Submitted:
25 April 2025
Posted:
27 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Collection
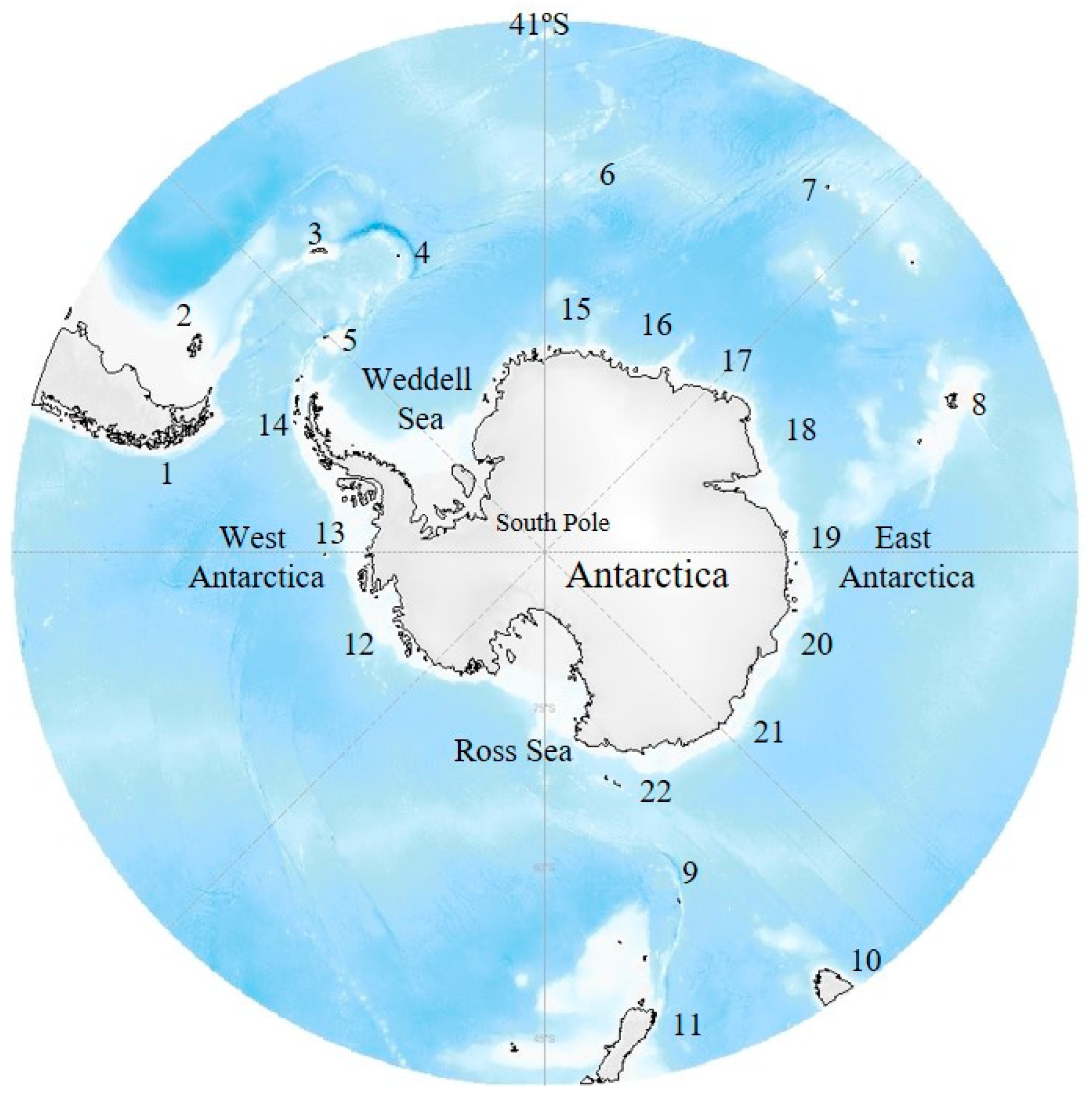
2.2. Geographic Areas and Zones
3. Results.
3.1. General Biodiversity Results
3.2. The Sea Slugs of Patagonia/Magellan Zone
3.3. The Sea Slugs of the Subantarctics Zones
3.4. The Sea Slugs of Tasmania
3.5. The Sea Slugs of Southern Island of New Zealand
3.6. The Antarctic Sea Slugs
3.7. The Pteropoda of the Southern Ocean
3.8. Taxonomy and Records








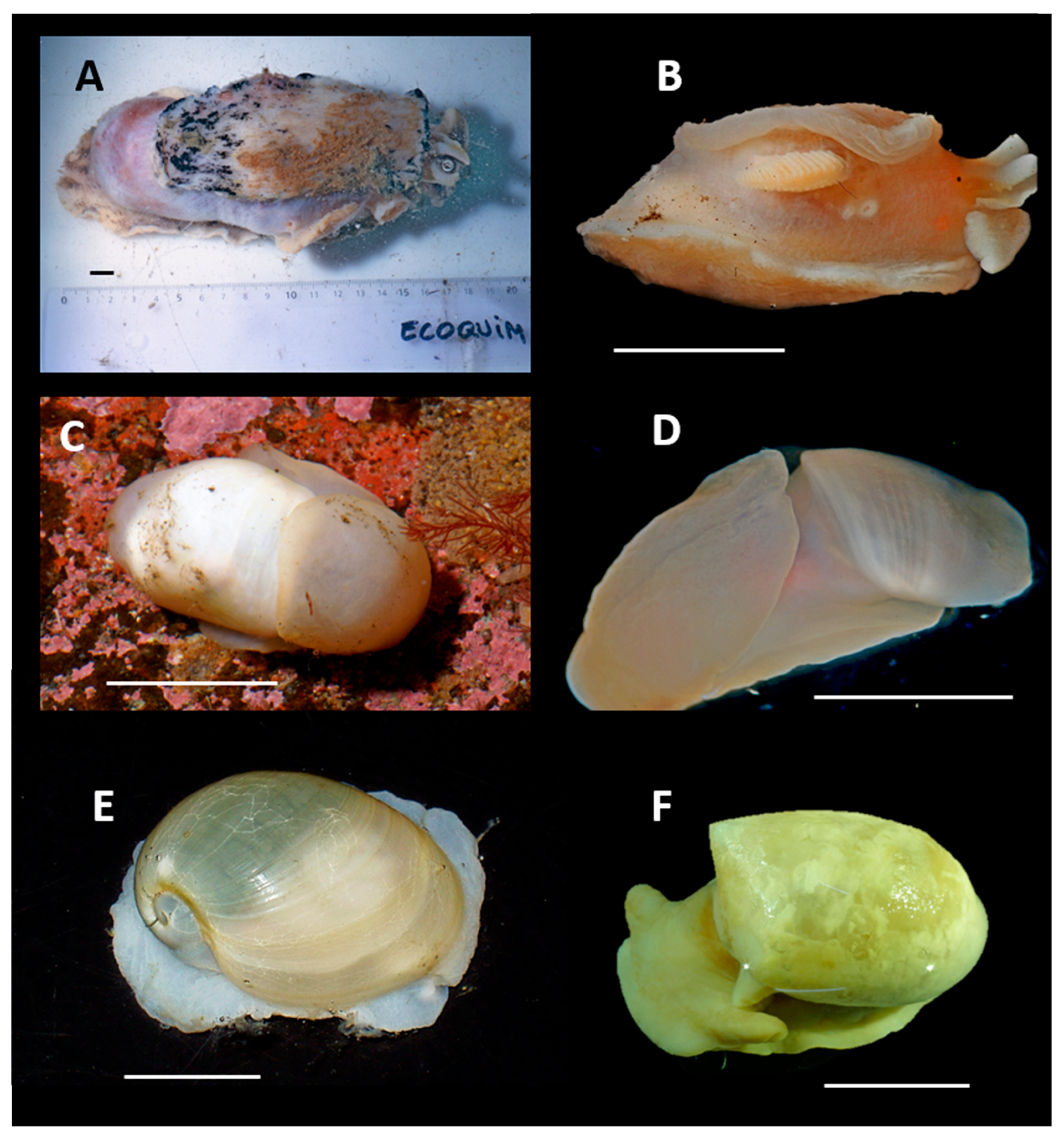




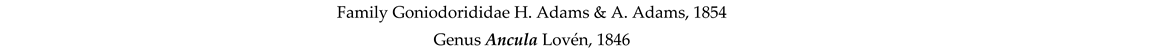

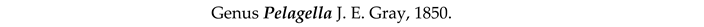
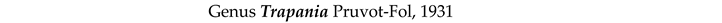
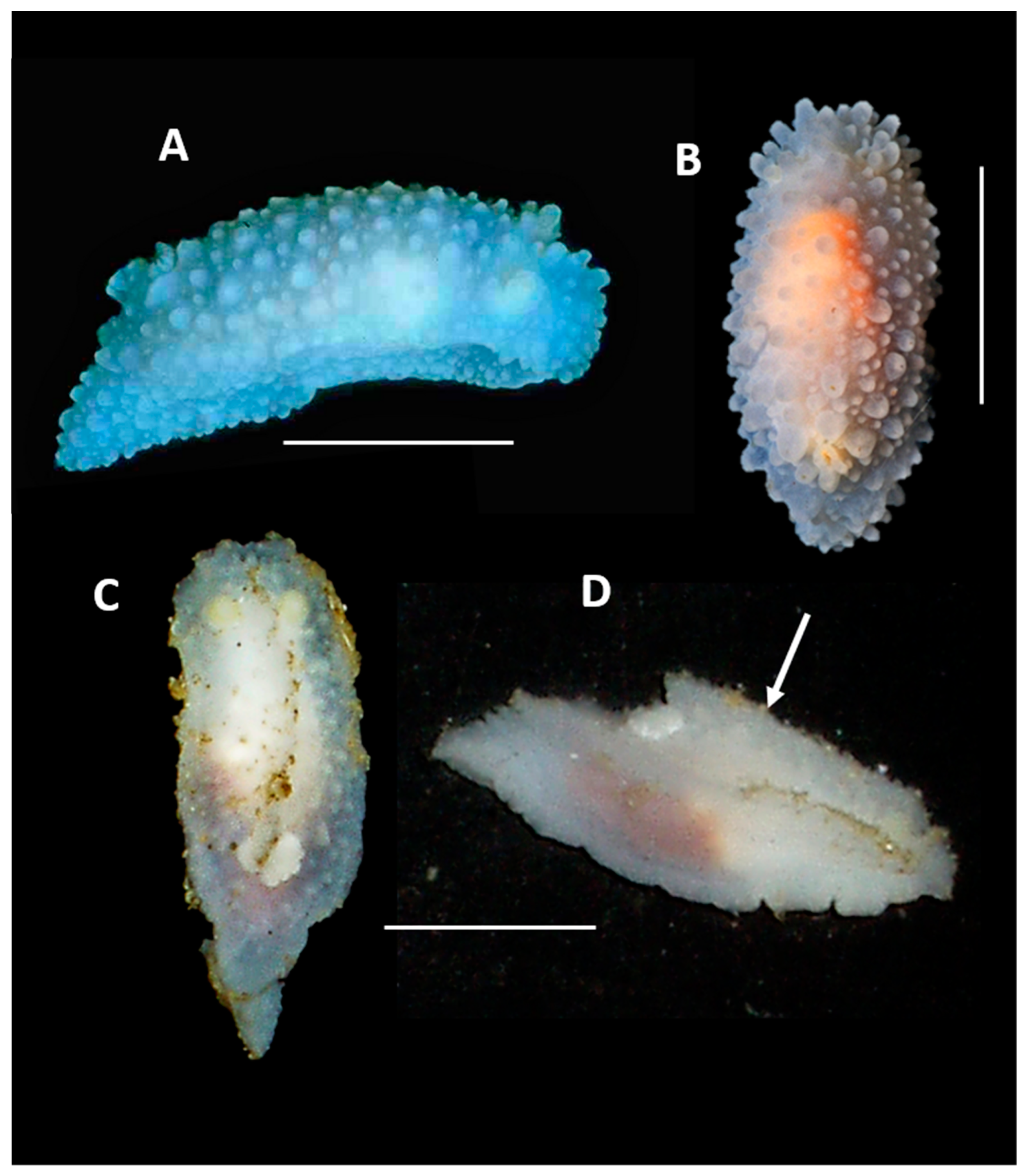




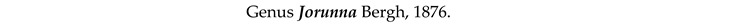
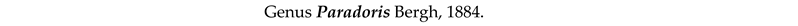
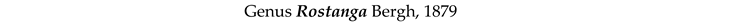
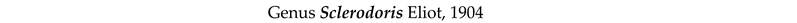





