1. Introduction
Axillary lymph node dissection (ALND) in breast cancer (BC) is becoming less common; however, it is still being necessary in certain cases like high axillary volume. When performed, a traditional approach is often followed in surgical technique and postoperative care. Despite evolving practices, certain aspects of this procedure continue to be handled in a conventional manner in most hospitals.
One of the most prevalent postoperative complications following ALND is seroma formation, reported in 15-90% of cases, with 0- 26% of presenting as a symptomatic seroma, after lymphadenectomy or sentinel lymph node dissection [
1,
2,
3,
4] Seroma results from an accumulation of serous fluid in the dead space created by tissue removal, leading to patient discomfort, increased risk of infection, and potential delays in adjuvant therapy. Seroma volume is one of the main factors that causes discomfort in patients undertaking axillary surgery for BC. However, many studies describe total seroma volume by measuring the accumulated milliliters, regardless of symptoms. However, the volume for itself should be considered less relevant than the symptoms and expressed by the patient discomfort. Patient symptoms should be the signal to indicate further clinical intervention.
With the aim to reduce seroma formation, surgical drains have traditionally been used to evacuate fluid and promote tissue adherence. However, in recent years, new approaches have challenged this standard practice, including early drain removal, no-drain techniques and the use of fibrin sealants or axillary padding.
Other critical aspects are the possibility of same day discharge which has been widely integrated into many surgical specialties but is less common after ALND. Similarly, minimal invasive surgery, although adopted in many fields years ago, have not yet been fully consolidated in BC surgery despite being described for some time.
In this context, quality of life (QoL), is a basic outcome to consider after BC surgery and especially after ALDN. ALDN impacts in arm function, pain and lymphedema, factors closely related to QoL. The instruments used to evaluate QoL after BC are: EORTC QLQ-C30, QLQ-BR23, FACT-B, and SF-36, these instruments asses physical, emotional and psychosocial domains. Interestingly, while ALND clearly increases arm-related symptoms compared to sentinel node biopsy, its overall impact on global QoL scores appears limited, likely due to the overlapping effects of systemic therapies and breast surgery. As surgical techniques evolve, improving QoL must remain a central objective in the management of BC patients undergoing axillary surgery.
This review sought to discuss the impact of QoL after ALDN, use of sealants or drains, same day discharge and minimal invasive surgery in BC.
2. Materials and Methods
We conducted a systematic keyword-based literature search across Scopus, PubMed, Web of Science, and Google Scholar, identifying studies that included the terms “axillary lymphadenectomy” OR “axillary dissection” ALND (“breast cancer” OR “surgical management” OR “oncological outcomes” OR “ambulatory surgery” OR “no drain” OR “sealant-free”).
To ensure a broad and balanced perspective, we included peer-reviewed articles, systematic reviews, meta-analyses, and clinical guidelines published in English. Additionally, we employed a snowballing technique, reviewing the reference lists of selected articles to identify further relevant studies. Each article was independently assessed by a member of the research team for relevance, with a focus on studies discussing the evolution of axillary surgery, de-escalation strategies, ambulatory lymphadenectomy, the absence of drains or sealants, and their impact on oncological safety and patient outcomes. Special attention was given to studies evaluating postoperative recovery, complications, and QoL following ALDN.
3. Ambulatory Surgery and Early Discharge Post ALND
Traditionally, all patients undergoing BC surgery required hospital admission. However, same-day discharge has progressively become more common, especially in procedures involving the breast such as lumpectomies and mastectomies. Nevertheless, some surgeries still have high hospitalization rates, such as immediate breast reconstructions and ALDN. Following this trend, a patient undergoing a mastectomy with ALDN is most likely to suffer an extended hospitalization. There is evidence in literature that same-day surgery after mastectomy and axillary clearance is feasible and does not increase postoperative complications. The authors emphasized that proper patient selection and education significantly reduce hospital stays and associated costs [
5].
One of the main barriers to ambulatory discharge in BC surgery is patient belief, with studies reporting up to 20% of patients refusing early discharge [
6]. This resistance could appear because of the lack of information given by the medical team, feelings of insecurity towards the diagnose or the procedure, or fear of managing postoperative care at home. Major complications within the first few hours’ post-surgery are luckily uncommon, being bleeding the most frequent serious complication requiring reintervention [
7]. Fear of such complications might lead some patients to request hospital admission instead of opting for ambulatory management.
Other common reasons for prolonged hospitalization include postoperative nausea and pain, although pain management has significantly improved with the use of regional anesthesia techniques such as the Breast Interfascial Regional Block (BRILMA) or Pectoral Blocks (PECS) [
8]. Effective multimodal pain control is instrumental for enabling same-day discharge in this scenario.
For a secure and efficient early discharge, certain criteria must be met, including: adequate home support (a caregiver for the first night), proximity to a hospital (living within 100 km or 1 hour from a medical facility) or access to a mobile phone for emergency communication [
6]. These measures are essential to ensure early detection and prompt management of potential complications.
3.1. Benefits of Ambulatory Surgery
Level I and II axillary dissection can be safely performed as an outpatient procedure with [
9] or without drains [
10]. Ambulatory surgery offers numerous advantages including: increased patient autonomy and QoL, lower healthcare costs [
11], improved physical recovery -including better shoulder mobility and reduced postoperative pain at three months [
12] and potentially lower seroma rates (18% vs. 34%,
p < 0.001), without increasing other complications [
13].
3.2. The Role of ERAS Protocols in Facilitating Early Discharge
Enhanced Recovery After Surgery (ERAS) protocols have played a pivotal role in enabling safe and effective ambulatory surgery for breast cancer (BC) patients. These protocols include evidence-based perioperative strategies such as minimized preoperative fasting, multimodal pain management with opioid reduction, optimized fluid management and early mobilization [
8,
14]. Regional anesthesia techniques such as PECS blocks and serratus plane blocks, combined with light general anesthesia are also included and help with further enhanced recovery.
3.3. Early Discharge Feasibility and Alternatives to Drains
Most studies evaluating ambulatory ALDN have involved patients discharged with a drain [
15,
16]. While drains are generally safe and effective, they can cause discomfort and challenges in daily activities and self-care.
A randomized clinical study by Horgan et al. compared early discharge on postoperative day 3 with a drain in situ versus standard 7-day hospitalization. Their findings revealed no significant differences in seroma formation, wound infection, or psychological outcomes. Moreover, patient satisfaction was high in the early discharge group, and this approach led to substantial healthcare cost savings [
16].
Given that symptomatic seroma is a common side effect of ALDN, some researchers have explored drain-free surgical techniques. The "Padding" technique, which reduces the axillary dead space without using drains, has shown promising results [
6,
17]. Classe et al. further evaluated this technique in a randomized trial that included an ambulatory group, achieving an ambulatory surgery rate of 84.5% with symptomatic seroma rates of 22.2%, comparable to previous studies (18%) [
6].
Today, less invasive surgical techniques continue to emerge, aiming to further minimize symptomatic seroma formation and improve recovery; this aspect will be discussed in later sections of the present review.
3.4. Economic Impact of Ambulatory Surgery
The economic impact of ambulatory surgery in BC treatment is significant, as reducing hospital stays leads to substantial cost savings, without compromising patient outcomes. In the United Kingdom, a study by Holcombe et al, estimated annual savings of £187,500, based on the assumption that a hospital bed costs £250 per night and that approximately 75% of patients could benefit from early discharge [
13]. Extending this analysis, a broader economic evaluation suggested that reducing hospital stays by 5–6 days could lower costs by approximately £1000 per patient, generating substantial savings for the National Health Service [
12]. Similarly, in Europe, a study conducted in the Netherlands identified postoperative hospitalization as the most significant expense after surgical BC treatment. By implementing early discharge protocols, hospitals were able to reduce costs by
$1320 per patient, which translated to a 30% decrease in total postoperative care costs [
11]. These findings highlight the financial viability of shifting towards ambulatory care models, reinforcing the need for further adoption of early discharge strategies in BC surgery.
4. Omission of Surgical Drains in Lymphadenectomy
The use of drains in ALDN for breast cancer remains a widely debated topic in surgical practice. The primary goal of drains is to control postoperative seroma formation, and avoid punctures in symptomatic seromas. Seroma formation rates vary between 3% and 85% across studies, with prolonged drainage potentially delaying adjuvant therapies and increasing infection risks [
18].
