Submitted:
24 April 2025
Posted:
24 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Characterization
2.2. Effect of the Isolated Compounds on Trypanosomes
2.3. Effect of the ISOLATED Compounds on Leishmania Mexicana
2.4. Effect of the Isolated Compounds on HEK
4. Materials and Methods
4.1. Plant Collection
4.2. Extraction and Isolation of Constituents
4.3. Spectroscopic Analysis
4.4. Parasite Culture
4.5. Antitrypanosomal and Antileishmanial Assays
4.6. Toxicity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet. 2017, 390, 2397–2409. [Google Scholar] [PubMed]
- Giordani, F.; Morrison, L. J.; Rowan, T. G.; De Koning, H. P.; Barrett, M. P. The animal trypanosomiasis and their chemotherapy: a review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [PubMed]
- Akazue, P.I.; Ebiloma, G.U.; Ajibola, O.; Isaac, C.; Onyekwelu, K.; Ezeh, C.O.; Eze, A.A. Sustainable Elimination (Zero Cases) of Sleeping Sickness: How Far Are We from Achieving This Goal? Pathogens 2019, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Anene, B.M.; Onah, D.N.; Nawa, Y. Drug resistance in pathogenic African trypanosomes: what hopes for the future? Vet Parasitol. 2001, 96, 83–100. [Google Scholar]
- Geerts, S.; Holmes, P.H.; Eisler, M.C.; Diall, O. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 2001, 17, 25–8. [Google Scholar]
- Ungogo, M.A.; De Koning, H.P. Drug resistance in animal trypanosomiases: Epidemiology, mechanisms and control strategies. Int. J. for Parasitology: Drugs and Drug Resistance 2024, 25, 1–30. [Google Scholar]
- Zheoat, A.M.; Alenezi, S.; Elmahallawy, E.K.; Ungogo, M.A.; Alghamdi, A.H.; Watson, D.G.; Igoli, J.O.; Gray, A.I.; de Koning, H.P.; Ferro, V.A. Antitrypanosomal and Antileishmanial Activity of Chalcones and Flavanones from Polygonum salicifolium. Pathogens 2021, 10, 1–9. [Google Scholar] [CrossRef]
- Desquesnes, M.; Dargantes, A.; Lai, D.H.; Lun, Z.R.; Holzmuller, P. and Jittapalapong, S. Trypanosoma evansi and surra: a review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Research International 2013, 321237. [Google Scholar]
- Fetene, E.; Leta, S.; Regassa, F.; Büscher, P. Global distribution, host range and prevalence of Trypanosoma vivax: a systematic review and meta-analysis. Parasit Vectors 2021, 14, 80. [Google Scholar]
- Graf, F.E.; Ludin, P.; Wenzler, T.; Kaiser, M.; Brun, R.; Pati Pyana, P.; Büscher, P.; De Koning, H.P.; Horn, D.; Mäser, P. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl. Trop. Dis. 2013, 7, e2475. [Google Scholar]
- Munday, J.C.; Eze, A.A.; Baker, N.; Glover, L.; Clucas, C.; Aguinaga Andrés, D.; Natto, M.J.; Teka, I.A.; McDonald, J.; Lee, R.S.; Graf, F.E.; Ludin, P.; Burchmore, R.J.S.; Turner, C.M.R.; Tait, A.; MacLeod, A.; Mäser, P.; Barrett, M.P.; Horn, D.; De Koning, H.P. Trypanosoma brucei Aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and is the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [Google Scholar] [PubMed]
- De Koning, H.P. The drugs of sleeping sickness: their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Ungogo, M.A.; Campagnaro, G.D.; Alghamdi, A.H.; Natto, M.J.; De Koning, H.P. Differences in transporters rather than drug targets are the principal determinants of the different innate sensitivities of Trypanosoma congolense and Trypanozoon subgenus trypanosomes to diamidines and melaminophenyl arsenicals. Int. J. Mol. Sci. 2022, 23, 2844. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Crioft, S.L. Boelaert, M. Leishmaniasis. The Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar]
