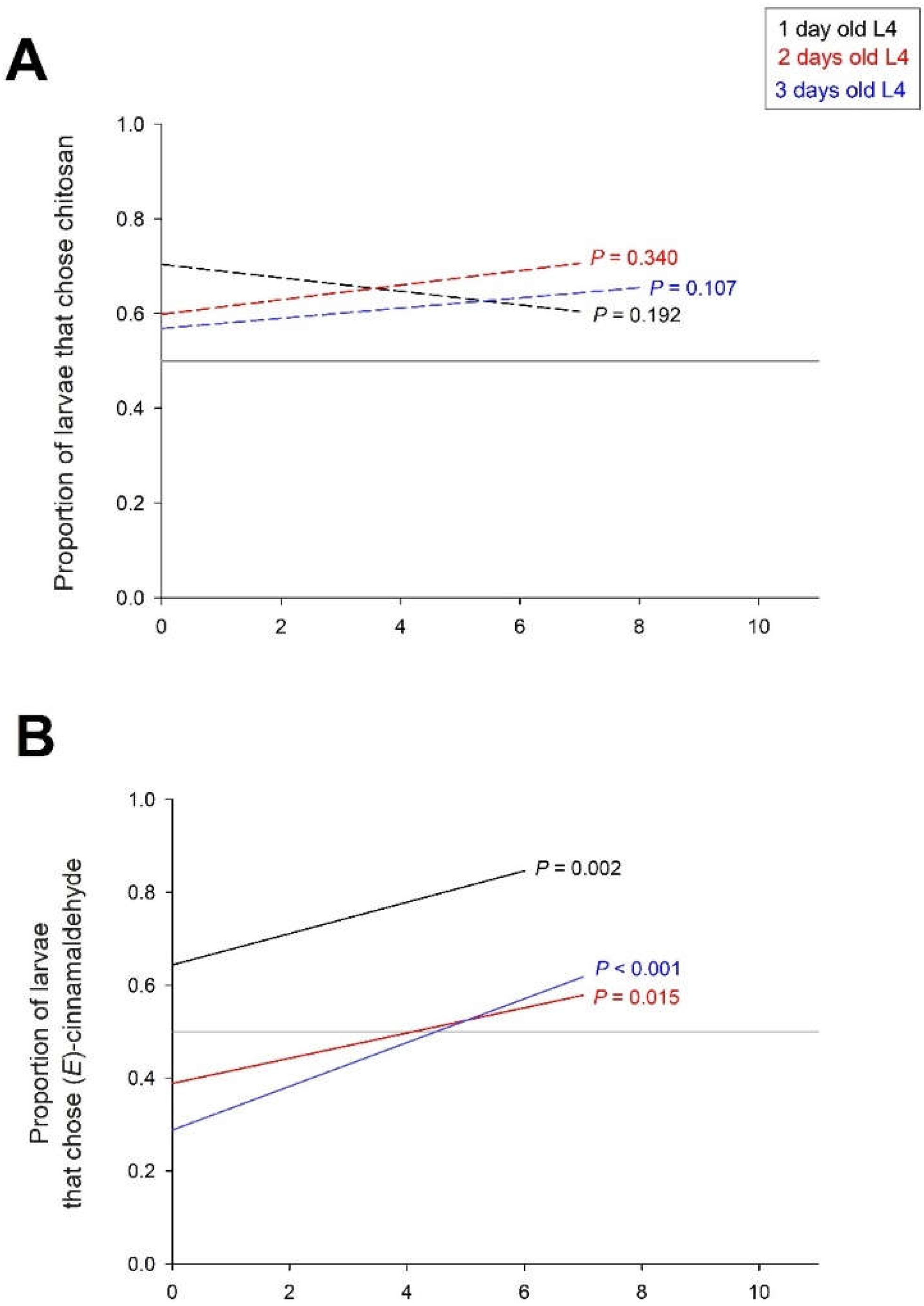
In a final experiment, we tested the persistence of the effects of the treatments over a period of 10 days. Tomatoes were treated on day 0 with chitosan alone, with chitosan and (E)-cinnamaldehyde, or with CH and cinnamon EO. Choice tests were performed on L4 larvae (one, two or three days old) between 1 and 10 days after treatment. The frequency of larvae that chose the trap with the tomato treated with CH over the control tomato was not significantly dependent on the time after treatment (X21 = 1.0 P = 0.328), the age of the larvae (X22 = 1.5 P = 0.476) and their interaction (X22 = 4.2 P = 0.122) (
Figure 6A).