4.1. Types of Drainage Protocols
The most commonly used drains in ALND are closed suction drains, which function by continuously evacuating fluid from the surgical site through a vacuum-assisted mechanism. Volume-controlled drainage protocols, where the drain is removed once daily output falls below 30-50 mL, has been the standard practice [
19], this may take up to 5 to 15 days. Comparatively, short-term drainage protocols advocate for removal within 24 to 48 hours postoperatively, regardless of output. One of the main issues with prolonged drainage placement is inflammation. In the presence of an inflammatory exudate, the drain itself may exacerbate the inflammatory response, potentially increasing seroma formation rather than preventing it. [
20]. In addition, prolonged drainage can cause patient discomfort and increase the risk of an infection.
4.2. Use of Drains
Omission of drains is not a new trend; in fact, it has been practiced since 1988. Cameron et al. (1988) [
21], Somers (1992) et al [
22], Zavotsky et al. (1998) [
23], and Jain et al. (2004) [
20] compared volume-controlled drainage with no drainage and reported a higher incidence of clinically significant seroma formation in patients who did not receive drains, this fact seems reasonable and logical, given that drainage promotes the elimination of the formed seroma, preventing its accumulation. Authors in 1990 reported the results of a study on 259 consecutive patients who underwent ALDN without drainage or sealants, performed as an outpatient procedure. In their series, symptomatic seroma occurred in only 4.2% of cases (Level I and II). These findings, dating back decades, demonstrate that symptomatic seroma without drainage has a low incidence and is entirely manageable [
10].
Despite the increased seroma incidence, these studies found no significant differences in wound infection rates between groups, concluding that drains may reduce seroma occurrence but they do not necessarily have an impact in infection risk. Additionally, avoiding drains was associated with a shorter hospital stay [
24,
25], an improved patient comfort and a more efficient healthcare resource utilization.
Avoiding the placement of a drain has also shown to improve pain control [
23], therefore this could help in achieving a better QoL and higher autonomy after ALDN.
Studies have shown that closed suction drainage can help prevent excessive seroma accumulation. A meta-analysis comparing volume-controlled drainage (<30–50 mL/24 h) to no or short-term drainage found that seroma formation was more common in patients without drains (RR 0.44, 95% CI 0.24–0.80). However, while drains reduced the need for repeated aspirations, their presence was associated with longer hospital stays [
19] and less infection [
26,
27]. A systematic review published in 2010 also found a reduction rate of seroma in drain group [
25] but, the previous systematic review and the meta-analysis include studies conducted before 2004, so its findings should be reassessed in light of 20 years of advancements in surgical techniques and technology. Similar results about mean seroma volume are found by Soon et al, they described a higher number of volumes in the no-drain group (856.7 mL) compared to the drain group (538.8 mL) but differences were not checked to be statistically significative. In all cases, the reported volumes in patients without drainage refer to seromas that required drainage due to being symptomatic. However, there are few studies that compare axillary volume in patients without drainage using ultrasound [
28], showing a minimal volume after 15 days [
29].
Despite these findings, some research suggests that early removal of drains (within 24–48 hours) does not significantly increase seroma rates and may facilitate faster recovery [
18]. Furthermore, randomized trials have demonstrated that using additional techniques, such as axillary padding or flap fixation, can significantly reduce seroma formation, making drains unnecessary in select case [
17,
30,
31]. This padding or flap fixation consist of only 3 stitches between the skin subcutaneous flap and the pectoral and serratus fascia with the objective or reduce the dead space.
4.3. Alternative Techniques to Reduce Seroma
Several authors studied the seroma after BC surgery. In 2009, Droeser and colleagues did not find a reduction in symptomatic seroma between drain and no-drain groups [
19]. They also include a group with sealant application and they observed a significant reduction in seroma formation associated with sealant use in patients who underwent mastectomy and ALND (P = 0.012). Other authors found similar results in seroma production using a new technique of suturing flaps without drains [
32].
However, previously quoted studies are outdated and since then, the electro thermal bipolar vessel sealing system has emerged. These devices have demonstrated a significant reduction in seroma formation [
33,
34]. The surgical technique and technological advancements in surgical instruments also contribute to the progressive decrease in seroma formation, as they allow for better tissue control and preservation. Additionally, there has been an improvement in techniques with more conservative lymphadenectomies, now limited to levels I and II, in contrast to the early 2000s when level III was also included.
To date, all these improvements contribute to a significant reduction in seroma formation compared to previous years. As a result, studies advocating no-drain approaches as a standard practice have started to emerge [
28].
4.4. Recent Scientific Evidence
The Cochrane review, published in 2013, provides a comprehensive analysis of the use of drains in ALDN. However, it includes previously mentioned studies, being the most recent Soon et al, published in 2005. The conclusion of this review is that the use of a drain does not eliminate the risk of developing seroma or the need for subsequent aspiration. While drains were associated with a lower incidence of seroma formation (OR 0.46, 95% CI 0.23–0.91, P = 0.03), they did not significantly reduce the total volume of seroma aspirated. Additionally, the presence of a drain did not impact infection rates (P = 0.14), but it was associated with a longer hospital stay (mean increase of 1.47 days, P = 0.0003). These findings suggest that while drains can help manage seroma formation, they do not fully prevent it, and alternative strategies should be considered in modern surgical practice [
35].
Two recent studies have been found that include patients without axillary drain: the GALA trial [
28] and the
REDHEMOPACH trial [
36]. We will have to wait for the final results of GALA study to obtain more up-to-date conclusions.
Despite the lack of new comparative studies regarding the non-use of drains after ALDN, their routine placement remains common. This is primarily due to the fear of symptomatic seroma, which may require aspiration, or simply because it has traditionally been the standard practice. After an ALDN, surgeons must balance the comfort of not using drains for the patient against the possibility of needing aspiration in cases of symptomatic seroma. This decision ultimately leads to variations in clinical practice depending on the center and the treating surgeon.
In cases where drains are not placed, proper postoperative monitoring is essential, and symptoms should be managed effectively with analgesics if needed, aiming to reduce symptomatic seroma caused by pain. If aspiration is required, it should always be performed under sterile conditions to prevent secondary infections.
5. Use of Tissue Sealants in ALDN
With the aim of reducing seroma after ALDN, some authors have advocated the use of different tissue sealants either to close the axillary space or to decrease seroma formation. Depending of the type of sealant, they can either seal lymphatic vessels or facilitate tissue adhesion.
We find in literature studies assessing various sealants, being either used alone or in conjunction with drains. Some authors suggest that combining drains with sealants may not offer the same advantages as using sealants alone, as drains could hinder the stabilization of the sealant in the lymphadenectomy bed and disrupt tissue adherence.
The main disadvantage of using sealants is the cost, but it should be evaluated whether their use reduces other types of costs, such hospitalization time, additional medical consulting or avoiding the use of drains.
5.1. Types of Sealants
5.1.1. Fibrin-Based Sealants
Fibrin glue is one of the most commonly studied biological sealants, composed of fibrinogen and thrombin, previously of bovine but now human origin. Fibrin sealants function as biological hemostatic agents, interacting with tissues during surgery to support hemostasis, stimulate fibroblast growth and proliferation, and facilitate the closure of lymphatic vessels. These products are easy to apply with rapid polymerization (60 seconds) [
31]. Some examples of this fibrin sealants are: Tisseel ®, Evicel ®, Artiss ®, Tissucol and others. This type of sealant is used in many surgical scenarios [
37].
There are different randomized clinical studies that assess the outcomes after the application of fibrin sealant in patients undergoing either mastectomy or breast-conserving surgery. These studies conclude that the total volume of seroma production is lower with fibrin sealant [
38,
39,
40]. However, there are studies that do not show these differences in volumes but can be criticized for the small number of patients (30-40 patients) [
40,
41,
42] or for their methodology [
20].
Conversano et al, associate fibrin sealant to the closure process "padding22" previously described, and a reduction in hospital stay duration was observed (p<0.001), achieving a mean cost reduction per patient of 2,619 euros [
31]. This study did not show differences in seroma formation or the number of punctures required. However, it has been previously described those studies, including more recent ones, do find volume differences in favor of the sealant group [
43]. Nevertheless, this latter study evaluates the placement of drainage plus sealant, unlike the previous one where no drains were used. Therefore, drawing clear conclusions regarding the benefit of the sealant in patients with or without drainage may present a significant methodological bias.
In the most recent meta-analysis published by Chang et al. on the use of these sealants, some studies included patients who underwent mastectomy, which is already known to typically present with larger seroma volumes. In most of them, patients in both groups had drains, and it was described that in the fibrin sealant group, there was a lower seroma volume and shorter drainage duration, while the results regarding the incidence of symptomatic seroma, length of hospital stay, or infection remained the same [
44].