- García-Hernández, R.; Manzano, J.I.; Castanys, S.; Gamarro, F. Leishmania donovani develops resistance to drug combinations. PLoS Negl. Trop. Dis. 2012, 6, e1974. [Google Scholar]
- Nwodo, N.J.; Ibezim, A.; Ntie-Kang, F.; Adikwu, M.U.; and Chika, J. Anti-Trypanosomal Activity of Nigerian Plants and Their Constituents. Molecules 2015, 20, 7750–7771. [Google Scholar] [CrossRef]
- Ungogo, M.A.; Ebiloma, G.U.; Ichoron, N.; Igoli, J.O.; De Koning, H.P.; Balogun, E.O. A review of the antimalarial, antitrypanosomal and antileishmanial activities of natural compounds isolated from Nigerian flora. Front. Chem. 2020, 8, 617448. [Google Scholar]
- Ebiloma, G.U.; Ichoron, N.; Watson, D.G.; Igoli, J.O.; De Koning, H.P. The strong anti-kinetoplastid properties of bee propolis: Composition, identification of the active agents and their biochemical targets. Molecules 2020, 25, 5155. [Google Scholar] [CrossRef]
- Alotaibi, A.; Ebiloma, G.U.; Williams, R.; Alenezi, S.; Donachie, A.M.; Guillaume, S.; Igoli, J.O.; Fearnley, J.; De Koning, H.P.; Watson, D.G. European propolis is highly active against trypanosomatids including Crithidia fasciculata. Sci. Rep. 2019, 9, 11364. [Google Scholar]
- Siheri, W.; Ebiloma, G.U.; Igoli, J.O.; Gray, A.I.; Biddau, M.; Akrachalanont, P.; Alenezi, S.; Edrada-Ebel, R.; Muller, S.; Lawrence, C.; Fearnley, J.; Watson, D.G.; De Koning, H.P. Isolation of a novel flavanonol and an alkylresorcinol with highly potent anti-trypanosomal activity from Libyan propolis. Molecules 2019, 24, 1041. [Google Scholar] [CrossRef] [PubMed]
- Cerone, M.; Uliassi, E.; Prati, F.; Ebiloma, G.U.; Lemgruber, L.; Bergamini, C.; Watson, D.G.; de, A.M.; Ferreira, T.; Roth Cardoso, G.S.H.; Soares Romeiro, L.A.; de Koning, H.P.; Bolognesi, M.L. Discovery of Sustainable Drugs for Neglected Tropical Diseases: Cashew Nut Shell Liquid (CNSL)-Based Hybrids Target Mitochondrial Function and ATP Production in Trypanosoma brucei. ChemMedChem. 2019, 14, 621–635. [Google Scholar] [PubMed]
- Rossi, M.; Martinengo, B.; Diamanti, E.; Salerno, A.; Rizzardi, N.; Fato, R.; Bergamini, C.; Souza de Oliveira, A.; de Araújo Marques Ferreira, T.; Andrade Holanda, C.; Roeiro, L.; Soeiro, M.N.; Nunes, K.; Ferreira de Almeida Fiuza, L.; Meuser Batista, M.; Fraga, C.; Alkhalaf, H.E.A.; Elmahallawy, E.K.; Ebiloma, G.U.; De Koning, H.; Vittorio, S.; Vistoli, G.; Blanquart, C.; Bertrand, P.; Bolognesi, M.L. Benign-by-design SAHA analogues. ACS Med Chem Lett. 2024, 15, 1506–1515. [Google Scholar] [PubMed]
- Agishi, E.C. Tiv, Idoma, Etulo, Igede, Akweya, Hausa, English and Scientific Names of Plants, 2nd ed.; Agitab Publishers ltd: Makurdi, Nigeria, 2010; p. 139. [Google Scholar]
- Ogbonna, A.I.; Adepoju, S.O.; Ogbonna, C.I.C.; Yakubu, T.; Itelima, J.U.; Dajin, V.Y. Root tuber of Tacca leontopetaloides L. (Kunze) for food and nutritional security. Microbiology: Current Research 2017, 1, 5–11. [Google Scholar]
- Ogunwusi, A.A. and Ibrahim, H.D. Prospects for Industrial Utilization of Tacca (Tacca involucrata) in Nigeria. Journal of Natural Sciences Research 2023, 14, 10–20. [Google Scholar]
- Jiang, J-H.; Yang, H-M.; Wang, Y-L. and Chen, Y.G. Phytochemical and Pharmacological Studies of the Genus Tacca: A Review. Tropical Journal of Pharmaceutical Research 2014, 13, 635–648.