The frequency of larvae choosing the (E)-cinnamaldehyde-treated tomato trap over the control tomato trap depended on post-treatment time (X21 = 41.3 P < 0.001) and larval age (X22 = 121.1 P < 0.001), with no interaction between post-treatment time and larval age (X22 = 2.2 P = 0.330). The attractiveness of (E)-cinnamaldehyde increased over time in the three age classes of L4 larvae, and the attractiveness of (E)-cinnamaldehyde was higher in one-day-old larvae than in old larvae (
Figure 6B).
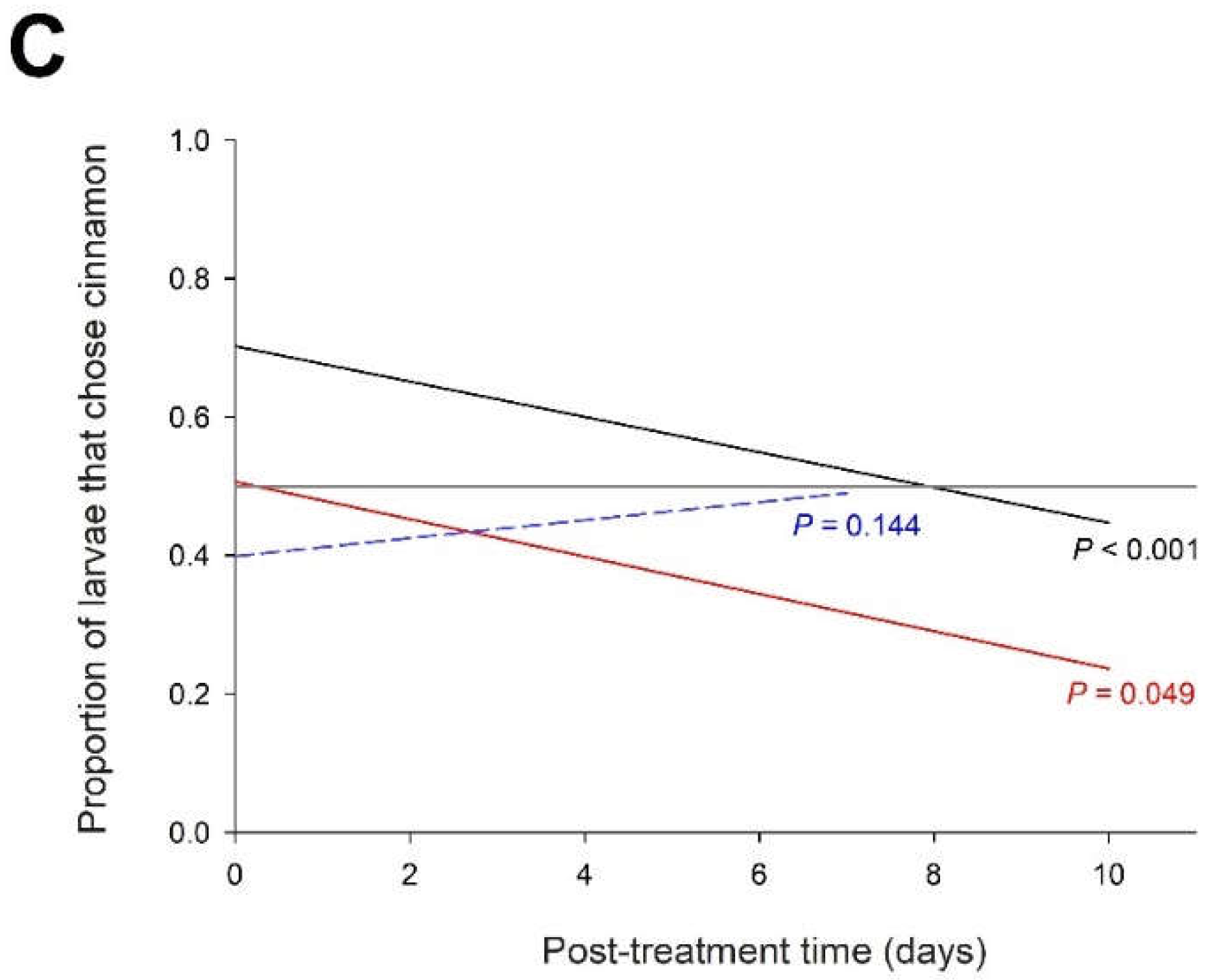
The number of larvae that chose the trap with the tomato treated with cinnamon EO over the control tomato depended on the time after treatment (X21 = 5.3 P = 0.021), the age of the larvae (X22 = 41.5 P < 0.001) and their interaction (X22 = 14.4 P < 0.001). The frequency of larvae selecting the cinnamon EO trap decreased significantly over time in one- and two-day-old L4 larvae, but not in three-day-old L4 larvae (
Figure 6C). In one-day-old L4 larvae, cinnamon EO was attractive at the beginning of the treatment but became neutral after 10 days. In two-day-old L4 larvae, cinnamon EO was neutral at the beginning of the treatment but became repellent after a few days.
4. Discussion
The study tested the use of EOs combined with CH to protect tomato fruit from S. littoralis, an important insect pest in many parts of the world (CABI, 2020). Few published studies have investigated this use of mixtures of EOs and CH on insect pests (Abenaim et al., 2023; Parichanon et al., 2023; Soltani et al., 2023; Djebbi et al., 2024). We demonstrated a repellent effect of cinnamon EO combined with CH on S. littoralis larvae. No effect on larval attractiveness or repellency was found for the other four EOs tested and for CH alone. No overall repellent effect on larvae was found for (E)-cinnamaldehyde, but this major compound in cinnamon EO had specific effects when considering larval age and body mass, and time after treatment.
The Repellent Effect of Cinnamon EO
S. littoralis larvae were not sensitive to tea tree, mandarin, lemon and orange EO, nor to CH alone. However, cinnamon EO had a repellent effect on larvae as observed in our behavioral test (
Figure 2). The consumption of tomatoes by larvae over 48 hours was also reduced by cinnamon EO (
