Submitted:
24 April 2025
Posted:
24 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Bacterial Arsenal of Antimicrobial Weapons
2.1. Diffusible Toxins
2.2. Contact-Dependent Systems
3. The Type VI Secretion System (T6SS)
3.1. General Background
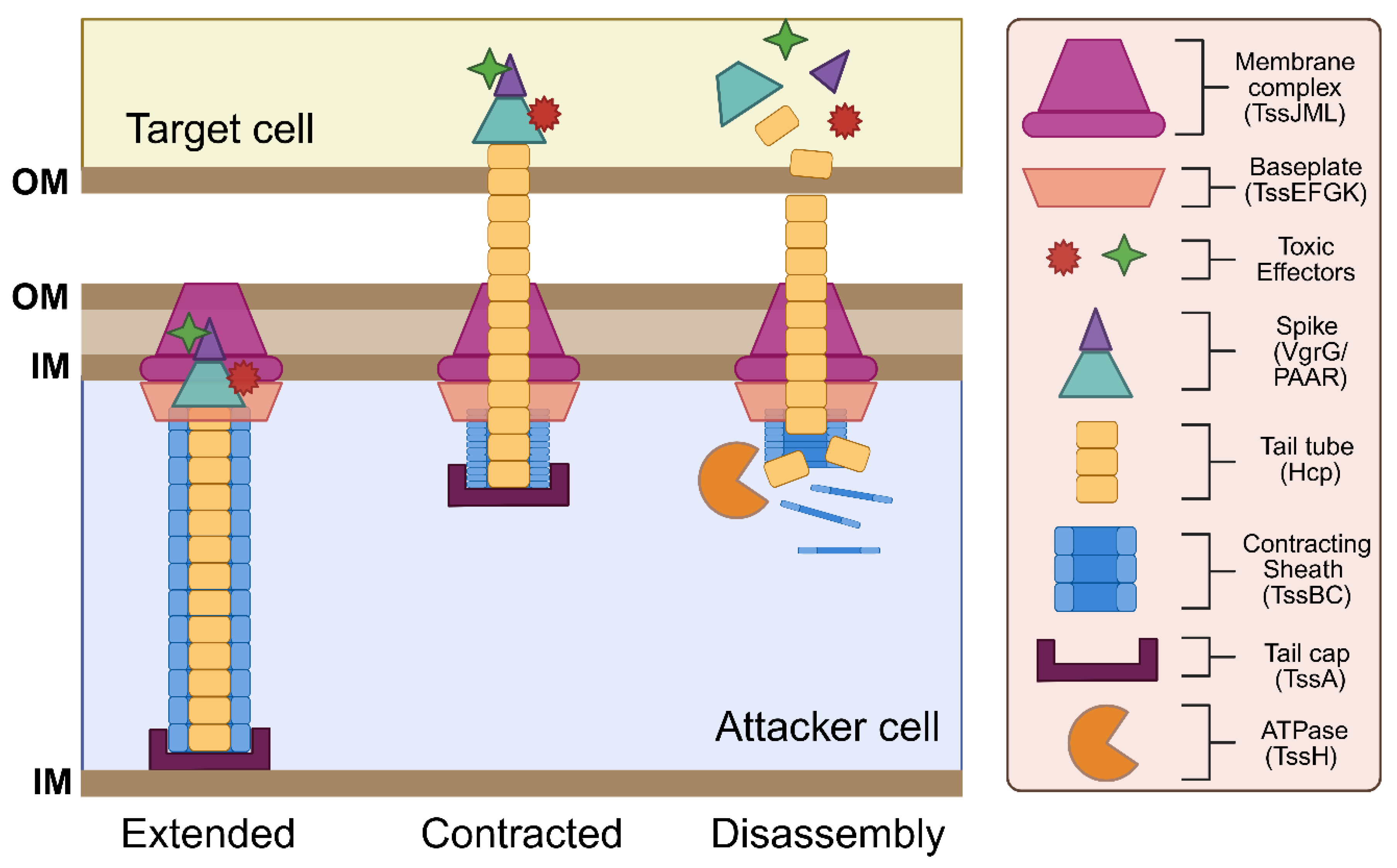
3.2. Assembly and Function of T6SS Proteins
3.2.1. Contractile Sheath-Tube Proteins
3.2.2. Baseplate-Associated Proteins
3.2.3. Membrane-Associated Proteins
3.2.4. Effector Proteins
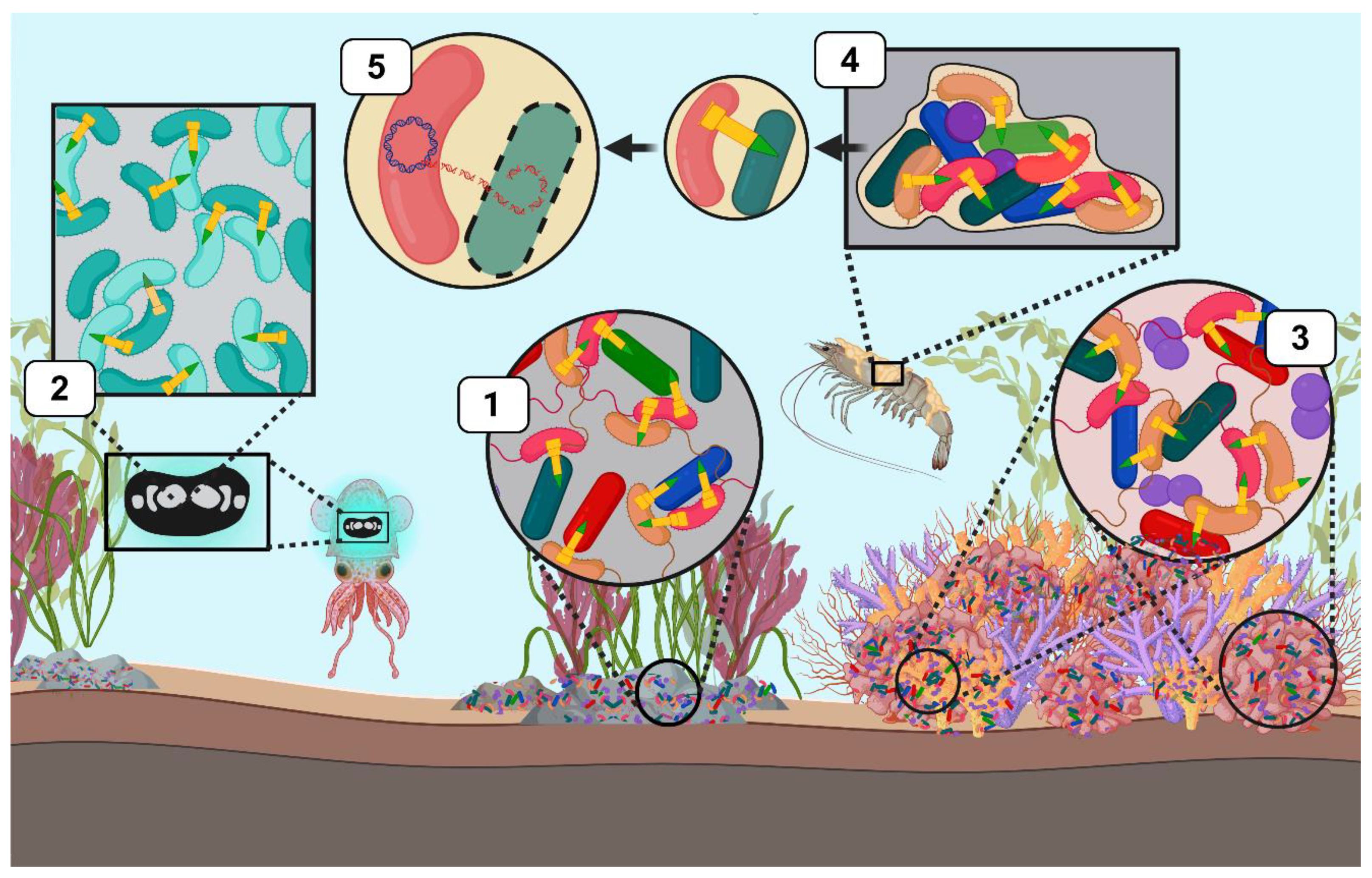
4. T6SS in Vibrio: Impact on Marine Free-Living and Host-Associated Microbial Communities
4.1. T6SS of V. cholerae in Marine Microbial Communities
4.2. T6SS of Vibrio parahaemolyticus in Marine Microbial Communities
4.4. T6SS of Vibrio coralliilyticus in Coral Symbiosis
4.3. T6SS of Vibrio fischeri in Squid Symbiosis
5. Emerging role of T6SS in Vibrio: Shaping Biofilm Dynamics
6. Emerging role of T6SS in Vibrio: Facilitating Gene Acquisition via HGT
Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PG | Peptidoglycan |
| T6SS | Type VI Secretion System |
| LO | Light Organ |
| HGT | Horizontal Gene Transfer |
References
- Stubbendieck, R.M.; Vargas-Bautista, C.; Straight, P.D. Bacterial communities: Interactions to scale. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- García-Bayona, L.; Comstock, L.E. Bacterial antagonism in host-associated microbial communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [PubMed]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Long, R.A.; Azam, F. Antagonistic interactions among marine pelagic bacteria. Appl Environ Microbiol 2001, 67, 4975–4983. [Google Scholar] [CrossRef]
- Ghoul, M.; Mitri, S. The ecology and evolution of microbial competition. Trends Microbiol 2016, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Granato, E.T.; Meiller-Legrand, T.A.; Foster, K.R. The evolution and ecology of bacterial warfare. Curr. Bio 2019, 29, R521–R537. [Google Scholar] [CrossRef]
- Cianfanelli, F.R.; Monlezun, L.; Coulthurst, S.J. Aim, load, fire: The type VI secretion system, a bacterial nanoweapon. Trends Microbiol 2016, 24, 51–62. [Google Scholar] [CrossRef]
- Dix, S.R.; Owen, H.J.; Sun, R.; Ahmad, A.; Shastri, S.; Spiewak, H.L.; Mosby, D.J.; Harris, M.J.; Batters, S.L.; Brooker, T.A.; et al. Structural insights into the function of type VI secretion system TssA subunits. Nat Commun 2018, 9, 4765. [Google Scholar] [CrossRef]
- Allsopp, L.P.; Bernal, P. Killing in the name of: T6SS structure and effector diversity. Microbiol 2023, 169. [Google Scholar] [CrossRef]
- Basler, M.; Pilhofer, M.; Henderson, G.P.; Jensen, G.J.; Mekalanos, J.J. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 2012, 483, 182–186. [Google Scholar] [CrossRef]
- Dyrma, S.; Pei, T.-T.; Liang, X.; Dong, T. Not just passengers: effectors contribute to the assembly of the type VI secretion system as structural building blocks. J. Bacteriol 2025, 207, e00455–24. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Cascales, E. Antibacterial weapons: Targeted destruction in the microbiota. Trends Microbiol 2018, 26, 329–338. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins — a viable alternative to antibiotics? Nat. Rev. Microbiol 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Willey, J.M.; Van Der Donk, W.A. Lantibiotics: Peptides of diverse structure and function. Annu. Rev. Microbiol. 2007, 61, 477–501. [Google Scholar] [CrossRef]
- McAuliffe, O.; Ross, R.P.; Hill, C. Lantibiotics: Structure, biosynthesis and mode of action. FEMS Microbiol Rev 2001, 25, 285–308. [Google Scholar] [CrossRef]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol 2024, 22, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Fimland, N.; Rogne, P.; Fimland, G.; Nissen-Meyer, J.; Kristiansen, P.E. Three-dimensional structure of the two peptides that constitute the two-peptide bacteriocin Plantaricin EF. BBA Proteins Proteom. 2008, 1784, 1711–1719. [Google Scholar] [CrossRef]
- Duquesne, S.; Destoumieux-Garzón, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from Enterobacteria. Nat. Prod. Rep. 2007, 24, 708. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Nuccio, S.-P.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, R.A.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef]
