Submitted:
23 April 2025
Posted:
24 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Methods
2.3. Data Analysis
3. Results
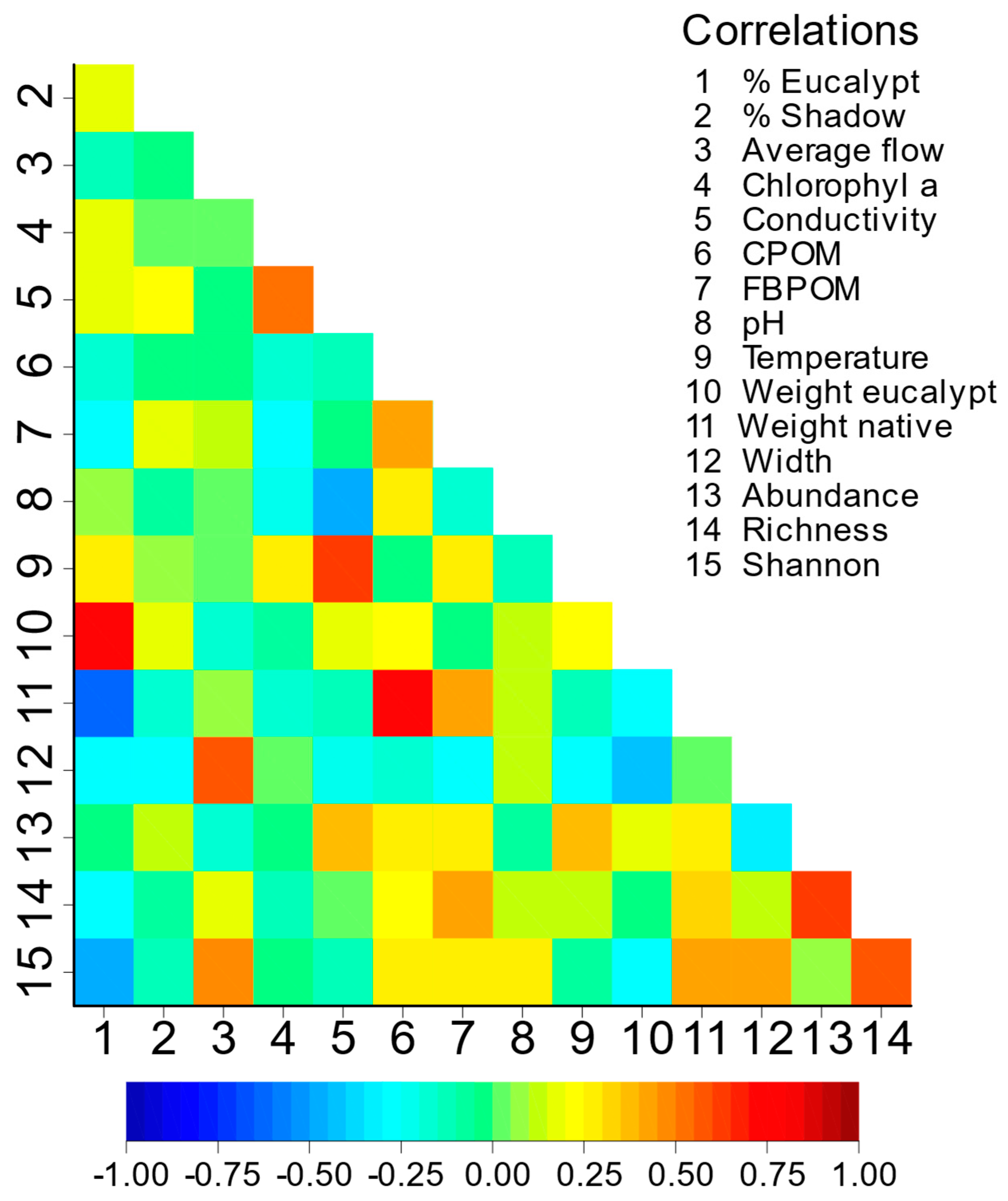
3.1. Stream Site Characterization
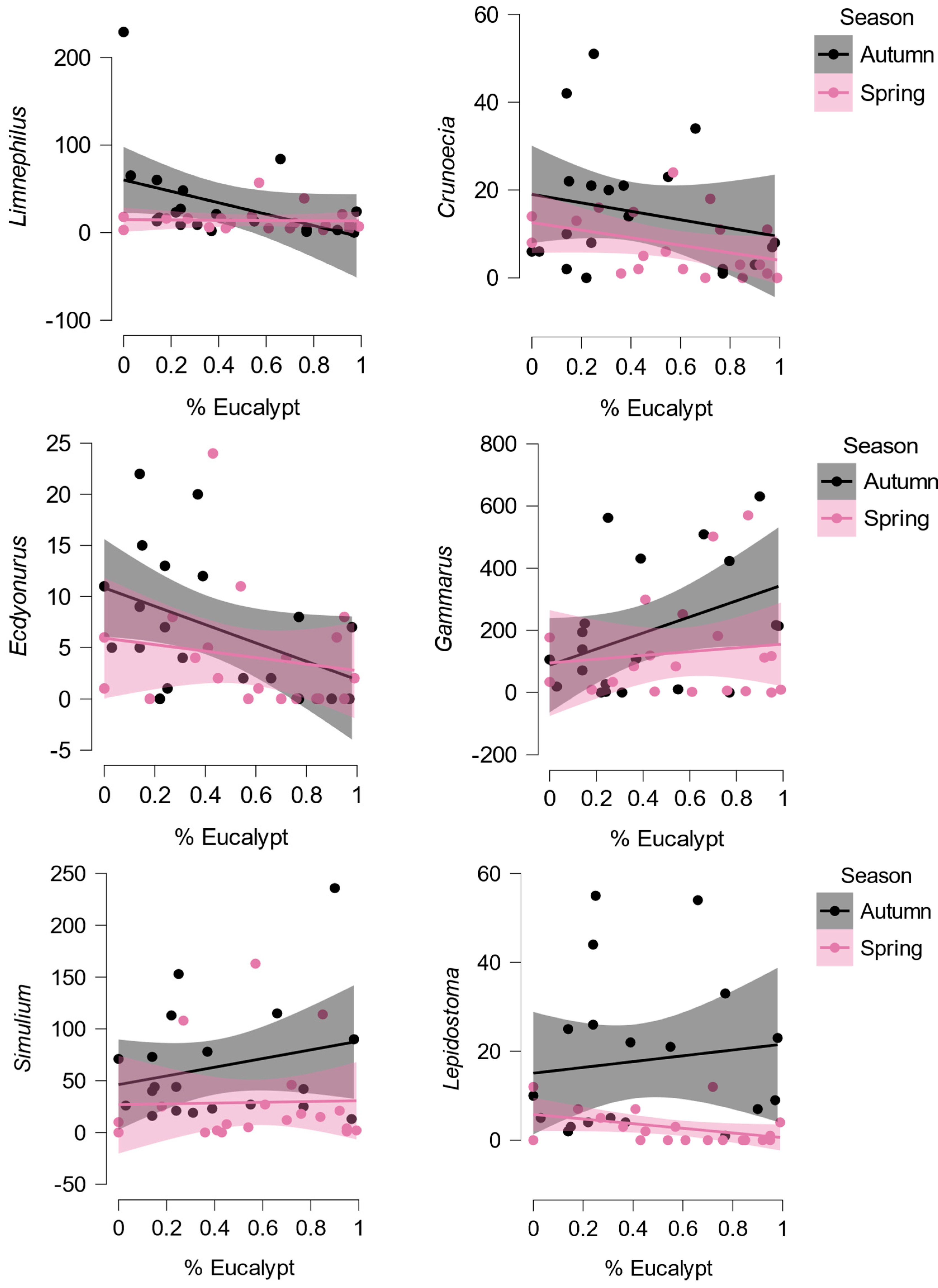
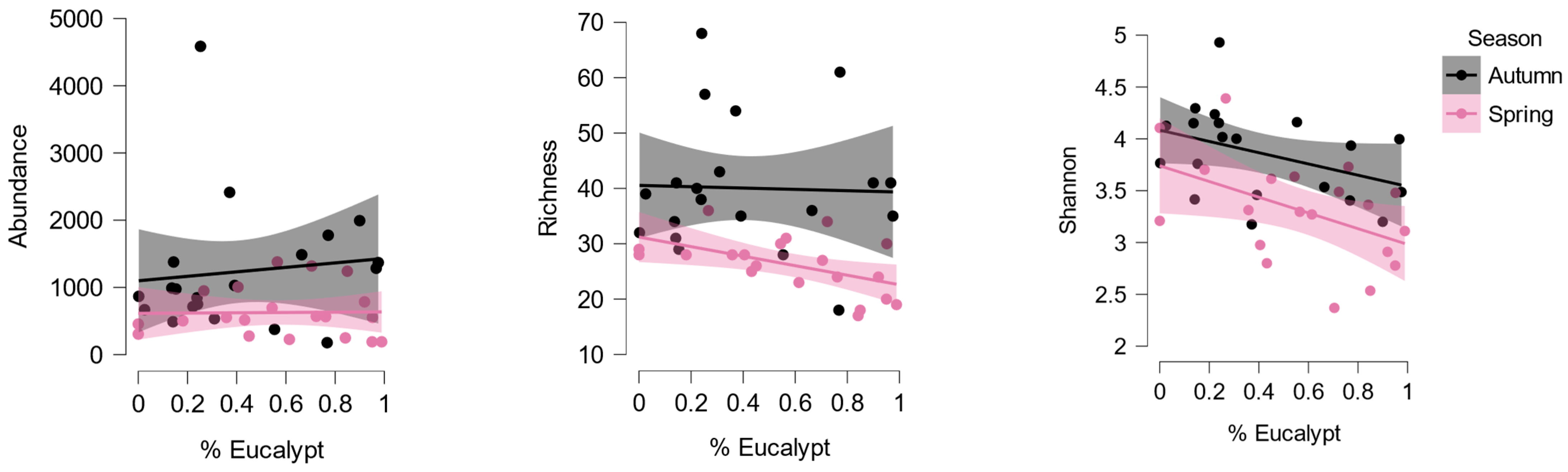
3.2. Macroinvertebrate Abundance, Richness and Diversity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike’s Information Criterion |
| CPOM | Coarse Particulate Organic Matter |
| FBOPM | Fine Benthic Particulate Organic Matter |
Appendix A
| Autumn | |||||||||||
| Locality and stream | Latitude | Longitude | T (ºC) | Conductivity | pH | %Shadow | Width | Flow | FBPOM | CPOM | Chlorophyl a |
| Aldán,Bouzas | 42.29872 | -8.81164 | 14.30 | 171.00 | 5.98 | 70 | 0.65 | 2.93 | 0.3133 | 82.62 | 0.502 |
| Aldán, Orxas | 42.28328 | -8.81056 | 14.70 | 153.50 | 6.21 | 70 | 2.17 | 158.45 | 0.3433 | 172.95 | 3.267 |
| Marín, Agrela | 42.38064 | -8.66778 | 11.20 | 54.40 | 6.77 | 90 | 0.48 | 2.55 | 0.4383 | 463.80 | 0.241 |
| Marín, Gorgadas | 42.33878 | -8.72917 | 11.70 | 51.90 | 6.31 | 60 | 1.47 | 75.26 | 0.3983 | 380.65 | 1.969 |
| Vilaboa, Portiño | 42.33289 | -8.66764 | 13.80 | 43.80 | 6.71 | 75 | 1.55 | 92.78 | 0.7470 | 549.32 | 0.672 |
| Vilaboa, Maior | 42.33211 | -8.66083 | 14.40 | 39.60 | 7.37 | 50 | 1.90 | 11.76 | 0.8233 | 357.73 | 0.634 |
| Coruxo, Gontade | 42.17664 | -8.78444 | 14.80 | 70.40 | 6.81 | 100 | 0.20 | 1.14 | 1.0250 | 399.39 | 0.020 |
| Coruxo, Saiáns | 42.16736 | -8.79714 | 14.10 | 113.80 | 6.47 | 100 | 0.54 | 2.49 | 0.9767 | 583.88 | 0.201 |
| Gondomar, Peitieiros | 42.08669 | -8.73131 | 12.40 | 34.80 | 6.77 | 40 | 1.30 | 58.81 | 0.2900 | 491.47 | 11.829 |
| Gondomar, Mordagáns | 42.11244 | -8.72339 | 12.20 | 49.80 | 6.76 | 70 | 1.73 | 121.26 | 0.7833 | 293.04 | 27.557 |
| Mondariz, da Vida | 42.17842 | -8.58033 | 12.80 | 48.00 | 6.50 | 70 | 1.10 | 63.07 | 0.0633 | 0.0441 | 9.882 |
| Mondariz, Cillarga | 42.21158 | -8.56094 | 13.20 | 36.00 | 6.50 | 75 | 0.98 | 75.53 | 0.5583 | 565.71 | 10.385 |
| Porriño, da Fraga | 42.21444 | -8.58953 | 12.60 | 31.20 | 6.60 | 90 | 1.93 | 279.24 | 0.8400 | 278.37 | 0.052 |
| Porriño, Louro | 42.17589 | -8.60692 | 13.70 | 66.50 | 5.80 | 75 | 0.52 | 31.13 | 1.8333 | 483.12 | 0.024 |
| Campo Lameiro, Grande | 42.53258 | -8.48572 | 12.40 | 23.00 | 6.74 | 85 | 1.63 | 25.01 | 0.5217 | 249.23 | 0.695 |
| Campo Lameiro, Teixoeiras | 42.54658 | -8.54872 | 12.00 | 28.90 | 6.81 | 60 | 1.56 | 40.96 | 0.4150 | 871.20 | 0.932 |
| Pontevedra, Seixiña | 42.53839 | -8.46836 | 12.30 | 29.10 | 6.31 | 60 | 0.83 | 6.58 | 0.4883 | 281.18 | 0.878 |
| Pontevedra, As Laceiras | 42.53042 | -8.41822 | 10.00 | 25.00 | 6.32 | 90 | 1.63 | 44.51 | 0.4467 | 175.05 | 0.667 |
| Ponteareas, Xabriña | 42.20158 | -8.42372 | 12.80 | 34.70 | 6.32 | 50 | 2.23 | 108.62 | 0.2867 | 315.99 | 7.848 |
| Ponteareas, Fragón | 42.19333 | -8.45972 | 13.50 | 61.10 | 6.20 | 65 | 1.64 | 81.58 | 0.0650 | 153.54 | 15.115 |
| mean | 12.95 | 58.33 | 6.51 | 72.25 | 1.30 | 64.18 | 0.58 | 357.41 | 4.67 | ||
| Spring | |||||||||||
| Aldán,Bouzas | 42.29872 | -8.81164 | 13.90 | 191.30 | 5.98 | 90 | 0.65 | 3.28 | 0.0000 | 282.41 | 75.115 |
| Aldán, Orxas | 42.28328 | -8.81056 | 14.20 | 123.90 | 6.21 | 70 | 2.17 | 68.17 | 0.0000 | 127.45 | 82.897 |
| Marín, Agrela | 42.38064 | -8.66778 | 12.80 | 43.60 | 6.77 | 65 | 0.48 | 6.13 | 0.0000 | 342.79 | 7.393 |
| Marín, Gorgadas | 42.33878 | -8.72917 | 13.40 | 67.10 | 6.31 | 80 | 1.47 | 68.68 | 0.8650 | 224.58 | 25.298 |
| Vilaboa, Portiño | 42.33289 | -8.66764 | 13.70 | 46.50 | 6.71 | 90 | 1.55 | 100.75 | - | 110.57 | 0.690 |
| Vilaboa, Maior | 42.33211 | -8.66083 | 14.30 | 42.30 | 7.37 | 60 | 1.90 | - | 0.0000 | 57.64 | 0.188 |
| Coruxo, Gontade | 42.17664 | -8.78444 | 14.00 | 66.00 | 6.81 | 95 | 0.20 | 2.88 | 0.4950 | 328.28 | 9.333 |
| Coruxo, Saiáns | 42.16736 | -8.79714 | 14.10 | 106.40 | 6.47 | 90 | 0.54 | 10.52 | 0.0000 | 81.52 | 33.864 |
| Gondomar, Peitieiros | 42.08669 | -8.73131 | 13.00 | 37.90 | 6.77 | 45 | 1.30 | 13.10 | 0.0717 | 152.83 | 29.101 |