Figure 3). The repellent property of cinnamon EO has previously been highlighted in other insect pests (Nerio et al., 2010). More recently, the efficacy of EO from cinnamon leaves and flowers was also evaluated against two common pests, Sitophilus oryzae and Callosobruchus maculatus (Narayanankutty et al., 2021). Leaf EO was shown to repel these pests even at low concentrations. Cinnamon EO also repels the invasive species Cydalima perspectalis (Szelenyi et al., 2020), adults of Lasioderma serricorne (Ren et al., 2022), and has been shown to be an effective deterrent to oviposition in Culex pipiens (Farag et al., 2024). Specifically, we have shown that the repellent effect of cinnamon EO is maintained when it is associated with CH. Therefore, our results suggest that the combination of CH with cinnamon EO has good potential as a biocontrol strategy against the pest moth S. littoralis.
Lack of overall effect of the compound (E)-cinnamaldehyde
EOs are complex mixtures of different molecules (Narayanankutty et al., 2021; Djebbi et al., 2024; Farag et al., 2024). It has long been assumed that the effects of these complex mixtures on animal behavior are due to their major compounds, as observed in pheromone mixtures. EOs are often described as rich in active compounds such as (E)-cinnamaldehyde or limonene (Fouad and da Camara, 2017; Narayanankutty et al., 2021; Djebbi et al., 2024). (E)-cinnamaldehyde is the major compound in cinnamon EO, and limonene is the major compound in the EOs of the three Citrus species studied (
Table 1). Because cinnamon EO had a repellent effect on S. littoralis larvae, in contrast to the studied Citrus species EOs, we tested the effect of (E)-cinnamaldehyde on larval feeding and behavior. Although (E)-cinnamaldehyde is the major compound in cinnamon EO, we did not find a repellent effect of this molecule on larvae. Szelényi et al. (2020) also showed that (E)-cinnamaldehyde did not induce electrophysiological responses at the antennae of the box tree moth and did not explain a repellent effect of cinnamon EO. The most intense electrophysiological response of the box tree moth was induced by benzaldehyde, a less abundant compound in cinnamon EO. In contrast to the box tree moth study, Deletre et al. (2015) found that (E)-cinnamaldehyde induced a deterrent effect and an electrophysiological response at the antennae of the mosquito Anopheles gambiae. Thus, repellent and olfactory responses can vary between species, and the major component of cinnamon EO is not necessarily its active compound.
