1. Introduction
A healthy, functional gut barrier is a hallmark of good gut health. The gut barrier is a collection of layers, including intestinal mucus and epithelial cells, that separates the lumen from the rest of the intestinal tissues and broader circulatory system [
1]. It is the barrier between the inner workings of the human body and the environment. When functioning optimally, the gut barrier only allows transport of desirable nutrients and compounds into the cells or adjacent paracellular space. With impaired function, undesirable elements can enter the intercellular space to cause intestinal dysbiosis and move into the circulatory systems to cause systemic effects. A weakened gut barrier increases susceptibility to a range of gut-mediated effects. This can include low-grade inflammation due to translocation of bacteria-derived lipopolysaccharides from the lumen to the interstitial space via “leaky” tight junctions [
2]. A weakened gut barrier is common in conditions like irritable bowel syndrome (IBS).
In the United States (US), conditions related to irritable bowel are highly prevalent, reportedly affecting up to 15% of the population [
3]. IBS is divided into four subtypes: IBS-C (constipation predominant), IBS-D (diarrhea predominant), IBS-M (IBS with mixed bowel habits) and unclassified. Of these, IBS-D is the most common, affecting almost half of those with IBS [
4]. Persistent GI-related symptoms in IBS-D, such as frequent diarrhea and abdominal discomfort, are disruptive of daily activities and can have a profoundly negative effect on quality of life [
5]. Recent treatments focus on symptom management using dietary modifications, probiotics, pharmacological interventions, and psychological therapies, however, many people report only partial relief [
6]. Emerging evidence supports the role of nutrition-based strategies in IBS-D management, particularly supplements and dietary interventions that target intestinal barrier function [
7,
8].
Bioactives have been shown to impart several human health benefits and are rising considerably in popularity among consumers. Bioactives, a class of health-benefiting compounds found in plants, fungi, and bacteria, are now being explored for their potential role in improved gut barrier function. N-trans caffeoyltyramine (NCT) and N-trans feruloyltyramine (NFT) are naturally occurring bioactive compounds with potential therapeutic benefits related to gut disorders. These compounds have been associated with anti-inflammatory and gut-barrier-enhancing properties in preclinical models, including improvements in tight junction integrity and reductions in intestinal permeability [
9,
10]. Given the growing evidence linking gut permeability with IBS-D pathophysiology, investigating the effects of NCT and NFT in this population is of significant interest. Further, the overt nature of IBS-D symptomology allows for comprehensive evaluation of both objective and subjective improvements in gut barrier function.
