1. Introduction
As the incidence of anterior cruciate ligament (ACL) reconstructions continues to rise alongside sustained demands for high-level athletic performance, the frequency of revision ACL reconstruction is expected to increase accordingly [
1,
2]. Anterior cruciate ligament (ACL) reconstructions have been reported to fail in approximately 12% of cases [
3]. Among the technical causes of failure, femoral tunnel malposition is the most frequently cited, accounting for 63% to 90% of such cases [
4]. Additionally, graft impingement against the intercondylar notch has been identified as a significant contributor to graft deterioration and postoperative limitations in range of motion [
5].
Graft impingement can lead to several complications, including anterior knee pain, joint effusions, loss of extension, and recurrent instability. Anterior knee pain typically results from the graft impinging against the intercondylar notch when the knee approaches full extension. Effusions may develop due to wear particles and fractured graft fibers caused by graft abrasion. Loss of extension can occur when the graft acts as a checkrein against the intercondylar notch. In cases of severe impingement, graft failure may ensue, ultimately leading to recurrent instability [
5,
6].
There is some variability among previous studies regarding the precise localization of the anatomic center of the ACL femoral footprint [
7,
8]. The anatomy of the ACL footprint exhibits considerable variability in both size and shape [
9]. Therefore, the optimal tunnel position in anatomic single-bundle ACL reconstruction may vary depending on the graft diameter as well as the size and morphology of the ACL footprint [
10,
11]. With the development of surgical techniques that allow independent creation of the femoral tunnel from the tibial tunnel, it has become possible to minimize graft impingement by considering the morphology of the femoral notch as well as the femoral and tibial footprints [
11] Placement of the femoral tunnel in a more proximal and anterior position is correlated with a higher likelihood of graft impingement [
12,
13]. With regard to the tibial tunnel, graft impingement in full knee extension remains a concern associated with anterior tunnel placement. However, a limited number of biomechanical and clinical studies have demonstrated that anterior tibial tunnel placement can improve stability without compromising knee extension [