To explain that the major compound of EO is not necessarily its active compound, it is possible that interactions between molecules induce synergies or antagonisms. This has been raised in studies questioning the ecological importance of interactions between pheromones and volatile plant compounds, as perturbations in odorant detection have been reported with these mixtures (Badeke et al., 2016). Insect olfactory systems may have evolved to cope with such complex olfactory landscapes (Hansson & Stensmyr, 2011), but this remains poorly understood (Conchou et al., 2021). Potential synergies and antagonisms between molecules increase the importance of studying isolated molecules from complex mixtures. However, the single molecule approach is often studied due to the regulatory context of marketing and regulatory dossiers, which are more difficult to conduct on complex mixtures (EFSA et al., 2023). Whatever the reason for the divergence of our results between the mixture cinnamon EO and its main compound (E)-cinnamaldehyde, this divergence shows the interest in comparing their respective effects. As discussed below, our study also shows that the effects of EOs and their compounds may depend on individual variations.
Age- and weight-dependent effects of cinnamon EO and (E)-cinnamaldehyde
The lack of an overall effect of (E)-cinnamaldehyde does not mean that it has no effect. In fact, we detected its effects when we considered the age and body mass of S. littoralis larvae. These effects related to ontogeny and interindividual variation are expected because age and body mass can alter species responses to toxic and olfactory substances (Malbert-Colas et al., 2020; Bagni et al., 2024). Studies investigating the influence of interindividual variation on sensitivity to EOs are rare (Pineda et al., 2023; Guesmi et al., 2024). Guesmi et al. (2024) found no difference in the repellent effect of Anethum graveolens EO between larvae and adults of Tribolium confusum, but found higher and faster mortality in larvae than adults. Pineda et al. (2023) showed that Drosophila suzukii responded differently to Eucalyptus EO depending on sex and age-mating status by comparing newly emerged virgin adults and 5-7 days old mated adults. To our knowledge, no study has examined the influence of body mass on the response to EOs. Interestingly, our study showed that body mass and age of S. littoralis larvae influenced responses to cinnamon EO and (E)-cinnamaldehyde.
The attractiveness of cinnamon EO was dependent on the interaction between larval body mass and age. This interaction was caused by a decrease in attractiveness of cinnamon EO with larval body mass, which was observed only in 1-day-old L4 larvae (
Figure 5C). Cinnamon EO was only attractive to the smallest larvae. An interaction between body mass and larval age was even observed in the chitosan/control choice experiment, with a decrease in attractiveness of chitosan with larval body mass observed only in two-day-old L4 larvae (
Figure 5A). The attractiveness of (E)-cinnamaldehyde also decreased with larval body mass. This decrease was found in the three age classes of L4 larvae studied, but resulted in contrasting attractiveness-repellency profiles. In one-day-old L4 larvae, (E)-cinnamaldehyde was attractive to the smallest larvae and neutral to the largest larvae (
Figure 5B). In three-day-old L4 larvae, it was neutral in the smallest larvae and repulsive in the largest. An intermediate pattern around neutrality of (E)-cinnamaldehyde was observed in two-day-old L4 larvae.
The attractiveness of cinnamon EO and (E)-cinnamaldehyde decreased with larval age. They were only attractive to one-day-old L4 larvae (
Figure 5 and
Figure 6). This could be explained by a difference in physiology between larvae that have just molted (one day old) and older larvae that are resuming feeding activity. An alternative hypothesis is a change in their peripheral olfactory system, as observed in S. littoralis larvae between their first and fourth instars (Revadi et al., 2021). Revadi et al. demonstrated that this instar-specific behavioral plasticity was mediated by an odorant receptor, and therefore that the ability of insects to detect odors can change during development.