- Rebuffat, S. Microcins in action: Amazing defence strategies of Enterobacteria. Biochem Soc Trans 2012, 40, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Pilsl, H.; Gro�, P. Colicins: Structures, modes of action, transfer through membranes, and evolution. Arch. Microbiol. 1994, 161, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cascales, E.; Buchanan, S.K.; Duché, D.; Kleanthous, C.; Lloubès, R.; Postle, K.; Riley, M.; Slatin, S.; Cavard, D. Colicin biology. Microbiol Mol Biol Rev 2007, 71, 158–229. [Google Scholar] [CrossRef]
- Klein, T.A.; Ahmad, S.; Whitney, J.C. Contact-dependent interbacterial antagonism mediated by protein secretion machines. Trends Microbiol 2020, 28, 387–400. [Google Scholar] [CrossRef]
- Garcia, E.C. Contact-dependent interbacterial toxins deliver a message. Curr Opin Microbiol 2018, 42, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.S.; Aoki, S.K.; Low, D.A. Bacterial contact-dependent delivery systems. Annu. Rev. Genet. 2010, 44, 71–90. [Google Scholar] [CrossRef]
- Aoki, S.K.; Pamma, R.; Hernday, A.D.; Bickham, J.E.; Braaten, B.A.; Low, D.A. Contact-dependent inhibition of growth in Escherichia coli. Science 2005, 309, 1245–1248. [Google Scholar] [CrossRef]
- Hayes, C.S.; Koskiniemi, S.; Ruhe, Z.C.; Poole, S.J.; Low, D.A. Mechanisms and biological roles of contact-dependent growth inhibition systems. Cold Spring Harb Perspect Med 2014, 4, a010025–a010025. [Google Scholar] [CrossRef]
- Aoki, S.K.; Diner, E.J.; De Roodenbeke, C. t’Kint; Burgess, B.R.; Poole, S.J.; Braaten, B.A.; Jones, A.M.; Webb, J.S.; Hayes, C.S.; Cotter, P.A.; et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 2010, 468, 439–442. [Google Scholar] [CrossRef]
- Willett, J.L.E.; Gucinski, G.C.; Fatherree, J.P.; Low, D.A.; Hayes, C.S. Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 11341–11346. [Google Scholar] [CrossRef]
- Aoki, S.K.; Malinverni, J.C.; Jacoby, K.; Thomas, B.; Pamma, R.; Trinh, B.N.; Remers, S.; Webb, J.; Braaten, B.A.; Silhavy, T.J.; et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol 2008, 70, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, Z.C.; Nguyen, J.Y.; Xiong, J.; Koskiniemi, S.; Beck, C.M.; Perkins, B.R.; Low, D.A.; Hayes, C.S. CdiA effectors use modular receptor-binding domains to recognize target bacteria. mBio 2017, 8, e00290–17. [Google Scholar] [CrossRef] [PubMed]
- Morse, R.P.; Nikolakakis, K.C.; Willett, J.L.E.; Gerrick, E.; Low, D.A.; Hayes, C.S.; Goulding, C.W. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 21480–21485. [Google Scholar] [CrossRef]
- Beck, C.M.; Willett, J.L.E.; Cunningham, D.A.; Kim, J.J.; Low, D.A.; Hayes, C.S. CdiA effectors from uropathogenic Escherichia coli use heterotrimeric osmoporins as receptors to recognize target bacteria. PLoS Pathog 2016, 12, e1005925. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; Crook, D.W.; Hood, D.W. Type IV secretion systems: Tools of bacterial horizontal gene transfer and virulence. Cell Microbiol 2008, 10, 2377–2386. [Google Scholar] [CrossRef]
- Waksman, G.; Fronzes, R. Molecular architecture of bacterial type IV secretion systems. Trends Biochem Sci 2010, 35, 691–698. [Google Scholar] [CrossRef]
- Cascales, E.; Christie, P.J. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 2004, 304, 1170–1173. [Google Scholar] [CrossRef]
- Wallden, K.; Rivera-Calzada, A.; Waksman, G. Microreview: Type IV secretion systems: Versatility and diversity in function. Cell Microbiol 2010, 12, 1203–1212. [Google Scholar] [CrossRef]
- Alvarez-Martinez, C.E.; Christie, P.J. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev 2009, 73, 775–808. [Google Scholar] [CrossRef]
- Souza, D.P.; Oka, G.U.; Alvarez-Martinez, C.E.; Bisson-Filho, A.W.; Dunger, G.; Hobeika, L.; Cavalcante, N.S.; Alegria, M.C.; Barbosa, L.R.S.; Salinas, R.K.; et al. Bacterial killing via a type IV secretion system. Nat Commun 2015, 6, 6453. [Google Scholar] [CrossRef]
- Singh, R.P.; Kumari, K. Bacterial type VI secretion system (T6SS): An evolved molecular weapon with diverse functionality. Biotechnol Lett 2023, 45, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Abby, S.S.; Cury, J.; Guglielmini, J.; Néron, B.; Touchon, M.; Rocha, E.P.C. Identification of protein secretion systems in bacterial genomes. Sci Rep 2016, 6, 23080. [Google Scholar] [CrossRef] [PubMed]
