Submitted:
27 December 2023
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
- History of a gastrointestinal disorder
- Lactose intolerant
- High fiber consumer (≥15 g per day)
- Use of pre-and probiotics in the past 90 days
- High protein consumer (i.e. vegetarians or those who follow diets high in protein such as paleo)
- History of psychological illness or conditions that may interfere with subjects ability to understand study directions
- Use of antibiotics or signs of active systemic infection in the last 6 months. Subjects who are on hypo/hypercaloric diet aiming for weight loss or weight gain
- History or presence of cancer in the prior 2 years (except for non-melanoma skin cancer).
- Currently pregnant, lactating or planning to be pregnant during the study period
- Regular use of dietary supplements (ex: fish oil, riboflavin, etc.), 90 days prior to study inclusion
- Exposure to any non-registered drug product within the last 30 days prior to screening visit
- History of or strong potential for alcohol or substance abuse (within 12 months of screening visit). Alcohol abuse is defined as >60g (men)/40g (women) pure alcohol per day (1.5 L/ 1 l beer resp. 0.75l/0.5l wine).
- Current smoker or use of tobacco products in the past 90 days
- Concurrent or recent participation (30 days) in a dietary intervention trial
2.2. Material and Characteristics
2.3. Supplementation and dosages
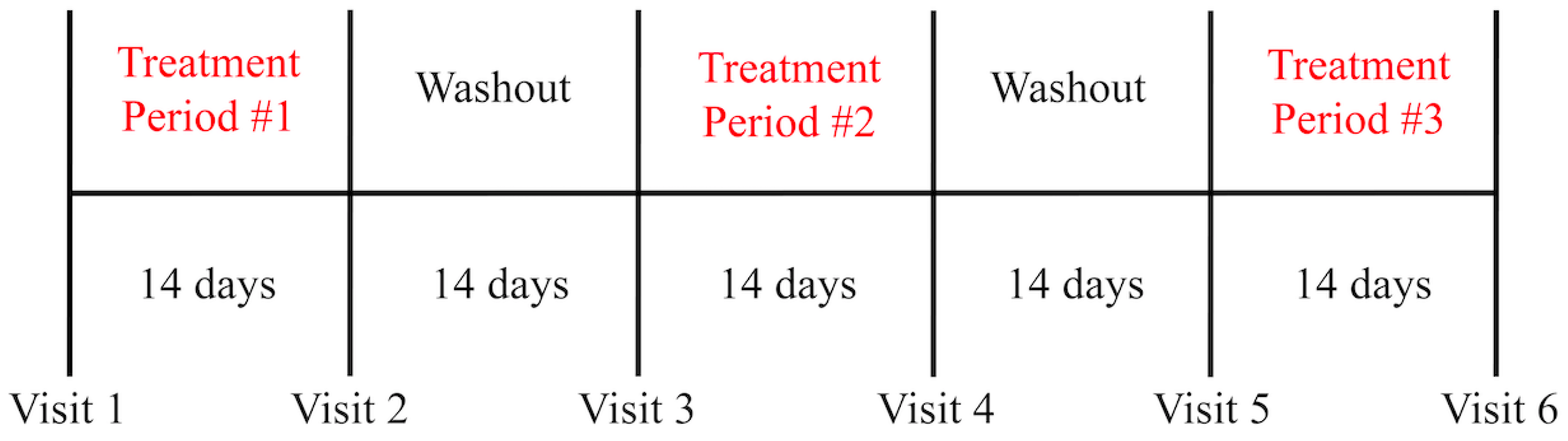
2.4. Study Design and Protocol
2.5. 16S rRNA Sequencing
2.6. Salivary cortisol
2.7. Bristol Stool Form Scale
2.8. Statistical Analysis
3. Results
3.1. Microbiome Composition
| ● | Mean Change (Day 0-Day 14) | p-value | ||||
| ● | Treatment 1 (6 g PHGG) |
Treatment 2 (3 g PHGG) |
Treatment 3 (Control) |
● 1 vs 2 | ● 1 vs 3 | ● 2 vs 3 |
| ● Phylum Relative | ||||||
| Verrucomicrobia | ● 0.0089 + 0.0324 | ● 0.0015 + 0.0258 | ● -0.0082 + 0.0318 | ● 0.0102 | ||
| ● Class Relative | ||||||
| Verrucomicrobiae | ● 0.0092 + 0.0328 | ● 0.0011 + 0.0256 | ● -0.0082 + 0.0322 | ● 0.0092 | ||
| ● Order Relative | ||||||
| Verrucomicrobiales | ● 0.0094 + 0.0335 | ● 0.0012 + 0.0262 | ● -0.0083 + 0.0328 | ● 0.0093 | ||
| ● Family Relative | ||||||
| ● Verrucomicrobiaceae | ● 0.0099 + 0.0357 | ● 0.0012 + 0.0278 | ● -0.0087 + 0.0347 | ● 0.0108 | ||
| ● Genus Relative | ||||||
| Faecalibacterium | ● -0.0166 + 0.0529 | ● -0.0371 + 0.0458 | ● -0.0014 + 0.0541 | ● 0.0054 | ||
| Oscillospira | ● -0.0102 + 0.0345 | ● 0.0059 + 0.0247 | ● -0.0128 + 0.0340 | ● 0.0374 | ● 0.0156 | |
| Akkermansia | ● 0.0094 + 0.0342 | ● 0.0015 + 0.0275 | ● -0.0083 + 0.0333 | ● 0.0116 | ||
| ● | Mean Change (Day 0-Day 14) | p-value | ||||
| ● | Treatment 1 (6 g PHGG) |
Treatment 2 (3 g PHGG) |
Treatment 3 (Control) |
● 1 vs 2 | ● 1 vs 3 | ● 2 vs 3 |
| ● Class Counts | ||||||
| Erysipelotrichi | -92.5 + 429.2 | 9.8 + 134.0 | 143.3 + 463.1 | ● 0.0092 | ||
| ● Order Counts | ||||||
| Erysipelotrichales | -92.5 + 429.2 | 9.8 + 134.0 | 143.3 + 463.1 | ● 0.0092 | ||
| ● Genus Counts | ||||||