5.1.2. Polyethylene Glycol (PEG)-Based Sealants
Polyethylene glycol (PEG)-coated patches, such as Hemopatch™, have been introduced as an alternative to drains. Some studies have reported increased seroma formation but a reduction in emergency visits and outpatient aspirations in the intervention group. This discrepancy may be attributed to complications associated with drains rather than the effect of Hemopatch itself, as described in their findings [
36].
5.1.3. Cyanoacrylate-Based Sealants
Cyanoacrylate adhesives, such as Glubran 2 ®, are synthetic adhesives with rapid polymerization properties, creating a strong bond over tissue.
For this reason, cyanoacrylate has been used in various surgical procedures as an adhesive and for sealing structures. Among its approved uses is its application after inguinal or ALDN to reduce seroma formation.
There are studies suggesting that the use of cyanoacrylate sealants reduces seroma formation. However, most of these studies include patients undergoing mastectomy, which involves a larger dissection surface, and report a decrease in seroma volume and drainage duration [
45]. Conversely, other studies have not found significant differences in seroma volumes in patients with both drainage and sealant application [
42]. However, these studies often have a small sample size and methodological limitations that may influence their outcomes [
46,
47]. A recent study with a more robust methodological design also failed to demonstrate differences in seroma volumes following sealant application [
46].
There are also studies that have found a higher seroma volume after the application of Glubran 2[
48].
In general, these studies include both patients undergoing breast-conserving surgery and mastectomy, all of whom receive drainage. To directly assess whether the sealant is associated with a reduction in seroma volume, studies with stricter selection criteria will be necessary to evaluate its effectiveness independently in mastectomy or lymphadenectomy. Additionally, it will be essential to analyze studies without drainage to determine whether its presence influences the sealant's effectiveness. For this, we must wait for the results of the GALA study [
28].
5.1.4. Thrombin and Fibrinogen Combipatches
TachoSil ®, a combination patch containing thrombin and fibrinogen, has been evaluated for its lymphostatic properties.
Studies comparing TachoSil with standard drainage techniques have yielded mixed results. While some research suggests that TachoSil may help reduce hospital stay duration, it does not appear to significantly prevent seroma formation or decrease the volume of drained lymph fluid [
49,
50].
Table 1.
Comparative Outcomes: Sealants vs Drainage.
Table 1.
Comparative Outcomes: Sealants vs Drainage.
| Outcome |
Drainage |
Sealants |
| Seroma formation |
Common |
Variable (higher in some studies, lower in others) |
| Hospital Stay |
Prolonged |
Reduced in some sealant groups |
| Need for postoperative aspirations |
Frequent |
Reduced in some sealant groups |
| Pain and discomfort |
Higher due to drains |
Lower in most sealant groups |
| Cost-effectiveness |
Increased outpatients’ visits |
Potential cost savings |
6. Minimally Invasive and Endoscopic Surgical Approaches
Throughout these years, ALDN has been a key component in the staging of BC, with the majority of cases resulting in negative lymphadenectomies. After 2010, ALDN shifted from a staging procedure to a curative treatment, no longer performed in patients with luminal tumors and T1 or T2 stage. Today, axillary lymphadenectomy is only indicated in cases of high axillary burden, with more than three positive axillary lymph nodes, or in the context of persistent node positivity after neoadjuvant therapy. However, this indication remains a topic of ongoing debate in the current clinical landscape.
The conventional and classic technique for performing an ALDN is an open surgery. However, minimally invasive or endoscopic surgery for performing endoscopic lymphadenectomies dates back to the 1990s [
51]. These techniques emerged as alternatives to open surgery with the goal of reducing morbidity without compromising oncological efficacy. Most of the published studies on this topic date back to before 2010 and include a small number of cases.
There are more recent meta-analyses, published in 2020, that demonstrate no significant differences between the endoscopic or called MALND (Mastoscopic Axillary Lymph Node Dissection) and conventional or called CALND (Conventional Axillary Lymph Node Dissection) groups in terms of the number of lymph nodes removed, tumor recurrence rate, axillary drainage, postoperative hospitalization time, and tumor size [
52] but most of the studies included are performed before 2017, therefore, the evidence of axillary endoscopic lymphadenectomy for BC should be re-evaluated in light of the new current surgical indications and technology.
6.1. Endoscopic Axillary Lymphadenectomy
In the early stages of endoscopic lymphadenectomy, some authors described the technique by combining lymphadenectomy with liposuction to facilitate axillary dissection [
53,
54]. However, liposuction presented several drawbacks, such as tissue fragmentation, poor preservation of lymph nodes, and can increase vascular trauma. For this reason, authors like Kamprath et al [
55] and Tagaya et al [
51] later described the technique without added liposuction, correctly identifying the vascular and nervous bundles. These latter authors (2002) achieved an adequate number of extracted lymph nodes and a lower incidence of neuromuscular complications.
Recently, in 2023, studies have emerged regarding the endoscopic sentinel lymph node detection, highlighting some benefits such as a lower seroma rate and improved aesthetic outcomes, although at the expense of a longer surgical time [
56,
57].
Several comparative studies have analyzed the benefits of endoscopic axillary lymphadenectomy in relation to the conventional technique. The systematic review conducted by Aponte-Rueda et al, which examines 12 studies with case series ranging from 1997 to 2007, describes a longer operative time in endoscopic surgery, with no differences in the quality of lymph node extraction (>10 nodes) and a low complication rate [
58].
As we previously mentioned, the most recent meta-analysis published by Xiong et al. found that endoscopic surgery is associated with less intraoperative bleeding, shorter drainage duration, and a lower incidence of postoperative complications, although with longer surgical time [
52].
De Wilde et al. (2003) and Chengyu Luo, confirmed that endoscopic lymphadenectomy does not compromise the quality of axillary staging and reduces the incidence of lymphedema and sensory alterations in the arm[
59,
60] but better aesthetic appearance [
60].
Seroma has also been found different between the two types of approach being lower in endoscopic compared to conventional (10 vs 45%) [
55] and also in endoscopic BSGC [
57].
Surgical time, described as longer than classical surgery can be reduced after experience, Kamprath et al reported that the first 16 patients had significantly longer operative times, but after 17 cases, surgical time was reduced, suggesting that experience and training play a crucial role [
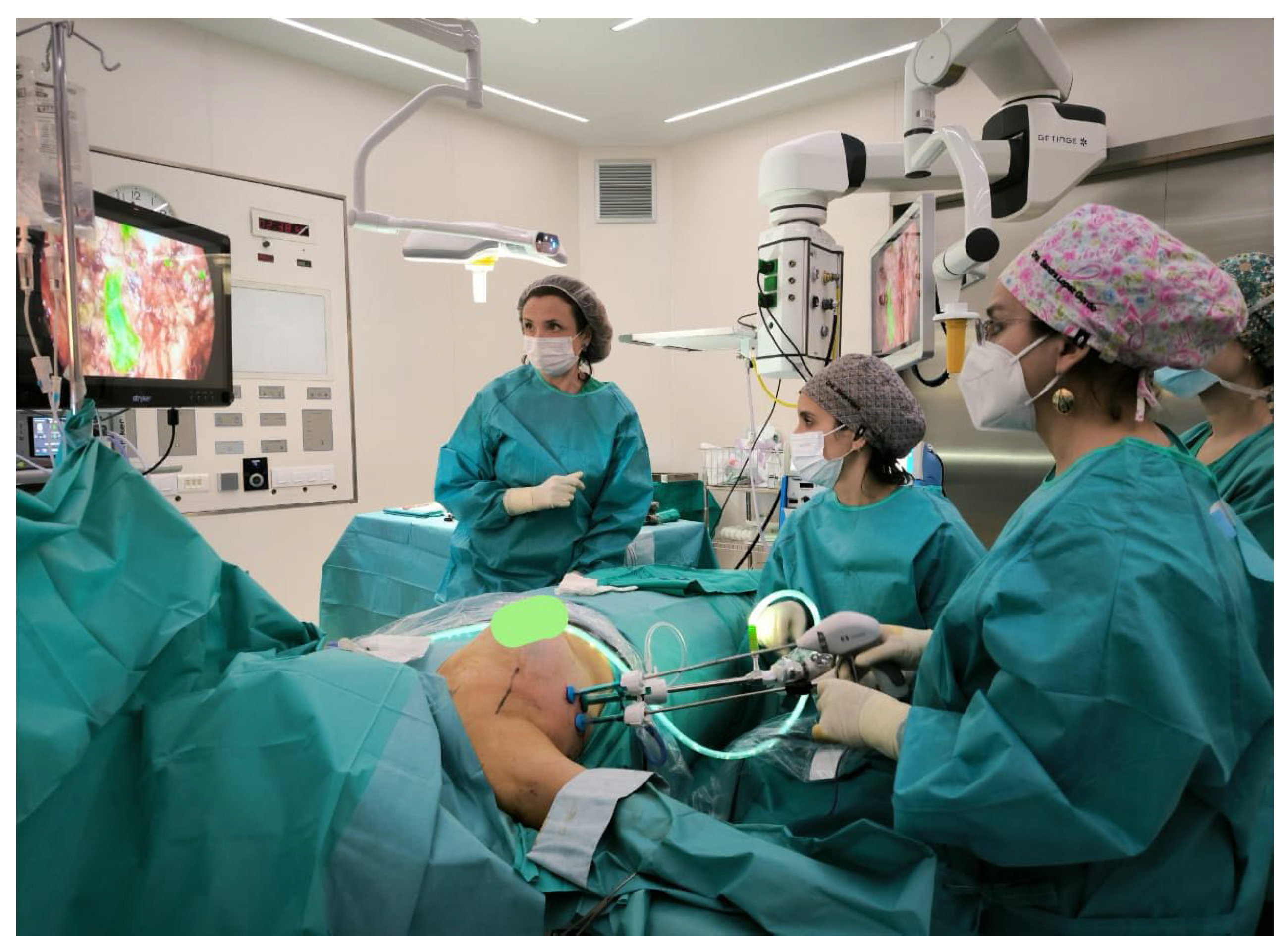
55]. It is suggested that with adequate training, a surgeon may become proficient in this technique after performing a limited number of cases. Now the endoscopic technique has been performed and modify by the new technological advancements (
Figure 1). The injection of the colorant indocyanine green in the areola, helps to identify all the lymph nodes from the territory of the mammary gland. This method it has being used for lymph node detection [
61] but also in endoscopic ALDN can facilitate the identification of all the axillary lymph nodes easily (
Figure 1).