| Gondomar, Mordagáns | 42.11244 | -8.72339 | 13.00 | 53.10 | 6.76 | 70 | 1.73 | 117.64 | 0.0000 | 133.89 | 5.024 |
| Mondariz, da Vida | 42.17842 | -8.58033 | 13.40 | 51.00 | 6.50 | 60 | 1.10 | 21.22 | 0.0900 | 271.78 | 0.276 |
| Mondariz, Cillarga | 42.21158 | -8.56094 | 13.00 | 34.20 | 6.50 | 45 | 0.98 | 3.03 | 0.2650 | 196.92 | 0.374 |
| Porriño, da Fraga | 42.21444 | -8.58953 | 13.10 | 31.70 | 6.60 | 90 | 1.93 | 18.63 | 0.1017 | 229.85 | 0.176 |
| Porriño, Louro | 42.17589 | -8.60692 | 14.00 | 61.90 | 5.80 | 70 | 0.52 | 8.63 | 1.4317 | 138.39 | 0.182 |
| Campo Lameiro, Grande | 42.53258 | -8.48572 | 11.50 | 20.20 | 6.74 | 70 | 1.63 | 22.45 | 0.0000 | 205.02 | 0.095 |
| Campo Lameiro, Teixoeiras | 42.54658 | -8.54872 | 11.10 | 26.10 | 6.81 | 90 | 1.56 | 11.17 | 0.0000 | 205.24 | 0.091 |
| Pontevedra, Seixiña | 42.53839 | -8.46836 | 12.50 | 26.40 | 6.31 | 80 | 0.83 | 3.07 | 0.0000 | 270.02 | 0.160 |
| Pontevedra, As Laceiras | 42.53042 | -8.41822 | 11.30 | 24.00 | 6.32 | 60 | 1.63 | 30.26 | 0.0000 | 50.92 | 0.962 |
| Ponteareas, Xabriña | 42.20158 | -8.42372 | 11.80 | 36.80 | 6.32 | 80 | 2.23 | 23.69 | 0.2867 | 238.97 | 0.161 |
| Ponteareas, Fragón | 42.19333 | -8.45972 | 12.30 | 61.60 | 6.20 | 90 | 1.64 | 27.09 | 0.0650 | 87.74 | 0.000 |
| Mean | 13.02 | 57.60 | 6.51 | 74.50 | 1.30 | 29.49 | 0.19 | 186.84 | 13.57 |
| Rank | Model | Deviance | AIC | n | K | AICc | deltaAICc | relative Likelihood | Akaike Weight |
| 1 | Season+Flow+Temperature | 509.00 | 551.50 | 39 | 3 | 552.19 | 0.00 | 1.00 | 0.35 |
| 2 | Season+Flow+Temperature+Conductivity | 505.50 | 552.46 | 39 | 4 | 553.64 | 1.45 | 0.48 | 0.17 |
| 3 | Season+Flow+Temperature+Weigth native | 504.61 | 553.16 | 39 | 4 | 554.34 | 2.15 | 0.34 | 0.12 |
| 4 | Season+Flow+Temperature+% Eucalypt | 496.92 | 553.39 | 39 | 4 | 554.57 | 2.38 | 0.30 | 0.11 |
| 5 | Season+Flow+Temperature+Width | 497.86 | 553.45 | 39 | 4 | 554.63 | 2.44 | 0.30 | 0.10 |
| 6 | Season+Flow+Temperature+Weigth eucalypt | 504.48 | 553.62 | 39 | 4 | 554.80 | 2.61 | 0.27 | 0.10 |
| 7 | Season+Flow | 524.69 | 556.48 | 39 | 2 | 556.81 | 4.63 | 0.10 | 0.03 |
| 20 | Season+FBPOM | 516.69 | 558.66 | 39 | 2 | 558.99 | 6.81 | 0.03 | 0.01 |
| 29 | FBPOM | 530.91 | 560.22 | 39 | 1 | 560.33 | 8.14 | 0.02 | 0.01 |
| 8 | Flow | 546.18 | 565.63 | 39 | 1 | 565.74 | 13.55 | 0.00 | 0.00 |
| 9 | Season+Temperature | 528.63 | 567.99 | 40 | 2 | 568.31 | 16.13 | 0.00 | 0.00 |
| 10 | Season+Conductivity | 536.15 | 568.43 | 40 | 2 | 568.75 | 16.57 | 0.00 | 0.00 |
| 11 | Season+Width | 528.70 | 569.55 | 40 | 2 | 569.87 | 17.69 | 0.00 | 0.00 |
| 12 | Season | 543.21 | 571.49 | 40 | 1 | 571.60 | 19.41 | 0.00 | 0.00 |
| 13 | Season+Weigth eucalypt | 537.91 | 572.87 | 40 | 2 | 573.19 | 21.01 | 0.00 | 0.00 |
| 14 | Season+CPOM | 543.25 | 572.88 | 40 | 2 | 573.20 | 21.02 | 0.00 | 0.00 |
| 15 | Season+Weigth native | 538.62 | 573.06 | 40 | 2 | 573.38 | 21.20 | 0.00 | 0.00 |
| 16 | Season+Shadow | 538.78 | 573.12 | 40 | 2 | 573.44 | 21.26 | 0.00 | 0.00 |
| 17 | Season+pH | 530.86 | 573.15 | 40 | 2 | 573.47 | 21.29 | 0.00 | 0.00 |
| 18 | Season+Chlorophyl | 539.43 | 573.40 | 40 | 2 | 573.72 | 21.54 | 0.00 | 0.00 |
| 19 | Season+% Eucalypt | 531.17 | 573.54 | 40 | 2 | 573.86 | 21.68 | 0.00 | 0.00 |
| 21 | Conductivity | 555.16 | 574.95 | 40 | 1 | 575.06 | 22.87 | 0.00 | 0.00 |
| 22 | Temperature | 548.32 | 575.31 | 40 | 1 | 575.42 | 23.23 | 0.00 | 0.00 |
| 23 | Weigth native | 554.01 | 575.67 | 40 | 1 | 575.78 | 23.59 | 0.00 | 0.00 |
| 24 | CPOM | 558.93 | 575.88 | 40 | 1 | 575.99 | 23.80 | 0.00 | 0.00 |
| 25 | Width | 547.85 | 576.21 | 40 | 1 | 576.32 | 24.13 | 0.00 | 0.00 |
| 26 | Weigth eucalypt | 556.22 | 578.77 | 40 | 1 | 578.88 | 26.69 | 0.00 | 0.00 |
| 27 | Shadow | 557.78 | 579.72 | 40 | 1 | 579.83 | 27.64 | 0.00 | 0.00 |
| 28 | pH | 550.01 | 579.82 | 40 | 1 | 579.93 | 27.74 | 0.00 | 0.00 |
| 30 | % Eucalypt | 550.04 | 579.98 | 40 | 1 | 580.09 | 27.90 | 0.00 | 0.00 |