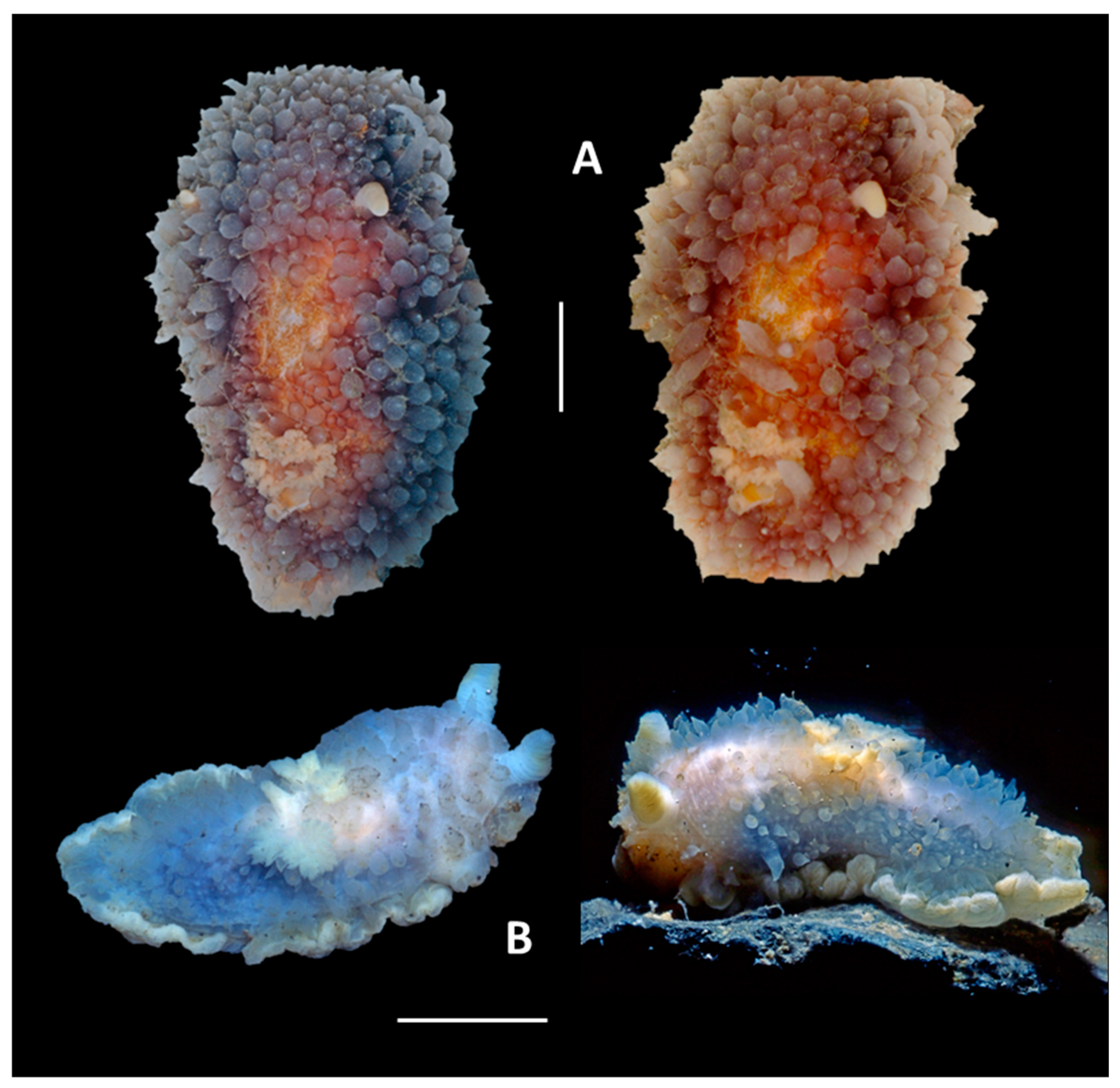





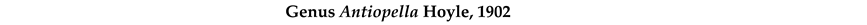

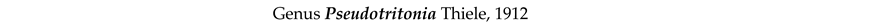





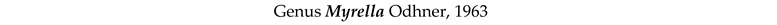
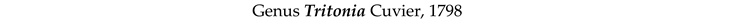
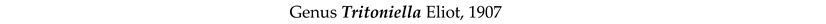

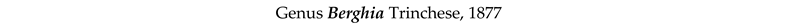
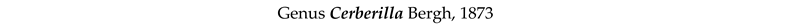
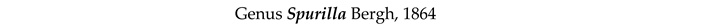
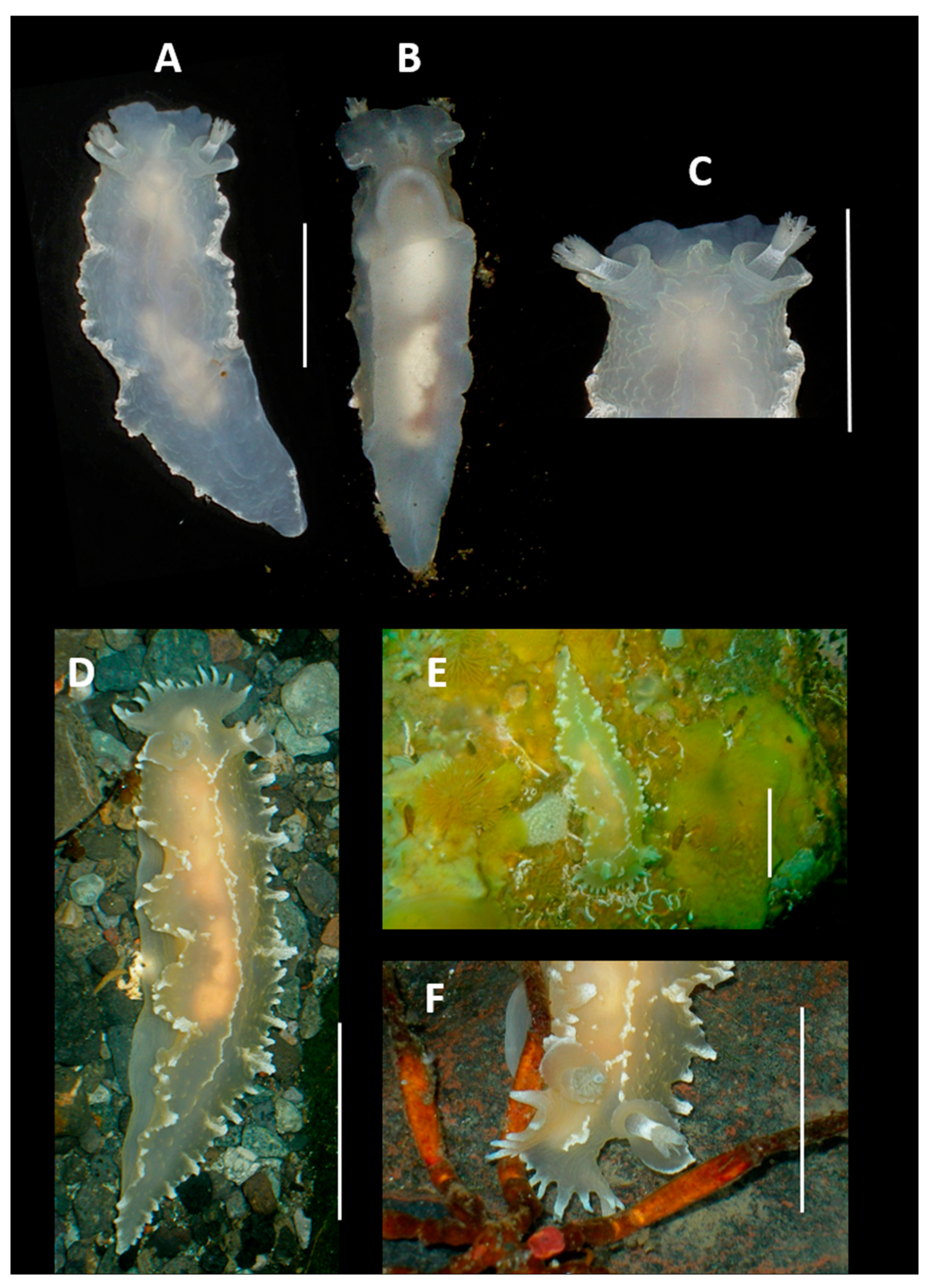
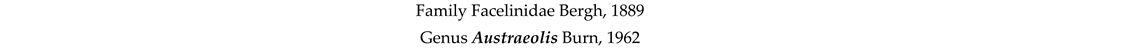
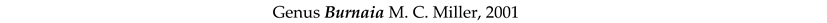
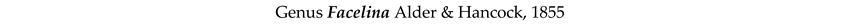
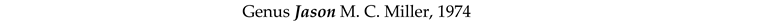






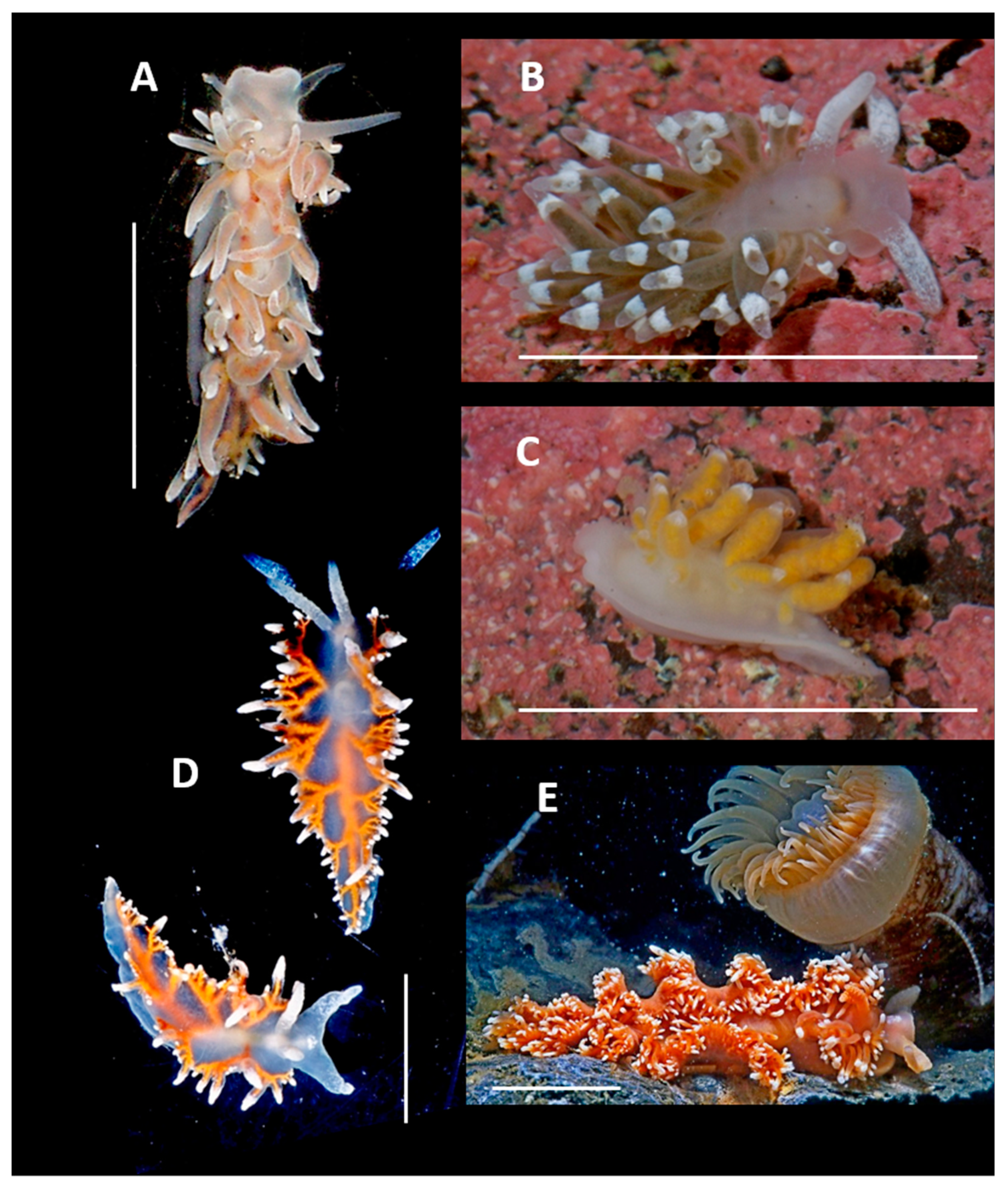
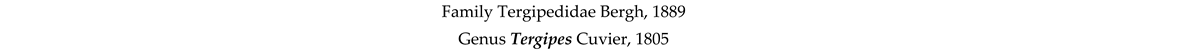
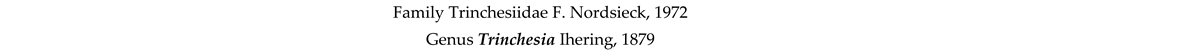







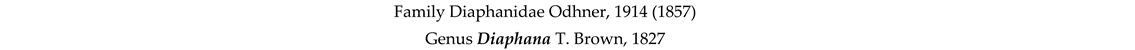

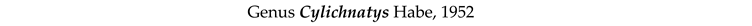
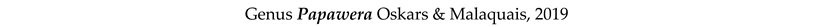
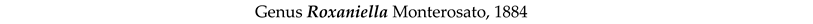
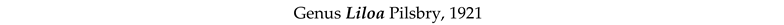

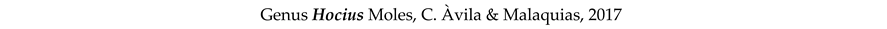
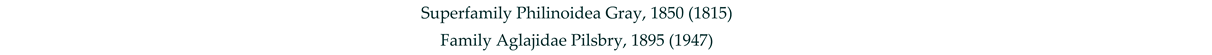
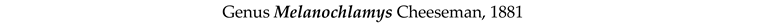




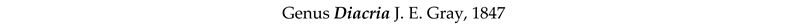
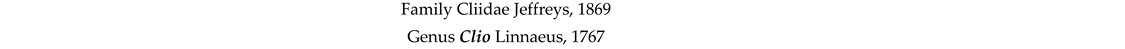
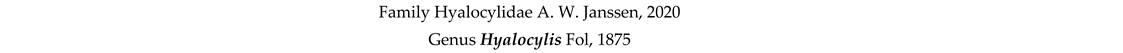
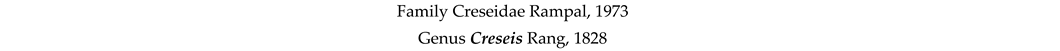