14,
15,
16].
The purpose of the present study was to identify the optimal combination of tibial and femoral tunnel positions that minimizes impingement between the ACL graft and the femoral intercondylar notch in anatomical single-bundle ACL reconstruction. It was hypothesized that more anterior positioning of both the tibial and femoral tunnels would be associated with increased impingement volume.
2. Materials and Methods
2.1. 3D Model Reconstruction
Computed tomography (CT) images of the knee from nine subjects were included in this study. Three-dimensional CT scans were acquired using a 16-channel CT scanner (GE Healthcare) with 0.625 mm slice thickness at four distinct knee flexion angles (0°, 45°, 90°, and 120°) in healthy volunteers. Subjects were selected based on the following inclusion criteria: (1) absence of femoral or tibial fractures; (2) no osseous deformities; (3) no ligamentous injuries; (4) no history of surgical intervention; and (5) absence of osteoarthritis exceeding Kellgren-Lawrence grade I [
17]. Digital Imaging and Communications in Medicine (DICOM) data were extracted from the picture archiving and communication system (Centricity PACS, GE Medical System Information Technologies, Milwaukee, Wisconsin). These data were subsequently imported into Mimics software (version 21.0; Materialise, Belgium) for three-dimensional (3D) reconstruction of the femoral and tibial models.
In the present study, the anterior cruciate ligament (ACL) graft was simplified to a virtual cylinder in the three-dimensional (3D) model. Based on previous research indicating that double-looped semitendinosus and gracilis grafts have an average diameter of 8 mm (range 7-9 mm) [
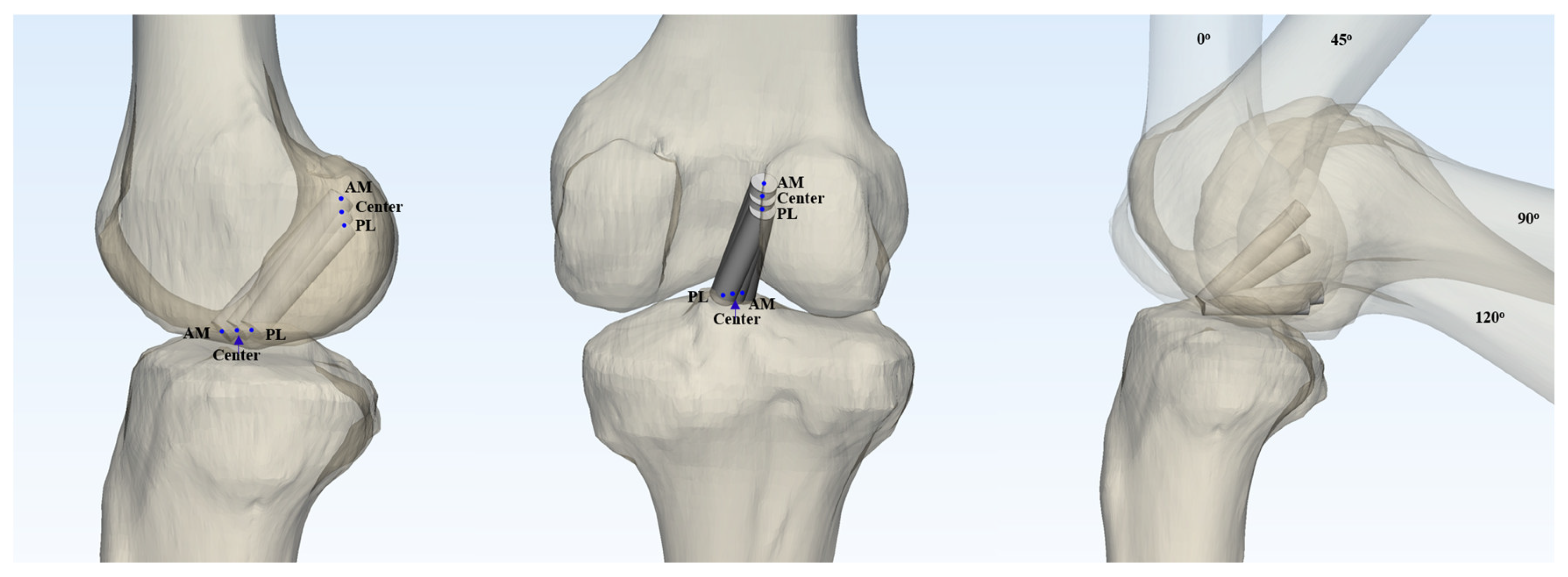
18], virtual grafts with diameters of 7 mm and 9 mm were modeled. Nine different graft configurations were investigated by varying the femoral and tibial footprint locations (anteromedial, central, and posteromedial) in all possible combinations. Taking into account the two graft diameters and four knee flexion angles (0°, 45°, 90°, and 120°), a total of 72 virtual ACL cylinders were created and analyzed (
Figure 1).
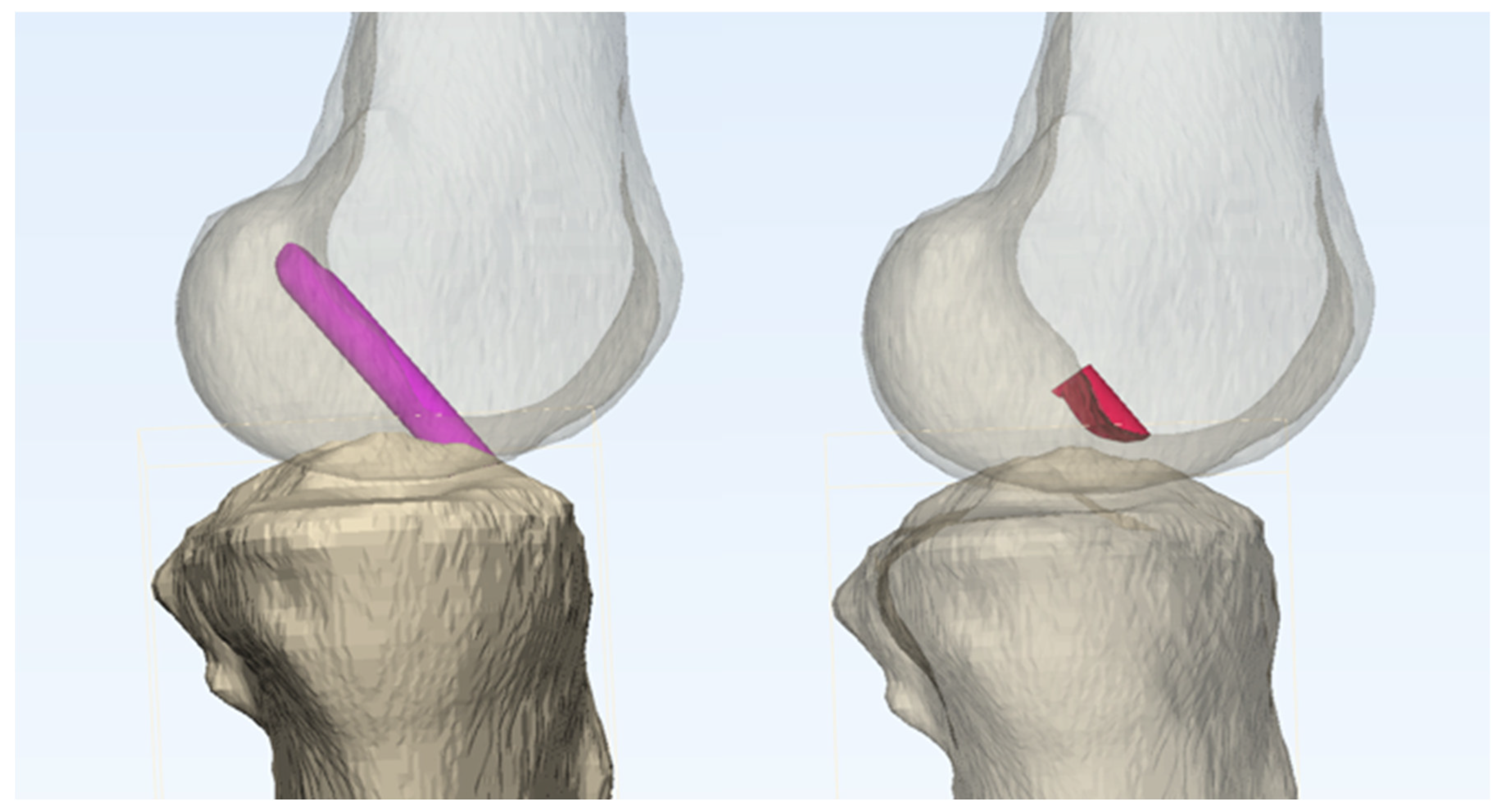
For each configuration, the impingement volume was quantified by measuring the overlap between the intercondylar notch of the femoral condyle and the virtual graft. This measurement was performed using Boolean operators in the Mimics 3D simulation program, which has been validated to provide measurement accuracy within 1 mm.[