| 31 | Chlorophyl | 558.50 | 580.15 | 40 | 1 | 580.26 | 28.07 | 0.00 | 0.00 |
| Rank | Model | Deviance | AIC | n | K | AICc | deltaAICc | relative Likelihood | Akaike Weight |
| 1 | Season+FBPOM | 203.10 | 218.86 | 39 | 2 | 219.19 | 0.00 | 1.00 | 0.32 |
| 2 | % eucalyp+Season+FBPOM | 198.93 | 220.05 | 39 | 3 | 220.74 | 1.54 | 0.46 | 0.15 |
| 3 | Season+Flow | 214.90 | 220.86 | 39 | 2 | 221.19 | 2.00 | 0.37 | 0.12 |
| 4 | Season+Flow+Temperature | 212.95 | 221.62 | 39 | 3 | 222.31 | 3.11 | 0.21 | 0.07 |
| 5 | % Eucalypt+Season+Flow | 210.39 | 221.67 | 39 | 3 | 222.36 | 3.16 | 0.21 | 0.06 |
| 6 | Season | 212.38 | 223.25 | 40 | 1 | 223.36 | 4.16 | 0.12 | 0.04 |
| 7 | % Eucalypt+Season+Flow+Weight native | 213.62 | 222.85 | 39 | 4 | 224.03 | 4.83 | 0.09 | 0.03 |
| 8 | Season+Temperature | 210.42 | 223.90 | 40 | 2 | 224.22 | 5.03 | 0.08 | 0.03 |
| 9 | % Eucalypt+Season | 207.97 | 224.04 | 40 | 2 | 224.36 | 5.17 | 0.08 | 0.02 |
| 10 | % Eucalypt+Season+Temperature | 205.18 | 223.84 | 40 | 3 | 224.51 | 5.31 | 0.07 | 0.02 |
| 11 | % Eucalypt+Season+Flow+Weight eucalypt | 212.80 | 223.42 | 39 | 4 | 224.60 | 5.40 | 0.07 | 0.02 |
| 12 | Season+Width | 209.86 | 224.70 | 40 | 2 | 225.02 | 5.83 | 0.05 | 0.02 |
| 13 | Weigth eucalypt+Season | 216.11 | 224.87 | 40 | 2 | 225.19 | 6.00 | 0.05 | 0.02 |
| 14 | Season+pH | 209.10 | 225.06 | 40 | 2 | 225.38 | 6.19 | 0.05 | 0.01 |
| 15 | Season+CPOM | 221.56 | 225.18 | 40 | 2 | 225.50 | 6.31 | 0.04 | 0.01 |
| 16 | Season+Conductivity | 218.83 | 225.28 | 40 | 2 | 225.60 | 6.41 | 0.04 | 0.01 |
| 17 | Season+Shadow | 217.07 | 225.30 | 40 | 2 | 225.62 | 6.43 | 0.04 | 0.01 |
| 18 | Season+Chlorophyl | 217.39 | 225.32 | 40 | 2 | 225.64 | 6.45 | 0.04 | 0.01 |
| 19 | Weigth native+Season | 216.95 | 225.32 | 40 | 2 | 225.64 | 6.45 | 0.04 | 0.01 |
| 20 | % Eucalypt+Season+Weight native | 211.55 | 225.48 | 40 | 3 | 226.15 | 6.95 | 0.03 | 0.01 |
| 21 | % Eucalypt+Season+Weight eucalypt | 210.44 | 225.66 | 40 | 3 | 226.33 | 7.13 | 0.03 | 0.01 |
| 22 | FBPOM | 217.21 | 229.37 | 39 | 1 | 229.48 | 10.28 | 0.01 | 0.00 |
| 23 | Flow | 231.86 | 233.85 | 39 | 1 | 233.96 | 14.76 | 0.00 | 0.00 |
| 24 | Weigth native | 232.69 | 237.07 | 40 | 1 | 237.18 | 17.98 | 0.00 | 0.00 |
| 25 | % Eucalypt | 225.88 | 238.16 | 40 | 1 | 238.27 | 19.07 | 0.00 | 0.00 |
| 26 | CPOM | 238.95 | 238.74 | 40 | 1 | 238.85 | 19.65 | 0.00 | 0.00 |
| 27 | Temperature | 229.67 | 239.25 | 40 | 1 | 239.36 | 20.16 | 0.00 | 0.00 |
| 28 | Width | 228.88 | 239.71 | 40 | 1 | 239.82 | 20.62 | 0.00 | 0.00 |
| 29 | pH | 228.12 | 240.07 | 40 | 1 | 240.18 | 20.98 | 0.00 | 0.00 |
| 30 | Chlorophyl | 235.84 | 240.11 | 40 | 1 | 240.22 | 21.02 | 0.00 | 0.00 |
| 31 | Weigth eucalypt | 235.28 | 240.14 | 40 | 1 | 240.25 | 21.05 | 0.00 | 0.00 |
| 32 | Conductivity | 237.82 | 240.27 | 40 | 1 | 240.38 | 21.18 | 0.00 | 0.00 |
| 33 | Shadow | 235.84 | 240.31 | 40 | 1 | 240.42 | 21.22 | 0.00 | 0.00 |
| Rank | Model | Deviance | AIC | n | K | AICc | deltaAICc | relative Likelihood | Akaike Weight |
| 1 | % Eucalypt+Season+Width+pH | -22.50 | -29.68 | 40 | 4 | -28.54 | 0.00 | 1.00 | 0.43 |
| 2 | % Eucalypt+Season+Width | -22.57 | -27.88 | 40 | 3 | -27.21 | 1.32 | 0.52 | 0.22 |
| 3 | % Eucalypt+Season+Width+Weight eucalypt | -13.92 | -26.23 | 40 | 4 | -25.09 | 3.45 | 0.18 | 0.08 |
| 4 | % Eucalypt+Season+Width+Weight native | -12.28 | -25.96 | 40 | 4 | -24.82 | 3.72 | 0.16 | 0.07 |
| 5 | % Eucalypt+Season+Width+Flow | -9.53 | -25.94 | 39 | 4 | -24.76 | 3.77 | 0.15 | 0.07 |
| 6 | % Eucalypt+Season+Flow | -11.01 | -25.31 | 39 | 3 | -24.62 | 3.91 | 0.14 | 0.06 |
| 7 | % Eucalypt+Season+pH | -20.35 | -24.22 | 40 | 3 | -23.55 | 4.98 | 0.08 | 0.04 |
| 8 | % Eucalypt+Flow | -9.78 | -22.14 | 39 | 2 | -21.81 | 6.73 | 0.03 | 0.01 |
| 9 | % Eucalypt+Season | -19.36 | -21.70 | 40 | 2 | -21.38 | 7.16 | 0.03 | 0.01 |
| 10 | % Eucalypt+Season+Weight eucalypt | -10.39 | -19.71 | 40 | 3 | -19.04 | 9.49 | 0.01 | 0.00 |
| 11 | % Eucalypt+Season+Weight native | -9.05 | -19.64 | 40 | 3 | -18.97 | 9.56 | 0.01 | 0.00 |