| Faecalibacterium | ● -799.2 + 1831.6 | ● -1586.5 + 2504.4 | ● -37.1 + 2864.3 | ● 0.0090 | ||
3.2. Salivary Cortisol
3.3. Stool Consistency
| Type 1-2 (Constipation) | Type 3-4 (Normal) | Type 5-7 (Loose Stool) | |
| Placebo | 21.2% | 39.4% | 39.4% |
| 3 g PHGG | 21.2% | 36.4% | 42.4% |
| 6 g PHGG | 25% | 50% | 25% |
3.4. Safety Aspects
4. Discussion
References
- Slavin JL, Greenberg NA. Partially hydrolyzed guar gum: Clinical nutrition uses. Nutrition. 2003 Jun 1;19(6):549–52. [CrossRef]
- Setayesh L, Pourreza S, Khosroshahi MZ, Asbaghi O, Bagheri R, Kelishadi MR, et al. The effects of guar gum supplementation on lipid profile in adults: a GRADE-assessed systematic review, meta-regression and dose–response meta-analysis of randomised placebo-controlled trials. British Journal of Nutrition. 2022 Jul 15;1–11. [CrossRef]
- Alaeian MJ, Pourreza S, Yousefi M, Golalipour E, Setayesh L, Khosroshahi MZ, et al. The effects of guar gum supplementation on glycemic control, body mass and blood pressure in adults: A GRADE-assessed systematic review and meta-analysis of randomized clinical trials. Diabetes Research and Clinical Practice. 2023 May 1;199:110604. [CrossRef]
- Giannini EG, Mansi C, Dulbecco P, Savarino V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition. 2006 Mar 1;22(3):334–42. [CrossRef]
- Cuomo R, Russo L, Sarnelli G, Savino I, Vozzella L, Zito F, et al. Partially hydrolyzed guar gum in the treatment of irritable bowel syndrome with constipation: Effects of gender, age, and body mass index. Saudi J Gastroenterol. 2015;21(2):104. [CrossRef]
- Niv E, Halak A, Tiommny E, Yanai H, Strul H, Naftali T, et al. Randomized clinical study: Partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr Metab (Lond). 2016 Feb 6;13:10. [CrossRef]
- Parisi GC, Zilli M, Miani MP, Carrara M, Bottona E, Verdianelli G, et al. High-Fiber Diet Supplementation in Patients with Irritable Bowel Syndrome (IBS): A Multicenter, Randomized, Open Trial Comparison Between Wheat Bran Diet and Partially Hydrolyzed Guar Gum (PHGG). Dig Dis Sci. 2002 Aug 1;47(8):1697–704. [CrossRef]
- Kapoor MP, Koido M, Kawaguchi M, Timm D, Ozeki M, Yamada M, et al. Lifestyle related changes with partially hydrolyzed guar gum dietary fiber in healthy athlete individuals – A randomized, double-blind, crossover, placebo-controlled gut microbiome clinical study. Journal of Functional Foods. 2020 Sep 1;72:104067. [CrossRef]
- Ohashi Y, Sumitani K, Tokunaga M, Ishihara N, Okubo T, Fujisawa T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef Microbes. 2015;6(4):451–5. [CrossRef]
- Okubo T, Ishihara N, Takahashi H, Fujisawa T, Kim M, Yamamoto T, et al. Effects of Partially Hydrolyzed Guar Gum Intake on Human Intestinal Microflora and Its Metabolism. Bioscience, Biotechnology, and Biochemistry. 1994 Jan 1;58(8):1364–9. [CrossRef]
- Yasukawa Z, Inoue R, Ozeki M, Okubo T, Takagi T, Honda A, et al. Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial. Nutrients. 2019 Sep 10;11(9):2170. [CrossRef]