19] (
Figure 2).
The three-dimensional reconstructed femoral model was positioned in a true lateral view, with both femoral condyles superimposed. The medial femoral condyle was virtually removed from the 3D model to allow for a direct visualization of the medial wall of the lateral femoral condyle. The neutral position of the tibia, which provided an unobstructed view of the tibial joint surface, was established [
20]. To define the centers of the ACL femoral and tibial footprints for the anteromedial (AM), central, and posterolateral (PL) bundles, we adopted the classical grid system proposed by Bernard et al. [
21]. The reference frame was defined using a rectangular coordinate system, with the line passing through the highest point of the intercondylar notch as the superior border, and the outer margins of the lateral wall of the intercondylar notch serving as the lateral and medial borders. (
Figure 3A)
2.2. Foot Print Positioning
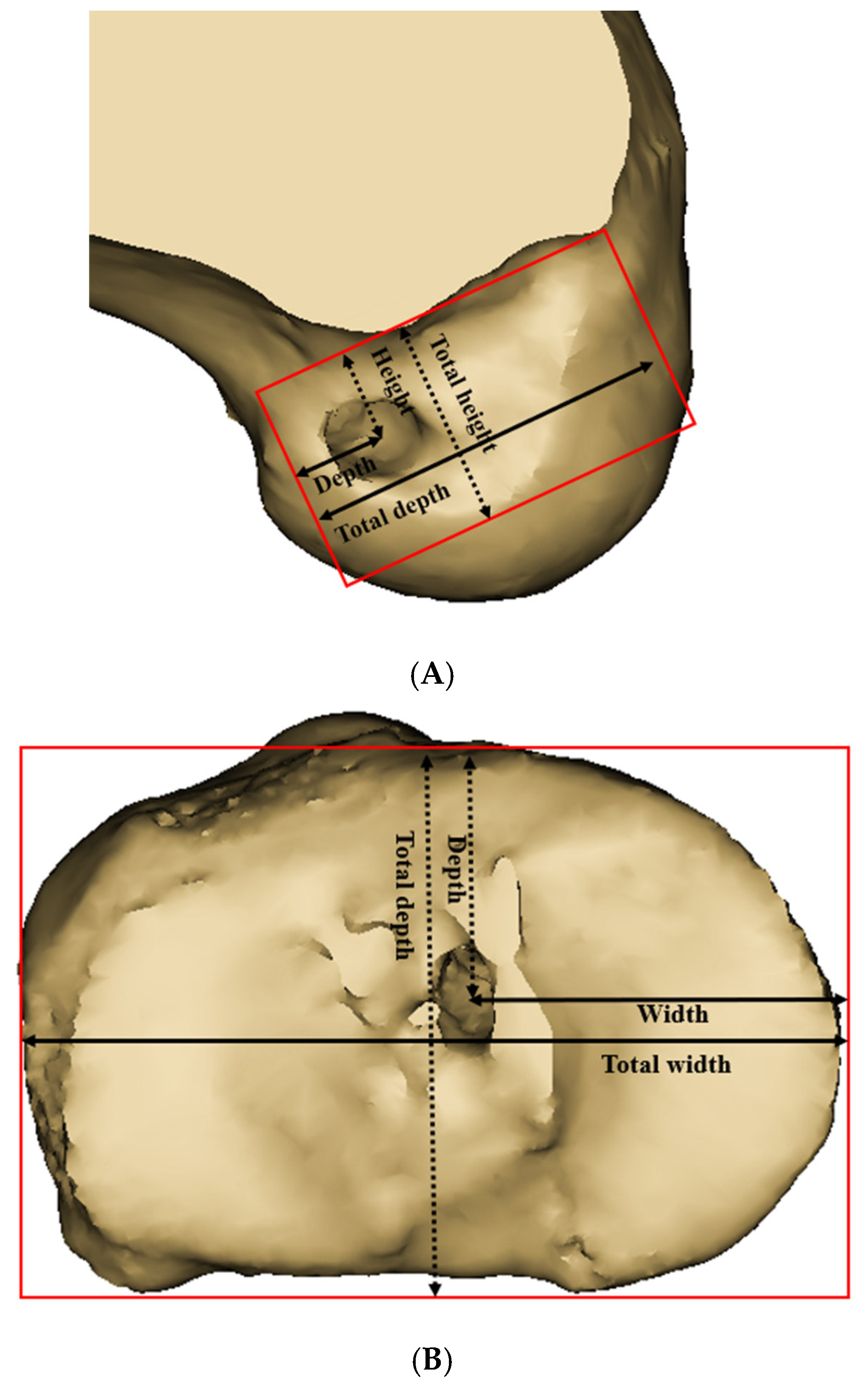
According to previous studies using 3D CT models, the femoral center of the AM bundle footprint was identified at a point located 25% of the total height, measured from the superior to inferior border of the medial wall of the lateral femoral condyle, and 33% of the total depth, measured from the anterior to posterior border [
22,
23]. The femoral center of the PL bundle footprint was identified at a point located 45.1% of the total height and 50% of the total depth, measured in the same manner.
The reference frame was established using the joint surface in the tibial neutral position with a top view, and the cortical outline was defined as the outer border. (
Figure 3B). The tibial center of the AM bundle was located at a point 35% of the total depth (measured from the anterior-to-posterior border) and 50.5% of the total width (measured from the medial-to-lateral border). The tibial center of the PM bundle was located at a point 46.4% of the total depth and 52.4% of the total width [
22,
23]. The central point was defined as the midpoint of the line connecting the AM and PL bundle footprints. In both the tibia and femur, the central point was established at the midpoint between the AM and PL bundle footprints, as defined above.
2.3. Statistical Analysis