Therefore, the purpose of this study was to investigate the effects of a proprietary dietary supplement containing bioactive compounds NCT and NFT on intestinal barrier function in individuals with IBS-D. The findings from this study will contribute to existing evidence in the field and help support the design of larger-scale clinical trials exploring dietary interventions that support gut barrier health.
2. Materials and Methods
2.1. Study Population
Study participants included male and female adults ages 18 to 70 years, body mass index (BMI) of 18.5 – 39.9kg/m
2, diagnosed with IBS-D based on ROME IV criteria [
11] confirmed by bowel diary, and IBS-SSS score above 175. Included participants presented LPS levels greater than 0.21 ng/ml, indicating increased intestinal permeability [
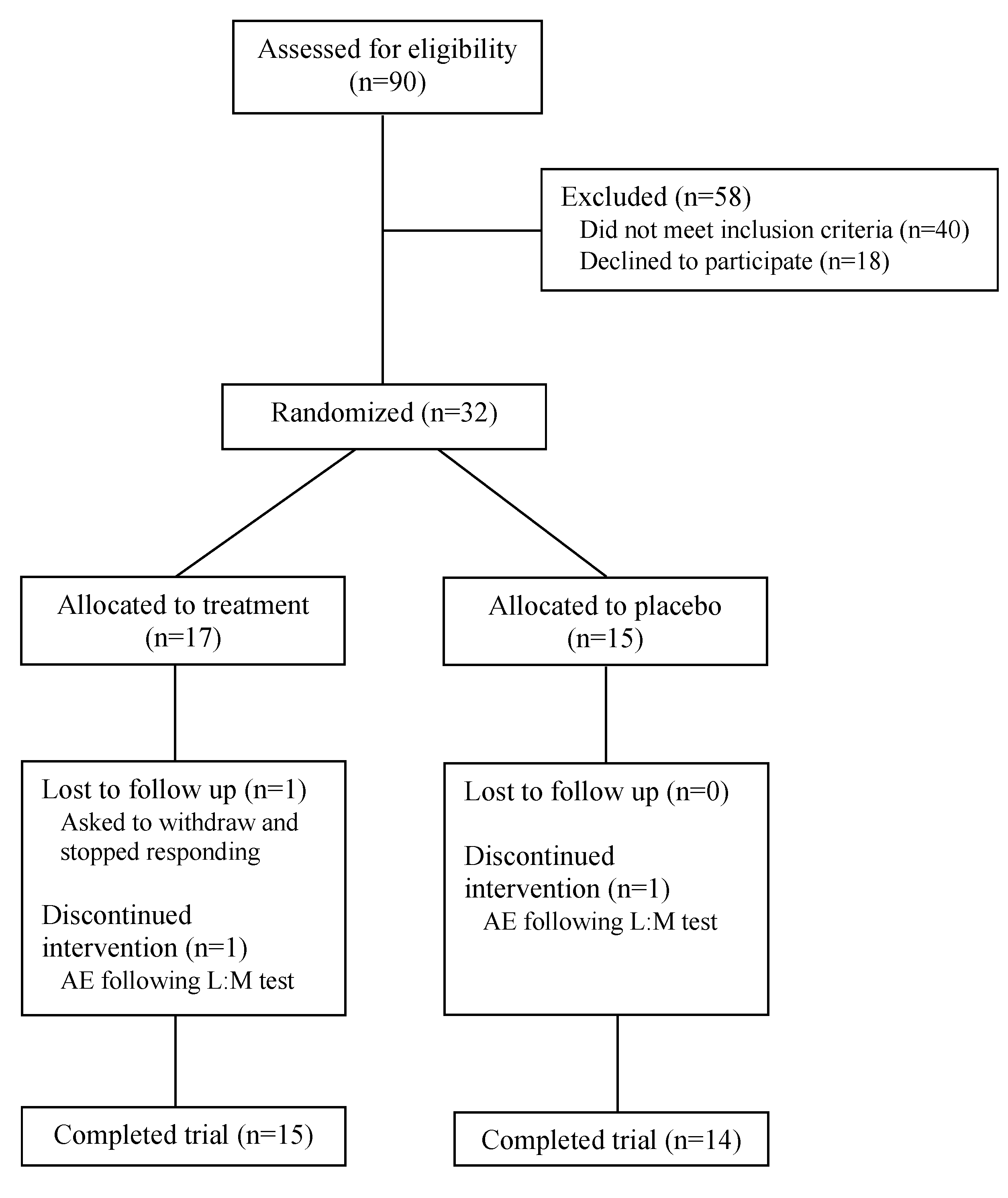
12]. Participants were excluded if they had a history of inflammatory bowel disease, recent antibiotic use (within prior 30 days), chronic NSAID (nonsteroidal anti-inflammatory drugs) use, major dietary changes, or significant comorbidities that could affect gut function. Participants were screened through medical history obtained from their general practitioner, symptom assessment, and baseline biomarker evaluation. After screening 90 potential participants for eligibility, 32 were enrolled and 29 completed the study protocol (
Figure 1). Informed consent was obtained from all persons involved. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Chelsea Research Ethics Committee, London, UK (Approval Number: 344580). This trial was registered on ClinicalTrials.gov (ID: NCT06543498).
2.2. Study Design
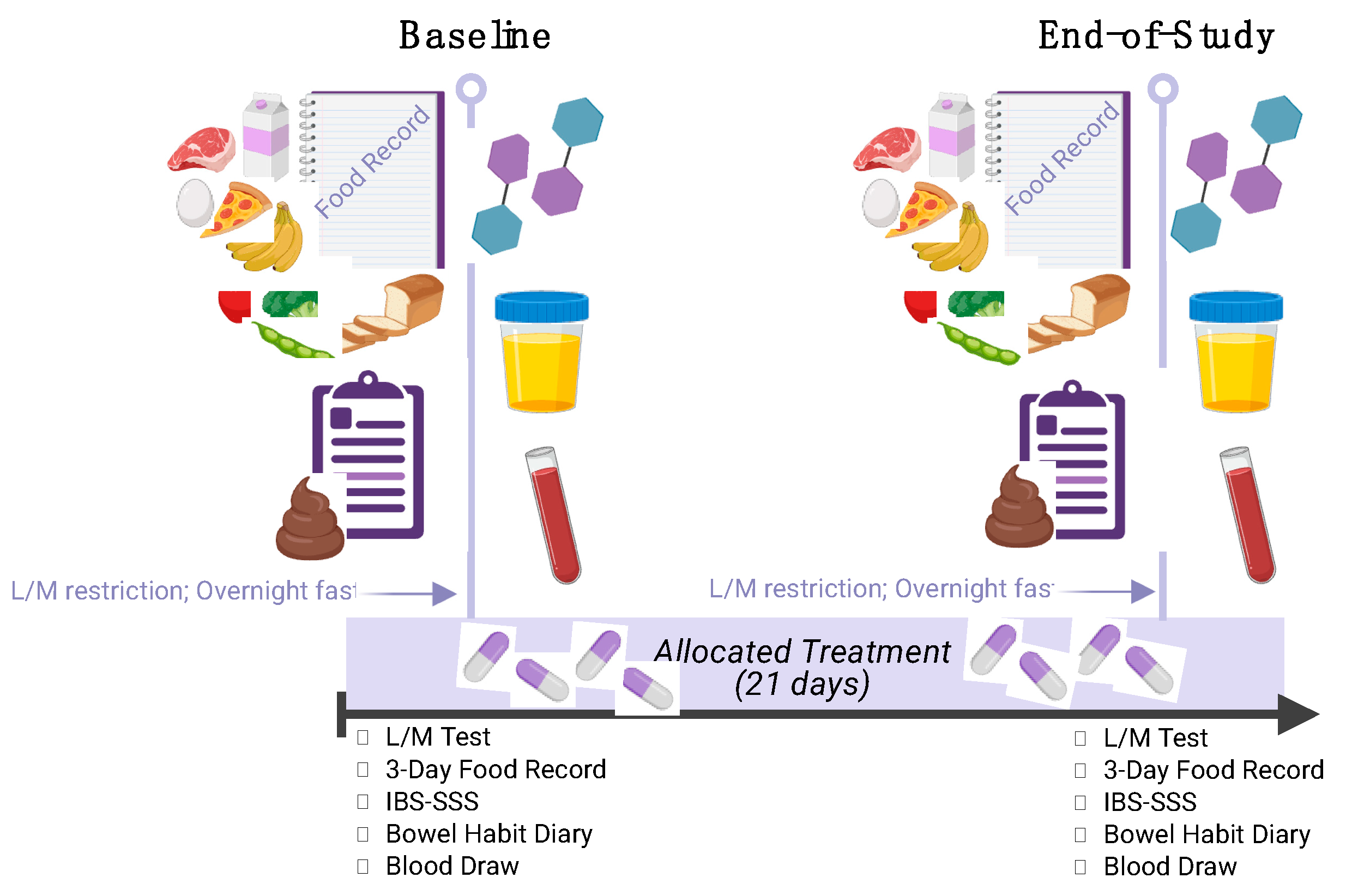
This study utilized a double-blinded, randomized, placebo-controlled parallel design with a 3-week intervention period. The active intervention and placebo were identical in appearance. This study included 3 visits at the Functional Gut Clinic (FGC) of either London or Manchester in the United Kingdom (
Figure 2). The purpose of this study was to investigate the effects of a proprietary NCT/NFT supplement (Brightseed
® Bio Gut Core) on markers of gut barrier function, including Lactulose/Mannitol ratio, Lipopolysaccharide (LPS), Lipopolysaccharide Binding Protein (LBP), sCD14, Zonulin, and LCN2, and the severity of symptoms related to IBS-D. Data collection was completed between August 2024 and January 2025.
Participants were recruited through advertisements on social media, newspapers, and the FGC website, as well as through direct contact with those who had previously consented to being approached for research opportunities. Those who were interested completed a virtual informed consent document and received a screening phone call to verify basic demographic details, medical history and current medication list to confirm suitability for the study. After suitability was confirmed, a screening visit (Visit 1) was scheduled.
During Visit 1, in-person informed consent was obtained, and a comprehensive medical history and medication intake was recorded. Participants completed the ROME IV [