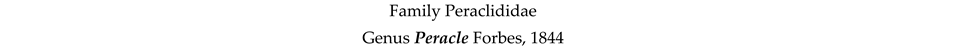

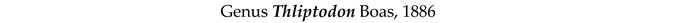
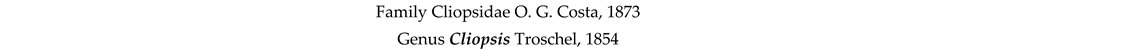
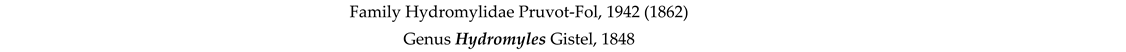
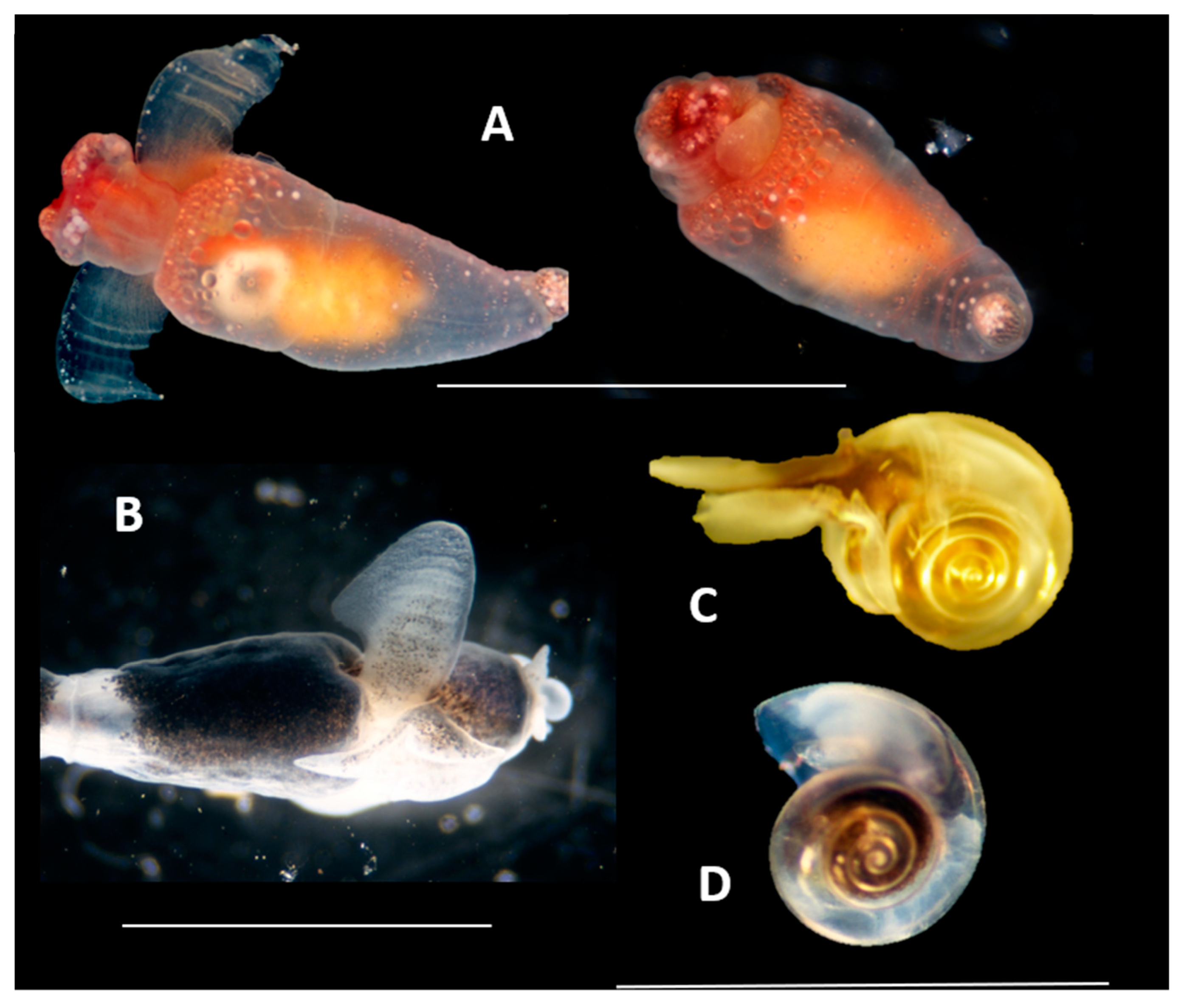