| 12 | % Eucalypt+Season+CPOM | -3.88 | -19.61 | 40 | 3 | -18.94 | 9.59 | 0.01 | 0.00 |
| 13 | Season | -17.08 | -18.28 | 40 | 1 | -18.17 | 10.36 | 0.01 | 0.00 |
| 14 | % Eucalypt+Width | -14.95 | -17.96 | 40 | 2 | -17.64 | 10.90 | 0.00 | 0.00 |
| 15 | % Eucalypt+pH | -14.18 | -15.84 | 40 | 2 | -15.52 | 13.02 | 0.00 | 0.00 |
| 16 | % Eucalypt | -13.09 | -13.18 | 40 | 1 | -13.07 | 15.46 | 0.00 | 0.00 |
| 17 | % Eucalypt+Weight eucalypt | -5.72 | -12.88 | 40 | 2 | -12.56 | 15.98 | 0.00 | 0.00 |
| 18 | % Eucalypt+CPOM | 0.71 | -12.79 | 40 | 2 | -12.47 | 16.07 | 0.00 | 0.00 |
| 19 | % Eucalypt+Weight native | -4.28 | -12.66 | 40 | 2 | -12.34 | 16.20 | 0.00 | 0.00 |
| 20 | Flow | -1.65 | -12.04 | 39 | 1 | -11.93 | 16.61 | 0.00 | 0.00 |
| 22 | Width | -9.83 | -11.32 | 40 | 1 | -11.21 | 17.32 | 0.00 | 0.00 |
| 21 | % Eucalypt+FBPOM | -10.52 | -11.44 | 39 | 2 | -11.11 | 17.43 | 0.00 | 0.00 |
| 27 | Weigth native | -3.24 | -11.02 | 40 | 1 | -10.91 | 17.62 | 0.00 | 0.00 |
| 23 | % Eucalypt+Shadow | -2.57 | -11.22 | 40 | 2 | -10.90 | 17.64 | 0.00 | 0.00 |
| 24 | % Eucalypt+Chlorophyl | -2.22 | -11.22 | 40 | 2 | -10.90 | 17.64 | 0.00 | 0.00 |
| 25 | % Eucalypt+Temperature | -7.96 | -11.22 | 40 | 2 | -10.90 | 17.64 | 0.00 | 0.00 |
| 26 | % Eucalypt+Conductivity | -0.69 | -11.21 | 40 | 2 | -10.89 | 17.65 | 0.00 | 0.00 |
| 33 | FBPOM | -7.84 | -7.55 | 39 | 1 | -7.44 | 21.10 | 0.00 | 0.00 |
| 28 | Weigth eucalypt | 1.02 | -6.32 | 40 | 1 | -6.21 | 22.32 | 0.00 | 0.00 |
| 29 | CPOM | 6.71 | -5.44 | 40 | 1 | -5.33 | 23.20 | 0.00 | 0.00 |
| 30 | pH | -5.24 | -5.02 | 40 | 1 | -4.91 | 23.62 | 0.00 | 0.00 |
| 31 | Shadow | 4.50 | -2.79 | 40 | 1 | -2.68 | 25.85 | 0.00 | 0.00 |
| 32 | Conductivity | 6.36 | -2.72 | 40 | 1 | -2.61 | 25.92 | 0.00 | 0.00 |
| 34 | Chlorophyl | 5.41 | -2.13 | 40 | 1 | -2.02 | 26.51 | 0.00 | 0.00 |
| 35 | Temperature | -0.22 | -2.10 | 40 | 1 | -1.99 | 26.54 | 0.00 | 0.00 |
References
- Parker, J.D.; Torchin, M.E.; Hufbauer, R.A.; Lemoine, N.P.; Alba, C.; Blumenthal, D.M.; Bossdorf, O.; Byers, J.E.; Dunn, A.M.; Heckman, R.W.; et al. Do Invasive Species Perform Better in Their New Ranges? Ecology 2013, 94, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien Species as a Driver of Recent Extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic Invasive Species: Challenges for the Future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic Homogenization: A Few Winners Replacing Many Losers in the next Mass Extinction. Trends in Ecology & Evolution 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Mooney, H.A.; Mack, R.N.; Neville, L.E.; Schei, P.J.; Waage, J.K. Invasive Alien Species: A New Synthesis; Island Press: Washington DC, 2005. [Google Scholar]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biological Reviews 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Vance, E.D.; Fox, T.R.; Kirst, M. Eucalyptus beyond Its Native Range: Environmental Issues in Exotic Bioenergy Plantations. International Journal of Forestry Research 2013, 2013, e463030. [Google Scholar] [CrossRef]
- Tomé, M.; Almeida, M.H.; Barreiro, S.; Branco, M.R.; Deus, E.; Pinto, G.; Silva, J.S.; Soares, P.; Rodríguez-Soalleiro, R. Opportunities and Challenges of Eucalyptus Plantations in Europe: The Iberian Peninsula Experience. Eur J Forest Res 2021, 140, 489–510. [Google Scholar] [CrossRef]
- Iglesias-Carrasco, M.; Torres, J.; Cruz-Dubon, A.; Candolin, U.; Wong, B.B.M.; Velo-Antón, G. Global Impacts of Exotic Eucalypt Plantations on Wildlife. Biological Reviews 2025, brv70022. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M.C. Historical Development of the Portuguese Forest: The Introduction of Invasive Species. Forests 2019, 10, e974. [Google Scholar] [CrossRef]
- Ferreira, V.; Boyero, L.; Calvo, C.; Correa, F.; Figueroa, R.; Gonçalves, J.F.; Goyenola, G.; Graça, M.A.S.; Hepp, L.U.; Kariuki, S.; et al. A Global Assessment of the Effects of Eucalyptus Plantations on Stream Ecosystem Functioning. Ecosystems 2019, 22, 629–642. [Google Scholar] [CrossRef]