- Unni, R.; Pintor, K.L.; Diepold, A.; Unterweger, D. Presence and absence of type VI secretion systems in bacteria. Microbiol 2022, 168. [Google Scholar] [CrossRef]
- Zheng, J.; Leung, K.Y. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol 2007, 66, 1192–1206. [Google Scholar] [CrossRef]
- Pukatzki, S.; Ma, A.T.; Revel, A.T.; Sturtevant, D.; Mekalanos, J.J. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 15508–15513. [Google Scholar] [CrossRef]
- Pukatzki, S.; Ma, A.T.; Sturtevant, D.; Krastins, B.; Sarracino, D.; Nelson, W.C.; Heidelberg, J.F.; Mekalanos, J.J. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 1528–1533. [Google Scholar] [CrossRef]
- Bladergroen, M.R.; Badelt, K.; Spaink, H.P. Infection-blocking genes of a symbiotic Rhizobium leguminosarum strain that are involved in temperature-dependent protein secretion. MPMI 2003, 16, 53–64. [Google Scholar] [CrossRef]
- Srinivasa Rao, P.S.; Yamada, Y.; Tan, Y.P.; Leung, K.Y. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol Microbiol 2004, 53, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordoñez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef]
- Schell, M.A.; Ulrich, R.L.; Ribot, W.J.; Brueggemann, E.E.; Hines, H.B.; Chen, D.; Lipscomb, L.; Kim, H.S.; Mrázek, J.; Nierman, W.C.; et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol 2007, 64, 1466–1485. [Google Scholar] [CrossRef]
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.S.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef]
- Escoll, P.; Mondino, S.; Rolando, M.; Buchrieser, C. Targeting of host organelles by pathogenic bacteria: A sophisticated subversion strategy. Nat Rev Microbiol 2016, 14, 5–19. [Google Scholar] [CrossRef]
- Russell, A.B.; Peterson, S.B.; Mougous, J.D. Type VI secretion system effectors: Poisons with a purpose. Nat Rev Microbiol 2014, 12, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.G.; Flaugnatti, N.; Lugo, K.A.; Lam, L.H.; Jacobson, A.; Baylot, V.; Durand, E.; Journet, L.; Cascales, E.; Monack, D.M. Salmonella typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl. Acad. Sci. U.S.A. 2016, 113. [Google Scholar] [CrossRef]
- Joshi, A.; Kostiuk, B.; Rogers, A.; Teschler, J.; Pukatzki, S.; Yildiz, F.H. Rules of engagement: The type VI secretion system in Vibrio cholerae. Trends Microbiol 2017, 25, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.A.; Blondel, C.J.; Barros-Infante, M.F.; Rivera, D.; Moreno-Switt, A.I.; Santiviago, C.A.; Pezoa, D. Identification of type VI secretion systems effector proteins that contribute to interbacterial competition in Salmonella dublin. Front. Microbiol. 2022, 13, 811932. [Google Scholar] [CrossRef]
- Unterweger, D.; Miyata, S.T.; Bachmann, V.; Brooks, T.M.; Mullins, T.; Kostiuk, B.; Provenzano, D.; Pukatzki, S. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat Commun 2014, 5, 3549. [Google Scholar] [CrossRef] [PubMed]
- Unterweger, D.; Kitaoka, M.; Miyata, S.T.; Bachmann, V.; Brooks, T.M.; Moloney, J.; Sosa, O.; Silva, D.; Duran-Gonzalez, J.; Provenzano, D.; et al. Constitutive type VI secretion system expression gives Vibrio cholerae intra- and interspecific competitive advantages. PLoS ONE 2012, 7, e48320. [Google Scholar] [CrossRef]
- Speare, L.; Cecere, A.G.; Guckes, K.R.; Smith, S.; Wollenberg, M.S.; Mandel, M.J.; Miyashiro, T.; Septer, A.N. Bacterial Symbionts use a type VI secretion system to eliminate competitors in their natural host. Proc. Natl. Acad. Sci. U.S.A. 2018, 115. [Google Scholar] [CrossRef]
- Brooks, T.M.; Unterweger, D.; Bachmann, V.; Kostiuk, B.; Pukatzki, S. Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J. Biol Chem 2013, 288, 7618–7625. [Google Scholar] [CrossRef]
- Silverman, J.M.; Brunet, Y.R.; Cascales, E.; Mougous, J.D. Structure and regulation of the type VI secretion system. Annu. Rev. Microbiol. 2012, 66, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Lacourse, K.D.; Cambillau, C.; DiMaio, F.; Mougous, J.D.; Veesler, D. Structure of the type VI secretion system TssK–TssF–TssG baseplate subcomplex revealed by Cryo-Electron microscopy. Nat Commun 2018, 9, 5385. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Brackmann, M.; Castaño-Díez, D.; Kudryashev, M.; Goldie, K.N.; Maier, T.; Stahlberg, H.; Basler, M. Cryo-EM structure of the extended type VI secretion system sheath–tube complex. Nat Microbiol 2017, 2, 1507–1512. [Google Scholar] [CrossRef]