Time-dependent effect of cinnamon EO and (E)-cinnamaldehyde
Over 10 days, we found opposite temporal variations in attractiveness and repellency profiles between cinnamon EO and (E)-cinnamaldehyde. The frequency of larvae choosing tomato traps treated with cinnamon EO decreased over time in one- and two-day-old L4 larvae, resulting in attractiveness of cinnamon at the beginning of treatment in one-day-old L4 larvae and repellency of cinnamon after 10 days in two-day-old L4 larvae (
Figure 6C). In contrast, the attractiveness of (E)-cinnamaldehyde increased over time in the three age classes of L4 larvae studied (
Figure 6B).
At the hourly scale, from post-treatment tests between 1 and 24 h, a decrease in repellency over time was found for 21 out of 28 EOS tested on the pest Sitophilus zeamais (Yang et al., 2020). Cinnamon EO was tested on this pest and the high repellency observed at 1 and 3 h after treatment disappeared after 24 h. Yang et al. explained the decreased repellency of EOS over time by the high volatility of most of their low molecular weight molecules, although the volatility of EOS may also depend on the type and structure of the test surface and the EO formulation. Variation in the effects of EOs has also been described in the mosquito Culex pipiens (Farag et al., 2024). Farag et al. suggested that the variation in response to EOs depends on their ratios of monoterpenoids, phenylpropanoids, and fatty acids. A variation in such ratios over time could also explain the decrease in attractiveness of cinnamon EO over 10 days that we observed in one- and two-day-old L4 larvae. The variation in these ratios may have been caused by differences in the volatility of the molecules and interactions between the molecules of EO and the treated tomatoes.
5. Conclusions
In conclusion, our study highlights the potential of the combination of CH and cinnamon essential oil as an environmentally friendly solution to protect tomatoes from S. littoralis attack. However, we also show that the efficacy of essential oils may depend on the persistence of the effect over time, as well as the variation in age and body mass of the pests. Although the influence of inter-individual variation on sensitivity to essential oils is rarely considered, our results encourage studies to investigate its influence on the efficacy of new molecules for crop protection and food preservation.
Author Contributions
Conceptualization: TD, PC, DS; Chemical analysis: BC, RA; Data and statistical analysis: PC, MM, DS; Writing—original draft preparation: DS, MM; Writing—review and editing: TD, MM, BC, RA; Supervision: DS, TD; Funding acquisitions, BC; All authors have read and agreed to the published version of the manuscript..
Funding
This research has been supported by the PRIMA program, under project FEDKITO. The PRIMA program is supported by the European Union.
Data Availability Statement
Data is available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abenaim, L.; Conti, B. Chitosan as a Control Tool for Insect Pest Management: A Review. Insects 2023, 14, 949. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. (2007). Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Pub. Corp, Carol Stream, IL, USA.
- Adenuga A., H. , Muhammad-Lawal A., Rotimi O. A (2013). Economics and Technical Efficiency of Dry Season Tomato Production in Selected Areas in Kwara State, Nigeria. Agris on-line Papers in Economics and Informatics. 1.
- Ascrizzi, R. , Flamini G., Bedini S., Tani C., Giannotti P., Lombardi T., Conti B., Fraternale D (2021). Ferulago campestris Essential Oil as Active Ingredient in Chitosan Seed-Coating: Chemical Analyses, Allelopathic Effects, and Protective Activity against the Common Bean Pest Acanthoscelides obtectus. Agronomy. ;11:1578. [CrossRef]
- Badeke, E.; Haverkamp, A.; Hansson, B.S. ; Sachse, S (2016). A challenge for a male noctuid moth? Discerning the female sex pheromone against the background of plant volatiles. Front. Physiol., 7, 143.
- Bagni, T. , Siaussat D. ( 2024). The effect of developmental temperature on olfaction in a moth revealed by its interaction with body mass. Communications Biology 7, 1133. [CrossRef]
- Burdock, G. A (2009). Fenaroli’s Handbook of Flavor Ingredients; VI.; CRC Press: Boca Raton, FL (USA), ISBN 9781420090772.