As This study was an experimental design, and sample size calculation was not performed as nine pre-determined knee models were analyzed. Statistical analyses were conducted using SPSS Statistics version 23.0 (IBM Corp). The mean impingement volume based on cylinder diameter was compared using an independent t-test. The mean impingement volume according to knee flexion angle was compared and analyzed using a paired t-test. The main effect of tibial and femoral footprint positions on impingement volume, based on data from 72 simulations across the nine knee models, was analyzed using one-way analysis of variance (ANOVA), comparing three types of footprints (AM, central, PL) for both tibia and femur, respectively [
24]. The interaction effect between tibial and femoral footprint positions was analyzed using two-way analysis of variance (ANOVA) [
25]. The level of significance was set at P < 0.05. Partial eta squared was calculated using post hoc tests.
3. Results
Table 1 presents the impingement volumes (mean ± standard deviation) for different graft configurations across various femoral and tibial footprint locations. Analysis was conducted for both 7 mm and 9 mm graft diameters.
For the 7 mm graft diameter, the highest impingement volume was observed in the femoral anteromedial (AM) - tibial anteromedial (AM) configuration (338.3 ± 117.5 mm3), while the lowest was seen in the femoral posterolateral (PL) - tibial posterolateral (PL) configuration (26.6 ± 43.4 mm3). There was a statistically significant difference in impingement volumes across tibial footprint locations (P<0.001), with AM positions consistently showing higher impingement than central and PL positions. However, differences between femoral footprint locations were not statistically significant (P=0.174).
For the 9 mm graft diameter, impingement volumes were consistently higher compared to the 7 mm grafts. The femoral AM - tibial AM configuration again demonstrated the highest impingement volume (577.8 ± 171.3 mm3), while the femoral PL - tibial PL configuration showed the lowest (73.5 ± 85.6 mm3). Similar to the 7 mm graft results, significant differences were observed across tibial footprint locations (P<0.001), but not across femoral footprint locations (P=0.140).
Across all configurations, tibial footprint position had a greater influence on impingement volume than femoral footprint position. Moving from anteromedial to posterolateral positions on the tibia consistently resulted in significant reductions in impingement volume (P<0.001).