- Cordero-Rivera, A. O Eucalipto é Como o Estado: Chupa e Leva Todo Para El. Recursos Rurais 2019, 15, 19–33. [Google Scholar] [CrossRef]
- Graça, M.A.S. The Role of Invertebrates on Leaf Litter Decomposition in Streams - a Review. Internat. Rev. Hydrobiol. 2001, 86, 383–393. [Google Scholar] [CrossRef]
- Graça, M.A.; Pozo, J.; Canhoto, C.; Elosegi, A. Effects of Eucalyptus Plantations on Detritus, Decomposers, and Detritivores in Streams. The Scientific World 2002, 2, 1173–1185. [Google Scholar] [CrossRef]
- González, J.M.; Graça, M.A.S. Conversion of Leaf Litter to Secondary Production by a Shredding Caddis-fly. Freshwater Biology 2003, 48, 1578–1592. [Google Scholar] [CrossRef]
- Ferreira, V.; Koricheva, J.; Pozo, J.; Graça, M.A.S. A Meta-Analysis on the Effects of Changes in the Composition of Native Forests on Litter Decomposition in Streams. Forest Ecology and Management 2016, 364, 27–38. [Google Scholar] [CrossRef]
- Pereira, M.; Greet, J.; Jones, C.S. Native Riparian Plant Species Dominate the Soil Seedbank of In-Channel Geomorphic Features of a Regulated River. Environmental Management 2021, 67, 589–599. [Google Scholar] [CrossRef]
- Oester, R.; Altermatt, F.; Bruder, A. Riparian Forests Shape Trophic Interactions in Detrital Stream Food Webs. Functional Ecology 2024, 38, 2196–2206. [Google Scholar] [CrossRef]
- Cordero-Rivera, A.; Martínez Álvarez, A.; Álvarez, M. Eucalypt Plantations Reduce the Diversity of Macroinvertebrates in Small Forested Streams. Animal Biodiversity and Conservation 2017, 40, 87–97. [Google Scholar] [CrossRef]
- Abelho, M.; Graça, M.A.S. Effects of Eucalyptus Afforestation on Leaf Litter Dynamics and Macroinvertebrate Community Structure of Streams in Central Portugal. Hydrobiologia 1996, 324, 195–204. [Google Scholar] [CrossRef]
- Canhoto, C.; Graça, M.A.S. Food Value of Introduced Eucalypt Leaves for a Mediterranean Stream Detritivore: Tipula Lateralis. Freshwater Biology 1995, 34, 209–214. [Google Scholar] [CrossRef]
- Canhoto, C.; Graça, M.A.S. Leaf Barriers to Fungal Colonization and Shredders (Tipula Lateralis) Consumption of Decomposing Eucalyptus Globulus. Microbial Ecology 1999, 37, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Graça, M.A.S. A Laboratory Study on Feeding Plasticity of the Shredder Sericostoma Vittatum Rambur (Sericostomatidae). Hydrobiologia 2007, 575, 353–359. [Google Scholar] [CrossRef]
- O’Connell, A.M.; Menagé, P.M.A. Litter Fall and Nutrient Cycling in Karri (Eucalyptus Diversicolor F. Muell.) Forest in Relation to Stand Age. Australian Journal of Ecology 1982, 7, 49–62. [Google Scholar] [CrossRef]
- Pozo, J. Leaf Litter Processing of Alder and Eucalyptus in the Agüera Stream System (North Spain) I. Chemical Changes. archiv_hydrobiologie 1993, 127, 299–317. [Google Scholar] [CrossRef]
- Molinero, J.; Pozo, J.; Gonzalez, E. Litter Breakdown in Streams of the Agüera Catchment: Influence of Dissolved Nutrients and Land Use. Freshwater Biology 1996, 36, 745–756. [Google Scholar] [CrossRef]
- Pozo, J.; Basaguren, A.; Elósegui, A.; Molinero, J.; Fabre, E.; Chauvet, E. Afforestation with Eucalyptus Globulus and Leaf Litter Decomposition in Streams of Northern Spain. In Oceans, rivers and lakes: energy and substance transfers at interfaces; Amiard, J.-C., Le Rouzic, B., Berthet, B., Bertru, G., Eds.; Springer Netherlands: Dordrecht, 1998; pp. 101–109. ISBN 978-94-010-6216-9. [Google Scholar]
- Ferreira, V.; Larrañaga, A.; Gulis, V.; Basaguren, A.; Elosegi, A.; Graça, M.A.S.; Pozo, J. The Effects of Eucalypt Plantations on Plant Litter Decomposition and Macroinvertebrate Communities in Iberian Streams. Forest Ecology and Management 2015, 335, 129–138. [Google Scholar] [CrossRef]
- Pozo, J.; Gonzalez, E.; Diez, J.R.; Molinero, J.; Elosegui, A. Inputs of Particulate Organic Matter to Streams with Different Riparian Vegetation. Journal of the North American Benthological Society 1997, 16, 602–611. [Google Scholar] [CrossRef]