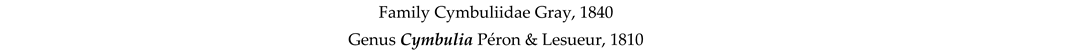

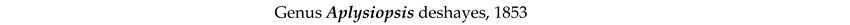


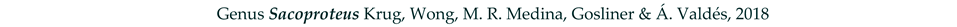




4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Abraham, P. S. Notes on some genera of nudibranchiate Mollusca, with notices of a new genus and some hitherto undescribed species, in the collection of the British Museum. Annals and Magazine of Natural History 1876, 18(104), 132–146. [Google Scholar] [CrossRef]
- Aldea, C.; Césped, T.; Rosenfeld, S. sea slugs from Bernardo O´Higgings National Park (S. Chile). Thalassas 2011, 27(2), 37–48. [Google Scholar]
- Aldea, C.; Olabarria, C.; Troncoso, J. S. Bathymetric zonation and diversity gradient of gastropods and bivalves in West Antarctica from the South Shetland Islands to the Bellingshausen Sea. Deep-Sea Research Part I: Oceanographic Research Papers 2008, 55(3), 350–368. [Google Scholar] [CrossRef]
- Aldea, C.; Olabarria, C.; Troncoso, J.S. Spatial patterns of benthic diversity in molluscs from West Antarctica. Antarctic Science 2009, 21(4), 341–353. [Google Scholar] [CrossRef]
- Aldea, C.; Troncoso, J. S. Systematic and distribution of shelled mollusks (Gastropoda, Bivalvia and Scaphopoda) from the South Shetland Island to the Bellingshausen Sea, West Antarctica. Iberus 2008, 26(2), 43–117. [Google Scholar]
- Aldea, C. & Troncoso, J.S. (2010). Moluscos del Mar de Bellinghausen (Antártica), Vigo, Spain, 250 pp.
- Apte, D. Opisthobranch fauna of Lakshadweep Islands, India, with 52 new records to Lakshadweep and 40 new records to India: part 1. Journal of the Bombay Natural History Society 2009, 106(2), 162–175. [Google Scholar]
- Apte, D.; Bhave, V.; Parasharya, D. An annotated and illustrated checklist of the opisthobranch fauna of Gulf of Kutch, Gujarat, India, with 21 new records for Gujarata and 13 new records from India: Part 1. Journal of the Bombay Natural History Society 2010, 107(1), 14–23. [Google Scholar]
- Arias, A.; Crocetta, F. Umbraculum umbraculum (Gastropoda: Heterobranchia) spreading northwards: additional evidence to the “tropicalization” of the Bay of Biscay. Cahiers de Biologie Marine 2016, 57, 285–286. [Google Scholar]
- Arnaud, P.M.; Troncoso, J.S.; Ramos, A. Species diversity and assemblage of macrobenthic Mollusca from the South Shetland Islands and Bransfield Strait (Antarctica). Polar Biology 2001, 24, 105–112. [Google Scholar] [CrossRef]
- Arntz, W. E.; Thatje, S.; Linse, K.; Avila, C.; Ballesteros, M.; Barnes, D. K.; Teixido, N. Missing link in the Southern Ocean: sampling the marine benthic fauna of remote Bouvet Island. Polar Biology 2006, 29(2), 83–96. [Google Scholar] [CrossRef]
- Australian Antarctic Data Centre. Occurrence data set. 2024. Available online: https://data.aad.gov.au/aadc/biodiversity/ (accessed on 4 September 2024).
- Avila, C.; Iken, K.; Fontana, A.; Cimino, G. Chemical ecology of the Antarctic nudibranch Bathydoris hodgsoni Eliot, 1907: defensive role and origin of its natural products. Journal of experimental marine biology and ecology 2000, 252(1), 27–44. [Google Scholar] [CrossRef] [PubMed]
- Bakker, H, Gill. Naturalis Biodiversity Center (NL) - Mollusca. Naturalis Biodiversity Center. Occurrence dataset. 2024. Available online: https://www.gbif.org/occurrence/4863291182. [CrossRef]
- Ballesteros, M. “Biological cycle of two outstanding nudibranchs from Antarctica” in OPK-Opistobranquis. Published: 08/12/2024. 2024. Available online: http://opistobranquis.info/en/?p=45501 (accessed on 8 December 2024).
- Ballesteros, M.; Avila, C. A new tritoniid species (Mollusca: Opisthobranchia) from Bouvet Island. Polar Biology 2006, 29(2), 128–136. [Google Scholar] [CrossRef]
- Barnes, D. K.; Bullough, L. W. Some observations on the diet and distribution of nudibranchs at Signy Island, Antarctica. Journal of Molluscan Studies 1996, 62(3), 281–287. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; _Downey, R.V. Chapter 5.23. Bryzoa. In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Scientific Committee on Antarctic Research; Cambridge, 2014; pp. 195–199. [Google Scholar]
- Basher, Z.; Costello, M.J. Chapter 5.22. Shrimps (Crustacea: Decapoda). In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Scientific Committee on Antarctic Research; Cambridge, 2014; pp. 190–194. [Google Scholar]
- Bastida, R.; Roux, A.; Martinez, D. E. Benthic communities of the Argentine continental shelf. Oceanologica Acta 1992, 15(6), 687–698. [Google Scholar]
- Battini, N.; Giachetti, C. B.; Castro, K. L.; Bartolus, A.; Schwindt, E. New invasive predator reduces the abundance of native prey in a cold-temperate marine fouling community. Aquatic Conservation Marine and Freshwater Ecosystems 2021, 1–13. [Google Scholar] [CrossRef]
- Beesley, P.; Ross, G.; Wells, A. Mollusca: The Southern Synthesis; Australian Biological Resources, CSIRO Publishing; Melbourne, Australia, 1998. [Google Scholar]
- Behrens, D. W.; Hermosillo, A.; Fletcher, K.; Jensen, G. C. Eastern pacific nudibranchs; Sea Challengers, 2022. [Google Scholar]
- Bergh, R. Report on the Nudibranchiata. Challenger reports, Zoology 10(26), 1–154.
- Bergh, R. Die Opisthobranchier der Sammlung Plate. Zoologische Jahrbücher, Supplement 4, 481–582.
- Bergh, R. Malacologische Untersuchungen. In Reisen im Archipel der Philippinen von Dr. Carl Gottfried Semper; Zweiter Theil. Wissenschaftliche Resultate, 1904; Vol. 9, Chapter 6, Part 1-56, pp. 1–56. [Google Scholar]
- Bergh, R. Die Opisthobranchiata der Siboga-expedition. Siboga-Expeditie. 1905, 50, 1–248. [Google Scholar]
- Bouchet, P. Toledonia lymnaeaeformis (E. A. Smith, 1879). In: MolluscaBase (2017). 2010. Available online: http://marinespecies.org/aphia.php?p=taxdetails&id=197475 (accessed on 7 February 2018).
- Bouchet, P.; Decock, W.; Lonneville, B.; Vanhoorne, B.; Vandepitte, L. Marine biodiversity discovery: the metrics of new species descriptions. Frontiers in Marine Science 2023, 10, 929989. [Google Scholar] [CrossRef]
- Bowen, M. M.; Wilkin, J. L.; Emery, W. J. Variability and forcing of the East Australian Current. Journal of Geophysical Research: Oceans 2005, 110, C03019. [Google Scholar] [CrossRef]
- Branch, M.L.; Arnaud, P.M.; Cantera, J.; Gianakouras, D. The Benthic Mollusca and Brachiopoda of Subantarctic Marion and Prince Edward Islands: 1) Illustrated Keys to the Species 2) Records of the 1982-1989 University of Cape Town Surveys. South African Journal of Antarctic Research 1991, 21(1), 45–64. [Google Scholar]
- Brandt, A.; De Broyer, C.; De Mesel, I.; Ellingsen, K. E.; Gooday, A. J.; Hilbig, B.; Tyler, P. A. The biodiversity of the deep Southern Ocean benthos. Philosophical Transactions of the Royal Society B: Biological Sciences 2007, 362(1477), 39–66. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.C.; Bowden, B.W. A realignment of the marine biogeographic provinces with particular reference for fish distributions. Journal of biogeography 2012, 39, 12–30. [Google Scholar] [CrossRef]
- Brueggeman, P.; Wu, N.; Bosch, I.; Bowser, S. Underwater Field Guide to Ross Island & McMurdo Sound, Antarctica; Scripps Institution of Oceanography Library, 1999. [Google Scholar]
- Burn, R. Notes on some opisthobranchs mainly from South Australia. Records of the South Australian Museum 1966, 15(2), 329–352. [Google Scholar]
- Burn, R. Opisthobranch molluscs from the Australian sub-Antarctic territories of Macquarie and Heard Islands. Proceedings of the Royal Society of Victoria 1973, 86, 39–46. [Google Scholar]
- Burn, R. A checklist and bibliography of the Opisthobranchia (Mollusca: Gastropoda) of Victoria and the Bass Strait area, south-eastern Australia. Museum Victoria Science Reports 2006, 10, 1–42. [Google Scholar] [CrossRef]
- Burn, R. (2015). Nudibranchs and related molluscs. Museum Victoria Field Guides. Melbourne, Australia.
- Burridge, A. K.; Goetze, E.; Wall-Palmer, D.; Le Double, S. L.; Huisman, J.; Peijnenburg, K. T. C. A. Diversity and abundance of pteropods and heteropods along a latitudinal gradient across the Atlantic Ocean. Progress in Oceanography 2017, 158, 213–223. [Google Scholar] [CrossRef]
- Butler, A. J.; Rees, T.; Beesley, P.; Bax, N. J. Marine Biodiversity in the Australian Region. PLoS ONE 2010, 5(8), e11831. [Google Scholar] [CrossRef]
- Carcelles, A. Catálogo de la Malacofauna Antártica Argentina. Anales del Museo del Nahuel Huapi 1953, 3, 155–250. [Google Scholar]
- Carcelles, A. R.; Williamson, S. I. Catálogo de los moluscos marinos de la provincia magallánica. Revista del Instituto Nacional de Investigación de Ciencias Naturales, Ciencias Zoológicas 1951, 2(5), 225–383. [Google Scholar]
- Cárdenas, J.; Aldea, C.; Valdovinos, C. Chilean marine mollusca of northern Patagonia collected during the CIMAR-10 Fjords Cruise. Gayana 2008, 72(2), 202–240. [Google Scholar] [CrossRef]
- Carmona, L.; Lei, B. R.; Pola, M.; Gosliner, T. M.; Valdés, A.; Cervera, J. L. Untangling the Spurilla neapolitana (Delle Chiaje, 1841) species complex: a review of the genus Spurilla Bergh, 1864 (Mollusca: Nudibranchia: Aeolidiidae). Zoological Journal of the Linnean Society 2014, 170(1), 132–154. [Google Scholar] [CrossRef]
- Castellanos, Z. A.; Bartolotta, S.; Rolán, E. Aportes a la malacofauna del talud superior del Atlántico Sur. Thalassas 1987a, 5, 57–70. [Google Scholar]
- Castellanos, Z. A.; Rolán, E.; Bartolotta, S. Nuevos micromoluscos de la plataforma inferior Argentina y talud superior (Moll. Gastropoda). Revista del Museo de la Plata, Zoología 1987b, (2)(14 (156)), 93–107. [Google Scholar]
- Castellanos, Z. A.; Landoni, N. A.; Dadon, J. R. Catálogo descriptivo de la malacofauna marina Magallánica 12. Opisthobranchia. Comisión de Investigaciones Científicas, Buenos Aires. 1993, 1–299. [Google Scholar]
- Cattaneo-Vietti, R. Nudibranch Molluscs from the Ross Sea, Antarctica. Journal of Molluscan Studies 1991, 57 Supplement Part 4, 223–228. [Google Scholar] [CrossRef]
- Cattaneo-Vietti, R.; Chiantore, M; Schiaparelli, S.; Albertelli, G. Shallow-and deep-water mollusc distribution at Terra Nova Bay (Ross Sea, Antarctica). Polar Biology 2000, 23, 173–182. [Google Scholar] [CrossRef]
- Chaban, E.M. New genus. Ruthenica 2016, 26(1), 49–56. [Google Scholar]
- Chaban, E. M.; Ekimova, I. A.; Chernyshev, A. V. Philinopsis gigliolii (Gastropoda: Heterobranchia: Aglajidae) from the Sea of Japan: validity, synonymy and biogeography. Invertebrate Zoolology 2024, 21(2), 157–169. [Google Scholar] [CrossRef]
- Challis, D. A. New species of Pseudovermis (Opisthobranchia: Aeolidacea) from New Zealand and the Solomon Islands. Transactions of the Royal Society of New Zealand 1969, 11(10), 153–165. [Google Scholar]
- Cheeseman, T. On a new genus of Opisthobranchiate Mollusca. Transactions and Proceedings of the Royal Society of New Zealand 13, 224.
- Churchill, C. K.; Valdés, A.; Foighil, D. Ó. Molecular and morphological systematics of neustonic nudibranchs (Mollusca: Gastropoda: Glaucidae: Glaucus), with descriptions of three new cryptic species. Invertebrate Systematics 2014, 28(2), 174–195. [Google Scholar] [CrossRef]
- Cimino, G.; Ghiselin, M. T. Chemical Defense and Evolution of Opisthobranch Gastropods. California Academy of Sciences. 2009. [Google Scholar]
- Clark, K. B. Nudibranch life cycles in the Northwest Atlantic and their relationship to the ecology of fouling communities. Helgoländer Wissenschaftliche Meeresuntersuchungen 1975, 27(1), 28–69. [Google Scholar] [CrossRef]
- Cobb, G.; Willan, R. C. Undersea Jewels: A colour guide to Nudibranchs. Australian Biological Resources Study, Canberra, Australia . 2006. [Google Scholar]
- Coleman, N. 1001 Nudibranchs: catalogue of Indo-Pacific sea slugs; Neville Coleman’s Underwater Geographic Pty Ltd, 2001. [Google Scholar]
- Coleman, N. (2008). Nudibranchs encyclopedia (New). Neville Coleman’s Underwater Geographic.
- Coleman, N. Nudibranchs encyclopedia-catalogue of Asia/Indo Pacific sea slugs, second edition (New); Neville Coleman’s Underwater Geographic, 2015. [Google Scholar]
- Cresswell, G. R.; Peterson, J. L.; Pender, L. F. The East Australian Current, upwellings and downwellings off eastern-most Australia in summer. Marine and Freshwater Research 2016, 68(7), 1208–1223. [Google Scholar] [CrossRef]
- Dadon, J. R.; Chauvin, S. F. Distribution and abundance of Gymnosomata (Gastropoda: Opisthobranchia) in the Southwest Atlantic. Journal of Molluscan Studies 1998, 64, 345–354. [Google Scholar] [CrossRef]