11] questionnaire and completed a 14-day bowel habit diary using the Bristol Stool Chart to confirm IBS-D status. A blood sample was collected to assess gut barrier dysfunction, with eligibility confirmed by LPS levels > 0.21 ng/ml. Females with childbearing potential underwent a urine pregnancy test to confirm non-pregnancy. Upon meeting all eligibility criteria, participants received instructions to follow a specific diet avoiding foods with lactulose and mannitol one day before Visit 2 and were provided with a 3-day food record to complete on three consecutive days prior to Visit 2. Also at this visit, participants were randomized to their allocated treatment group using a pre-determined block randomization code. Both the participants and research team were blinded to treatment allocation.
During Visit 2, study participants arrived at the FGC in an overnight fasted state, returned the 3-day food record, and completed questionnaires including the IBS-SSS. A blood sample was collected. Participants then ingested a lactulose:mannitol test solution prepared with two sugar probes (5g mannitol and 10g lactulose) in ~200 mL water. Following solution ingestion, participants completed an in-clinic 8 h urine collection. Water consumption was permitted ad libitum during the collection period. After samples were collected, participants received a bottle with a 3-week supply of NCT/NFT dietary supplement capsules with instructions to consume 1 capsule daily. Study participants were also given another bowel diary with Bristol Stool Chart and 3-day food record to be completed over the next 3-week treatment period prior to Visit 3. Urine samples were aliquoted and stored in a -80°C freezer until analysis.
During Visit 3, participants returned completed bowel habit diary and 3-day food record. Supplement bottles were collected, and remaining capsules were counted to verify compliance. Participants who met compliance requirements repeated all procedures performed during Visit 2.
2.3. Assessment of Intestinal Permeability
Lactulose/Mannitol Ratio: The Lactulose/Mannitol ratio test was used to assess gut permeability. This is the gold standard for such assessment [
13]. Participants were instructed to follow a specific diet on the day before the study to improve the accuracy of the test by reducing background mannitol occurring in foods and fasted for 12 hours prior to the study. Participants provided a fasted urine void before ingesting a dual sugar probe: Lactulose (a synthetic non-absorbable disaccharide) and Mannitol (a naturally occurring poorly absorbed monosaccharide). Total urine was then collected over 0-8h time period. Concentration of both sugars molecules were detected with high-performance liquid chromatography (HPLC) using a resin-based cation exchange column with a refractive index detector for recovery. The ratio of lactulose/mannitol excretion determines gut barrier dysfunction.