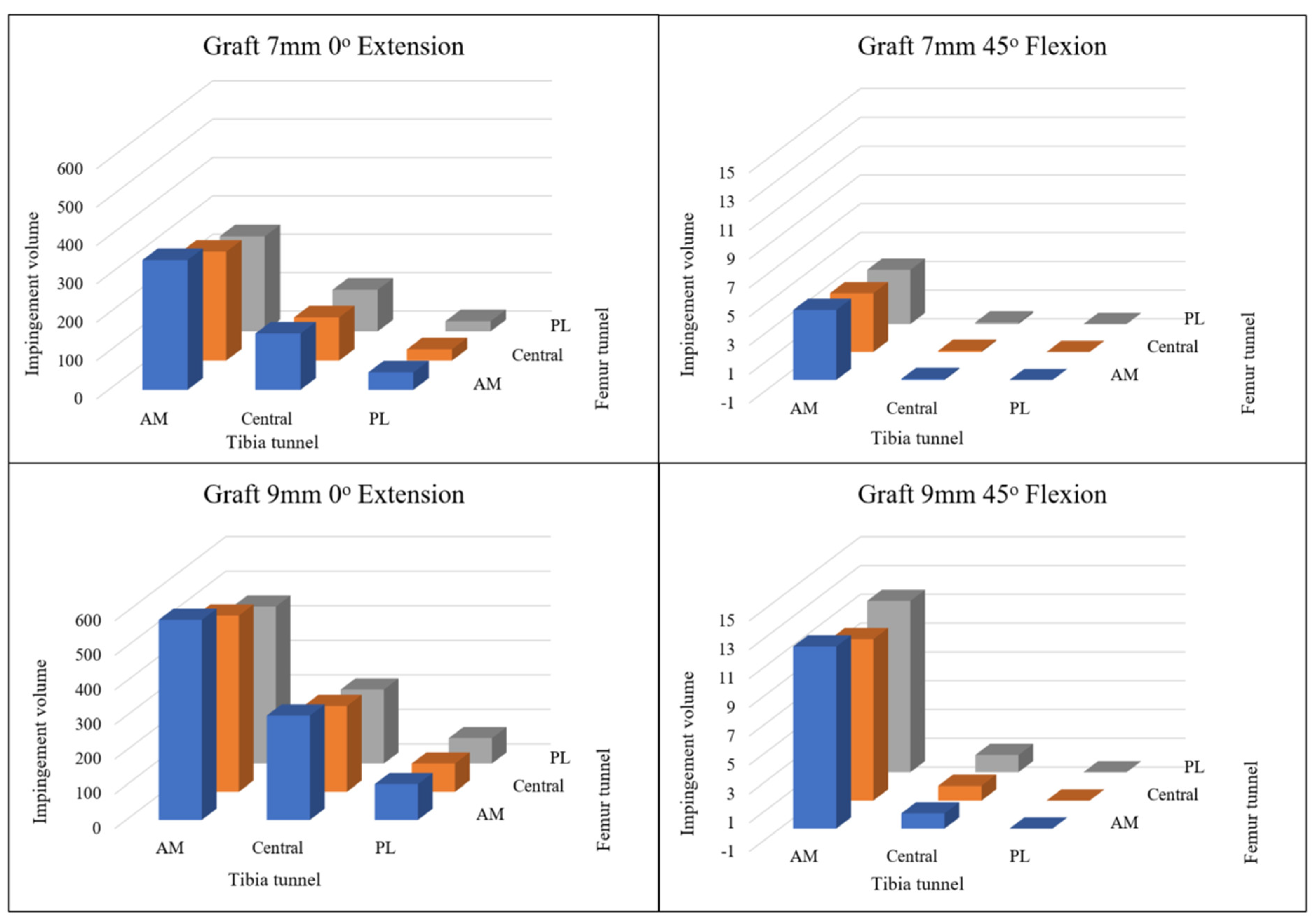
Figure 4 illustrates the impingement volumes where the virtual ACL grafts overlap with the femoral intercondylar notch, comparing two different graft diameters (7 mm and 9 mm) across three distinct footprint positions at both 0° knee extension and 45° knee flexion.
At 0° knee extension, impingement volumes were consistently higher for the 9 mm diameter grafts compared to the 7 mm grafts across all footprint positions. The anteromedial footprint position demonstrated the greatest impingement volume, followed by the central position, with the posterolateral position showing the least impingement for both graft diameters.
When the knee was flexed to 45°, a notable reduction in impingement volume was observed across all configurations compared to full extension. (P <0.01) However, the pattern of impingement volume distribution remained consistent, with the anteromedial position continuing to exhibit the highest impingement values and the posterolateral position showing the lowest values.
At 90 degrees and 120 degrees of knee flexion, impingement volumes were negligible across all graft configurations.