- Rapisarda, C.; Cherrak, Y.; Kooger, R.; Schmidt, V.; Pellarin, R.; Logger, L.; Cascales, E.; Pilhofer, M.; Durand, E.; Fronzes, R. In situ and high-resolution Cryo-Em structure of a bacterial type VI secretion system membrane complex. EMBO J. 2019, 38, e100886. [Google Scholar] [CrossRef]
- Nazarov, S.; Schneider, J.P.; Brackmann, M.; Goldie, K.N.; Stahlberg, H.; Basler, M. Cryo-EM reconstruction of type VI secretion system baseplate and sheath distal end. EMBO J. 2018, 37, e97103. [Google Scholar] [CrossRef]
- Chang, Y.; Rettberg, L.A.; Ortega, D.R.; Jensen, G.J. In Vivo Structures of an intact type VI secretion system revealed by electron cryotomography. EMBO Rep. 2017, 18, 1090–1099. [Google Scholar] [CrossRef]
- Leiman, P.G.; Basler, M.; Ramagopal, U.A.; Bonanno, J.B.; Sauder, J.M.; Pukatzki, S.; Burley, S.K.; Almo, S.C.; Mekalanos, J.J. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 4154–4159. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Douzi, B.; Durand, E.; Roussel, A.; Cascales, E.; Cambillau, C. Towards a complete structural deciphering of type VI secretion system. Curr. Opin. Struct. Biol. 2018, 49, 77–84. [Google Scholar] [CrossRef]
- Bingle, L.E.; Bailey, C.M.; Pallen, M.J. Type VI secretion: A beginner’s guide. Curr Opin Microbiol 2008, 11, 3–8. [Google Scholar] [CrossRef]
- Cascales, E. The type VI secretion toolkit. EMBO Rep. 2008, 9, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Morgado, S.; Vicente, A.C. Diversity and distribution of type VI secretion system gene clusters in bacterial plasmids. Sci Rep 2022, 12, 8249. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Smith, S.N.; Kanso, E.; Septer, A.N.; Rycroft, C.H. A Subcellular biochemical model for T6SS dynamics reveals winning competitive strategies. PNAS Nexus 2023, 2, pgad195. [Google Scholar] [CrossRef] [PubMed]
- Förster, A.; Planamente, S.; Manoli, E.; Lossi, N.S.; Freemont, P.S.; Filloux, A. Coevolution of the ATPase ClpV, the sheath proteins TssB and TssC, and the accessory protein TagJ/HsiE1 distinguishes type VI secretion classes. J. Bio. Chem. 2014, 289, 33032–33043. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.M.; Santillana, E.; Spínola-Amilibia, M.; Torreira, E.; Culebras, E.; Romero, A. Crystal structure of Hcp from Acinetobacter Baumannii: A component of the type VI secretion system. PLoS ONE 2015, 10, e0129691. [Google Scholar] [CrossRef]
- Cascales, E.; Cambillau, C. Structural biology of type VI secretion systems. Phil. Trans. R. Soc. B 2012, 367, 1102–1111. [Google Scholar] [CrossRef]
- Bönemann, G.; Pietrosiuk, A.; Diemand, A.; Zentgraf, H.; Mogk, A. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J. 2009, 28, 315–325. [Google Scholar] [CrossRef]
- Hernandez, R.E.; Gallegos-Monterrosa, R.; Coulthurst, S.J. Type VI secretion system effector proteins: Effective weapons for bacterial competitiveness. Cell. Microbiol. 2020, 22. [Google Scholar] [CrossRef]
- Brunet, Y.R.; Espinosa, L.; Harchouni, S.; Mignot, T.; Cascales, E. Imaging type VI secretion-mediated bacterial killing. Cell Rep. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Zoued, A.; Durand, E.; Santin, Y.G.; Journet, L.; Roussel, A.; Cambillau, C.; Cascales, E. TssA: The cap protein of the type VI secretion system tail. BioEssays 2017, 39, 1600262. [Google Scholar] [CrossRef]
- Planamente, S.; Salih, O.; Manoli, E.; Albesa-Jové, D.; Freemont, P.S.; Filloux, A. TssA forms a Gp6-like ring attached to the type VI secretion sheath. EMBO J. 2016, 35, 1613–1627. [Google Scholar] [CrossRef]
- Lin, L.; Capozzoli, R.; Ferrand, A.; Plum, M.; Vettiger, A.; Basler, M. Subcellular localization of type VI secretion system assembly in response to cell–cell contact. EMBO J. 2022, 41, e108595. [Google Scholar] [CrossRef] [PubMed]