In terms of oncological outcomes, the previously described studies reported an axillary recurrence rate of 0.5%, with no significant differences between groups regarding local recurrence, either in the axilla or the breast. Regarding the number of lymph nodes, all studies demonstrated the removal of more than 10 lymph nodes [
58,
62].
6.2. Robotics and the New Frontier of Axillary Surgery
To date, robot-assisted ALDN for the treatment of BC is not widely approved and remains limited to research scenarios. In regions such as Europe and Asia, studies suggest that robotic surgery for mastectomy may offer advantages, including smaller incisions and improved aesthetic outcomes. However, is still being evaluated in clinical trials.
More recently, robotics has revolutionized minimally invasive surgery, offering additional benefits in precision and ergonomics. Studies on the da Vinci system have shown that robot-assisted ALDN can be a safe and effective procedure, reducing surgical trauma while enhancing cosmetic results. Despite its potential, further clinical validation is required before robotic axillary lymphadenectomy becomes a standard practice in BC treatment.
Only a few studies have been found on robot-assisted ALDN, as it must be performed under clinical trials and in centers with prior experience in robotic breast surgery, as previously mentioned [
63,
64]. These studies have been conducted mainly in Korea and China where the experience by robot surgery is higher.
Both studies were associated with mastectomy, but Chen et al. also incorporated axillary lipolysis during the same surgical procedure.
The rate of infection, fat necrosis, and lymphedema (6.67% vs. 26.67%), was lower with the robotic technique according to Chen et al, with no significant differences in other parameters such as bleeding, surgical time, or recurrence. A later study performed in Korea [
63], also found no differences between the two techniques in terms of perioperative outcomes, except for smaller incision sizes in the robotic approach. The study further highlights that since robotic surgery may require a longer operative time, technical training is essential to develop adequate surgical skills.
Regarding robot-assisted endoscopic lymphadenectomy, we are still in the early stages of the technique. In fact, the introduction of the Da Vinci Single Port system may add an advanced technology respect the classical one in axillary dissection, due to the limited space in the cavity. However, due to its high cost, this single port robot is currently available in a limited number of centers.
7. Breast Cancer Axillary Lymphadenectomy and Quality of Life
7.1. Introduction
In developed countries, most breast cancer patients survive for long periods of time, with an overall survival at five years of 91.7% (SEER 21). Surgery remains the only curative treatment for breast cancer. Nevertheless, the improvement in survival is mainly due to the development of systemic treatments and the early diagnosis of cancer by screening programs. In this context, surgeons have adjusted the aggressiveness of surgery to reduce the post-surgical sequelae. This means that new objectives are becoming increasingly important, such as the assessment of quality of life, health status and cosmetic impact in long-term survivors. Patient-reported Outcomes (PROs) help to reduce the gap between the information provided by clinicians and the patient's own experience, leading to better communication and improved information on the care provided to patients. In addition, they allow the selection of treatments that provide the most benefit to patients, not only in terms of survival or reduction of recurrence, but also by including valuable patient choice, which clinicians may not fully perceived. PROs are defined as any information about the state of the patient's health condition that comes directly from the patient (or in some cases from the patient's caregiver), without interpretation of the response by healthcare personnel or any other person[
65].
7.2. Conservative Surgery of the Axilla
The ACOSOG Z0011 [
66,
67] and AMAROS [
68] studies have produced a paradigm shift in axillary surgery in breast cancer patients: the possibility of avoiding axillary lymph nodes dissection/lymphadenectomy (ALND) in a specific group of patients with initial axillary nodes involvement. Omitting lymphadenectomy brings benefits to patients, especially by reducing the morbidity associated with the surgical technique, mainly lymphedema.
In recent years the ACOSOG Z1071 [
69], SENTINA [
70] and TSN FNAC [
71] trials have observed the possibility of avoiding lymphadenectomy in cN1 patients who treat with primary systemic treatment (PST) presented negativization of the initial axillary involvement. In order to assess the effect of PTS on axillary disease, the MD Anderson group [
72] designed the Targeted axillary dissection (TAD), that provides the possibility of evaluating the pathological response of axillary disease after PST, with an acceptable false negative rate (FNR) (<10%). TAD includes resection of the affected node and resection of 3 or more sentinel nodes.
We suppose that TAD and Sentinel node biopsy (SNB) reduces the impact of surgery on patients with breast cancer in breast cancer patients, but what the real impact does this de-escalation have on patients' quality of life?
7.3. Quality of Life and Breast Cancer Surgery
When we analyze the quality of life (QoL) in breast cancer patients, we must consider that surgical treatment usually includes two associated aims, local control of the cancer and axillary staging. The QoL of surgical breast cancer patients is influenced by the surgery performed on the breast and on the axilla. In addition, this QoL is also influenced by radiotherapy and associated systemic treatment.
Conservative surgery seems to have a lower overall impact on patients’ QoL [
73,
74]. Although recent studies show that reconstruction after mastectomy can offer a comparable benefit in some dimensions such as satisfaction with the breasts and physical well-being [
75]. The type of surgery performed on the breast also has a negative effect on the ipsilateral arm symptoms, Boechmer observed 5 times more arm symptomatology in patients with mastectomy, with or without reconstruction, in the regression model with respect to conservative surgery [
76], Laws, observed greater arm symptomatology in patients with mastectomy + reconstruction, especially in autologous reconstruction [
77], Enien, notes greater arm symptomatology in patients with modified radical mastectomy than in patients with conservative surgery [
78]. The IDEAL study also observed greater arm symptomatology in patients with mastectomy, independent effect of surgery adjusted for radiotherapy [
79]. However, in the UK START trial, about the effect of radiotherapy on the quality of life of surgical patients, observed that patients with conservative surgery had a worse quality of life than patients with mastectomy[
80]. Although it concluded that the most influential factors in these patients were chemotherapy and the age of the patients.
Regarding axillary surgery, the situation is complicated, since in pN1 patients in whom lymphadenectomy is obviated, axillary radiotherapy (RT) is administered with therapeutic intent. We know that RT itself has an impact on patients' quality of life and on arm-related symptoms. The main studies assessing the effect of ALND in breast cancer patients focus on the effect of this technique vs. SNB (
Table 2). Most of them do not use specific tools/questionnaires to assess the morbidity of the affected arm, and use subscales of general questionnaires such as the QLQ-BR23 that include 2-3 specific questions about arm involvement.