This visualization in
Figure 4 confirms that both graft diameter and footprint positioning significantly influence the degree of impingement, with larger diameter grafts and more anteriorly positioned footprints resulting in greater impingement volumes within the intercondylar notch.
4. Discussion
The present study provides valuable insights into the optimization of tunnel positioning in anatomical single-bundle ACL reconstruction to minimize graft impingement. Our findings demonstrate that both graft diameter and footprint location significantly influence the degree of impingement between the ACL graft and the femoral intercondylar notch.
Our results revealed a clear pattern of increasing impingement volume as footprint locations moved from posterolateral to anteromedial positions, particularly on the tibial side. Interestingly, while this trend was observed in both femoral and tibial footprints, statistical significance was only reached for variations in tibial positioning (P<0.001). This suggests that tibial tunnel placement may be more critical than femoral placement in minimizing graft impingement, a finding that has important clinical implications for surgical technique.
The impingement volumes were substantially higher at full knee extension (0°) and decreased significantly at 45° of flexion, becoming negligible at 90° and 120° of flexion. This pattern reflects the biomechanical reality that ACL grafts are most vulnerable to impingement when the knee approaches full extension, which is consistent with previous literature [
26,
27]. The dramatic reduction in impingement at greater flexion angles indicates that postoperative rehabilitation protocols emphasizing early flexion exercises may help to minimize graft stress during the critical healing period.
Graft diameter also played a significant role in impingement volume, with 9 mm grafts demonstrating consistently higher impingement volumes compared to 7 mm grafts across all footprint combinations. A. Orsi et al. reported that larger ACL grafts as well as high femoral and anterior tibia insertion site shifting would lead to increased contact area and impingement forces [
28]. The change in the amount of impingement according to the change of tibia was larger than that of femur which statistically proven. It was confirmed that the location of the tibia is a key factor relative to the femur in terms of the impingement volume. This finding suggests that surgeons should consider graft size when planning tunnel positions, potentially adopting more posterior tunnel placements for larger diameter grafts to reduce impingement risk. However, this must be balanced against the mechanical advantage of larger grafts, which may offer superior strength and stability [
29].
The femoral AM - tibial AM configuration demonstrated the highest impingement volume (577.8 ± 171.3 mm³ for 9 mm grafts), while the femoral PL - tibial PL configuration showed the lowest (73.5 ± 85.6 mm³). This nearly eight-fold difference highlights the critical importance of tunnel positioning in controlling graft impingement. These findings align with previous studies indicating that anterior placement of both femoral and tibial tunnels increases the risk of graft impingement [
28,
30,
31,
32].
It is noteworthy that while femoral footprint location variations did not reach statistical significance in affecting impingement volume, there was still a consistent trend of reduced impingement with more posterolateral positioning. This suggests that even small adjustments in femoral tunnel placement may contribute to reducing impingement risk, though the magnitude of this effect appears less substantial than that of tibial positioning. The anatomic center of the ACL femoral footprint exhibits considerable variability, with previous studies reporting variations of up to 6 mm proximally to distally and 2 mm anteriorly to posteriorly in ACL femoral origin size [
7,
9,
33]. This natural diversity in footprint anatomy suggests that the optimal tunnel position may vary depending on individual patient anatomy, graft diameter, and original ACL footprint shape and size, particularly when employing anatomic single-bundle ACL reconstruction with independent tunnel drilling techniques. The findings from our study regarding minimization of graft impingement should be carefully considered when selecting tunnel positions to optimize graft longevity.
Our study has several strengths, including the use of high-resolution 3D CT models from multiple subjects, evaluation across various knee flexion angles, and assessment of multiple footprint combinations with different graft diameters. The virtual simulation approach allowed for standardized measurements without the variability inherent in cadaveric studies or the ethical considerations of invasive testing in living subjects.
However, this study also has limitations. First, the simplified cylindrical model of the ACL graft does not fully capture the complex morphology and deformation characteristics of actual grafts. Second, our analysis focused solely on osseous impingement and did not consider soft tissue interactions or dynamic changes during functional activities. Third, while impingement volume provides a quantitative measure of potential graft-notch conflict, it does not directly translate to clinical outcomes or graft failure rates.
The clinical implications of our findings are substantial. Surgeons performing anatomical single-bundle ACL reconstruction should consider adopting more posterolateral tunnel positions, particularly on the tibial side, to minimize impingement risk. Additionally, in cases where larger diameter grafts are required, extra attention should be paid to tunnel positioning to avoid excessive impingement. Notchplasty may be considered for cases where anatomical positioning cannot avoid significant impingement, particularly with larger grafts [
34].