- Zoued, A.; Durand, E.; Bebeacua, C.; Brunet, Y.R.; Douzi, B.; Cambillau, C.; Cascales, E.; Journet, L. TssK is a trimeric cytoplasmic protein interacting with components of both phage-like and membrane anchoring complexes of the type VI secretion system. J. Biol. Chem. 2013, 288, 27031–27041. [Google Scholar] [CrossRef] [PubMed]
- Brunet, Y.R.; Zoued, A.; Boyer, F.; Douzi, B.; Cascales, E. The type VI secretion TssEFGK-VgrG phage-like baseplate is recruited to the TssJLM membrane complex via multiple contacts and serves as assembly platform for tail tube/sheath polymerization. PLoS Genet 2015, 11, e1005545. [Google Scholar] [CrossRef]
- Aschtgen, M.; Zoued, A.; Lloubès, R.; Journet, L.; Cascales, E. The C-tail anchored TssL subunit, an essential protein of the enteroaggregative Escherichia coli Sci-1 type VI secretion system, is inserted by YidC. MicrobiologyOpen 2012, 1, 71–82. [Google Scholar] [CrossRef]
- Aschtgen, M.-S.; Thomas, M.S.; Cascales, E. Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP... what else? Virulence 2010, 1, 535–540. [Google Scholar] [CrossRef]
- Durand, E.; Derrez, E.; Audoly, G.; Spinelli, S.; Ortiz-Lombardia, M.; Raoult, D.; Cascales, E.; Cambillau, C. Crystal structure of the VgrG1 actin cross-linking domain of the Vibrio Cholerae type VI secretion system. J. Biol. Chem. 2012, 287, 38190–38199. [Google Scholar] [CrossRef]
- Sexton, J.A.; Miller, J.L.; Yoneda, A.; Kehl-Fie, T.E.; Vogel, J.P. Legionella Pneumophila DotU and IcmF are required for stability of the Dot/Icm complex. Infect Immun 2004, 72, 5983–5992. [Google Scholar] [CrossRef]
- Durand, E.; Zoued, A.; Spinelli, S.; Watson, P.J.H.; Aschtgen, M.-S.; Journet, L.; Cambillau, C.; Cascales, E. Structural characterization and oligomerization of the TssI protein, a component shared by bacterial type VI and type IVb secretion systems. J. Biol. Chem. 2012, 287, 14157–14168. [Google Scholar] [CrossRef]
- Felisberto-Rodrigues, C.; Durand, E.; Aschtgen, M.-S.; Blangy, S.; Ortiz-Lombardia, M.; Douzi, B.; Cambillau, C.; Cascales, E. Towards a structural comprehension of bacterial Type VI secretion systems: Characterization of the TssJ-TssM complex of an Escherichia coli pathovar. PLoS Pathog 2011, 7, e1002386. [Google Scholar] [CrossRef]
- Liang, X.; Kamal, F.; Pei, T.-T.; Xu, P.; Mekalanos, J.J.; Dong, T.G. An onboard checking mechanism ensures effector delivery of the type VI secretion system in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 23292–23298. [Google Scholar] [CrossRef]
- Crisan, C.V.; Hammer, B.K. The Vibrio cholerae type VI secretion system: Toxins, regulators and consequences. Environ. Microbiol. 2020, 22, 4112–4122. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Altindis, E.; Dong, T.; Catalano, C.; Mekalanos, J. Secretome analysis of Vibrio cholerae type VI secretion system reveals a new effector-immunity pair. mBio 2015, 6, e00075–15. [Google Scholar] [CrossRef]
- Dong, T.G.; Ho, B.T.; Yoder-Himes, D.R.; Mekalanos, J.J. Identification of T6SS-dependent effector and immunity proteins by Tn-Seq in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 2623–2628. [Google Scholar] [CrossRef]
- Russell, A.B.; LeRoux, M.; Hathazi, K.; Agnello, D.M.; Ishikawa, T.; Wiggins, P.A.; Wai, S.N.; Mougous, J.D. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 2013, 496, 508–512. [Google Scholar] [CrossRef]
- Unterweger, D.; Kostiuk, B.; Ötjengerdes, R.; Wilton, A.; Diaz-Satizabal, L.; Pukatzki, S. Chimeric adaptor proteins translocate diverse type VI secretion system effectors in Vibrio cholerae. EMBO J. 2015, 34, 2198–2210. [Google Scholar] [CrossRef]
- Miyata, S.T.; Kitaoka, M.; Brooks, T.M.; McAuley, S.B.; Pukatzki, S. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium Discoideum. Infect Immun 2011, 79, 2941–2949. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.T.; Unterweger, D.; Rudko, S.P.; Pukatzki, S. Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae. PLoS Pathog 2013, 9, e1003752. [Google Scholar] [CrossRef]
- Geller, A.M.; Shalom, M.; Zlotkin, D.; Blum, N.; Levy, A. Identification of type VI secretion system effector-immunity pairs using structural bioinformatics. Mol Syst Biol 2024, 20, 702–718. [Google Scholar] [CrossRef]
- Alcoforado Diniz, J.; Liu, Y.; Coulthurst, S.J. Molecular weaponry: Diverse effectors delivered by the type VI secretion system. Cell Microbiol 2015, 17, 1742–1751. [Google Scholar] [CrossRef]