Gut Barrier Biomarkers: Serum biomarkers were also used to evaluate the strength of the gut barrier. Blood samples were collected at clinic visits 1, 2 & 3, by a phlebotomy certified member of the research team. Samples were centrifuged to aliquot viable serum and plasma which were frozen and stored at -80°C. LPS was detected using the Limulus amebocyte lysate quantitative end-point assay. The values for serum LBP, sCD14, Zonulin, and LCN2 were obtained using quantitative enzyme-linked immunosorbent assay (ELISA). LPS was also used during the screening phase as part of the eligibility assessment to verify evidence of increased intestinal permeability prior to randomization.
2.4. Assessment of GI-Symptoms
ROME IV: The ROME IV diagnostic criteria for IBS, a validated questionnaire used to identify functional GI disorders, was used for this purpose. The 89-point questionnaire contains questions relating to the esophagus, stomach and abdomen and can be used as a diagnostic tool, particularly in the research setting, to classify participants with one (or more) of the 33 recognised functional GI disorders in adults. For the purposes of the study, only the IBS specific questions (#40-67) were used to reduce participant burden.
IBS-SSS: The IBS-Symptom Severity Score (IBS-SSS), a validated questionnaire used to determine severity of abdominal and bowel symptoms, was used for this purpose. The questionnaire is comprised of 7 questions, 2 visual analogue and 5 numerical which are scored from 0 to 10. The total of the 5 numerical questions was calculated and multiplied by 10 to determine symptom severity. 75–174 indicates mild disease, 175–300 moderate disease and over 300 severe disease. The IBS-SSS was administered on each of the 3 scheduled study visits. Use of the IBS-SSS in this study was licensed by the Rome Foundation.
Bowel Habit Diary and Bristol Stool Chart: Participants were asked to complete a bowel habit diary throughout the study. The first 14 days were used to assess eligibility on the basis of IBS-D symptomatology. Completion of the diary through randomized treatment (21 days) was used to assess changes in bowel habit over time. The diary collected information on symptoms associated with bowel habit and the Bristol Stool Score grading of each bowel movement. The Bristol Stool Chart is a validated diagram, encompassing images and descriptions of 7 distinct stool types for participants to use to describe each of their bowel movements. Use of the Bristol Stool Chart in this study was licensed by the Rome Foundation.
2.5. Compliance
Food Records: Participants completed three day-food records to verify compliance with the protocol (including pre-visit dietary restrictions) and confirm maintenance of habitual diet throughout the intervention. Intake was recorded on three consecutive days prior to the baseline visit and end of treatment visit. Participants were asked to record all food and drink consumed, with quantities/descriptions in as much detail as possible, including brand names etc. Over-the-counter or prescribed pre- or probiotics were not permitted.
2.6. Safety Blood Assessments
Hematology and clinical chemistry analyses were performed at local laboratories in accordance with their protocols. Values were supplied to the Investigator to evaluate clinical significance and pathological changes.
2.7. Statistical Analysis
A sample size of approximately 26 participants (~13 per group) was needed to detect a minimal clinically important difference of 0.02, with a standard deviation of 0.025, at 80% power and an alpha level of 0.05 (two-tailed). Data are expressed as mean ± standard deviation and analyzed using SPSS (IBM SPSS Statistics, Version 27.0, IBM Corp, Armonk, NY, USA) or JASP 0.19.3. Normality was assessed using the Shapiro-Wilk test. Between-group differences were evaluated using independent t-tests for normally distributed variables and Mann-Whitney U tests for non-normally distributed variables. For the L/M data, a spaghetti plot was used to visually identify outliers, which were subsequently removed prior to analysis of that endpoint (one outlier removed per treatment group). A p-value of <0.05 was considered statistically significant. Effect sizes were calculated to determine the magnitude of observed differences.
4. Discussion
The gut barrier is the partition between the internal systems of the body and the external environment and is fundamental to gut and overall health. A weakened gut barrier increases susceptibility to gut-related health issues, including gut inflammation. A dysfunctional gut barrier also has adverse physiological implications, such as impaired nutrient absorption and endotoxemia [
15,
16]. Additionally, resulting symptoms such as frequent diarrhea, abdominal pain, and bloating impede activities of daily living and negatively impact overall quality of life [
5].
Conditions characterized by chronic, low-grade inflammation, such as obesity, are also often linked to an impaired intestinal barrier [
17,
18]. Recent evidence supports the theory that excess weight contributes to gut dysbiosis and is associated with symptoms of IBS [
18]. Indeed, the prevalence of IBS in those with obesity is higher than in the general population, with over 30% of obese persons being affected [
18]. Considering that almost three quarters (73.6%) of the adult population in the US is either overweight or obese [
19], it is likely that a substantial number of people are at increased risk for having a compromised gut barrier and/or associated symptomology.
In addition, some medications can also adversely impact GI symptoms and gut barrier. For instance, obesity treatments now include pharmacotherapy, namely, glucagon-like peptide-1 (GLP-1) receptor agonists. These medications have demonstrated substantial effectiveness and are quickly rising in popularity [
20]. One of the mechanisms by which GLP-1 receptor agonists work is by delaying gastric emptying [
21]. This slowed motility may mimic symptoms of IBS like abdominal distension and pain [
22] and is also associated with increased frequency of diarrhea [
23,
24].