- Danis, B.; Griffiths, H. J.; Jangoux, M. Chapter 5.24. Asteroidea. In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Scientific Committee on Antarctic Research; Cambridge, 2014; pp. 200–207. [Google Scholar]
- Dall, W. H. Illustrations and descriptions of new, unfigured, or imperfectly known shells, chiefly American in the U. S. National Museum. Proceedings of the United States National Museum 1902, 24(1264), 499–566. [Google Scholar] [CrossRef]
- Dayrat, B. A monographic revision of basal discodorid sea slugs (Gastropoda, Opisthobranchia, Nudibranchia, Doridina). Proceedings of the California Academy of Sciences. Series 4 2010, 61 suppl. I, 1–403. [Google Scholar]
- Dayton, P. K.; Robilliard, G. A.; Paine, R. T.; Dayton, L. B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecological Monographs 1974, 44(1), 105–128. [Google Scholar] [CrossRef]
- De Broyer, C.; Jazdzeska, A. Chapter 5.17. Biogeographic patterns of Southern Ocean benthic Amphipoda. In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Scientific Committee on Antarctic Research; Cambridge, 2014; pp. 155–165. [Google Scholar]
- Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H. J., et al., Eds.; Scientific Committee on Antarctic Research, Cambridge, 2014; 498 pp. [Google Scholar]
- Dell, R. K. Antarctic Mollusca: with special reference to the fauna of the Ross Sea. Bulletin Royal Society of New Zealand Bulletin . 1990, 27. [Google Scholar]
- de Vries, J.; Christa, G.; Gould, S. B. Plastid survival in the cytosol of animal cells. Trends in Plant Science 2014, 19(6), 347–350. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos Silva, F.; Pola, M.; Cervera, J. L. A stomach plate to divide them all: a phylogenetic reassessment of the family Tritoniidae (Nudibranchia: Cladobranchia). Zoological Journal of the Linnean Society 2023, 199(2), 445–476. [Google Scholar] [CrossRef]
- Doello-Jurado, M. Dos nuevas especies de moluscos marinos. Physis 1918, 4(17), 259–273. [Google Scholar]
- Edmunds, M. Protective mechanisms in the Eolidacea (Mollusca, Nudibranchia). Zoological Journal of the Linnean Society 1966, 46(308), 27–71. [Google Scholar] [CrossRef]
- Eléaume, M.; Hemery, L. G.; Roux, M.; Améziane, N. Chapter 5.25. Sothern Ocean Crinoids. In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Scientific Committee on Antarctic Research; Cambridge, 2014; pp. 208–212. [Google Scholar]
- Eliot, C. N. E. Notes on some new or little-known members of the family Dorididae. Journal of Molluscan Studies 1903, 5(5), 331–337. [Google Scholar] [CrossRef]
- Eliot, C. N. E. The Nudibranchiata of the Scottish National Antarctic Expedition. Transactions of the Royal Society of Edinburgh 1905, 41 (3), 519–532. [Google Scholar] [CrossRef]
- Eliot C.N.E. (1907a). Mollusca IV.—Nudibranchiata. National Antarctic Expedition 1901–1904. Zoology, 2: 1–28.
- Eliot; C.N.E. Nudibranchs from New Zealand and the Falkland Islands. Proceedings of the Malacological Society of London 1907b, 7, 327–361. [Google Scholar]
- Eliot, C.N.E. The Nudibranchiata of the Scottish National Antarctic Expedition. Report of the Scientific Results of the Voyage of S. Y. “Scotia” during the years 1902, 1903, and 1904, under the leadership of William S. Bruce, Volume V—Zoology, Part II. Nudibranchiata 1909, 11–24. [Google Scholar]
- Engl, W. Shells of Antarctica. ConchBooks . Hackenheim, Germany, 2012; 402 pp. [Google Scholar]
- Farias, N. E.; Obenat, S.; Goya, A. B. Outbreak of a neurotoxic side-gilled sea slug (Pleurobranchaea sp.) in Argentinian coasts. New Zealand Journal of Zoology 2015, 42:1, 51–56. [Google Scholar] [CrossRef]
- Forcelli, D. O. Moluscos Magallánicos; Vázquez Mazzini Editores; Buenos aires, Argentina, 2000. [Google Scholar]
- García, F. J.; Bertsch, H. Diversity and distribution of the Gastropoda Opisthobranchia from the Atlantic Ocean: a global biogeogaphic approach. Scientia Marina 2009, 73(1), 153–160. [Google Scholar] [CrossRef]
- García, F. J.; Troncoso, J. S.; García-Gómez, J. C.; Cervera, J. L. Anatomical and taxonomical studies of the Antarctic nudibranchs Austrodoris kerguelenensis (Bergh, 1884) and A. georgiensis n. sp. from the Scotia Sea. Polar Biology 1993, 13, 417–421. [Google Scholar] [CrossRef]
- García, F. J.; Troncoso, J. S.; Cervera, J. L.; García-Gómez, J. C. Description of the Antarctic notaspidean Polictenidia tomasi n. g. and n. sp. (Gastropoda, Opisthobranchia) from the Scotia Sea, proposing also a new notaspidean tribe. Polar Bíology 1996, 16, 79–85. [Google Scholar] [CrossRef]
- Garcia, F. J.; García-Gómez, J. C.; Troncoso, J. S.; Cervera, J. L. A descriptive study of some antarctic notaspidean opisthobanchs (Gastropoda) with a description of a new genus and species. Polar Biology 1994, 14, 261–268. [Google Scholar] [CrossRef]
- Ghiglione, C.; Alvaro, M. C.; Griffiths, H. J.; Linse, K.; Schiaparelli, S. Ross Sea Mollusca from the latitudinal gradient program: R/V Italica 2004 Rauschert dredge samples. ZooKeys 2013, 341, 37–48. [Google Scholar]
- Ghiglione, C.; Alvaro, M.C.; Piazza, P.; Bowden, D.; Griffiths, H. J.; Carota, C.; Nava, C. R.; Schiaparelli, S. Mollusc species richness from shelf to abyssal depths in the Ross Sea (Antarctica): results from the New Zealand IPY-CAML expedition and implications for future sampling. Polar Biology 2017, 40, 1989–2000. [Google Scholar] [CrossRef]
- Gilmer, R. W.; Lalli, C. M. Bipolar variation in Clione, a gymnosomatous pteropod. American Malacological Bulletin 1990, 8, 67–75. [Google Scholar]
- Gosliner, & T, M. Nudibranchs of Southern Africa: A Guide ot Opisthobranch Molluscs of Southern Africa. Sea Challerngers . 1987. [Google Scholar]
- Gosliner, T. M.; Draheim, R. Indo-Pacific opisthobranch gastropod biogeography: How do we know what we don’t know? Malacological Bulletin 1996, 12(1), 37–43. [Google Scholar]
- Gosliner, T. M.; Valdés, Á.; Behrens, D. W. Nudibranch and Sea Slug Identification: Indo-pacific; New World Publications, 2015; 408 pp. [Google Scholar]
- Gould, A. A. (1847). Descriptions of new shells, collected by the United States Exploring Expedition. Proceedings of the Boston Society of Natural History. 2: 196-198, 200-203, 204-208 [March]; 209, 210-212, 214-215, 222-224 [June]; 225, 237-239 [July]; 251-252 [December].
- Gould, A. Mollusca and Shells [in]: United States Exploring Expeditions, 1838, 1839, 1840, 1841, 1842 under the command of Charles Wilkes, U.S.N. Philadelphia, C. Sherman & son 12.
- Griffiths, H. J.; Linse, K.; Crame, J. A. SOMBASE–Southern Ocean Mollusc Database: a tool for biogeographic analysis in diversity and ecology. Organisms Diversity & Evolution 2003, 3(3), 207–213. [Google Scholar]
- Griffiths, H. J.; Barnes, D. K.; Linse, K. Towards a generalized biogeography of the Southern Ocean benthos. Journal of Biogeography 2008, 36(1), 162–177. [Google Scholar] [CrossRef]
- Griffiths, H. J.; Whittle, R. J.; Roberts, S. J.; Belchier, M.; Linse, K.; Thatje, S. Chapter 5.21. Decapoda: Crabs and Lobsters. In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Scientific Committee on Antarctic Research; Cambridge, 2014; pp. 185–189. [Google Scholar]
- Grove, S. The seashells of Tasmania: a comprehensive guide. Taroona Publications . 2011. [Google Scholar]
- Grove, S. A guide to the seashells and marine molluscs of Tasmania. 2015. Available online: http://www.molluscsoftasmania.net/Species.
- Grove, S. An annotated checklist of Tasmanian marine molluscs; 2018. [Google Scholar]
- Grove, S. J.; Kershaw, R. C.; Smith, B. J.; Turner, E. A systematic list of the marine molluscs of Tasmania; Queen Victoria Museum and Art Gallery, 2006; Vol. 8, 122 pp. [Google Scholar]
- Gutt, Sirenko, Arntz. Weddell Sea Macrozoobenthos. 2000. Available online: https://www.gbif.org/occurrence/240910901 (accessed on 4 September 2024). [CrossRef]
- Hain, S. Die beschalten benthischen Mollusken (Gastropoda und Bivalvia) des Weddellmeeres, Antarktis. Berichte zur Polarforschung 1990, 70, 1–181. [Google Scholar]
- Hedley, C. Australasian Antarctic Expedition 1911-1914. Scientific Reports, Series C. Zoology and botany Mollusca 1916, vol. IV, part 1, 1–80. [Google Scholar]
- Hunt, B. P. V.; Pakhomov, E. A.; Hosie, G. W.; Siegel, V.; Ward, P.; Bernard, K. Pteropods in Southern Ocean ecosystems. Progress in Oceanography 2008, 78(3), 193–221. [Google Scholar] [CrossRef]
- Hutton, F. W. Manual of the New Zealand Mollusca. A systematic and descriptive catalogue of the marine and land shells, and of the soft mollusks and Polyzoa of New Zealand and the adjacent islands; Dominium Museum, New Zealand.
- Ingels, J.; Hauquier, F.; Raes, M.; Vanreusel, A. Chapter, 5.3. Antarctic free-living marine nematodes. In Biogeographic Atlas of the Southern Ocean; De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Scientific Committee on Antarctic Research; Cambridge, 2014; pp. 83–87. [Google Scholar]
- Innabi, J.; Stout, C. C.; Valdés, Á. Seven new “cryptic species of Discodorididae (Mollusca, Gastropoda, Nudibranchia) from New Caledonia. Zookeys 2023, 1152, 45–95. [Google Scholar] [CrossRef]
- Janussen, D.; Downey, R. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5.5. Porifera. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 94–102. [Google Scholar]
- Jensen, K. R. Biogeography of the Sacoglossa (Mollusca, Opisthobrancia). Bonner zoologische Beiträge 2007, 55(3/4), 255–281. [Google Scholar]
- Johnson, R. F.; Gosliner, T. M. Traditional taxonomic groupings mask evolutionary history: A molecular phylogeny and new classification of the chromodorid nudibranchs. PLoS ONE 2012, 7(4), e33479. [Google Scholar] [CrossRef]
- Johnson, P. M.; Willows, A. Defense in sea hares (Gastropoda, Opisthobranchia, Anaspidea) multiple layers of protection from egg to adult. Marine and Freshwater Behaviour and Physiology 1999, 32, 147–180. [Google Scholar] [CrossRef]
- Jörger, K. M.; Schrödl, M.; Schwabe, E.; Würzberg, L. A glimpse into the deep of the Antarctic Polar Front–Diversity and abundance of abyssal molluscs. Deep Sea Research Part II: Topical Studies in Oceanography 2014, 108, 93–100. [Google Scholar] [CrossRef]
- Kaiser, P. Die Gattung Bathydoris Bergh 1884 in patagonischen Gewässern (Opisthobranchia, Nudibranchia). Spixiana (Zeitschrift für Zoologie) 1980, 3(1), 43–51. [Google Scholar]
- Kaiser, S. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5.18. Antarctic and sub-Antarctic Isopod Crustaceans (Peracarida: Malacostraca). In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 166–172. [Google Scholar]
- Kershaw, R. C. A systematic list of the Mollusca of Tasmania, Australia. Papers and Proceedings of the Royal Society of Tasmania 1955, 89, 289–355. [Google Scholar] [CrossRef]
- Kienberger, K.; Carmona, L.; Pola, M.; Padula, V.; Gosliner, T.M.; Cervera, J. L. Aeolidia papillosa (Linnaeus, 1761) (Mollusca: Heterobranchia: Nudibranchia), single species or a cryptic species complex? A morphologial and molecular study. Zoological Journal of the Linnean Society 2016, 177, 481–506. [Google Scholar] [CrossRef]
- Kiko, R.; Kramer, M.; Spindler, M.; Wägele, H. Tergipes antarcticus (Gastropoda, Nudibranchia): distribution, life cycle, morphology, anatomy and adaptation of the first mollusk known to live in Antarctic sea ice. Polar Biology 2008, 31, 1383–1395. [Google Scholar] [CrossRef]
- Knox, G. A. Biology of the Southern Ocean; CRC Press, 2006; 640 pp. [Google Scholar]
- Lawver, L. A.; Gahagan, L. M.; Daiziel, I. W. D. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 3.2. Reconstruction of the Southern Ocean and Antarctic regions. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 36–42. [Google Scholar]
- Linse, K. Mollusca of the Magellan region. A checklist of the species and their distribution. Scientia Marina 1999, 63 (supl. I), 399–407. [Google Scholar] [CrossRef]
- Linse, K. New records of shelled marine molluscs at Bouvet Island and preliminary assessment of their biogeographic affinities. Polar Biology 2006, 29(2), 120127. [Google Scholar] [CrossRef]
- Linse, K.; Griffiths, H. J.; Barnes, D. K.; Clarke, A. Biodiversity and biogeography of Antarctic and sub-Antarctic mollusca. Deep Sea Research Part II: Topical Studies in Oceanography 2006, 53(8), 985–1008. [Google Scholar] [CrossRef]