Barranger, observed greater symptomatology in the ipsilateral arm of patients with ALND but found no significant changes in the quality of life of patients (measured by QLQ-c30 and QLQ-BR23) and no significant differences in difficulties at work and/or with daily activities [
81]. The 10-year review of the study AMAROS, observed that 44.2% of the patient’s reported lymphedema at any time point after ALND plus RT, compared with 28.6% of the patients after SNB plus RT [
82]. For two items of the arm symptoms scale, a statistically significant difference was observed. For QoL, no statistical differences were observed between any of the selected scales (arm symptoms, pain, or body image) at 1, 3, and 5 years after treatment. Belmont has shown that relevant differences persisted between SNB and ALND groups for arm morbidity and its impact on functioning and QOL throughout the 1-year period after treatment [
83]. Arm volume change corrected for contralateral change was more frequent in patients with ALND (51.1% vs. 14.5% p=0.062). However, QoL between groups, measured by generic QoL questionnaires (SF36 and FACT-B+4), was negligible. Dabakuyo, in a multicenter cohort study, analyzed the QoL of patients undergoing ALND vs SNB according to surgeon preference. QoL was evaluated using the EORTC QLQ-C30 and the EORTC QLQ-BR- 23 to assess the global health status (GHS), and the arm (BRAS) and breast (BRBS) symptom scales. They observed the lowest BRAS QoL scores were recorded in the SNB group with no changes in the GHS and breast (BRBS) symptom [
84]. They observed the lowest BRAS QoL scores were recorded in the SNB group with no changes in the GHS and breast (BRBS) symptom. The randomized trial Del Bianco et al, observed that the SNB group had significantly less lymph-oedema, movement restrictions, pain and numbness with respect to the ALND group [
85]. The mean scores of the Psychological General Well-Being Index (PGWB) were significantly better in the SNB group than in the ALND group, but this difference was no longer significant at 24 months. Kootstra, studied the morbidity related to axillary surgery [
86]. Significant time effects were found in the difference scores for the 4 functional shoulder measurements. They observed that limitations in shoulder function and arm strength were most severe immediately after surgery for breast cancer and by the 2-year follow-up, all women had regained their preoperative level of arm muscle strength. Women in the ALND group showed a gradual progression of lymphedema during the 2 years of follow-up, whereas the women in the SNB group did not develop lymphedema. Al Nakib, observed greater arm symptomatology in patients with ALND. He did not observe significant differences in overall QoL (QLQ-BR23) but the global quality of life score was significantly correlated to the pain level, and to arm or breast scores [
87]. Rietman, observed that treatment related upper limb morbidity, perceived disabilities in ADL and worsening of QoL 2 years after surgery is significantly less after SNB compared to ALND [
88].
In summary, most of the studies that investigate the impact of lymphadenectomy on quality-of-life use tools to assess the general quality of life of breast cancer surgical patients. The use of specific probes or questionnaires focused on the morbidity of surgery in the ipsilateral arm is less frequent. It seems clear that axillary lymphadenectomy increases the frequency of arm symptoms and sequelae such as pain or lymphedema when compared to SNB, especially when associated with RT. These differences appear to reduce over time. Despite the sequelae, in general quality of life questionnaires, such as the QLQ-30 or QLQ-BR23, most studies do not observe a significant impact of the morbidity of axillary lymphadenectomy on the overall quality of life of patients. Some authors interpret this result as being due to the effect of systemic treatments, especially chemotherapy and hormone therapy, on patients' quality of life, which probably dampens the effect of surgery on overall quality of life. In addition, as we have seen above, the breast surgery itself is also a factor that affects quality of life and overlaps with the effect of axillary surgery. On the other hand, it is common when studying quality of life in cancer patients to observe these contradictory phenomena. Physicians measure the effects of treatments that they believe affect patients. Quality of life studies inform us about the real impact that these treatments have on patients.
8. Discussion
The evolution of ALDN in BC surgery has been marked by significant advancements in surgical techniques, perioperative care, and the optimization of patient outcomes. Our analysis highlights key aspects that influence the practice of ALDN, including ambulatory surgery feasibility, the role of surgical drains, the effectiveness of sealants, the development of minimally invasive approaches and QoL.
Ambulatory ALDN has emerged as a safe and cost-effective strategy that enhances patient autonomy, reduces hospital-related complications, and optimizes healthcare resources. ERAS protocols have played a pivotal role in facilitating early discharge, demonstrating that patients can achieve similar, if not superior, outcomes in the ambulatory setting compared to traditional inpatient care. However, challenges remain in ensuring patient selection criteria are appropriately defined to maximize safety. Future research should focus on refining surgical techniques to further reduce seroma formation and ensure optimal recovery while maintaining the safety standards of outpatient surgery.
The role of drains in ALDN continues to be debated, with studies demonstrating both benefits and drawbacks. While their primary function is to reduce seroma formation and the need for symptomatic aspirations, evidence suggests that their routine use may not be justified. The Cochrane review from 2013 concluded that although drains decrease seroma incidence, they do not eliminate it entirely nor reduce the need for aspiration. Moreover, drains do not impact in infection rates but do contribute to longer hospital stays and potential discomfort at home, impacting patients' QoL. Older studies from the late 20th century supported the necessity of drains, yet more recent findings challenge this notion, particularly with the advent of advanced surgical tools such as the electrothermal bipolar vessel sealing system and less extensive lymphadenectomy techniques. Given these developments, modern research increasingly supports no-drain approaches, aligning with efforts to improve postoperative recovery and minimize complications.
Sealants represent another important factor in ALDN, with studies suggesting they may play a role in reducing seroma formation. However, inconsistencies in study designs—particularly the inclusion of diverse patient populations undergoing varying types of breast surgery—have led to conflicting conclusions. Patients undergoing mastectomy have larger dissection areas, which inherently influence seroma formation, and the presence of a drain may interfere with sealant effectiveness. Among the available options, fibrin-based sealants appear to have the strongest supporting evidence. Given the increasing movement toward omitting drains, future studies should specifically assess the effectiveness of different sealants in patients managed without drainage. The upcoming results from the GALA study may provide further insights into the independent role of some sealants in seroma prevention.
Minimally invasive ALDN has demonstrated promising outcomes in reducing surgical complications while improving functional and aesthetic results. Endoscopic and robotic techniques have gained traction, with evidence suggesting comparable oncological safety and complication rates to conventional surgery. Additionally, these minimally invasive approaches have been associated with a lower incidence of sensory disturbances and seroma formation, likely due to reduced tissue trauma and better neurovascular preservation. However, challenges persist, including the need for specialized training and the learning curve associated with endoscopic approaches within the confined axillary space. While the relatively straightforward anatomy of the axilla may facilitate skill acquisition, widespread adoption will require high-quality multicenter studies to validate these findings. Furthermore, robotic-assisted lymphadenectomy, while promising, remains in the experimental phase and demands thorough cost analyses to determine its practicality for broader clinical application. Factors such as accessibility, cost-effectiveness, and technical expertise must be carefully considered before these approaches can be widely integrated into routine surgical practice.
Quality of life (QoL) considerations have become increasingly critical in the modern surgical management of breast cancer patients undergoing axillary lymphadenectomy. Although ALND is associated with higher rates of arm morbidity, including pain, reduced mobility, and lymphedema compared to sentinel node biopsy, its overall impact on global QoL scores appears surprisingly limited. This paradox may be explained by the overlapping influence of systemic therapies and breast surgery on patients' overall health perception. Nevertheless, minimizing surgical trauma through techniques such as minimally invasive lymphadenectomy, early drain removal, and outpatient surgery models has shown promise in preserving functional outcomes and improve postoperative recovery. As the field continues to evolve, integrating patient-reported outcome measures (PROMs) into routine clinical practice will be essential to better understand and address the real-world QoL impact of axillary surgery, ensuring that oncological safety is achieved without sacrificing the well-being of long-term breast cancer survivors.
9. Limitations
The studies reviewed are heterogeneous, with different series that, in some cases, include conservative surgeries with lymphadenectomy or patients undergoing mastectomy. This variability can lead to differing conclusions, as mastectomy generally results in higher seroma production and almost always requires drainage placement. To avoid the effect of mastectomy in the interpretation of the findings, priority was given to studies focusing on conservative surgery with lymphadenectomy, excluding findings from mastectomy patients. In consequence the conclusions of this review could not be directly applied to patients subjected to mastectomy.