- Carballeira, A.; Devesa, C.; Retuerto, R.; Santillán, E.; Ucieda, F. Bioclimatología de Galicia; Fundación Barrié de la Maza: A Coruña, 1983. [Google Scholar]
- García, X.R. Guía Das Plantas de Galicia; Edicións Xerais de Galicia: Vigo, 2008. [Google Scholar]
- Consellería do Medio Rural Primeiros Resultados Do Inventario Forestal Continuo de Galicia. Estimación de Superficies a Partires Do Mapa Forestal de Media Resolución; Xunta de Galicia: Santiago de Compostela, 2023. [Google Scholar]
- Lowe, R.L.; La Liberté, G.D. Benthic Stream Algae: Distribution and Structure. In Methods in stream ecology; Hauer, F.R., Lamberti, G.A., Eds.; Academic Press: San Diego, 2007; pp. 193–221. [Google Scholar]
- Anderson, D.R.; Burnham, K.P.; Thompson, W.L. Null Hypothesis Testing: Problems, Prevalence, and an Alternative. The Journal of Wildlife Management 2000, 64, 912–923. [Google Scholar] [CrossRef]
- Johnson, D.H. The Insignificance of Statistical Significance Testing. Journal of Wildlife Management 1999, 63, 763–772. [Google Scholar] [CrossRef]
- Moreno, C.E. Métodos Para Medir La Biodiversidad; Manuales y Tesis SEA; Manuales y Tesis SEA, vol. 1: Zaragoza, 2000. [Google Scholar]
- Anderson, D.R.; Link, W.A.; Johnson, D.H.; Burnham, K.P. Suggestions for Presenting the Results of Data Analyses. Journal of Wildlife Management 2001, 65, 373–378. [Google Scholar] [CrossRef]
- Molinero, J.; Pozo, J. Impact of a Eucalyptus (Eucalyptus Globulus Labill.) Plantation on the Nutrient Content and Dynamics of Coarse Particulate Organic Matter (CPOM) in a Small Stream. Hydrobiologia 2004, 528, 143–165. [Google Scholar] [CrossRef]
- Mah, A.N.M.M.A.; Puan, C.H.; Isa, M.F.M.; Saadun, N.; Razi, N.A. Response of Aquatic Fauna towards Eucalypt Plantations: A Review. Malaysian Forester 2022, 85, 141–151. [Google Scholar]
- Cordero-Rivera, A. Cuando Los Árboles No Dejan Ver El Bosque: Efectos de Los Monocultivos Forestales En La Conservación de La Biodiversidad. Acta biológica Colombiana 2011, 16, 247–268. [Google Scholar]
- Graça, M.A.S.; Ferreira, V.; Canhoto, C.; Encalada, A.C.; Guerrero-Bolaño, F.; Wantzen, K.M.; Boyero, L. A Conceptual Model of Litter Breakdown in Low Order Streams. Internat. Rev. Hydrobiol. 2015, 100, 1–12. [Google Scholar] [CrossRef]
- González-Paz, L.; Gestido, J.; Delgado, C.; Pedrol, N.; Pardo, I. Short-Term Effect of Eucalyptus Leachates on Green Food Webs in Headwaters. Water 2022, 15, 115. [Google Scholar] [CrossRef]
- Tubić, B.; Andjus, S.; Zorić, K.; Vasiljević, B.; Jovičić, K.; Čanak Atlagić, J.; Paunović, M. Aquatic Insects (Ephemeroptera, Plecoptera and Trichoptera) Metric as an Important Tool in Water Quality Assessment in Hilly and Mountain Streams. Water 2024, 16, 849. [Google Scholar] [CrossRef]
- do Amaral, P.H.M.; Rocha, C.H.B.; Alves, R.D.G. Effect of Eucalyptus Plantations on the Taxonomic and Functional Structure of Aquatic Insect Assemblages in Neotropical Springs. Studies on Neotropical Fauna and Environment 2023, 58, 35–46. [Google Scholar] [CrossRef]
- Peixoto, S.S.D.J.; Guimarães, L.P.; Alves, R.D.G. Influence of Eucalyptus Plantations on Benthic Macroinvertebrate Assemblages in Neotropical Springs. Acta Limnol. Bras. 2024, 36, e24. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Alimentación y Medio Ambiente Protocolo de Muestreo Y Laboratorio de Fauna Bentónica de Invertebrados En Ríos Vadeables. Código: ML-Rv-I-2013; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, 2013. [Google Scholar]
- Bonada, N.; Prat, N.; Munné, A.; Plans, M.; Solà, C.; Álvarez, M.; Pardo, I.; Moyà, G.; Ramon, G.; Toro, M.; et al. Intercalibración de La Metodología GUADALMED. Selección de Un Protocolo de Muestreo Para La Determinación Del Estado Ecológico de Los Ríos Mediterráneos. Limnética 2002, 21, 13–33. [Google Scholar] [CrossRef]
- MeteoGalicia Informe Climatolóxico Outono 2020; Xunta de Galicia: Santiago de Compostela, 2020.
- MeteoGalicia Informe Climatolóxico Primavera 2021; Xunta de Galicia: Santiago de Compostela, 2021; pp. 17 p.