Future research should explore the correlation between impingement volumes measured in virtual simulations and clinical outcomes such as graft failure rates, range of motion limitations, and patient-reported outcomes. Additionally, studies comparing the impingement characteristics of different graft types (e.g., bone-patellar tendon-bone versus hamstring) and fixation methods would provide valuable information for surgical planning.
5. Conclusion
The impingement volume progressively increased as tunnel position shifted from posterolateral to anteromedial orientation in both tibial and femoral components in three-dimensional virtual simulation of anatomical ACL reconstruction. Maximum impingement occurred at full extension and with larger diameter grafts, while becoming negligible at flexion angles exceeding 90°. Strategic optimization of tunnel placement, with particular attention to tibial positioning, may significantly reduce impingement.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
References
- Li, X.; Yan, L.; Li, D.; Fan, Z.; Liu, H.; Wang, G.; et al. Failure modes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. International Orthopaedics 2023, 1–16. [Google Scholar] [CrossRef]
- Ogunleye, P.; Jäger, H.; Zimmermann, F.; Balcarek, P.; Sobau, C.; Ellermann, A.; Zimmerer, A. Patients older than 55 years regain sporting and recreational activities after arthroscopic anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy 2022, 1-9.
- Crawford, S.N.; Waterman, M.B.R.; Lubowitz, J.H. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2013, 29, 1566–71. [Google Scholar]
- Allen, C.R.; Giffin, J.R.; Harner, C.D. Revision anterior cruciate ligament reconstruction. Orthopedic Clinics of North America 2003, 34, 79–98. [Google Scholar] [CrossRef]
- Howell, S.M. Principles for placing the tibial tunnel and avoiding roof impingement during reconstruction of a torn anterior cruciate ligament. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 1998, 6 (Suppl 1), S49-55.
- Howell, S.M.; Taylor, M.A. Failure of reconstruction of the anterior cruciate ligament due to impingement by the intercondylar roof. The Journal of bone and joint surgery American volume 1993, 75, 1044–55. [Google Scholar] [CrossRef]
- Piefer, J.W.; Pflugner, T.R.; Hwang, M.D.; Lubowitz, J.H. Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2012, 28, 872–81. [Google Scholar]
- Burkart, A.; Debski, R.E.; McMahon, P.J.; Rudy, T.; Fu, F.H.; Musahl, V.; et al. Precision of ACL tunnel placement using traditional and robotic techniques. Comput Aided Surg 2001, 6, 270–8. [Google Scholar] [CrossRef]
- Kopf, S.; Musahl, V.; Tashman, S.; Szczodry, M.; Shen, W.; Fu, F.H. A systematic review of the femoral origin and tibial insertion morphology of the ACL. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 2009, 17, 213–9. [Google Scholar] [CrossRef]
- Musahl, V.; Plakseychuk, A.; VanScyoc, A.; Sasaki, T.; Debski, R.E.; McMahon, P.J.; Fu, F.H. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. The American journal of sports medicine 2005, 33, 712–8. [Google Scholar] [CrossRef]
- Van der Bracht, H.; Bellemans, J.; Victor, J.; Verhelst, L.; Page, B.; Verdonk, P. Can a tibial tunnel in ACL surgery be placed anatomically without impinging on the femoral notch? A risk factor analysis. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 2014, 22, 291–7. [Google Scholar] [CrossRef]
- Iriuchishima, T.; Tajima, G.; Ingham, S.J.; Shen, W.; Smolinski, P.; Fu, F.H. Impingement pressure in the anatomical and nonanatomical anterior cruciate ligament reconstruction: a cadaver study. The American journal of sports medicine 2010, 38, 1611–7. [Google Scholar] [CrossRef]
- Maak, T.G.; Bedi, A.; Raphael, B.S.; Citak, M.; Suero, E.M.; Wickiewicz, T.; Pearle, A.D. Effect of Femoral Socket Position on Graft Impingement After Anterior Cruciate Ligament Reconstruction. The American journal of sports medicine 2011, 39, 1018–23. [Google Scholar] [CrossRef]
- Staubli, H.U.; Rauschning, W. Tibial attachment area of the anterior cruciate ligament in the extended knee position. Anatomy and cryosections in vitro complemented by magnetic resonance arthrography in vivo. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 1994, 2, 138–46. [Google Scholar]
- Bedi, A.; Maak, T.; Musahl, V.; Citak, M.; O’Loughlin, P.F.; Choi, D.; Pearle, A.D. Effect of tibial tunnel position on stability of the knee after anterior cruciate ligament reconstruction: is the tibial tunnel position most important? The American journal of sports medicine 2011, 39, 366–73. [Google Scholar] [CrossRef]