- Colautti, J.; Tan, H.; Bullen, N.P.; Thang, S.S.; Hackenberger, D.; Doxey, A.C.; Whitney, J.C. A widespread accessory protein family diversifies the effector repertoire of the type VI secretion system spike. Nat Commun 2024, 15, 10108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ho, B.; Mekalanos, J.J. Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae. PLoS ONE 2011, 6, e23876. [Google Scholar] [CrossRef]
- Unterweger, D.; Miyata, S.T.; Bachmann, V.; Brooks, T.M.; Mullins, T.; Kostiuk, B.; Provenzano, D.; Pukatzki, S. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat Commun 2014, 5, 3549. [Google Scholar] [CrossRef]
- Crisan, C.V.; Chande, A.T.; Williams, K.; Raghuram, V.; Rishishwar, L.; Steinbach, G.; Watve, S.S.; Yunker, P.; Jordan, I.K.; Hammer, B.K. Analysis of Vibrio cholerae genomes identifies new type VI secretion system gene clusters. Genome Biol 2019, 20, 163. [Google Scholar] [CrossRef]
- Crisan, C.V.; Hammer, B.K. The Vibrio cholerae type VI secretion system: Toxins, regulators and consequences. Environ. Microbiol. 2020, 22, 4112–4122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shin, O.S.; Cameron, D.E.; Mekalanos, J.J. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 21128–21133. [Google Scholar] [CrossRef]
- Kostiuk, B.; Unterweger, D.; Provenzano, E.; Pukatzki, S. T6SS intraspecific competition orchestrates Vibrio cholerae genotypic diversity. Int. Microbiol. 2017, 130–137. [Google Scholar] [CrossRef]
- Santoriello, F.; Pukatzki, S. Type VI secretion systems: Environmental and intra-host competition of Vibrio cholerae. In Vibrio spp. Infections; Almagro-Moreno, S., Pukatzki, S., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, 2023; ISBN 978-3-031-22996-1. [Google Scholar]
- Bernardy, E.E.; Turnsek, M.A.; Wilson, S.K.; Tarr, C.L.; Hammer, B.K. Diversity of clinical and environmental isolates of Vibrio cholerae in natural transformation and contact-dependent bacterial killing indicative of type VI secretion system activity. Appl Environ Microbiol 2016, 82, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Keppel, K.; Fridman, C.M.; Bosis, E.; Salomon, D. Multiple T6SS, mobile auxiliary modules, and effectors revealed in a systematic analysis of the Vibrio parahaemolyticus pan-genome. mSystems 2022, 7, e00723–22. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, H.; Li, J.; Zhang, P.; Wu, B.; Zhu, B.; Zhang, Y.; Fang, W. Putative type VI secretion systems of Vibrio parahaemolyticus contribute to adhesion to cultured cell monolayers. Arch Microbiol 2012, 194, 827–835. [Google Scholar] [CrossRef]
- Salomon, D.; Gonzalez, H.; Updegraff, B.L.; Orth, K. Vibrio parahaemolyticus type VI secretion system 1 is activated in marine conditions to target bacteria, and is differentially regulated from system 2. PLoS ONE 2013, 8, e61086. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, L.; Zhang, Y.; Sheng, H.; Fang, W. VgrG2 of type VI secretion system 2 of Vibrio parahaemolyticus induces autophagy in macrophages. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Krediet, C.J.; Ritchie, K.B.; Paul, V.J.; Teplitski, M. Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proc. R. Soc. B. 2013, 280, 20122328. [Google Scholar] [CrossRef]
- Webster, N.S.; Reusch, T.B.H. Microbial contributions to the persistence of coral reefs. ISME J. 2017, 11, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Tran, C. Coral–microbe interactions: Their importance to reef function and survival. Emer. Top. Life Sci. 2022, 6, 33–44. [Google Scholar] [CrossRef]
- Wang, W.; Tang, K.; Wang, X. High temperatures increase the virulence of Vibrio bacteria towards their coral host and competing bacteria via type VI secretion systems. PLoS Biol 2024, 22, e3002788. [Google Scholar] [CrossRef]
- Mass, S.; Cohen, H.; Podicheti, R.; Rusch, D.B.; Gerlic, M.; Ushijima, B.; Van Kessel, J.C.; Bosis, E.; Salomon, D. The coral pathogen Vibrio coralliilyticus uses a T6SS to secrete a group of novel anti-eukaryotic effectors that contribute to virulence. PLoS Biol 2024, 22, e3002734. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Cai, Z.; Cheng, K.; Chen, G.; Zhou, J. Mitigation of Vibrio coralliilyticus-induced coral bleaching through bacterial dysbiosis prevention by Ruegeria profundi. Appl Environ Microbiol 2024, 90, e02274–23. [Google Scholar] [CrossRef]