10. Conclusions
Axillary lymphadenectomy in breast cancer surgery continues to advance, with ongoing efforts aimed at improving patient outcomes through innovation in surgical techniques and perioperative management. The transition toward ambulatory surgery, the reconsideration of drainage necessity, the exploration of sealants, and the development of minimally invasive techniques all represent key advancements in the field. Although axillary lymphadenectomy increases arm morbidity compared to sentinel node biopsy, its overall impact on quality of life appears limited, likely due to the overlapping effects of systemic therapies and breast surgery.
Future research should focus on refining these strategies to further enhance surgical safety, reduce complications, and improve the overall QoL for patients undergoing axillary lymphadenectomy.
11. Future Directions
As surgical management of axillary disease in breast cancer continues to evolve, several key areas warrant further investigation. Future studies should focus on optimizing patient selection criteria for ambulatory ALND, including standardized protocols to ensure safe same-day discharge. High-quality randomized trials are needed to better define the role of drains, particularly in light of new surgical technologies and minimally invasive approaches that may reduce seroma formation independently. The impact of tissue sealants on seroma prevention also requires clarification through well-designed studies that specifically separate outcomes by surgical technique and the presence or absence of drainage. Furthermore, while early data on minimally invasive and robotic ALND are promising, larger prospective trials are necessary to confirm their oncological safety, cost-effectiveness, and long-term benefits in terms of functional recovery and QoL. As patient-centered care becomes increasingly prioritized, future research must also incorporate patient-reported outcome measures (PROMs) to better capture the real-world impact of surgical innovations on quality of life. Finally, ongoing technological advances, including the refinement of robotic platforms and the development of enhanced sealing devices, offer exciting opportunities to further minimize morbidity and improve outcomes and QoL for breast cancer patients requiring axillary surgery.
Author Contributions
López Gordo and Ramirez-Maldonado designed the research framework. López Gordo developed the initial structure of the review and paragraphs. López Gordo and J Jimeno conducted the literature search and select the reviewed studies analyzed. López Gordo Write the first version of the review and the rest of the authors critically reviewed and modify the content of the review adding additional insights and refining the content. G-Monferrer designed the graphical abstract. All the authors reviewed and edited the manuscript critically for important intellectual content after it is elaboration. All authors approved the final version of the manuscript and agree with all the sections.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
References
- A. J. M. Van Bemmel, C. J. H. Van De Velde, R. F. Schmitz, and G. J. Liefers, “Prevention of seroma formation after axillary dissection in breast cancer: A systematic review,” European Journal of Surgical Oncology, vol. 37, no. 10, pp. 829–835, 2011. [CrossRef]
- P. K. Jain, R. Sowdi, A. D. G. Anderson, and J. MacFie, “Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer,” British Journal of Surgery, vol. 91, no. 1, pp. 54–60, 2004. [CrossRef]
- J. C. Taylor, S. Rai, F. Hoar, H. Brown, and L. Vishwanath, “Breast cancer surgery without suction drainage: The impact of adopting a ‘no drains’ policy on symptomatic seroma formation rates,” European Journal of Surgical Oncology, vol. 39, no. 4, pp. 334–338, 2013. [CrossRef]
- J. Gunn, T. Gibson, Z. Li, N. Diehl, S. Bagaria, and S. McLaughlin, “Symptomatic Axillary Seroma after Sentinel Lymph Node Biopsy: Incidence and Treatment,” Ann Surg Oncol, vol. 23, no. 10, pp. 3347–3353, 2016. [CrossRef]
- Tamminen, T. Meretoja, and I. Koskivuo, “Same-day mastectomy and axillary lymph node dissection is safe for most patients with breast cancer,” J Surg Oncol, vol. 125, no. 5, pp. 831–838, 2022. [CrossRef]
- P. Karakousis, “Axillary Padding as an Alternative to Closed Suction Drain for Ambulatory Axillary Lymphadenectomy—Invited Critique,” Archives of Surgery, vol. 137, no. 2, p. 173, 2002. [CrossRef]
- Z. Al-Hilli, K. M. Thomsen, E. B. Habermann, J. W. Jakub, and J. C. Boughey, “Reoperation for Complications after Lumpectomy and Mastectomy for Breast Cancer from the 2012 National Surgical Quality Improvement Program (ACS-NSQIP),” Ann Surg Oncol, vol. 22, pp. 459–469, 2015. [CrossRef]
- M. Medarde-Ferrer et al., “Aplicación de un protocolo ERAS (enhanced recovery after surgery) para la práctica cirugía oncológica compleja de la mama en régimen ambulatorio,” Revista de Senología y Patología Mamaria, vol. 37, no. 4, p. 100614, 2024. [CrossRef]
- M. Barry, W. P. Weber, S. Lee, A. Mazzella, and L. M. Sclafani, “Enhancing the clinical pathway for patients undergoing axillary lymph node dissection,” Breast, vol. 21, no. 4, pp. 440–443, 2012. [CrossRef]
- M. Siegel, “Level I and II Axillary Dissection in the Treatment of Early-Stage Breast Cancer,” Archives of Surgery, vol. 125, no. 9, p. 1144, 1990. [CrossRef]
- J. Bonnema et al., “Cost of care in a randomised trial of early hospital discharge after surgery for breast cancer,” Eur J Cancer, vol. 34, no. 13, pp. 2015–2020, 1998. [CrossRef]
- A. Bundred N, Maguire P, Reynolds J, Grimshaw J, Morris J, Thomson L, Barr L, “Randomised controlled trial of effects of early discharge after surgery for breast cancer,” BMJ, vol. 7;317(7168, pp. 1275–9. [CrossRef]
- Holcombe, N. West, R. E. Mansel, and K. Horgan, “The satisfaction and savings of early discharge with drain in situ following axillary lymphadenectomy in the treatment of breast cancer,” European Journal of Surgical Oncology, vol. 21, no. 6, pp. 604–606, 1995. [CrossRef]
- K. Jogerst et al., “Same-Day Discharge After Mastectomy: Breast Cancer Surgery in the Era of ERAS®,” Ann Surg Oncol, vol. 27, no. 9, pp. 3436–3445, 2020. [CrossRef]
- R. G. Margolese and J. C. M. Lasry, “Ambulatory surgery for breast cancer patients,” Ann Surg Oncol, vol. 7, no. 3, pp. 181–187, 2000. [CrossRef]
- K. Horgan, E. A. Benson, A. Miller, and A. Robertson, “Early discharge with drain in situ following axillary lymphadenectomy for breast cancer,” Breast, vol. 9, no. 2, pp. 90–92, 2000. [CrossRef]
- J. M. Classe, D. Berchery, L. Campion, R. Pioud, F. Dravet, and S. Robard, “Randomized clinical trial comparing axillary padding with closed suction drainage for the axillary wound after lymphadenectomy for breast cancer,” British Journal of Surgery, vol. 93, no. 7, pp. 820–824, 2006. [CrossRef]
- V. Srivastava, S. Basu, and V. K. Shukla, “Seroma formation after breast cancer surgery: What we have learned in the last two decades,” J Breast Cancer, vol. 15, no. 4, pp. 373–380, 2012. [CrossRef]
- R. A. Droeser et al., “Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: A meta-analysis,” Breast, vol. 18, no. 2, pp. 109–114, 2009. [CrossRef]
- P. K. Jain, R. Sowdi, A. D. G. Anderson, and J. MacFie, “Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer,” British Journal of Surgery, vol. 91, no. 1, pp. 54–60, 2004. [CrossRef]
- M. B. A E Cameron 1, S R Ebbs, F Wylie, “Suction drainage of the axilla: a prospective randomized trial,” Br J Surg, vol. 75(12), p. 1211, 1998. [CrossRef]