- Pylkas AM, Juneja LR, Slavin JL. Comparison of Different Fibers for In Vitro Production of Short Chain Fatty Acids by Intestinal Microflora. Journal of Medicinal Food. 2005 Mar;8(1):113–6. [CrossRef]
- Inoue R, Sakaue Y, Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J Clin Biochem Nutr. 2019 May;64(3):217–23. [CrossRef]
- Kapoor MP, Sugita M, Fukuzawa Y, Okubo T. Impact of partially hydrolyzed guar gum (PHGG) on constipation prevention: A systematic review and meta-analysis. Journal of Functional Foods. 2017 Jun 1;33:52–66. [CrossRef]
- Polymeros D, Beintaris I, Gaglia A, Karamanolis G, Papanikolaou IS, Dimitriadis G, et al. Partially Hydrolyzed Guar Gum Accelerates Colonic Transit Time and Improves Symptoms in Adults with Chronic Constipation. Dig Dis Sci. 2014 Sep;59(9):2207–14. [CrossRef]
- Ustundag G, Kuloglu Z, Kirbas N, Kansu A. Can partially hydrolyzed guar gum be an alternative to lactulose in treatment of childhood constipation? Turk J Gastroenterol. 2010 Dec 1;21(4):360–4. [CrossRef]
- Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A, et al. Health benefits of dietary fiber. Nutrition Reviews. 2009 Apr 1;67(4):188–205. [CrossRef]
- Abe A, Morishima S, Kapoor MP, Inoue R, Tsukahara T, Naito Y, et al. Partially hydrolyzed guar gum is associated with improvement in gut health, sleep, and motivation among healthy subjects. J Clin Biochem Nutr. 2023 Mar;72(2):189–97. [CrossRef]
- Williams L, Slavin JL. Dietary Fiber and Other Alternative Therapies and Irritable Bowel Syndrome. Topics in Clinical Nutrition. 2009 Sep;24(3):262. [CrossRef]
- Kwa WT, Sundarajoo S, Toh KY, Lee J. Application of emerging technologies for gut microbiome research. Singapore Med J. 2023 Jan 19;64(1):45–52. [CrossRef]
- Swanson KS, de Vos WM, Martens EC, Gilbert JA, Menon RS, Soto-Vaca A, et al. Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: a review. Benef Microbes. 2020 Mar 27;11(2):101–29. [CrossRef]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl Environ Microbiol. 2007 Aug;73(16):5261–7. [CrossRef]
- Taylor AM, Holscher HD. A review of dietary and microbial connections to depression, anxiety, and stress. Nutritional Neuroscience. 2020 Mar 3;23(3):237–50. [CrossRef]
- Haarhuis JE, Kardinaal A, Kortman G a. M. Probiotics, prebiotics and postbiotics for better sleep quality: a narrative review. Benef Microbes. 2022 Aug 3;13(3):169–82. [CrossRef]
- Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PWJ. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl). 2015;232(10):1793–801. [CrossRef]
- Mysonhimer AR, Cannavale CN, Bailey MA, Khan NA, Holscher HD. Prebiotic Consumption Alters Microbiota but Not Biological Markers of Stress and Inflammation or Mental Health Symptoms in Healthy Adults: A Randomized, Controlled, Crossover Trial. The Journal of Nutrition. 2023 Apr 1;153(4):1283–96. [CrossRef]
- Shokouhi N, Mohammadi S, Ghanbari Z, Montazeri A. Development of a new version of the Bristol Stool Form Scale: translation, content validity, face validity, and reliability of the Persian version. BMJ Open Gastroenterol. 2022 Dec 23;9(1):e001017. [CrossRef]