- Linse, K.; Schiotte, T. A new species of Diaphana from bathyal depths in the Weddell Sea, Antarctica and first record of Diaphana inflata (Strebel, 1908) in the high Antarctic (Gastropoda: Opisthobranchia). Journal of Molluscan Studies 2002, 68, 147–153. [Google Scholar] [CrossRef]
- Linse, K.; Schrödl, M.; McCIain, C. R.; Allcock, L. Mollusca in the Antarctic deep sea-preliminary notes on their taxonomy, biogeography and diversity. Berichte zur Polar und Meeresforschung 2002, 470, 95–101. [Google Scholar]
- Marcus, E. Lamellariacea und Opisthobranchia. Reports of the Lund University Chile Expedition 1948-49, Lunds University, Årssk 1959, 55, 1–133. [Google Scholar]
- Marcus, Ev. A toxonomic survey of the genus. Toledonia (Opisthobranchia, Diaphanidae). Zoologica Scripta 1976, 5(1), 25–33. [Google Scholar] [CrossRef]
- Marcus, Ev.; Gosliner, T.M. Review of the family Pleurobranchaeidae (Mollusca, Opisthobranchia). Annals of the South African Museum 1984, 93(1), 1–41. [Google Scholar]
- Maroni, P. J.; Baker, B. J.; Moran, A. L.; Woods, H. A.; Avila, C.; Johnstone, G. J.; Stark, J. S.; Kocot, K. M.; Lockhart, S.; Saucède, T.; Rouse, G. W.; Wilson, N. G. One Antarctic slug to confuse them all: The underestimated diversity of Doris kerguelenensis. Invertebrate Systematics 2022, 36, 419–435. [Google Scholar] [CrossRef]
- Maroni, P. J.; Wilson, N. G. Multiple Doris “kerguelenensis” (Nudibranchia) species span the Antarctic Polar Front. Ecology and Evolution 2022, 12(9), e9333. [Google Scholar] [CrossRef]
- Martynov, A. V. Archaic Tergipedidae of the Arctic and Antarctic: Murmania antiqua gen. et sp. nov. from the Barents Sea and a revision of the genus Guyvalvoria Vayssière with descriptions of two new species. Ruthenica 2006, 16, 73–88. [Google Scholar]
- Martens, E von.; Pfeffer, G. (1886) Die Mollusken von Süd-Georgien nach der Ausbeute der Deutschen Station 1882–83. Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten 3, 63–135.
- May, W. L. A check-list of the Mollusca of Tasmania. John Vail, Governements Printer . 1921. [Google Scholar]
- Maynard, D. Range extension or was it always there and we weren´t looking? The Tasmanian Naturalist 2014, 136, 101–107. [Google Scholar]
- Mccarthy, J.; Krug, P. J.; Valdés, A. Integrative systematics of Placida cremoniana (Trinchese, 1892) (Gastropoda, Heterobranchia, Sacoglossa) reveals multiple pseudocryptic species. Marine Biodiversity 2017, 49(1), 357–371. [Google Scholar] [CrossRef]
- Melville, J. C.; Standen, R. The marine mollusca of the Scotish National Antarctic Expedition, Part II. Transactions of the Royal Society of Edinburgh 1912, 48, 333–366. [Google Scholar] [CrossRef]
- Menacho, A. Redescripción y distribución geográfica de especies de gasterópodos antárticos de los géneros Marseniopsis Bergh, 1886 y Bathyberthella Willan, 1983. Trabajo Fin de Máster en Ciencias del Mar. Universidad de Barcelona . 2011. [Google Scholar]
- Merilees, W.; Burn, R. Archidoris kerguelenensis Bergh, the first record of a nudibranch from Macquarie Island. Victorian Naturalist 1969, 86, 137–138. [Google Scholar]
- Millen, S.; Martynov, A.V. Redescriptions of the nudibranch genera Akiodoris Bergh, 1879 and Armodoris Minichev, 1972 (Suborder Doridacea) with a new species of Akiodoris and a new family Akiodorididae. Proceedings of the California Academy of Sciences 2005, 56, 1–22. [Google Scholar]
- Miller, M. C. Aeolid nudibranchs (Gastropoda: Opisthobranchia) of the family Tergipedidae from New Zealand waters. Zoological Journal of the Linnean Society 1977, 60(3), 197–222. [Google Scholar] [CrossRef]
- Minichev, Y. S. (1972) Opisthobranchiate molluscs of the Davis Sea. Issledovaniia Fauny Morei 11, 358–382.
- Moles, J.; Wägele, H.; Ballesteros, M.; Pujals, A.; Uhl, G.; Àvila, C. The end of the cold loneliness: 3D comparison between Doto antarctica and a new sympatric species of Doto (Heterobranchia: Nudibranchia). PLoS ONE 2016a, 11(7), e0157941. [Google Scholar] [CrossRef]
- Moles, J.; Wägele, H.; Uhl, G.; Avila, C. Bipolarity in sea slugs: a new species of Doridunculus (Mollusca: Nudibranchia: Onchidoridoidea) from Antarctica. Organisms Diversity and Evolution 2016b, 17(1). [Google Scholar] [CrossRef]
- Moles, J.; Avila, C.; Malaquias, M.A.E. Systematic revision of the Antarctic gastropod family Newnesiidae (Heterobranchia: Cephalaspidea) with the description of a new genus and a new abyssal species. Zoological Journal of the Linnean Society 2017a, XX, 1–13. [Google Scholar] [CrossRef]
- Moles, J.; Wägele, H.; Cutignano, A.; Fontana, A.; Ballesteros, M.; Avila, C. Giant embryos and hatchlings of Antarctic nudibranchs (Mollusca: Gastropoda: Heterobranchia). Marine Biology 2017b, 164, 114. [Google Scholar] [CrossRef]
- Moles, J.; Wägele, H.; Schrödl, M.; Avila, C. A new Antartic heterobranch clade is sister to all other Cephalaspidea (Mollusca: Gastropoda). Zoologica Scripta 2017c, 46(2). [Google Scholar] [CrossRef]
- Moles, J.; Avila, C.; Malaquias, M.A.E. Unmasking Antarctic mollusc lineages: novel evidence from philinoid snails (Gastropoda: Cephalaspidea). Cladistic 2019, 35(5), 487–513. [Google Scholar] [CrossRef] [PubMed]
- MolluscaBase (Ed.) MolluscaBase. Paradoris dubia (Bergh, 1904); Accessed through: World Register of Marine Species at; 2025a; Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=533981on 2025-04-05.
- MolluscaBase eds. (2025b). MolluscaBase. Aphelodoris luctuosa Bergh, 1905. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1732011on 2025-04-05.
- MolluscaBase (Ed.) MolluscaBase. Toledonia brevior Eales, 1923; Accessed through: World Register of Marine Species at; 2025c; Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=509720on 2025-04-08.
- MolluscaBase (Ed.) MolluscaBase. Toledonia circumrosa (Thiele, 1904); Accessed through: World Register of Marine Species at; 2025d; Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=509710on 2025-04-04.
- MolluscaBase (Ed.) MolluscaBase. Ringiculimorpha. Accessed through: World Register of Marine Species; 2025e; Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1059023Available at; on 2025-04-04.
- MolluscaBase (Ed.) MolluscaBase. Verconia aureopunctata (Rudman, 1987); Accessed through: World Register of Marine Species at; 2025f; Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=866650on 2025-04-08.
- MolluscaBase (Ed.) MolluscaBase. Verconia closeorum (Rudman, 1986); Accessed through: World Register of Marine Species at; 2025g; Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=866652on 2025-04-08.
- Moreau, C.; Linse, K.; Griffiths, H. J.; Barnes, D.; Kaiser, S.; Glover, A.; Geissler, P. Amundsen Sea Mollusca from the BIOPEARL II expedition. ZooKeys 2013, 294, 1. [Google Scholar] [CrossRef]
- Muniain, C.; Ortea, J.A. First records of the genus Berghia Trinchese, 1877 (Opisthobranchia: Aeolidiidae) from Argentina, with description of a new species. Avicennia 1999, 10/11, 143–150. [Google Scholar]
- Muniain, C.; Ardila, N. E.; Cervera, J. L. Pleurobranchaea inconspicua Bergh, 1897 (Opisthobranchia: Pleurobranchidae): Redescription and distribution from Argentina and Colombia. Bonner zoologische Beiträge 2007, 55(3/4), 291–300. [Google Scholar]
- Nimbs, M. J. Designation of a Neotype for the Dwarf Sea Hare Aplysia concava GB Sowerby I, 1833, and a Review of the Status of Aplysia norfolkensis GB Sowerby II, 1869 (Mollusca: Heterobranchia). Records of the Australian Museum 2021, 73(5), 147–151. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Hutton, I.; Davis, T. R.; Larkin, M. F.; Smith, S. D. A. The heterobranch sea slugs of Lord Howe Island, NSW, Australia (Mollusca: Gastropoda). Proceedings of the Royal Society of Victoria 2020, 132(1), 12–41. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Larkin, M.; Davis, T. R.; Harasti, D.; Willan, R. C.; Smith, S. D. A. Southern range extensions for twelve heterobranch sea slugs (Gastropoda: Heterobranchia) on the eastern coast of Australia. Marine Biodiversity Records 2016, 9, 27. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Smith, S. D. A. Welcome strangers: Southern range extensions for seven heterobranch sea slugs (Mollusca: Gastropoda) on the subtropical east Australian coast, a climate change hot spot. Regional Studies in Marine Science 2016, 8(1), 27–32. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Smith, S. D. A. An illustrated inventory of the sea slugs of New South Wales, Australia (Gastropoda: Heterobranchia). Proceedings of the Royal Society of Victoria 2017a, 128(2), 44–113. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Smith, S. D. A. Revision of the southern distribution limit for the tropical marine herbivore Syphonota geographica (A. Adams & Reeve, 1850) (Heterobranchia: Aplysiidae) in a global climate change hot-spot. Australian Zoologist 2017b, 38(4), 582–589. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Smith, S. Beyond Capricornia: Tropical Sea Slugs (Gastropoda, Heterobranchia) Extend Their Distributions into the Tasman Sea. Diversity 2018, 10(3), 99. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Willan, R. C.; Smith, S. D. A. Range extensions for heterobranch sea slugs (formerly opisthobranch) belonging to the families Diaphanidae, Plakobranchidae and Facelinidae on the eastern coast of Australia. Marine Biodiversity Records 2015, 8, e76. [Google Scholar] [CrossRef]
- Nimbs, M. J.; Willan, R. C.; Smith, S. D. A. An historical summary of the distribution and diet of the Australian sea hares (Gastropoda: Heterobranchia: Aplysiidae). Zoological Studies 2017, 56(35), 1–15. [Google Scholar]
- Nimbs, M. J; Wilson, N. G. Saved by the Shell: Molecular Analysis Detects the Cryptic Sea Hare, Aplysia concava G. B. Sowerby I, 1833 (Mollusca: Heterobranchia: Aplysiidae), from Oceania, with a Redescription. Taxonomy 2021, 1(2), 48–59. [Google Scholar] [CrossRef]
- Odhner, N. H. Die Opisthobranchien. Further Zoological Results of the Sweedish Antarctic Expedition 1901-1903 1926, 2, 1–100. [Google Scholar]
- Odhner, N. H. The Nudibranchata, British Antarctic ‘Terra Nova’ expedition, 1910. British museum of natural history reports, Zoology 1934, 7, 229–310. [Google Scholar]
- Orr, J. C.; Fabry, V. J.; Aumont, O.; Bopp, L.; Doney, S. C.; Feely, R. A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; Key, R.M.; Lindsay, K.; Maier-Reimer, E.; Matear, R.; Monfray, P.; Mouchet, A.; Najjar, R. G.; Plattner, G. K.; Rodgers, K. B.; Sabine, C. L.; Sarmiento, J. L.; Schlitzer, R.; Slater, R. D.; Totterdell, I. J.; Weirig, M. F.; Yamanaka, Y.; Yool, A. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Orrell, T. Informatics and Data Science Center-Digital Stewardship NMNH Extant Specimen Records (USNM, US). Version 1.86. National Museum of Natural History, Smithsonian Institution. Occurrence dataset. 2024. Available online: https://www.gbif.org/occurrence/1321404872. [CrossRef]
- Oskars, T. R.; Bouchet, P.; Malaquias, M. A. E. A new phylogeny of the Cephalaspidea (Gastropoda: Heterobranchia) based on expanded taxon sampling and gene markers. Molecular Phylogenetics and Evolution 2015, 89, 130–150. [Google Scholar] [CrossRef]
- Osorio, C.; Reid, D. G. Moluscos marinos intermareales y submareales entre la Boca del Guafo y el estero Elefantes, sur de Chile. Investigaciones Marinas, Valparaiso 2004, 32(2), 71–89. [Google Scholar] [CrossRef]
- Paz-Sedano, S.; Smirnoff, D.; Gosliner, T. M.; Pola, M. When a genus must become two: resurrection of Pelagella Gray, 1850 with the description of six new species. Journal of Molluscan Studies 2023, 89(2), eyad008. [Google Scholar] [CrossRef]
- Paz-Sedano, S.; Moles, J.; Smirnoff, D.; Gosliner, T. M.; Pola, M. A combined phylogenetic strategy illuminates the evolution of Goniodorididae nudibranchs (Mollusca, Gastropoda, Heterobranchia). Molecular Phylogenetics and Evolution 2024, 107990. [Google Scholar] [CrossRef] [PubMed]
- Peck, L. S.; Webb, K. E.; Bailey, D. M. Extreme sensitivity of biological function to temperature in Antarctic marine species. Functional Ecology 2004, 18(5), 625630. [Google Scholar] [CrossRef]
- Pelseneer, P. Report on the Pteropoda collected by H.M.S. Challenger during the Years 1873-76. First Part.-Gymnosomata. Zoology Part LVIII 19.
- Pelseneer, P. Zoologie: Mollusques (Amphineures, Gastropodes et Lamellibranches). J.-E. Buschmann, Antwerp, Expédition antarctique Belge: Résultats du voyage du S.Y. Belgica en 1897–1898-1899 sous le commandement de A. de Gerlache de Gomery: Rapports scientifiques (1901–1913) 1903. [Google Scholar]