- R. G. Somers, L. K. Jablon, M. J. Kaplan, G. L. Sandler, and N. K. Rosenblatt, “The use of closed suction drainage after lumpectomy and axillary node dissection for breast cancer: A prospective randomized trial,” Ann Surg, vol. 215, no. 2, pp. 146–149, 1992. [CrossRef]
- J. Zavotsky, R. C. Jones, M. B. Brennan, and A. E. Giuliano, “Evaluation of axillary lymphadenectomy without axillary drainage for patients undergoing breast-conserving therapy,” Ann Surg Oncol, vol. 5, no. 3, pp. 227–231, 1998. [CrossRef]
- P. S. H. Soon, J. Clark, and C. J. Magarey, “Seroma formation after axillary lymphadenectomy with and without the use of drains,” Breast, vol. 14, no. 2, pp. 103–107, 2005. [CrossRef]
- X. D. He, Z. H. Guo, J. H. Tian, K. H. Yang, and X. D. Xie, “Whether drainage should be used after surgery for breast cancer? A systematic review of randomized controlled trials,” Medical Oncology, vol. 28, no. SUPPL. 1, pp. 22–30, 2011. [CrossRef]
- M. S. Troost, C. J. Kempees, and M. A. J. De Roos, “Breast cancer surgery without drains: No influence on seroma formation,” International Journal of Surgery, vol. 13, pp. 170–174, 2015. [CrossRef]
- J. C. Taylor, S. Rai, F. Hoar, H. Brown, and L. Vishwanath, “Breast cancer surgery without suction drainage: The impact of adopting a ‘no drains’ policy on symptomatic seroma formation rates,” European Journal of Surgical Oncology, vol. 39, no. 4, pp. 334–338, 2013. [CrossRef]
- S. López Gordo et al., “Seroma control in axillary lymphadenectomy with Glubran 2® without drain. Multicenter, prospective, randomized, clinical trial. GALA-ND study (Glubran, Axillary Lymphadenectomy, Ambulatory, No Drain),” Trials, vol. 25, no. 1, pp. 1–12, 2024. [CrossRef]
- S. S. Jeffrey, W. H. Goodson, D. M. Ikeda, R. L. Birdwell, and M. S. Bogetz, “Axillary Lymphadenectomy for Breast Cancer Without Axillary Drainage,” Archives of Surgery, vol. 130, no. 8, pp. 909–913, 1995. [CrossRef]
- J. R. Garbay et al., “Axillary padding without drainage after axillary lymphadenectomy - A prospective study of 299 patients with early breast cancer,” Breast Care, vol. 7, no. 3, pp. 231–235, 2012. [CrossRef]
- Conversano et al., “Use of Low-Thrombin Fibrin Sealant Glue After Axillary Lymphadenectomy for Breast Cancer to Reduce Hospital Length and Seroma,” Clin Breast Cancer, vol. 17, no. 4, pp. 293–297, 2017. [CrossRef]
- D. Purushotham et al., “Randomized clinical trial of no wound drains and early discharge in the treatment of women with breast cancer,” British Journal of Surgery, vol. 89, no. 3, pp. 286–292, 2002. [CrossRef]
- T. Cortadellas et al., “Electrothermal bipolar vessel sealing system in axillary dissection: A prospective randomized clinical study,” International Journal of Surgery, vol. 9, no. 8, pp. 636–640, 2011. [CrossRef]
- Lumachi, S. M. M. Basso, D. A. Santeufemia, M. Bonamini, and G. B. Chiara, “Ultrasonic dissection system technology in breast cancer: A case-control study in a large cohort of patients requiring axillary dissection,” Breast Cancer Res Treat, vol. 142, no. 2, pp. 399–404, 2013. [CrossRef]
- D. Thomson DR, Sadideen H, “Wound drainage after axillary dissection for carcinoma of the breast.,” Syst Rev, vol. 20;2013(10. [CrossRef]
- E. Buch-Villa et al., “Clinical and cost outcomes of a polyethylene glycol (PEG)-coated patch versus drainage after axillary lymph node dissection in breast cancer: results from a multicentre randomized clinical trial,” British Journal of Surgery, vol. 110, no. 9, pp. 1180–1188, 2023. [CrossRef]
- Sierra DH., “Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications,” J Biomater Appl, vol. 7 (4), pp. 309–352, 1993. [CrossRef]
- M. Moore et al., “Fibrin sealant reduces the duration and amount of fluid drainage after axillary dissection: A randomized prospective clinical trial,” J Am Coll Surg, vol. 192, no. 5, pp. 591–599, 2001. [CrossRef]
- N. Gilly, Y. François, A. C. Sayag-Beaujard, O. Glehen, A. Brachet, and J. Vignal, “Prevention of lymphorrhea by means of fibrin glue after axillary lymphadenectomy in breast cancer: Prospective randomized trial,” European Surgical Research, vol. 30, no. 6, pp. 439–443, 1998. [CrossRef]
- R. Ruggiero et al., “Axillary lymphadenectomy for breast cancer and fibrin glue,” Ann Ital Chir, vol. 85, no. 1, pp. 88–92, 2014.
- P. K. Mustonen, M. A. Härmä, and M. J. Eskelinen, “THE EFFECT OF FIBRIN SEALANT COMBINED WITH FIBRINOLYSIS INHIBITOR ON REDUCING THE AMOUNT OF LYMPHATIC LEAKAGE AFTER AXILLARY EVACUATION IN BREAST CANCER A prospective randomized clinical trial Background and Aims : One third of women undergoing mastectomy,” no. 1, pp. 209–212, 2004.
- JF. Vaxman F, Kolbe A, Stricher F, Zund D, Volkmar P, Gros D, “Does fibrin glue improve drainage after axillary lymph node dissection? Prospective and randomized study in humans.,” Eur Surg Res., vol. 27(5), pp. 346–52, 1995.
- R. Benevento et al., “The effects of low-thrombin fibrin sealant on wound serous drainage, seroma formation and length of postoperative stay in patients undergoing axillary node dissection for breast cancer: A randomized controlled trial,” International Journal of Surgery, vol. 12, no. 11, pp. 1210–1215, 2014. [CrossRef]
- Y. T. Chang, S. L. Shih, E. W. Loh, and K. W. Tam, “Effects of Fibrin Sealant on Seroma Reduction for Patients with Breast Cancer Undergoing Axillary Dissection: Meta-Analysis of Randomized Controlled Trials,” Ann Surg Oncol, vol. 27, no. 13, pp. 5286–5295, 2020. [CrossRef]
- K. Vasileiadou, C. Kosmidis, G. Anthimidis, S. Miliaras, I. Kostopoulos, and E. Fahantidis, “Cyanoacrylate Adhesive Reduces Seroma Production After Modified Radical Mastectomy or Quadrantectomy With Lymph Node Dissection—A Prospective Randomized Clinical Trial,” Clin Breast Cancer, vol. 17, no. 8, pp. 595–600, 2017. [CrossRef]
- E. Esposito et al., “Cyanoacrylate glue in breast surgery: the GLUBREAST Trial,” Front Oncol, vol. 14, no. January, pp. 1–10, 2024. [CrossRef]
- M. Al-Masri et al., “Effectiveness of Cyanoacrylate in Reducing Seroma Formation in Breast Cancer Patients Post-Axillary Dissection: A Randomized Controlled Trial,” Front Oncol, vol. 10, no. January, pp. 1–8, 2021. [CrossRef]
- Z. Clement, P. Shin, C. Hoffmann, M. Eaton, and W. McLeay, “A Double-Blinded Prospective Randomised Controlled Trial to Assess the Efficacy of Glubran-2 in Reducing Seroma Formation after a Mastectomy with or without Axillary Dissection,” Adv Breast Cancer Res, vol. 06, no. 04, pp. 117–128, 2017. [CrossRef]
- M. Vinchant et al., “Intérêt d’un combipatch de thrombine et de fibrinogène dans la prévention des lymphocèles après curage axillaire,” Gynecologie Obstetrique et Fertilite, vol. 41, no. 10, pp. 583–587, 2013. [CrossRef]
- Lacoste, L. Ouldamer, G. Body, and H. Marret, “Does the use of TachoSil allow to reduce the morbidity of axillary dissection?,” Gynecologie Obstetrique et Fertilite, vol. 41, no. 2, pp. 141–143, 2013. [CrossRef]
- N. Tagaya and K. Kubota, “Experience with endoscopic axillary lymphadenectomy using needlescopic instruments in patients with breast cancer: A preliminary report,” Surgical Endoscopy and Other Interventional Techniques, vol. 16, no. 2, pp. 307–309, 2002. [CrossRef]
- Xiong et al., “Contrast of Mastoscopic and Conventional Axillary Lymph Node Dissection of Patients With Breast Cancer: Meta-Analysis,” Cancer Control, vol. 27, no. 2, pp. 1–8, 2020. [CrossRef]
- B. Suzanne F, Emering C, Wattiez A, Bournazeau JA, Bruhat MA, “Le curage axillaire par lipo-aspiration et prélèvement endoscopique. A propos de 72 cas [Axillary lymphadenectomy by lipo-aspiration and endoscopic picking. Apropos of 72 cases].,” Chirurgie, vol. 122(2), pp. 138–42, 1997.