- Hatayama, K.; Terauchi, M.; Saito, K.; Higuchi, H.; Yanagisawa, S.; Takagishi, K. The importance of tibial tunnel placement in anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2013, 29, 1072–8. [Google Scholar]
- Kellgren, J.; Lawrence, J. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases 1957, 16, 494. [Google Scholar] [CrossRef]
- Takenaga, T.; Yoshida, M.; Albers, M.; Nagai, K.; Nakamura, T.; Fu, F.H.; Onishi, K. Preoperative sonographic measurement can accurately predict quadrupled hamstring tendon graft diameter for ACL reconstruction. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 2019, 27, 797–804. [Google Scholar] [CrossRef]
- Boettner, F.; Sculco, P.K.; Lipman, J.; Saboeiro, G.; Renner, L.; Faschingbauer, M. The effect of a low radiation CT protocol on accuracy of CT guided implant migration measurement: A cadaver study. Journal of Orthopaedic Research 2016, 34, 725–8. [Google Scholar] [CrossRef]
- Moon, H.S.; Choi, C.H.; Jung, M.; Lee, D.Y.; Chang, H.; Kim, S.H. Do Rotation and Measurement Methods Affect Reliability of Anterior Cruciate Ligament Tunnel Position on 3D Reconstructed Computed Tomography? Orthopaedic journal of sports medicine 2019, 7, 2325967119885882. [Google Scholar] [CrossRef]
- Bernard, M.; Hertel, P.; Hornung, H.; Cierpinski, T. Femoral insertion of the ACL. Radiographic quadrant method. The American journal of knee surgery 1997, 10, 14–21; discussion. [Google Scholar] [PubMed]
- Tank, S.; Dutt, S.; Sehrawat, R.; Kumar, V.; Sabat, D. 3D CT evaluation of femoral and tibial tunnels in anatomic double bundle anterior cruciate ligament reconstruction. J Clin Orthop Trauma 2021, 15, 22–6. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.D.; Manaqibwala, M.I.; Brown, C.H., Jr.; Steiner, M.E. Height and Depth Guidelines for Anatomic Femoral Tunnels in Anterior Cruciate Ligament Reconstruction: A Cadaveric Study. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2016, 32, 1098–105. [Google Scholar]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Annals of cardiac anaesthesia 2019, 22, 407. [Google Scholar] [CrossRef]
- Wilcox, R. One-Way and Two-Way ANOVA: Inferences About a Robust, Heteroscedastic Measure of Effect Size. Methodology 2022, 18, 58–73. [Google Scholar] [CrossRef]
- Jackson, D.W.; Gasser, S.I. Tibial tunnel placement in ACL reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery 1994, 10, 124–31. [Google Scholar]
- Howell, M.S.M.; Clark, J.A. Tibial tunnel placement in anterior cruciate ligament reconstructions and graft impingement. Clinical Orthopaedics and Related Research (1976–2007) 1992, 283, 187–95. [Google Scholar] [CrossRef]
- Orsi, A.D.; Canavan, P.K.; Vaziri, A.; Goebel, R.; Kapasi, O.A.; Nayeb-Hashemi, H. The effects of graft size and insertion site location during anterior cruciate ligament reconstruction on intercondylar notch impingement. The Knee 2017, 24, 525–35. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Lawrence, J.T.R.; West, R.L.; Toth, A.P.; Taylor, D.C.; Garrett, W.E. Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2012, 28, 526–31. [Google Scholar]
- Iriuchishima, T.; Shirakura, K.; Fu, F.H. Graft impingement in anterior cruciate ligament reconstruction. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 2013, 21, 664–70. [Google Scholar] [CrossRef]
- van der List, J.P.; Zuiderbaan, H.A.; Nawabi, D.H.; Pearle, A.D. Impingement following anterior cruciate ligament reconstruction: comparing the direct versus indirect femoral tunnel position. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA 2017, 25, 1617–24. [Google Scholar] [CrossRef]
- Simmons, R.; Howell, S.M.; Hull, M. Effect of the angle of the femoral and tibial tunnels in the coronal plane and incremental excision of the posterior cruciate ligament on tension of an anterior cruciate ligament graft: an in vitro study. JBJS 2003, 85, 1018–29. [Google Scholar] [CrossRef]
- Forsythe, B.; Kopf, S.; Wong, A.K.; Martins, C.A.; Anderst, W.; Tashman, S.; Fu, F.H. The location of femoral and tibial tunnels in anatomic double-bundle anterior cruciate ligament reconstruction analyzed by three-dimensional computed tomography models. JBJS 2010, 92, 1418–26. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, Y.; Thorhauer, E.; Sasaki, Y.; Smolinski, P.; Tashman, S.; Fu, F.H. Quantitative in situ analysis of the anterior cruciate ligament: length, midsubstance cross-sectional area, and insertion site areas. The American journal of sports medicine 2016, 44, 118–25. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).