- Rubio-Portillo, E.; Martin-Cuadrado, A.B.; Caraballo-Rodríguez, A.M.; Rohwer, F.; Dorrestein, P.C.; Antón, J. Virulence as a side effect of interspecies interaction in Vibrio coral pathogens. mBio 2020, 11, e00201–20. [Google Scholar] [CrossRef]
- Cohen, H.; Fridman, C.M.; Gerlic, M.; Salomon, D. A Vibrio T6SS-mediated lethality in an aquatic animal model. Microbiol Spectr 2023, 11, e01093–23. [Google Scholar] [CrossRef]
- Septer, A.N. The Vibrio-squid symbiosis as a model for studying interbacterial competition. mSystems 2019, 4, e00108–19. [Google Scholar] [CrossRef] [PubMed]
- Visick, K.L.; Stabb, E.V.; Ruby, E.G. A lasting symbiosis: How Vibrio fischeri finds a squid partner and persists within its natural host. Nat Rev Microbiol 2021, 19, 654–665. [Google Scholar] [CrossRef]
- Nyholm, S.V.; McFall-Ngai, M.J. A lasting symbiosis: How the Hawaiian bobtail squid finds and keeps its bioluminescent bacterial partner. Nat Rev Microbiol 2021, 19, 666–679. [Google Scholar] [CrossRef]
- Jones, B.W.; Nishiguchi, M.K. Counterillumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda). Mar. Biol. 2004, 144, 1151–1155. [Google Scholar] [CrossRef]
- Lee, K.-H.; Ruby, E.G. Detection of the light organ symbiont, Vibrio fischeri, in Hawaiian seawater by using Lux gene probes. Appl Environ Microbiol 1992, 58, 942–947. [Google Scholar] [CrossRef]
- Montgomery, M.K.; McFall-Ngai, M. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 1994, 120, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Sycuro, L.K.; Ruby, E.G.; McFall-Ngai, M. Confocal microscopy of the light organ crypts in juvenile Euprymna scolopes reveals their morphological complexity and dynamic function in symbiosis. J. Morphol 2006, 267, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, M.S.; Ruby, E.G. Population structure of Vibrio fischeri within the light organs of Euprymna scolopes squid from two Oahu (Hawaii) populations. Appl Environ Microbiol 2009, 75, 193–202. [Google Scholar] [CrossRef]
- Sun, Y.; LaSota, E.D.; Cecere, A.G.; LaPenna, K.B.; Larios-Valencia, J.; Wollenberg, M.S.; Miyashiro, T. Intraspecific competition impacts Vibrio fischeri strain diversity during initial colonization of the squid light organ. Appl Environ Microbiol 2016, 82, 3082–3091. [Google Scholar] [CrossRef]
- Guckes, K.R.; Cecere, A.G.; Wasilko, N.P.; Williams, A.L.; Bultman, K.M.; Mandel, M.J.; Miyashiro, T. Incompatibility of Vibrio fischeri strains during symbiosis establishment depends on two functionally redundant Hcp genes. J. Bacteriol 2019, 201. [Google Scholar] [CrossRef]
- Bultman, K.M.; Cecere, A.G.; Miyashiro, T.; Septer, A.N.; Mandel, M.J. Draft genome sequences of type VI secretion system-encoding Vibrio fischeri strains FQ-A001 and ES401. Microbiol Resour Announc 2019, 8, e00385–19. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, S.V.; McFall-Ngai, M. The winnowing: Establishing the squid–Vibrio symbiosis. Nat Rev Microbiol 2004, 2, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Speare, L.; Smith, S.; Salvato, F.; Kleiner, M.; Septer, A.N. Environmental viscosity modulates interbacterial killing during habitat transition. mBio 2020, 11, e03060–19. [Google Scholar] [CrossRef]
- Speare, L.; Woo, M.; Bultman, K.M.; Mandel, M.J.; Wollenberg, M.S.; Septer, A.N. Host-like conditions are required for T6SS-mediated competition among Vibrio fischeri light organ symbionts. mSphere 2021, 6, e01288–20. [Google Scholar] [CrossRef] [PubMed]
- Suria, A.M.; Smith, S.; Speare, L.; Chen, Y.; Chien, I.; Clark, E.G.; Krueger, M.; Warwick, A.M.; Wilkins, H.; Septer, A.N. Prevalence and diversity of type VI secretion systems in a model beneficial symbiosis. Front. Microbiol. 2022, 13, 988044. [Google Scholar] [CrossRef]
- Guckes, K.R.; Yount, T.A.; Steingard, C.H.; Miyashiro, T.I. Quorum sensing inhibits interference competition among bacterial symbionts within a host. Curr. Biol 2023, 33, 4244–4251.e4. [Google Scholar] [CrossRef]
- Septer, A.N.; Sharpe, G.; Shook, E.A. The Vibrio Fischeri type VI secretion system incurs a fitness cost under host-like conditions. 2023.