- Peng Z, Yi J, Liu X. A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients. 2022 May 15;14(10):2072. [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients. 2013 Apr 22;5(4):1417–35. [CrossRef]
- Reider SJ, Moosmang S, Tragust J, Trgovec-Greif L, Tragust S, Perschy L, et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota—Results from the PAGODA Trial. Nutrients. 2020 Apr 28;12(5):1257. [CrossRef]
- Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms. 2018 Jul 23;6(3):75. [CrossRef]
- Belzer C, de Vos WM. Microbes inside—from diversity to function: the case of Akkermansia. ISME J. 2012 Aug;6(8):1449–58. [CrossRef]
- Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020 Sep;74(9):1251–62. [CrossRef]
- Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, et al. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS One. 2013 Aug 27;8(8):e71108. [CrossRef]
- Rajilić-Stojanović M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013 Mar;19(3):481–8. [CrossRef]
- Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, et al. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Official journal of the American College of Gastroenterology | ACG. 2010 Nov;105(11):2420. [CrossRef]
- Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020 Apr 15;8(4):573. [CrossRef]
- Crost EH, Tailford LE, Le Gall G, Fons M, Henrissat B, Juge N. Utilisation of Mucin Glycans by the Human Gut Symbiont Ruminococcus gnavus Is Strain-Dependent. PLoS One. 2013 Oct 25;8(10):e76341. [CrossRef]
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 2016 Dec 15;167(7):1897. [CrossRef]
- Kaakoush, NO. Sutterella Species, IgA-degrading Bacteria in Ulcerative Colitis. Trends in Microbiology. 2020 Jul 1;28(7):519–22. [CrossRef]
- Kaakoush, NO. Insights into the Role of Erysipelotrichaceae in the Human Host. Front Cell Infect Microbiol. 2015 Nov 20;5:84. [CrossRef]
- Chen W, Liu F, Ling Z, Tong X, Xiang C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS One. 2012 Jun 28;7(6):e39743. [CrossRef]
- Laursen MF, Laursen RP, Larnkjær A, Mølgaard C, Michaelsen KF, Frøkiær H, et al. Faecalibacterium Gut Colonization Is Accelerated by Presence of Older Siblings. mSphere. 2017 Nov 29;2(6):e00448-17. [CrossRef]
- Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine. 2017 Apr 8;15(1):73. [CrossRef]
- Moayyedi P, Quigley EMM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. The Effect of Fiber Supplementation on Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Official journal of the American College of Gastroenterology | ACG. 2014 Sep;109(9):1367. [CrossRef]
- Nagarajan N, Morden A, Bischof D, King EA, Kosztowski M, Wick EC, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. European Journal of Gastroenterology & Hepatology. 2015 Sep;27(9):1002. [CrossRef]
- So D, Gibson PR, Muir JG, Yao CK. Dietary fibres and IBS: translating functional characteristics to clinical value in the era of personalised medicine. Gut. 2021 Dec 1;70(12):2383–94. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