Athletes or those participating in endurance sports are also known to have a high incidence of GI-related issues [
25]. Data shows that strenuous exercise may induce intestinal hyperpermeability [
26]. Indeed, interventions targeting the gut barrier showed promising results [
27], suggesting that supplement intake aimed at improving gut barrier integrity have novel applications and can be extrapolated to this group, in addition to those suffering from inflammatory bowel conditions.
Many lifestyle-related and pharmacological treatments rightly focus on symptom management to provide acute relief [
6]. One such example is a synthetic antimicrobial drug, a non-systemic, broad-spectrum antibiotic that targets the gut/intestinal microbiota [
28]. In phase 3, double-blind, placebo-controlled trials, participants who had IBS without constipation were treated with either this antibiotic (550 mg, 3x/day) or placebo for 2 weeks. Indeed, treatment with the antibiotic provided significant relief of IBS symptoms, bloating, abdominal pain, and loose stools [
28], suggesting its usefulness in the short-term. As an antibiotic, however, its utility is limited in terms of ongoing support. At present, the long-term effects of these types of drugs have not been studied and they are not indicated for repeated treatments [
29]. Further, high costs associated with drugs may also be prohibitive to consumers [
29]. On the other hand, long-term management of IBS and related GI symptoms often involves lifestyle modification, including dietary intervention and supplement intake.
Bioactives, compounds naturally occurring in plants, are becoming increasingly recognized for their biological properties and potential human health benefit. NCT and NFT show particular promise in alleviating GI symptoms through their anti-inflammatory and gut microbiota-modulating properties [
9,
10]. These compounds act as agonists to hepatocyte nuclear factor 4 alpha (HNF4α), a nuclear transcription factor that influences intestinal permeability and gut barrier [
30]. In vitro, administration of NCT and NFT yielded a physiologically relevant rescue of inflammation-induced impairments in gut barrier function through reversal of increased intestinal permeability and of decreased transepithelial electrical resistance [
9]. In vivo evidence demonstrated the reversal of increased intestinal permeability in a diet-induced obesity mouse model with genetic deletion of HNF4α that predisposed the animals to IBD [
10]. Treatment with NCT mitigated the deleterious effects of a high-fat diet on HNF4α expression and on the genes critical to Paneth cell function thereby improving intestinal barrier integrity [
10]. These preclinical data suggest a crucial role for NCT and NFT in promoting gut barrier function.
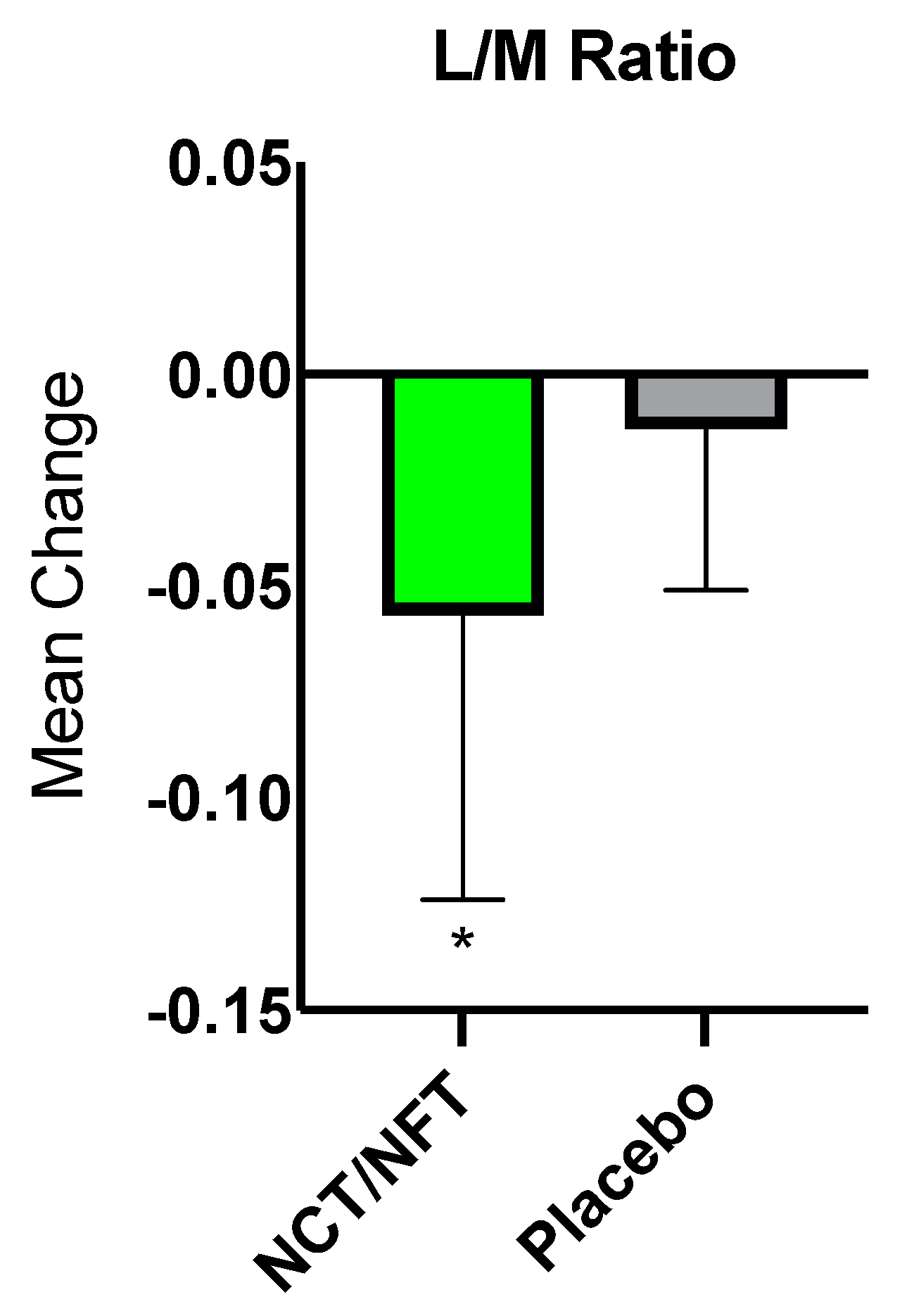
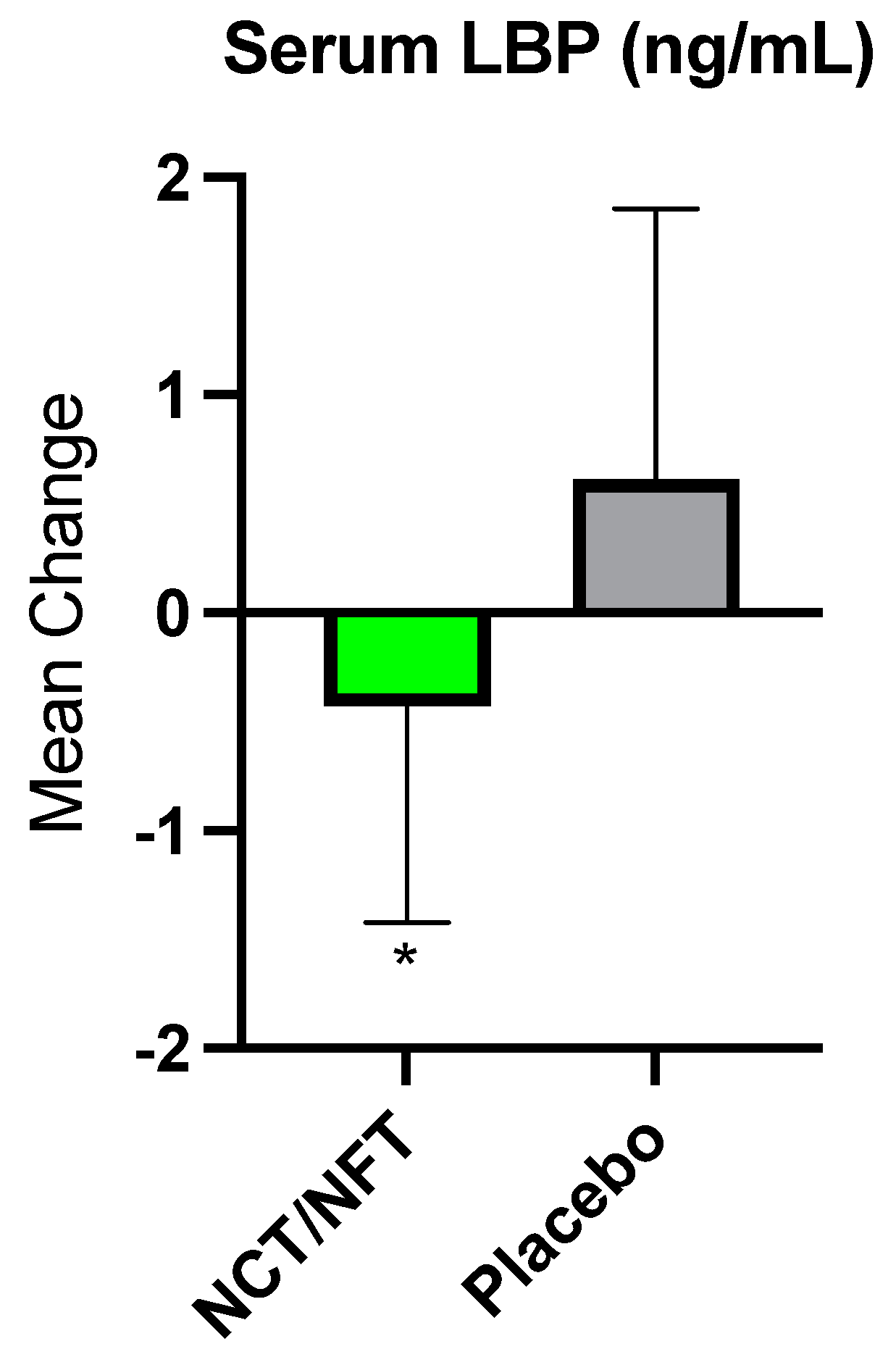
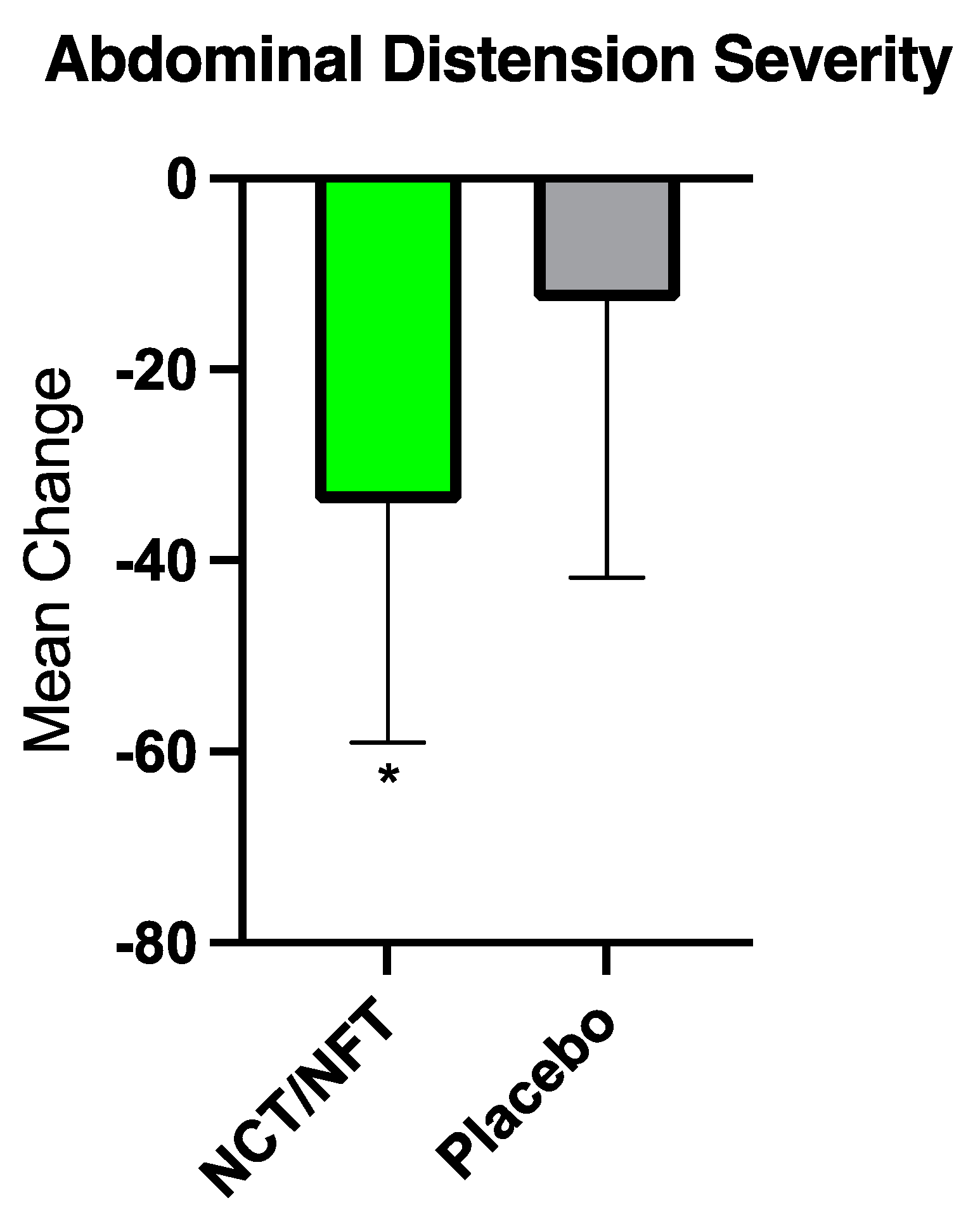
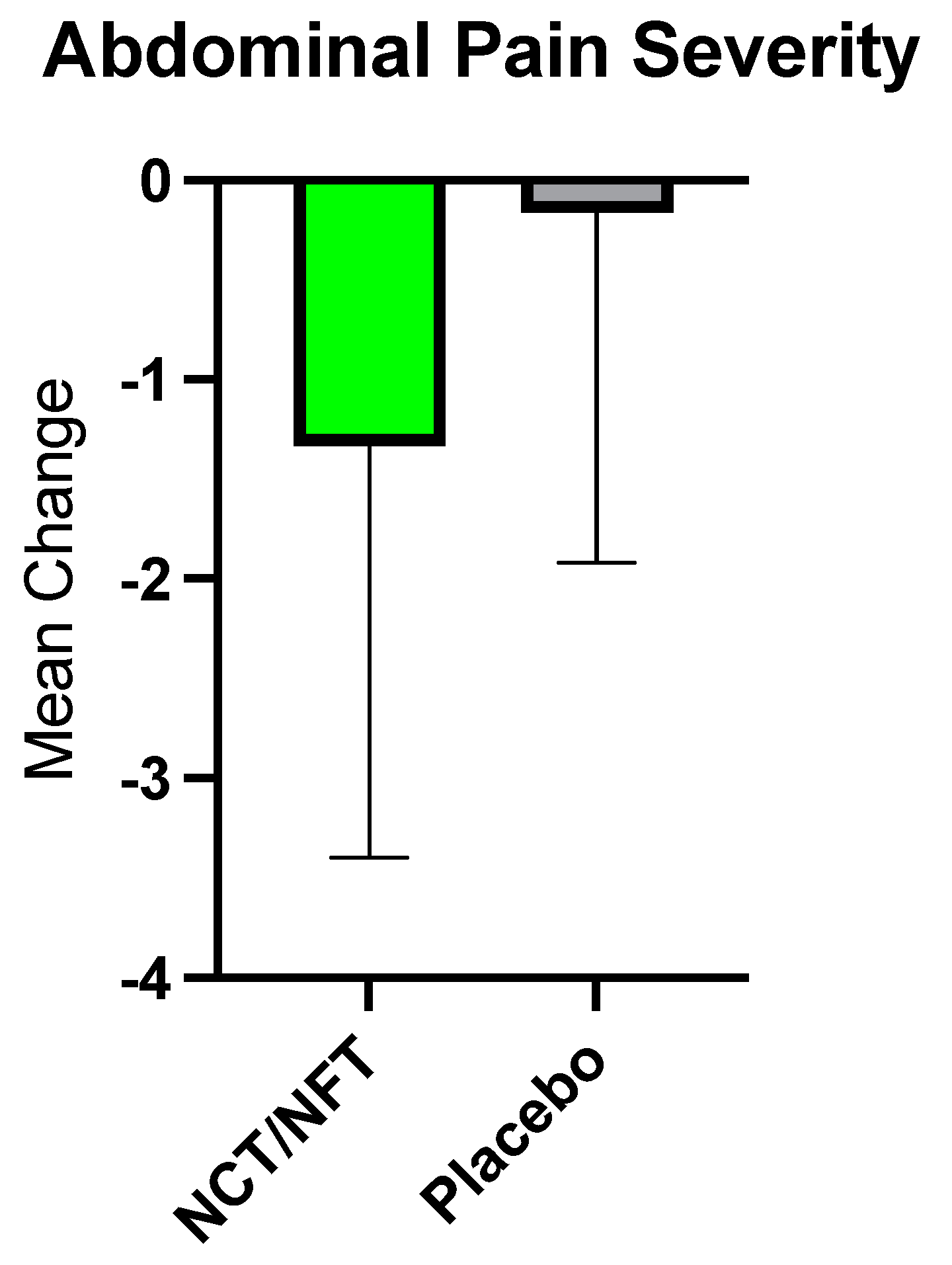