- B. Cangiotti L, Poiatti R, Taglietti L, Re P, “A mini-invasive technique for axillary lymphadenectomy in early breast cancer: a study of 15 patients,” J Exp Clin Cancer Res, vol. (3), no. 295–8, 1999.
- S. Kamprath, J. Bechler, R. Kühne-Heid, N. Krause, and A. Schneider, “Endoscopic axillary lymphadenectomy without prior liposuction: Development of a technique and initial experience,” Surg Endosc, vol. 13, no. 12, pp. 1226–1229, 1999. [CrossRef]
- Z. H. Wang et al., “Single-port endoscopic-sentinel lymph node biopsy combined with indocyanine green and carbon nanoparticles in breast cancer,” Surg Endosc, vol. 37, no. 10, pp. 7591–7599, 2023. [CrossRef]
- cai Yao, C. Liu, and J. Xian, “Comparison of single-pore non-liposuction near-infrared laparoscopy with conventional open surgery for axillary sentinel lymph node biopsy in patients with early breast cancer: a single-center, small-sample retrospective study,” World J Surg Oncol, vol. 21, no. 1, pp. 1–8, 2023. [CrossRef]
- M. E. Aponte-Rueda, R. A. Saade Cárdenas, and M. J. Saade Aure, “Endoscopic axillary dissection: A systematic review of the literature,” Breast, vol. 18, no. 3, pp. 150–158, 2009. [CrossRef]
- R. L. De Wilde, E. H. Schmidt, M. Hesseling, R. Mildner, V. Frank, and M. Tenger, “Comparison of classic and endoscopic lymphadenectomy for staging breast cancer,” Journal of the American Association of Gynecologic Laparoscopists, vol. 10, no. 1, pp. 75–79, 2003. [CrossRef]
- C. Luo et al., “Comparison of mastoscopic and conventional axillary lymph node dissection in breast cancer: Long-term results from a randomized, multicenter trial,” Mayo Clin Proc, vol. 87, no. 12, pp. 1153–1161, 2012. [CrossRef]
- S. López Gordo et al., “Indocyanine green for axillary sentinel lymph node biopsy in patients with breast cancer (INSEAN study),” Cir Esp, 2025. [CrossRef]
- Chengyu et al., “A standardized surgical technique for mastoscopic axillary lymph node dissection,” Surg Laparosc Endosc Percutan Tech, vol. 15, no. 3, pp. 153–159, 2005. [CrossRef]
- J. H. Ahn et al., “Early experience of robotic axillary lymph node dissection in patients with node-positive breast cancer,” Breast Cancer Res Treat, vol. 198, no. 3, pp. 405–412, 2023. [CrossRef]
- Chen et al., “Efficacy of da Vinci robot-assisted lymph node surgery than conventional axillary lymph node dissection in breast cancer – A comparative study,” International Journal of Medical Robotics and Computer Assisted Surgery, vol. 17, no. 6, pp. 1–9, 2021. [CrossRef]
- Basch, P. Torda, and K. Adams, “Standards for patient-reported outcome-based performance measures,” Jul. 10, 2013. [CrossRef]
- E. Giuliano et al., “Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial,” JAMA - Journal of the American Medical Association, vol. 318, no. 10, pp. 918–926, Sep. 2017. [CrossRef]
- E. Giuliano et al., “Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial,” JAMA - Journal of the American Medical Association, vol. 305, no. 6, pp. 569–575, Feb. 2011. [CrossRef]
- Donker et al., “Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial,” Lancet Oncol, vol. 15, no. 12, pp. 1303–1310, Nov. 2014. [CrossRef]
- J. C. Boughey et al., “Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (alliance) clinical trial,” JAMA - Journal of the American Medical Association, vol. 310, no. 14, pp. 1455–1461, 2013. [CrossRef]
- T. Kuehn et al., “Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study,” Lancet Oncol, vol. 14, no. 7, pp. 609–618, Jun. 2013. [CrossRef]
- J. F. Boileau et al., “Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study,” Journal of Clinical Oncology, vol. 33, no. 3, pp. 258–263, Jan. 2015. [CrossRef]
- S. Caudle et al., “Improved axillary evaluation following neoadjuvant therapy for patientswith node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection,” Journal of Clinical Oncology, vol. 34, no. 10, pp. 1072–1078, Apr. 2016. [CrossRef]
- S. Alvarez-Pardo et al., “Quality of Life in Breast Cancer Survivors in Relation to Age, Type of Surgery and Length of Time since First Treatment,” Int J Environ Res Public Health, vol. 19, no. 23, Dec. 2022. [CrossRef]
- Marinkovic, N. Djordjevic, L. Djordjevic, N. Ignjatovic, M. Djordjevic, and V. Karanikolic, “Assessment of the quality of life in breast cancer depending on the surgical treatment,” Supportive Care in Cancer, vol. 29, no. 6, pp. 3257–3266, Jun. 2021. [CrossRef]
- D. Smith et al., “Long-Term Quality of Life in Patients with Breast Cancer after Breast Conservation vs Mastectomy and Reconstruction,” JAMA Surg, vol. 157, no. 6, Jun. 2022. [CrossRef]
- U. Boehmer, M. Glickman, M. Winter, and M. A. Clark, “Long-term breast cancer survivors’ symptoms and morbidity: Differences by sexual orientation?,” Journal of Cancer Survivorship, vol. 7, no. 2, pp. 203–210, Jun. 2013. [CrossRef]
- Laws et al., “Long-Term Patient-Reported Arm Symptoms in Breast Cancer Survivors,” Ann Surg Oncol, vol. 31, no. 3, pp. 1623–1633, Mar. 2024. [CrossRef]
- M. Enien, N. Ibrahim, W. Makar, D. Darwish, and M. Gaber, “Health-related quality of life: Impact of surgery and treatment modality in breast cancer,” J Cancer Res Ther, vol. 14, no. 5, pp. 957–963, Jul. 2018. [CrossRef]
- M. Kool et al., “Long term effects of extended adjuvant endocrine therapy on quality of life in breast cancer patients,” Breast, vol. 24, no. 3, pp. 224–229, Jun. 2015. [CrossRef]
- Hopwood, J. Haviland, J. Mills, G. Sumo, and J. M Bliss, “The impact of age and clinical factors on quality of life in early breast cancer: An analysis of 2208 women recruited to the UK START Trial (Standardisation of Breast Radiotherapy Trial),” Breast, vol. 16, no. 3, pp. 241–251, Jun. 2007. [CrossRef]
- Barranger, G. Dubernard, J. Fleurence, M. Antoine, E. Darai, and S. Uzan, “Subjective morbidity and quality of life after sentinel node biopsy and axillary lymph node dissection for breast cancer,” J Surg Oncol, vol. 92, no. 1, pp. 17–22, Oct. 2005. [CrossRef]
- S. A. L. Bartels et al., “Radiotherapy or Surgery of the Axilla after a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial,” Journal of Clinical Oncology, vol. 41, no. 12, pp. 2159–2165, Apr. 2023. [CrossRef]
- R. Belmonte, O. Garin, M. Segura, A. Pont, F. Escalada, and M. Ferrer, “Quality-of-life impact of sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer patients,” Value in Health, vol. 15, no. 6, pp. 907–915, Sep. 2012. [CrossRef]
- T. S. Dabakuyo et al., “A multicenter cohort study to compare quality of life in breast cancer patients according to sentinel lymph node biopsy or axillary lymph node dissection,” Annals of Oncology, vol. 20, no. 8, pp. 1352–1361, 2009. [CrossRef]
- Del Bianco et al., “Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: Results of the sentinella-GIVOM Italian randomised clinical trial,” European Journal of Surgical Oncology, vol. 34, no. 5, pp. 508–513, May 2008. [CrossRef]
- J. J. Kootstra et al., “A longitudinal comparison of arm morbidity in stage I-II breast cancer patients treated with sentinel lymph node biopsy, sentinel lymph node biopsy followed by completion lymph node dissection, or axillary lymph node dissection,” Ann Surg Oncol, vol. 17, no. 9, pp. 2384–2394, Sep. 2010. [CrossRef]
- M. Al Nakib et al., “Quality of life at 2 years follow-up after sentinel lymph node biopsy, immediate or delayed axillary dissection for breast cancer,” Sep. 2010, Breast J. [CrossRef]
- J. S. Rietman et al., “Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer,” European Journal of Surgical Oncology, vol. 32, no. 2, pp. 148–152, Mar. 2006. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).