- Erdozain, M.; Kidd, K.; Kreutzweiser, D.; Sibley, P. Linking Stream Ecosystem Integrity to Catchment and Reach Conditions in an Intensively Managed Forest Landscape. Ecosphere 2018, 9, e02278. [Google Scholar] [CrossRef]
- Da Silva, E.C.; De Azevedo, K.D.F.S.; De Carvalho, F.G.; Juen, L.; Da Rocha, T.S.; Oliveira-Junior, J.M.B. Impacts of Oil Palm Monocultures on Freshwater Ecosystems in the Amazon: A Case Study of Dragonflies and Damselflies (Insecta: Odonata). Aquat Sci 2025, 87, in press. [Google Scholar] [CrossRef]
- Cunha, E.J.; Juen, L. Impacts of Oil Palm Plantations on Changes in Environmental Heterogeneity and Heteroptera (Gerromorpha and Nepomorpha) Diversity. Journal of Insect Conservation 2017, 21, 111–119. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Begot, T.O.; Da Silveira Prudente, B.; Juen, L.; De Assis Montag, L.F. Effects of Oil Palm Plantations on Habitat Structure and Fish Assemblages in Amazon Streams. Environ Biol Fish 2018, 101, 547–562. [Google Scholar] [CrossRef]
- Correa, F.S.; Juen, L.; Rodrigues, L.C.; Silva-Filho, H.F.; Santos-Costa, M.C. Effects of Oil Palm Plantations on Anuran Diversity in the Eastern Amazon. Animal Biology 2015, 65, 321–335. [Google Scholar] [CrossRef]
- Goded, S.; Ekroos, J.; Domínguez, J.; Azcárate, J.G.; Guitián, J.A.; Smith, H.G. Effects of Eucalyptus Plantations on Avian and Herb Species Richness and Composition in North-West Spain. Global Ecology and Conservation 2019, 19. [Google Scholar] [CrossRef]
- García-Fernández, F.; Vidal, M.; Regos, A.; Domínguez, J. Eucalyptus Cover as the Primary Driver of Native Forest Bird Reductions: Evidence from a Stand-Scale Analysis in NW Iberia. Forest Ecology and Management 2025, 586, e122714. [Google Scholar] [CrossRef]
- Xunta de Galicia. LEI 3/2018, do 26 de decembro, de Medidas Fiscais e Administrativas. Diario oficial de Galicia 2018, 247, 54319–54435. [Google Scholar]
- Hickey, M.B.C.; Doran, B. A Review of the Efficiency of Buffer Strips for the Maintenance and Enhancement of Riparian Ecosystems. Water Quality Research Journal of Canada 2004, 39, 311–317. [Google Scholar] [CrossRef]
- Braun, B.M.; Pires, M.M.; Stenert, C.; Maltchik, L.; Kotzian, C.B. Effects of Riparian Vegetation Width and Substrate Type on Riffle Beetle Community Structure. Entomological Science 2018, 21, 66–75. [Google Scholar] [CrossRef]
- Raitif, J.; Plantegenest, M.; Roussel, J.M. From Stream to Land: Ecosystem Services Provided by Stream Insects to Agriculture. Agriculture, Ecosystems and Environment 2019, 270–271, 32–40. [Google Scholar] [CrossRef]
- Rivas-Torres, A.; Cordero-Rivera, A. A Review of the Density, Biomass, and Secondary Production of Odonates. Insects 2024, 15, 510. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-Species versus Monocultures in Plantation Forestry: Development, Benefits, Ecosystem Services and Perspectives for the Future. Global Ecology and Conservation 2018, 15, e00419. [Google Scholar] [CrossRef]
- Manson, D.G.; Schmidt, S.; Bristow, M.; Erskine, P.D.; Vanclay, J.K. Species-Site Matching in Mixed Species Plantations of Native Trees in Tropical Australia. Agroforest Syst 2013, 87, 233–250. [Google Scholar] [CrossRef]
- Schnabel, F.; Beugnon, R.; Yang, B.; Richter, R.; Eisenhauer, N.; Huang, Y.; Liu, X.; Wirth, C.; Cesarz, S.; Fichtner, A.; et al. Tree Diversity Increases Forest Temperature Buffering via Enhancing Canopy Density and Structural Diversity. Ecology Letters 2025, 28, e70096. [Google Scholar] [CrossRef]
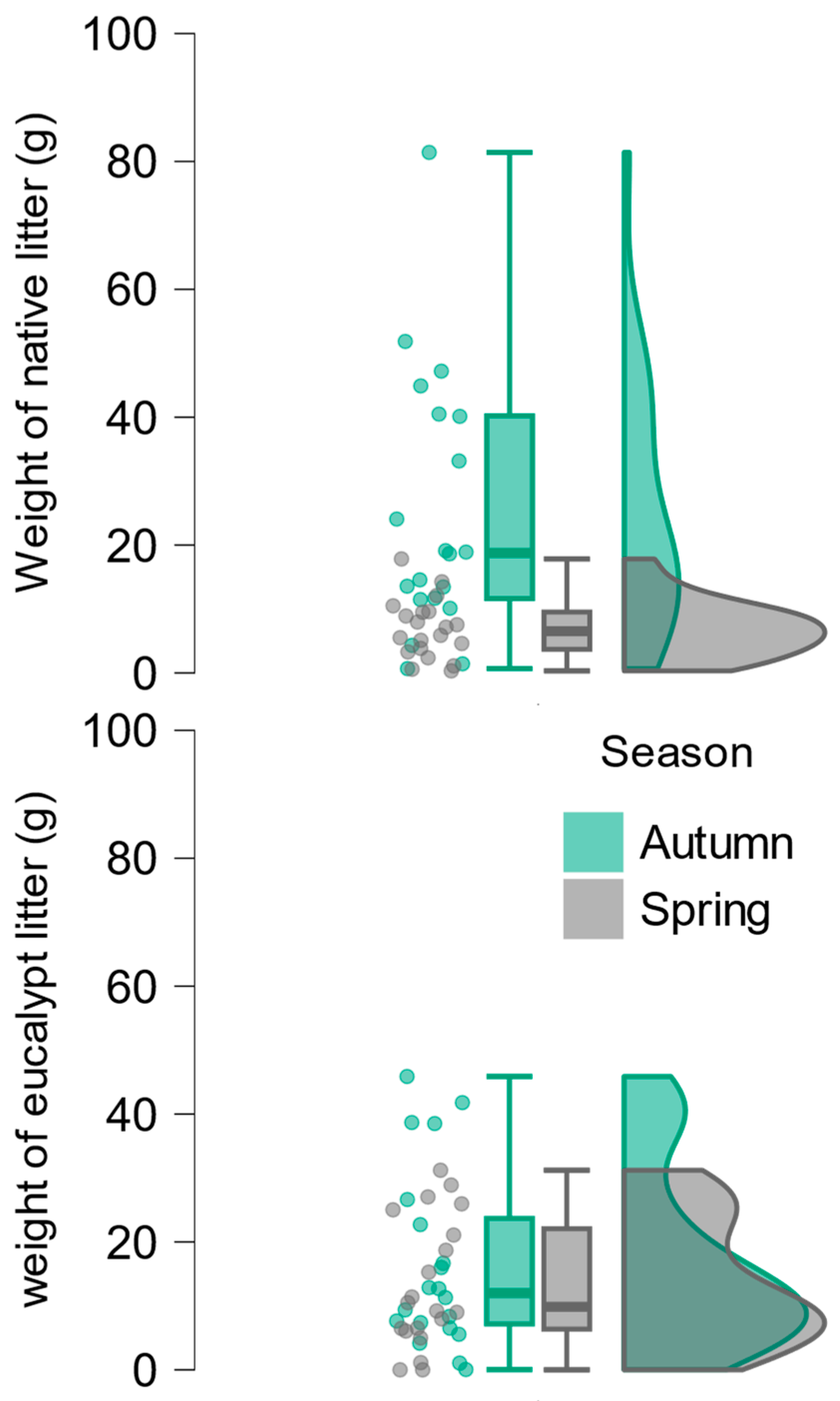

| Autunm | |||||||
| Locality and stream | Eucalypt (g) | Native (g) | % eucalypt | Abundance | Richness | Shannon | |
| Aldán,Bouzas | 38.67 | 4.32 | 90 | 1993 | 41 | 3.20 | |
| Aldán, Orxas | 4.16 | 14.56 | 22 | 714 | 40 | 4.24 | |
| Marín, Agrela | 38.51 | 11.67 | 77 | 178 | 18 | 3.40 | |
| Marín, Gorgadas | 1.06 | 40.13 | 3 | 669 | 39 | 4.13 | |
| Vilaboa, Portiño | 45.87 | 13.56 | 77 | 1776 | 61 | 3.93 | |
| Vilaboa, Maior | 5.56 | 33.15 | 14 | 1378 | 41 | 4.30 | |
| Coruxo, Gontade | 41.78 | 1.43 | 97 | 1285 | 41 | 4.00 | |
| Coruxo, Saiáns | 15.98 | 47.20 | 25 | 4586 | 57 | 4.02 | |
| Gondomar, Peitieiros | 12.69 | 40.49 | 24 | 845 | 38 | 4.15 | |
| Gondomar, Mordagáns | 7.64 | 24.07 | 24 | 751 | 68 | 4.93 | |