The current clinical study was conducted to further validate the efficacy of Brightseed® Bio Gut Core, a proprietary NCT- and NFT-based dietary supplement, on intestinal barrier function and severity of symptoms in individuals with IBS-D. Due to the overt nature of their symptoms, the IBS-D population is an ideal model for the assessment of gut barrier function and related GI symptoms. Twenty-nine participants completed the present trial and were randomized to either 120 mg/day of NCT/NFT or placebo for 3-weeks. Intestinal permeability as assessed by L/M ratio was significantly decreased in the NCT/NFT group compared to the placebo group (p<0.05). Supplementation with NCT/NFT also significantly decreased LBP, a surrogate marker of endotoxemia due to gut barrier dysfunction, and reduced the severity of abdominal distension as assessed by IBS-SSS (p<0.05). Further, trends in improvement were reported for subjective symptoms of bloating, abdominal pain, and impact on quality of life. The product appeared to be safe, and no adverse events related to supplementation with NCT/NFT were reported.
While the findings of this study are encouraging, there are several limitations worthy of consideration. First, the study sample is relatively small and larger studies would be helpful to replicate these findings. As a novel bioactive compound, the biological processes associated with the physiological improvements seen with NCT and NFT intake can be explored further. To explore potential mechanisms of action, multiple endpoints representing distinct biological pathways were evaluated, with the understanding that not all would necessarily yield positive findings. In this regard, lack of statistical significance for certain outcomes (serum LPS, sCD14, Zonulin, and LCN2) was expected and provides deeper insight into underlying mechanisms driving physiological response. Although some of the subjective endpoints (eg. abdominal pain) did not reach statistical significance, they demonstrated a positive trend in that direction, emphasizing the potential of NCT and NFT in strengthening the gut barrier and providing GI-symptom relief. The current study showed a placebo response rate of 57% for the IBS-SSS, which is typical of trials in IBS and other disorders involving gut-brain interactions [
31]. Although the current study showcased robust results for several parameters, including the primary endpoint of lactulose/mannitol ratio, conducting the study in a larger study population may help reach statistical significance for these endpoints.
In conclusion, supplementation with Brightseed® Bio Gut Core (containing 120 mg/day proprietary NCT/NFT blend) for 3 weeks significantly improved physiologically relevant measures of gut barrier integrity and subjective measures of symptom-induced GI discomfort/pain. These results emphasize the restorative potential of NCT and NFT to provide gut health barrier support and symptom relief.