- Peña, A. L. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5.6. Benthic Hydroids (Cnidaria, Hydrozoa). In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 103–106. [Google Scholar]
- Peñas, A.; Rolán, E. Deep water Pyramidelloidea of the Tropical South Pacific: Turbonilla and related genera. Tropical Deep Sea Benthos . 2010, vol 26. MNHN, Paris. [Google Scholar]
- Peñas, A.; Rolán, E. Deep water Pyramidelloidea from the Central and South Pacific: 3. The Tribes Eulimellini and Syrnolini; Universidade de Santiago de Compostela, Spain, 2016; 304 pp. [Google Scholar]
- Peñas, A.; Rolán, E. Deep Water Pyramidelloidea from the Central and South Pacific, Part. 4: The Tribe Chrysallidini. Sociedad Española de Malacología. ECIMAT. Universidade de Vigo, Spain . 2017. [Google Scholar]
- Peralta-Serrano, M.; Schrödl, M.; Wilson, N. G.; Moles, J. Revealing hidden diversity and cryptic speciation in Antarctic marine gastropods (Heterobranchia: Cephalaspidea). Antarctic Science 2025, 1–13. [Google Scholar] [CrossRef]
- Phillips, J. A. Marine macroalgal biodiversity hotspots: why is there high species richness and endemism in southern Australian marine benthic flora? Biodiversity and Conservation 2001, 10(9), 1555–1577. [Google Scholar] [CrossRef]
- Pitt, N. R.; Poloczanska, E. S.; Hobday, A. J. Climate-driven range changes in Tasmanian intertidal fauna. Marine and Freshwater Research 2010, 61(9), 963–970. [Google Scholar] [CrossRef]
- Pola, M.; Camacho-Garcia, Y. E.; Gosliner, T. M. Molecular data illuminate cryptic nudibranch species: the evolution of the Scyllaeidae (Nudibranchia: Dendronotina) with a revision of Notobryon. Zoological Journal of the Linnean Society 2012, 165, 311–336. [Google Scholar] [CrossRef]
- Pontes, M. “Polycera hedgpethi” in OPK-Opistobranquis. Published: 14/01/2024. 2023. Available at. Available online: http://opistobranquis.info/en/?p=14413.
- Powell, A. W. B. New species of nudibranchiate Mollusca from Auckland waters. Records of the Auckland Institute and Museum 1937, 2, 119–124. [Google Scholar]
- Powell, A. W. B. Antarctic and subantarctic Mollusca: Gastropoda and Pelecypoda. Discovery Repports 1951, 26, 47–196. [Google Scholar] [CrossRef]
- Powell, A. W. B. (1955): Mollusca of the southern islands of New Zealand. Cape Exped.–Science Results NZ sub-Antarctic Expedition, 1941-45.
- Powell, A. W. B. Mollusca of Kerguelen and Macquarie Islands. Reports British, Australian, and New Zealand Antarctic Research Expedition (1929-1931). Series B, Zoology and Botany 1957, 6(7), 107–149. [Google Scholar]
- Powell, A. W. B. Mollusca from the Victoria-Ross quadrants of Antarctica. Reports British, Australian, and New Zealand Antarctic Research Expedition (1929-1931). Series B, Zoology and Botany 1958, 6, 165–215. [Google Scholar]
- Powell, A. W. B. Antarctic and Subantarctic Mollusca. Records of the Auckland Institute and Museum 1960, 5, 117–193. [Google Scholar]
- Powell, A. W. B. New Zealand Mollusca. In Marine, Land and Freshwater Shells; William Collins Publishers; Auckland, 1979; 500 pp. [Google Scholar]
- Price, R. M.; Gosliner, T. M.; Valdés, A. Systematics and phylogeny of Philine (Gastropoda: Opisthobranchia), with emphasis on the Philine aperta species complex. The Veliger 2011, 51(2), 1–58. [Google Scholar]
- Primo, C.; Vázquez, E. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5.27. Ascidian fauna south of the Sub-Tropical Front. In Biogographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 221–228. [Google Scholar]
- Ramos, J. E.; Pecl, G. T.; Semmens, J. M.; Strugnell, J. M.; León, R. I.; Moltschaniwskyj, N. A. Reproductive capacity of a marine species (Octopus tetricus) within a recent range extension area. Marine and Freshwater Research 2015, 66(11), 999–1008. [Google Scholar] [CrossRef]
- Rang, S. Histoire naturelle des Aplysiens, première famille de l´ordre des Tectibranches. In De Ferrusac, Histoire naturelle, Generale et particuliere des Mollusques; Arthur Bertrand Libraire, Paris; Vol. 1.
- Rauschert, M.; Arntz, W. Antarctic Macrobenthos. A field Guide of the invertebrates living at the Antarctic seafloor. Bremen, Germany . 2015. [Google Scholar]
- Reid, D. G.; Osorio, C. The shallow-water marine Mollusca of the Estero Eleantes and Laguna San Rafael, southern Chile. Bulletin of the Natural Histtory Museum of London (Zool.) 2000, 66(2), 109–146. [Google Scholar]
- Richmond, M. H. Tasmanian sea shells; Devonport, Tasmania, 1992; Vol 2. Richmond Printers, 111 pp. [Google Scholar]
- Ridgway, K.; Hill, K. Poloczanska, E.S., Hobday, A.J., Richardson, A.J., Eds.; The East Australian Current. In A Marine Climate Change Impacts and Adaptation Report Card for Australia; NCCARF Publication 05/09, 2009. [Google Scholar]
- Rochebrune, A. T.; Mabille, J. Mollusques Mission Scientifique du Cap Horn. VI. Zoologie, Paris.
- Rodriguez, E.; Fautin, D. G. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Antarctic Hexacorals (Cnidaria, Anthozoa, Hexacorallia). In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 113–116. [Google Scholar]
- Romani, L.; Rolán, E.; Simone, L.R.L.; Crocetta, F. Emarginula poppeorum and Acteon fasuloi: replacement names for the preoccupied taxa Emarginula gigantea Poppe, 2008 and Acteon elongatus Castellanos, Rolán & Bartolotta, 1987 (Mollusca: Gastropoda). Zootaxa 2017, 4300(1), 149–150. [Google Scholar]
- Rosenfeld, S.; Aldea, C. An unknown opisthobranch (Mollusca: Gastropoda) in the Magellan region. Toledonia parelata Dell, 1990: new records and similar species. Anales Instituto Patagonia (Chile) 2011, 39(2), 133–136. [Google Scholar] [CrossRef]
- Rudman, W. B. Aeolid opisthobranch molluscs (Glaucidae) from the Indian Ocean and the south-west Pacific. Zoological Journal of the Linnean Society 1980, 68(2), 139–172. [Google Scholar] [CrossRef]
- Rudman, W. B. The Chromodorididae (Opisthobranchia: Mollusca) of the Indo-West Pacific: a review of the genera. Zoological Journal of the Linnean Society 1984, 81(2-3), 115–273. [Google Scholar] [CrossRef]
- Rudman, W. B. The Chromodorididae (Opisthobranchia: Mollusca) of the Indo-West Pacific: the genus Glossodoris Ehrenbergh (= Casella, H. & A. Adams). Zoological Journal of the Linnean Society 1986, 86(2), 101–184. [Google Scholar]
- Rudman, W. B. The Chromodorididae (Opisthobranchia: Mollusca) of the Indo-West Pacific: the genus Ceratosoma JE Gray. Zoological Journal of the Linnean Society 1988, 93(2), 133–185. [Google Scholar] [CrossRef]
- Rudman, W. B.; Willan, R. C. Opisthobranchia. The southern synthesis. In Fauna of Australia; CSIRO, Melbourne, 1998. [Google Scholar]
- Rudman, W. B. Pseudotritonia quadrangularis Thiele, 1912. [In] Sea Slug Forum. Australian Museum, Sydney. 2007. Available from. Available online: http://www.seaslugforum.net/factsheet/pseuquad.
- Salvador, R. B.; Cunha, C. Lanayrella, a new Acteonidae genus (Gastropoda, Heterobranchia) from Tierra del Fuego. Journal of Natural History 2020, 54(15-16), 1009–1018. [Google Scholar] [CrossRef]
- Saucède, T.; Pierrat, B.; David, B. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5.26. Echinoids. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 213–220. [Google Scholar]
- Schächinger, P. M.; Schrödl, M.; Wilson, N. G.; Moles, J. Crossing the Polar Front-Antarctic species discovery in the nudibranch genus Tritoniella (Gastropoda). Organisms Diversity & Evolution 2022, 22, 431–456. [Google Scholar] [CrossRef]
- Schiaparelli, S.; Ghiglione, C.; Alvaro, M. C.; Griffiths, H. J.; Linse, K. Diversity, abundance and composition in macrofaunal molluscs from the Ross Sea (Antarctica): results of fine-mesh sampling along a latitudinal gradient. Polar biology 2014, 37(6), 859–877. [Google Scholar] [CrossRef]
- Schiaparelli, S.; Linse, K. De Broyer, C., Koubbi, P., Griffiths, H.J., Raymond, B., Udekem d’Acoz, C.d’, Eds.; Chapter 5.10. Gastropoda. In Biogeographic Atlas of the Southern Ocean; <i>Scientific Committee on Antarctic Research</i>; Cambridge, 2014; pp. 122–125. [Google Scholar]
- Schiaparelli, S.; Lorz, A. N.; Cattaneo-Vietti, R. Diversity and distribution of mollusk assemblages on the Victoria Land coast and the Balleny Islands, Ross Sea, Antarctica. Antarctic Science 2006, 18, 615–631. [Google Scholar] [CrossRef]
- Schrödl, M. The genus Berthella Blainville, 1825 (Notaspidea: Pleurobranchidae) from Magellanic waters. Journal of Molluscan Studies 1999a, 65(4), 399409. [Google Scholar] [CrossRef]
- Schrödl, M. Zoogeographic relationships of Magellan Nudibranchia (Mollusca: Opisthobranchia) with particular reference to species from adjacent regions. Scientia Marina 1999b, 63(S1), 409–416. [Google Scholar] [CrossRef]
- Schrödl, M. Revision of the nudibranch genus Cadlina (Gastropoda: Opisthobranchia) from the Southern Ocean. Journal of the Marine Biological Association of the UK 2000a, 80(2), 299–309. [Google Scholar] [CrossRef]
- Schrödl, M. Revision of Dorid Nudibranchia Collected during the French Cape Horn Expedition in 1882-1883, with Discussion of the Genus Geitodoris Bergh, 1891. The Veliger 2000b, 43(3), 197–209. [Google Scholar]
- Schrödl, M. Sea slugs of southern South America: systematics, biogeography and biology of Chilean and Magellanic Nudipleura (Mollusca--Opisthobranchia). ConchBooks . 2003. [Google Scholar]
- Schrödl, M.; Alarcón, M. A.; Bedriñana, L. R.; Bravo, F. J.; Bustamante, C. M.; Carvalho, R.; Forsterra, G.; Gallardo, C.; Haussermann, V.; Salmen, A. Nudipleura from the southern Chilean Comau Fjord with redescription of Polycera priva Er. Marcus, 1959. Vita Malacologica 2005, 3, 23–33. [Google Scholar]
- Schrödl, M.; Grau, J. Nudibranchia from the remote southern Chilean Guamblin and Ipún Islands (Chonos Archipelago, 44º-45º S), with re-description of Rostanga pulchra MacFarland, 1905. Revista Chilena de Historia Natural 2006, 79(1), 3–12. [Google Scholar] [CrossRef]
- Schrödl, M.; Engl., W.; Aldea, C.; Kohlberg, G.; Schories, D. Schories, D., Kohlberg, G., Eds.; Gastropods, Gastropoda, pp. 128-153. In Marine wildlife, King George Island, Antarctica; Dirk Schories Publications, 2016; 348 pp. [Google Scholar]
- Schüller, M.; Ebbe, B. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5-13. Polychaetes. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 134–137. [Google Scholar]
- Seager, J.R. A Redescription of the Antarctic opisthobranch Piline gibba Strebel, 1908 from the type locality, South Georgia, South Atlantic. Journal of Molluscan Studies 1978, 44(2), 171–179. [Google Scholar]
- Smith, E. A. Report on the Collections of Natural History made in the Antarctic regions during the voyage of the Southern Cross. VII Mollusca. British museum, London 1902, 24-25, 201–2013. [Google Scholar]
- Smith, S. D. A.; Nimbs, M. J. Citizen Scientists Record Significant Range Extensions for Tropical Sea Slug Species in Subtropical Eastern Australia. Diversity 2022, 14(4), 244. [Google Scholar] [CrossRef]
- SOMBASE British Antarctic Survey–Southern Ocean mollusc database: a tool for biogeographic analysis in diversity and evolution. Available online: https://www.gbif.org/occurrence/2270439982 (accessed on 2024-09-04). [CrossRef]
- Soler-Membrives, A.; Munilla, T.; Arango, P.; Griffiths, H. J. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5.14. Southern Ocean biogeographic patterns in Pycnogonida. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research, Cambridge, 2014; Volume pp, pp. 138–141. [Google Scholar]
- Spencer, H. J.; Willan, R. C. The Marine Fauna of New Zealand: Index to the Fauna 3. Mollusca. New Zealand Oceanographic Institute Memoir 1996, 105, 126. [Google Scholar]
- Spencer, H. G.; Marshall, B. A.; Willan, R. C. Gordon, D.P., Ed.; Checklist of New Zealand living Mollusca. In New Zealand inventory of biodiversity. Volume one. Kingdom Animalia: Radiata, Lophotrochozoa, Deuterostomia; Canterbury University Press; Christchurch, 2009; pp. 196–219. [Google Scholar]
- Spencer, H. G.; Willan, R. C.; Marshall, B.; Murray, T. J. Checklist of the Recent Mollusca recorded from the New Zealand Exclusive Economic Zone. 2016. Available online: http://www.molluscs.otago.ac.nz/index.html.
- Strebel, H. (1905): BeiträgezurKenntnis der Molluskenfauna der Magalhaen-Provinz III. Zoologische Jahrbucher Abteilung für Systematik, Geographie and Biologie, Jena 22, 575–666. Available online: https://www.biodiversitylibrary.org/page/9987352.