| Mondariz, da Vida | 26.63 | 0.68 | 98 | 1369 | 35 | 3.49 | |
| Mondariz, Cillarga | 9.37 | 51.84 | 15 | 976 | 29 | 3.76 | |
| Porriño, da Fraga | 16.68 | 13.44 | 55 | 376 | 28 | 4.16 | |
| Porriño, Louro | 7.38 | 44.89 | 14 | 489 | 31 | 3.42 | |
| Campo Lameiro, Grande | 8.35 | 18.62 | 31 | 534 | 43 | 4.00 | |
| Campo Lameiro, Teixoeiras | 12.86 | 81.41 | 14 | 990 | 34 | 4.15 | |
| Pontevedra, Seixiña | 11.29 | 19.13 | 37 | 2414 | 54 | 3.18 | |
| Pontevedra, As Laceiras | 0.04 | 18.90 | 0 | 867 | 32 | 3.77 | |
| Ponteareas, Xabriña | 22.71 | 11.48 | 66 | 1486 | 36 | 3.54 | |
| Ponteareas, Fragón | 6.50 | 10.11 | 39 | 1029 | 35 | 3.46 | |
| Mean | 16.69 | 25.05 | 41 | 1235.25 | 40.05 | 3.86 | |
| Spring | |||||||
| Aldán,Bouzas | 25.95 | 4.60 | 85 | 1241 | 18 | 2.54 | |
| Aldán, Orxas | 10.50 | 3.29 | 76 | 565 | 24 | 3.73 | |
| Marín, Agrela | 31.21 | 5.88 | 84 | 249 | 17 | 3.36 | |
| Marín, Gorgadas | 6.47 | 17.83 | 27 | 948 | 36 | 4.39 | |
| Vilaboa, Portiño | 11.38 | 0.58 | 95 | 554 | 30 | 3.48 | |
| Vilaboa, Maior | 1.13 | 5.11 | 18 | 503 | 28 | 3.70 | |
| Coruxo, Gontade | 25.02 | 10.50 | 70 | 1319 | 27 | 2.37 | |
| Coruxo, Saiáns | 4.98 | 3.84 | 57 | 1379 | 31 | 3.30 | |
| Gondomar, Peitieiros | 8.99 | 7.54 | 54 | 695 | 30 | 3.64 | |
| Gondomar, Mordagáns | 6.52 | 7.96 | 45 | 276 | 26 | 3.61 | |
| Mondariz, da Vida | 27.04 | 2.37 | 92 | 785 | 24 | 2.91 | |
| Mondariz, Cillarga | 9.21 | 12.10 | 43 | 516 | 25 | 2.80 | |
| Porriño, da Fraga | 15.27 | 9.60 | 61 | 227 | 23 | 3.27 | |
| Porriño, Louro | 6.07 | 8.91 | 41 | 1006 | 28 | 2.98 | |
| Campo Lameiro, Grande | 21.08 | 1.11 | 95 | 191 | 20 | 2.78 | |
| Campo Lameiro, Teixoeiras | 7.95 | 14.25 | 36 | 553 | 28 | 3.31 | |
| Pontevedra, Seixiña | 28.89 | 0.32 | 99 | 191 | 19 | 3.11 | |
| Pontevedra, As Laceiras | 0.00 | 5.51 | 0 | 306 | 28 | 4.10 | |
| Ponteareas, Xabriña | 18.70 | 7.16 | 72 | 570 | 34 | 3.49 | |
| Ponteareas, Fragón | 0.00 | 9.49 | 00 | 455 | 29 | 3.21 | |
| Mean | 13.32 | 6.90 | 58 | 626.45 | 26.25 | 3.30 | |
|
Taxon |
Autumn |
Spring |
Total |
% of total |
%Eucalypt F, p |
Season F, p |
| Protonemura | 3330 | 3383 | 6713 | 0.180 | 1.39, 0.246 | 0.16, 0.697 |
| Gammarus | 3885 | 2599 | 6484 | 0.174 | 0.08, 0.774 | 4.51, 0.046 |
| Tanytarsini | 3318 | 154 | 3472 | 0.093 | 0.35, 0.557 | 3.57, 0.074 |
| Hydropsyche | 1101 | 956 | 2057 | 0.055 | 0.97, 0.332 | 1.19, 0.289 |
| Simulium | 1269 | 581 | 1850 | 0.050 | 1.03, 0.316 | 10.88, 0.003 |
| Orthocladiinae | 1424 | 129 | 1553 | 0.042 | 0.66, 0.423 | 5.77, 0.026 |
| Chironomini | 810 | 281 | 1091 | 0.029 | 0.63, 0.433 | 1.92, 0.181 |
| Leuctra | 737 | 328 | 1065 | 0.029 | 0.86, 0.361 | 4.55, 0.045 |
| Baetis | 674 | 381 | 1055 | 0.028 | 1.71, 0.201 | 1.41, 0.249 |
| Cordulegaster | 485 | 544 | 1029 | 0.028 | 0.05, 0.818 | 0.39, 0.540 |
| Limnephilus | 667 | 278 | 945 | 0.025 | 4.73, 0.039 | 1.53, 0.231 |
| Atherix | 450 | 447 | 897 | 0.024 | 0.91, 0.345 | 0.22, 0.641 |
| Elodes | 402 | 183 | 585 | 0.016 | 1.11, 0.299 | 1.79, 0.195 |
| Hydrocyphon | 455 | 119 | 574 | 0.015 | 1.85, 0.184 | 2.74, 0.113 |
| Ceratopogoninae | 409 | 112 | 521 | 0.014 | 1.27, 0.269 | 1.41, 0.249 |
| Helicopsyche | 319 | 177 | 496 | 0.013 | 0.04, 0.846 | 2.73, 0.113 |
| Crunoecia | 301 | 153 | 454 | 0.012 | 4.71, 0.038 | 3.55, 0.074 |
| Elmis | 300 | 142 | 442 | 0.012 | 3.22, 0.085 | 0.60, 0.447 |
| Lepidostoma | 355 | 56 | 411 | 0.011 | 0.83, 0.371 | 14.21, 0.001 |
| Diplectrona | 398 | 0 | 398 | 0.011 | 0.67, 0.419 | 6.10, 0.023 |
| Hemerodromiidae | 182 | 171 | 353 | 0.009 | 1.84, 0.182 | 0.00, 0.985 |
| Rhyacophila | 216 | 103 | 319 | 0.009 | 3.30, 0.078 | 7.47, 0.013 |
| Oligochaeta | 281 | 26 | 307 | 0.008 | 0.38, 0.542 | 18.88, <0.001 |
| Limnius | 159 | 127 | 286 | 0.008 | 0.03, 0.872 | 1.26, 0.276 |
| Philopotamus | 166 | 119 | 285 | 0.008 | 2.36, 0.133 | 1.15, 0.295 |
| Nemoura | 122 | 120 | 242 | 0.006 | 0.29, 0.596 | 0.02, 0.877 |
| Calopteryx | 112 | 126 | 238 | 0.006 | 0.30, 0.587 | 0.25, 0.623 |
| Ecdyonurus | 143 | 82 | 225 | 0.006 | 5.21, 0.030 | 1.60, 0.220 |
| Hexatoma | 151 | 50 | 201 | 0.005 | 2.24, 0.145 | 7.53, 0.012 |
| Oulimnius | 136 | 47 | 183 | 0.005 | 1.88, 0.180 | 2.35, 0.975 |
| Other taxa | 1948 | 555 | 2503 | 0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