- CABI (2020). Spodoptera littoralis (cotton leafworm), in: CAB International. Wallingford, UK.
- Casariego, A.; Souza, B.W.S.; Vicente, A.A.; Teixeira, J.A.; Cruz, L. ; Díaz, R (2008). Chitosan coating surface properties as affected by plasticizer, surfactant and polymer concentrations in relation to the surface properties of tomato and carrot. Food Hydrocoll. 1452; 22. [Google Scholar]
- Conchou L, Lucas P, Deisig N, Demondion E, Renou M (2021). Effects of Multi-Component Backgrounds of Volatile Plant Compounds on Moth Pheromone Perception Insects ;12(5):409. 1 May. [CrossRef]
- Deletre, E. , Chandre F. ( Cuminum cyminum and Cinnamomum zeylanicum essential oils against Anopheles gambiae and prospects for their use as bednet treatments. Parasites Vectors 8, 316. [CrossRef]
- Djebbi, T. , Ascrizzi R., Bedini S., Farina P., Sanmartin C., Mediouni Ben Jemâa J., Bozzini M.F., Flamini G., Conti B. (2024). Physicochemical and repellent properties of chitosan films loaded with essential oils for producing an active packaging effective against the food pest Sitophilus oryzae. Journal of Stored Products Research 106, 102297. [CrossRef]
- EFSA (European Food Safety Authority), Barthélémy E, Bolognesi C, Castle L, Crebelli R, Di Consiglio E, Franz R, Grob K, Hellwig N, Lambré C, Lampi E, Merkel S, Milana MR and Rivière G (2023). Principles that could be applicable to the safety assessment of the use of mixtures of natural origin to manufacture food contact materials. EFSA supporting publication 2023:EN-8409. 32 pp. [CrossRef]
- FAO1-https://www.fao.
- FAO2-https://www.fao.org/3/I8167EN/i8167en.
- FAO3-https://www.fao.org/fileadmin/user_upload/sap/docs/Post-harvest%20losses%20along%20value%20and%20supply%20chains%20in%20the%20Pacific%20Island%20Countries.
- Farag SM, Moustafa MAM, Fónagy A, Kamel OMHM, Abdel-Haleem DR (2024). Chemical composition of four essential oils and their adulticidal, repellence, and field oviposition deterrence activities against Culex pipiens L. (Diptera: Culicidae). Parasitol Res. Jan 25;123(1):110. [CrossRef]
- Farina, P.; Ascrizzi, R.; Bedini, S.; Castagna, A.; Flamini, G.; Macaluso, M.; Mannucci, A.; Pieracci, Y.; Ranieri, A.; Sciampagna, M.C.; et al. (2022). Chitosan and Essential Oils Combined for Beef Meat Protection against the Oviposition of Calliphora vomitoria,Water Loss, Lipid Peroxidation, and Colour Changes. Foods 11, 3994. [CrossRef]
- Fouad, H.A. , da Camara C.A.G. (2017). Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae). Journal of Stored Products Research 73, 30-36. [CrossRef]
- FrEsh fooD sustainable pacKaging In The circular economy https://fedkito.agr.unipi.
- Goka, M. G. , Dufrechou, M. ( 3(5), 40–45. [CrossRef]
- Guesmi, F. , Amari R., Ajmi I.S., Athmouni K., Hfaiedh N., Allagui M.S., Mediouni Ben Jemâa J., Landouls A. (2024). Promising bioinsecticidal effect of Tunisian Anethum graveolens L. (dill) (Umbelliferae) essential oil against confused flour beetle, Tribolium confusum Jaquelin du Val. 1863 (Coleoptera: Tenebrionidae), Journal of Stored Products Research 106, 102273. [CrossRef]
- Hansson, B.S.; Stensmyr, M. C (2011). Evolution of insect olfaction. 72.