- Strebel, H. Die Gastropoden. Wissenschaftliche Ergebnisse der Schwedischen Südpolar Expedition 1908, 6(1), 1–112. [Google Scholar]
- Suter, H. Description of new species and subspecies of New Zealand Mollusca, with notes on a few species. Proceedings of the Malacological Society of London 1909, 8, 253–265. [Google Scholar]
- Suter, H. Manual of the New Zealand Mollusca with an atlas of quarto plates; John Mackay, Government Printer, Wellington, New Zealand, 1913; 1120 pp. [Google Scholar]
- Suthers, I. M.; Young, J. W.; Baird, M. E.; Roughan, M.; Everett, J. D.; Brassington, G. B.; Byrne, M.; Condie, S. A.; Hartog, J. R.; Hassler, C. S. The strengthening East Australian Current, its eddies and biological effects—an introduction and overview. Deep Sea Research Part II: Topical Studies in Oceanography 2011, 58(5), 538–546. [Google Scholar] [CrossRef]
- Tate, R.; May, W. L. (1901). A revised census of the marine Mollusca of Tasmania. Proceedings of the Linnean Society of New South Wales 26, 344–471.
- Tenison Woods, J. E. Census; with brief descriptions of the marine shells of Tasmania and the adjacent islands. Papers & Proceedings and Report of the Royal Society of Tasmania; pp. 26–57.
- Thiele, J. The Antarctic snails and mussels. German South Polar Expedition 1901–1903 1912, 13, 183–286. [Google Scholar]
- Todd, C. D. Barnes, H.B., Ed.; The ecology of nudibranch molluscs. In Oceanography and Marine Biology: An Annual Review; Aberdeen University Press; London, 1981; Volume 19, pp. 141–234 655 pp. [Google Scholar]
- Toonen, R. J.; Bowen, B. W.; Iacchei, M.; Briggs, J. C. Kliman, R.M., Ed.; Biogeography, Marine. In Encyclopedia of Evolutionary Biology; Academic Press; Oxford, 2016; vol. 1, pp. 166–178. [Google Scholar]
- Troncoso, J. S.; Aldea, C. Macrobenthic mollusc assemblages and diversity in the West Antarctica from the South Shetland Islands to the Bellingshausen Sea. Polar Biology 2008, 31, 1253–1265. [Google Scholar] [CrossRef]
- Troncoso, J. S.; García, F. J.; García, J. F. Gastropoda Opisthobranchia colected during the Spanish expeditions to the Scotia Sea. Thalassas 1997, 13, 11–33. [Google Scholar]
- Troncoso, N.; Van Goethem, J.; Troncoso, J. S. Contribution to the marine molluscan fauna of Kerguelen Islands, South Indian Ocean. Iberus 2001, 19(1), 83–114. [Google Scholar]
- Troncoso, J. S.; García, F. J.; Backeljau, T.; Urgorri, V. Faunistic and anatomical data on the Antarctic Opisthobranchia (Mollusca, Gastropoda) in the collections of the Royal Belgian Institute of Natural Sciences. Bulletin Institut royal Sciences Naturelles de Belgique, Biologie 1996, 66, 29–40. [Google Scholar]
- Troncoso, J. S.; García García, F. J.; García Gómez, J. C.; Ballesteros, M. Moluscos Opistobranquios capturados durante las Expediciones Españolas BENTART 94 y 95, ampliando la distribución y rango batimétrico de Bathydoris hodgsoni. Nova Acta Compostelana (Bioloxía) 1999, 9, 321–323. [Google Scholar]
- Turner, J.; Bindschadler, R.; Convey, P.; Di Prisco, G.; Fahrbach, E.; Gutt, J.; Summerhayes, C. Antarctic climate change and the environment. Antarctic Science 2009, 21(06), 541–563. [Google Scholar]
- Tynan, C. T. Ecological importance of the southern boundary of the Antarctic Circumpolar Current. Nature 1998, 392(6677), 708–710. [Google Scholar] [CrossRef]
- Van der Spoel, S.; Schalk, P. H.; Bleeker, J. Clio piatkowskii, a mesopelagic pteropod new to science (Gastropoda, Opisthobranchia). Beaufortia 1992, 43(1), 1–6. [Google Scholar]
- Valdés, A.; Hammon, J.; Behrens, D.; Dupont, A. Caribbean Sea Slugs. Sea Challengers . 2006. [Google Scholar]
- Valdés, Á.; Munian, C. Revision and taxonomic reassessment of magellanic species assigned to Anisodoris Bergh, 1898 (Nudibranchia: Doridoidea). Journal of Molluscan Studies 2002, 68(4), 345–351. [Google Scholar] [CrossRef]
- Valdés, Á.; Moran, A. L.; Woods, H. A. A new species of. Polar Biology 2011, 34(3), 459–463. [Google Scholar] [CrossRef]
- Valdés, Á.; Moran, A. L.; Woods, H. A. Revision of several poorly known Antarctic aeolid nudibranch species (Mollusca: Gastropoda), with the description of a new species. Journal of the Marine Biological Association of the United Kingdom 2012, 92(05), 1161–1174. [Google Scholar] [CrossRef]
- Valdés, A.; McLean, J. On two abyssal species of Scaphandridae G.O. Sars, 1878 (Gastropoda: Cephalaspidea) from the eastern Pacific. The Nautilus 2015, 129(3), 118–125. [Google Scholar]
- Vayssière, A. Diagnoses of new generic gastropod molluscs reported by Dr. Charcot Antarctic Expedition. Natural History Museum of Paris 1906a, 1, 47–149. [Google Scholar]
- Vayssière, A. Mollusques Nudibranches et Marséniadés. Expédition antarctique française commandée par le Dr. Jean Charcot (1903-1905) 1906b, 1–51. [Google Scholar]
- Vayssière, A. Zoological and anatomical research on Amphineura and gastropod molluscs (opisthobranch and prosobranch). Twelth french Antarctic expedition 1908-1910, Paris 1917, 1–50. [Google Scholar]
- Vicente, N. Nudibranches des Îles Kerguelen. Tethys 1973, 5, 629–634. [Google Scholar]
- Vicente, N.; Arnaud, P. M. Invertebrés marins des XIIe et XVe Expéditions Antarctiques Francaises en Terre Adélie. 12. Gastéropodes Opisthobranches. Tethys 1974, 5, 531–547. [Google Scholar]
- Wägele, H. The distribution of some Antarctic nudibranchs (Opisthobranchia). Journal of Molluscan Studies 1987, 53, 179–188. [Google Scholar] [CrossRef]
- Wägele, H. On the Anatomy and Zoogeography of Tritoniella belli Eliot, 1907 (Opisthobranchia, Nudibranchia) and athe synonymy of T. sinuata Eliot, 1907. Polar Biology 1989, 9, 235–243. [Google Scholar] [CrossRef]
- Wägele, H. Revision of the Antarctic genus Notaeolidia (Gastropoda, Nudibranchia), with a description of a new species. Zoologica Scripta 1990a, 19(3), 309–330. [Google Scholar] [CrossRef]
- Wägele, H. Revision of the genus Austrodoris Odhner, 1926 (Gastropoda, Opisthobranchia). Journal of Molluscan Studies 1990b, 56(2), 163–180. [Google Scholar] [CrossRef]
- Wägele, H. The morphology and taxonomy of the Antarctic species of Tritonia Cuvier, 1797 (Nudibranchia: Dendronotoidea). Zoological Journal of the Linnean Society 1995, 113(1), 21–46. [Google Scholar] [CrossRef]
- Wägele, H.; Ballesteros, M.; Avila, C. Defensive glandular structures in opisthobranch molluscs: from histology to ecology. Oceanography and Marine Ecology 2006, 44, 197–276. [Google Scholar]
- Wägele, H.; Hain, S. Description of a new notaspidean genus and species (Opisthobranchia: Notaspidea) from the Antarctic Ocean. Journal of Molluscan Studies 1991, 57 Supplement Part 4, 229–242. [Google Scholar] [CrossRef]
- Wägele, H.; Klusmann-Kolb, A. Opisthobranchia (Mollusca, Gastropoda)–more than just slimy slugs. Shell reduction and its implications on defence and foraging. Frontiers in Zoology 2005, 2, 3. [Google Scholar] [CrossRef]
- Wägele, H.; Barnes, D. K.; Bullough, L. W. Redescription of Charcotia granulosa Vayssiere, 1906 (Nudibranchia: Arminoidea: Charcotiidae) from Signy Island, Antarctica. Journal of Molluscan Studies 1995, 61(2), 197–207. [Google Scholar] [CrossRef]
- Wägele, H.; Willan, E.C. The morphology and anatomy of the Antarctic gastropod Bathyberthella antarctica (Opisthobranchia, Notaspidea, Pleurobranchidae). Zoologica Scripta 1994, 23(4), 313–324. [Google Scholar] [CrossRef]
- Wahidullah, S.; Guo, Y. W.; Fakhr, I. M. I.; Mollo, E. Cimino, G., Gavagnin, M., Eds.; Chemical diversity in opisthobranch molluscs from scarcely investigated Indo-Pacific areas. In Progress in Molecular and subcellular biology. Molluscs; Springer-Verlag; Berlin, Heidelberg, 2006; pp. 175–198. [Google Scholar]
- Watson, R. B. Mollusca of H.M.S. “Challenger” Expedition. Part XVIII. Journal of the Linnean Society of London, Zoology 17, 284–293. [CrossRef]
- Wiencke, C.; Ansler, C. D.; Clayton, M. N. De Broyer, C., Koubbi, P., Griffiths, H.J., et al., Eds.; Chapter 5.1. Macroalgae. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research; Cambridge, 2014; Volume pp, pp. 66–73. [Google Scholar]
- Willan, R. C. Marine Molluscs: Opisthobranchia, Part 2. Leigh Marine Laboratory, University of Auckland 1984. [Google Scholar]
- Willan, R. C.; Beesley, P.L.; Ross, G.J.B.; Wells, A. Order Notaspidea. In Mollusca: The Southern Synthesis. Fauna of Australia.; CSIRO Publishing, 1998; Vol. 5B, pp. 977–980. [Google Scholar]
- Willan, R. C.; Bertsch, H. Description of a new Pleurobranch (Opisthobranchia, Notaspidea) from Antarctic waters, with a review of Notaspideans from Southern Polar Seas. The Veliger 1987, 29, 292–302. [Google Scholar]
- Willan, R.C.; Davey, N.; Kelly, Michelle; Mills, NIWA Technical Editor Sadie. Super sea slugs, a guide to the sea slugs of New Zealand. Version 1, 52; NIWA Series Design by TCMedia Ltd, 2020; Available online: http://www.niwa.co.nz/coasts-and-oceans/marine-identification-guides-and-fact-sheets.Series and Managing.
- Willan, R. C.; Morton, J. Marine Molluscs, Part 2; Opisthobranchia. University of Auckland, Leigh Marine Laboratory. Auckland, New Zealand . 1984; 106 pp. [Google Scholar]
- Wilson, N. G.; Schrödl, M.; Halanych, K. M. Ocean barriers and glaciation: evidence for explosive radiation of mitochondrial lineages in the Antarctic sea slug Doris kerguelenensis (Mollusca, Nudibranchia). Molecular Ecology 2009, 18, 965–984. [Google Scholar] [CrossRef]
- Würzberg, L.; Peters, J.; Schwabe, E.; Rodkina, S.; Brandt, A. Pteropods (Gastropoda: Opisthobranchia) in the Southern Ocean: First results from fatty acid and stable isotope analyses on the SYSTCO material. ICES CM/A:16, 2009.
- Yonow, N. Red Sea Opisthobranchia 4: the orders Cephalaspidea, Anaspidea, Notaspidea and Nudibranchia: Dendronotacea and Aeolidacea. Fauna of Arabia 2000, 18, 87–132. [Google Scholar]
- Yonow, N. Opisthobranchs from the western Indian Ocean, with descriptions of two new species and ten new records (Mollusca, Gastropoda). ZooKeys 2012, 197, 1–129. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3376724/. [CrossRef] [PubMed]
- Yonow, N. Rasul, N-M-A., Stewart, I.C., Eds.; Sea slugs: unespected biodiversity and distribution. In The Red Sea; 2015; pp. 531–550. [Google Scholar]
- Zelaya, D. G. Systematics and biogeography of marine gastropod molluscs from South Georgia. Spixiana 2005, 28(2), 109. [Google Scholar]
- Zelaya, D. G.; Schejter, L.; Ituarte, C. Neacteonina argentina, new species, and family placement of the genus Neacteonina Thiele, 1912 (Mollusca: Gastropoda). Malacologia 2011, 53(2), 251–263. [Google Scholar] [CrossRef]
- Zhuo, J.; Gill, J. P.; Jansen, E. D.; Jenkins, M. W.; Chiel, H. J. Use of an invertebrate animal mode (Aplysia californica) to develop novel neural interfaces for neuromodulation. Frontiers in Neuroscience 2022, 16, 1080027. [Google Scholar] [CrossRef]
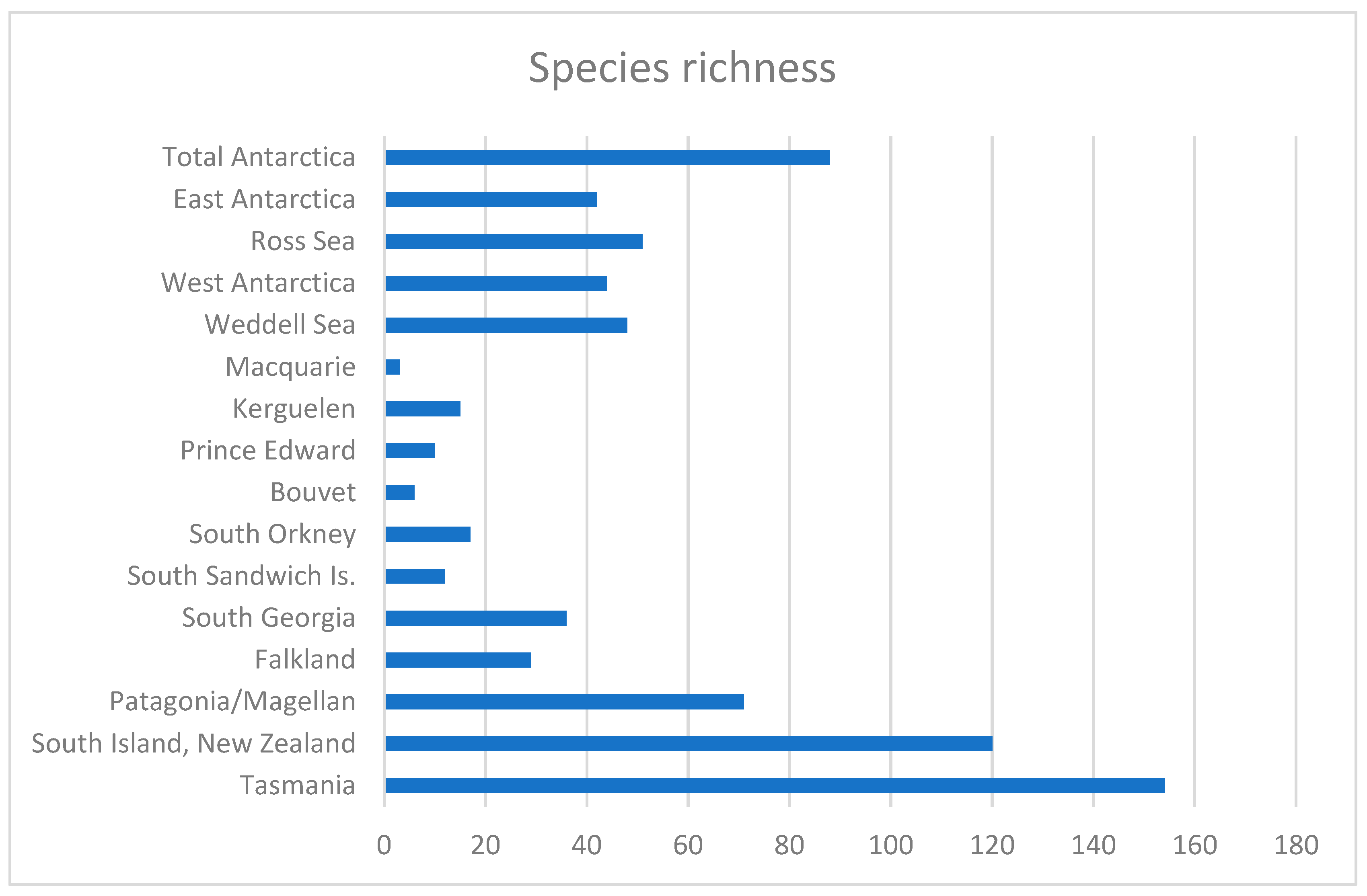
| Zone | Acteonimorpha | Ringiculimorpha | Nudibranchia | Pleurobranchida | Umbraculida | Pteropoda | Cephalaspidea | Runcinacea | Aplysiida | Sacoglossa | Total species |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patagonia/Magellan | 4 | 0 | 39 | 3 | 0 | 8 | 13 | 0 | 0 | 4 | 71 |
| Falkland (Malvinas) Is. | 1 | 0 | 15 | 1 | 0 | 3 | 9 | 0 | 0 | 0 | 29 |
| South Georgia. Is. | 3 | 0 | 15 | 1 | 0 | 7 | 10 | 0 | 0 | 0 | 36 |
| South Sandwich Is. | 1 | 0 | 2 | 1 | 0 | 5 | 3 | 0 | 0 | 0 | 12 |
| South Orkney.Is | 1 | 0 | 9 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | 17 |
| Bouvet Is. | 0 | 0 | 3 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 6 |
| Prince Edward/Crozet Is. | 0 | 0 | 2 | 0 | 0 | 6 | 2 | 0 | 0 | 0 | 10 |
| Kerguelen Is. | 2 | 0 | 5 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 15 |
| Macquarie Is. | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Tasmania | 3 | 0 | 93 | 4 | 2 | 2 | 32 | 1 | 5 | 12 | 154 |
| South Island, NewZealand | 4 | 1 | 47 | 4 | 0 | 32 | 23 | 1 | 5 | 3 | 120 |
| Weddell Sea | 1 | 0 | 19 | 4 | 0 | 8 | 16 | 0 | 0 | 0 | 48 |
| West Antarctica | 3 | 0 | 19 | 1 | 0 | 13 | 9 | 0 | 0 | 0 | 45 |
| Ross Sea | 2 | 0 | 26 | 1 | 0 | 6 | 16 | 0 | 0 | 0 | 51 |
| East Antarctica | 2 | 0 | 22 | 1 | 0 | 8 | 9 | 0 | 0 | 0 | 42 |
| Total Antarctica | 4 | 0 | 40 | 4 | 0 | 14 | 27 | 0 | 0 | 0 | 89 |
| Total by groups | 14 | 1 | 209 | 13 | 2 | 40 | 90 | 2 | 6 | 17 | 394 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