- Lin, Y.L.; Smith, S.N.; Kanso, E.; Septer, A.N.; Rycroft, C.H. A subcellular biochemical model for T6SS dynamics reveals winning competitive strategies. PNAS Nexus 2023, 2, pgad195. [Google Scholar] [CrossRef]
- Yildiz, F.H.; Visick, K.L. Vibrio biofilms: so much the same yet so different. Trends Microbiol 2009, 17, 109–118. [Google Scholar] [CrossRef]
- Visick, K.L.; Schembri, M.A.; Yildiz, F.; Ghigo, J.-M. Biofilms 2015: Multidisciplinary approaches shed light into microbial life on surfaces. J. Bacteriol 2016, 198, 2553–2563. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat Rev Microbiol 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 2016, 80, 91–138. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wan, W.; Ding, B.; Cai, M.; Lu, M.; Liu, W. Type VI secretion system drives bacterial diversity and functions in multispecies biofilms. Microbiol. Res. 2024, 279, 127570. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat Rev Microbiol 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Sutherland, I. The biofilm matrix – an immobilized but dynamic microbial environment. Trends Microbiol 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Teschler, J.K.; Jiménez-Siebert, E.; Jeckel, H.; Singh, P.K.; Park, J.H.; Pukatzki, S.; Nadell, C.D.; Drescher, K.; Yildiz, F.H. VxrB influences antagonism within biofilms by controlling competition through extracellular matrix production and type 6 secretion. mBio 2022, 13, e01885–22. [Google Scholar] [CrossRef]
- Toska, J.; Ho, B.T.; Mekalanos, J.J. Exopolysaccharide protects Vibrio cholerae from exogenous attacks by the type 6 secretion system. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 7997–8002. [Google Scholar] [CrossRef]
- Bai, X.; Liu, P.; Wang, W.; Jin, Y.; Wang, Q.; Qi, Y.; Zhang, X.; Sun, W.; Fang, W.; Han, X.; et al. TssL2 of T6SS2 is required for mobility, biofilm formation, wrinkly phenotype formation, and virulence of Vibrio parahaemolyticus SH112. Appl Microbiol Biotechnol 2024, 108, 537. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, M.; Zhang, Y.; Li, X.; Luo, X.; Ji, S.; Lu, R. IcmF2 of the type VI secretion system 2 plays a role in biofilm formation of Vibrio parahaemolyticus. Arch Microbiol 2024, 206, 321. [Google Scholar] [CrossRef]
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347. [Google Scholar] [CrossRef]
- Ringel, P.D.; Hu, D.; Basler, M. The role of type VI secretion system effectors in target cell lysis and subsequent horizontal gene transfer. Cell Rep. 2017, 21, 3927–3940. [Google Scholar] [CrossRef] [PubMed]
- Meibom, K.L.; Blokesch, M.; Dolganov, N.A.; Wu, C.-Y.; Schoolnik, G.K. Chitin induces natural competence in Vibrio cholerae. Science 2005, 310, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Lo Scrudato, M.; Blokesch, M. A transcriptional regulator linking quorum sensing and chitin induction to render Vibrio cholerae naturally transformable. Nucleic Acids Res. 2013, 41, 3644–3658. [Google Scholar] [CrossRef]
- Debnath, A.; Miyoshi, S.-I. Chitin degradation and its effect on natural transformation: A systematic genetic study in Vibrio parahaemolyticus. Can. J. Microbiol. 2022, 68, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Debnath, A.; Mizuno, T.; Miyoshi, S. Regulation of chitin-dependent growth and natural competence in Vibrio parahaemolyticus. Microorganisms 2020, 8, 1303. [Google Scholar] [CrossRef]
- Souza, C.P.; Almeida, B.C.; Colwell, R.R.; Rivera, I.N.G. The importance of chitin in the marine environment. Mar Biotechnol 2011, 13, 823–830. [Google Scholar] [CrossRef]
- Jeuniaux, C.; Voss-Foucart, M.F. Chitin biomass and production in the marine environment. Biochem. Syst. Ecol. 1991, 19, 347–356. [Google Scholar] [CrossRef]
- Borgeaud, S.; Metzger, L.C.; Scrignari, T.; Blokesch, M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science 2015, 347, 63–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