- Khan, A.A., G. Qadar, A.A. Abro and M. Awais. (2023). Tomato yield losses due to attack of Hinks, C.F., Byers, J.R., 1976. Biosystematics of the genus Euxoa (Lepidoptera: Noctuidae): V. Rearing procedures, and life cycles of 36 species. Can. Entomol. 108, 1345–1357. 10.4039/Ent1081345-12 insects/pests in Pakkhal Valley of District Mansehra Khyber Pakhtunkhwa, Pakistan. Sarhad Journal of Agriculture, 39(1): 21-28.
- Malbert-Colas, A. , Drozdz T., Massot M., Bagni T. Chertemps T., Maria A., Maïbèche M., Siaussat D. (2020). Effects of low concentrations of deltamethrin are dependent on developmental stages and sexes in the pest moth Spodoptera littoralis. Environmental Science and Pollution Research 27, 41893-41901. [CrossRef]
- Naika, S. , de Jeude, J., de Goffau, M., Hilmi, M. and van Dam, B. (2005) Cultivation of tomato: Production, processing and marketing. Agromisa Foundation and CTA, Wageningen, Netherlands.
- Narayanankutty A, Kunnath K, Alfarhan A, Rajagopal R, Ramesh V (2021). Chemical Composition of Cinnamomum verum Leaf and Flower Essential Oils and Analysis of Their Antibacterial, Insecticidal, and Larvicidal Properties. Molecules. Oct 19;26(20):6303. [CrossRef]
- National Institute of Standards and Technology, NIST (2014). NIST/EPA/NIH Mass Spectral Library, NIST Standard Reference Database Number 69. The NIST Mass Spectrometry Data Center, Gaithersburg, MD, USA.
- Nerio, L. S. , Olivero-Verbel, J., & Stashenko, E. (2010). Repellent activity of essential oils: A review. Bioresource Technology, 101(1), 372-378.
- Parichanon, P. , Ascrizzi, R. ( Current Opinion in Green and Sustainable Chemistry, 103132. [CrossRef]
- Peng, Y. ; Li, Y (2014). Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll., 36, 287–293.
- Pineda, M. , Alves E.L.d.A., Antunes J.A., Carvalho V.d.C., Haddi K. (2023). Low concentrations of eucalyptus essential oil induce age, sex, and mating status-dependent stimulatory responses in drosophila suzukii. Agriculture 13, 404. [CrossRef]
- Ren Y, Wang T, Jiang Y, Chen D, Zuo W, Guo J, Jin D (2022). Behavioral Response, Fumigation Activity, and Contact Activity of Plant Essential Oils Against Tobacco Beetle (Lasioderma serricorne (F.). Front Chem. Mar 24:10:880608. eCollection 2022. [CrossRef]
- Revadi SV, Giannuzzi VA, Rossi V, Hunger GM, Conchou L, Rondoni G, Conti E, Anderson P, Walker WB, Jacquin-Joly E, Koutroumpa F, Becher PG (2021). Stage-specific expression of an odorant receptor underlies olfactory behavioral plasticity in Spodoptera littoralis larvae. BMC Biol. Oct 28;19:231. [CrossRef]
- Soltani A, Haouel-Hamdi S, Sadraoui Ajmi I, Djebbi T, Ben Abada M, Yangui I, Chouachi N, Hassine K, Majdoub H, Messaoud C, Mediouni Ben Jemâa (2023). Insights for the control of dried-fruit beetle Carpophilus hemipterus (Nitidulidae) using rosemary essential oil loaded in chitosan nanoparticles. J. Int J Environ Health Res. Dec;33(12):1243-1253. Epub 2022 Jun 2. [CrossRef]
- Szelényi, M. O, Erdei A.L, Jósvai J.K, Radványi D, Sümegi B, Vétek G, Molnár B.P, Kárpáti Z (2020). Essential Oil Headspace Volatiles Prevent Invasive Box Tree Moth (Cydalima perspectalis) Oviposition-Insights from Electrophysiology and Behaviour Insects. Jul 23;11(8):465. [CrossRef]
- Yang Y, Isman M, Tak JH (2020). Insecticidal Activity of 28 Essential Oils and a Commercial Product Containing Cinnamomum cassia Bark Essential Oil against Sitophilus zeamais Motschulsky. Insects. Jul 27;11(8):474. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).