- Habila, J.D.; Bello, I.A.; Dzikwe, A.A.; Ladan, Z. and Sabiu, I.M. Comparative Evaluation of Phytochemicals, Antioxidant and Antimicrobial Activity of Four Medicinal Plants Native to Northern Nigeria. Australian Journal of Basic and Applied Sciences 2011, 5, 537–543. [Google Scholar]
- Vu, Q.T.H.; Le, P.T.K.; Vo, H.P.H.; Nguyen, T.T.; Nguyen, T.K.M. Characteristics of Tacca leontopetaloides L. Kuntze Collected from a Giang in Vietnam. International Conference on Chemical Engineering, Food and Biotechnology. AIP Conf. Proc. 2017, 1878, 020022-1–020022-6. [Google Scholar] [CrossRef]
- Ahmed, S.; Rakib, A.; Islam, Md. A.; Khanam, B.H.; Faiz, F.B.; Arkajyoti, P. ; Md. Nazim Uddin Chy, Md. N.U.; Bhuiya, N.M.M.A.; Uddin, M.M.N.; Ullah, S.M.A.; Rahman, Md. A. and Emran, T.B. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clinical Phytoscience 2019, 5, 1–13. [Google Scholar]
- Yen, P.H.; Chi, V.T.Q.; Kiem, P.V.; Tai, B.H.; Quang, T.H.; Nhiem, N.X.; Anh, H.L.T.; Ban, N.K.; Thanh, B.V.; Minh, C.V.; Park, S.J.; Kim, S.H. Spirostanol saponins from Tacca veitnamensis and their inflammatory activity. Bioorganic and Medicinal Chemistry Letters 2016, 26, 3780–3784. [Google Scholar]
- Li, L.; Ni, W.; Li, X.R.; Hua, Y.; Fang, P.L.; Kong, L.M.; Pan, L.L.; Li, Y.; Chen, C.X.; Liu, H.Y. Taccasubosides A-D, four new steroidal glycosides from Tacca subflabellata. Steroids 2011, 76, 1037–1042. [Google Scholar] [PubMed]
- Li. Y.; Du, Y-F.; Gao, F.; Xu, J-B.; Zheng, L-L.; Liu, G. and Lei, Y. Taccalonolides: Structure, semi-synthesis, and biological activity. Frontiers in Pharmacology 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Dike, V.T.; Burbwa, V.; Bosha, J.A.; Yin, T.M.; Ebiloma, G.U.; de Koning, H.P.; Igoli, J.O. and Gray, A.I. Antitrypanosomal Activity of a Novel Taccalonolide from the Tubers of Tacca leontopetaloides. Phytochemical Analysis 2016, 27, 217–221. [Google Scholar] [PubMed]
- Peng, J.; Jackson, E. M.; Babinski, D. J.; Risinger, A. L.; Helms, G.; Frantz, D. E.; Mooberry, S. L. Evelynin, a Cytotoxic Benzoquinone-type retro-Dihydrochalcone from Tacca chantrieri. J. Nat. Prod. 2010, 73, 1590–1592. [Google Scholar]
- Peng, J.; Risinger, A.L.; Da, C.; Fest, G.A.; Kellogg, G.E. and Mooberry, S.L. Structure−Activity Relationships of Retro-dihydrochalcones Isolated from Tacca sp. Journal of Natural Products 2013, 76, 2189–2194. [Google Scholar]
- Nna., P.J.; Tor-Anyiin, T.A. Nna. P.J.; Tor-Anyiin, T.A. and Igoli, J.O. Fagaramide and Pellitorine from the Stem Bark of Zanthoxylum zanthoxyloides and Their Antimicrobial Activities. South Asian Research Journal of Natural Products 2019, 2, 1–8. [Google Scholar]
- Balekar, N.; Nakpheng, T. and Chiang, T.S. Wedelia trilobata L.: A Phytochemical and Pharmacological Review. Mai J. Sci. 2014, 41, 590–605, http://epg.science.cmu.ac.th/ejournal/Contributed Paper. [Google Scholar]
- Ikome, H.N.; Tamfu, A.N.; Abdou, J.P.; Fouotsa, H.; Nangmo, P.K.; Lah, F.C.W.; Tchinda, A.T.; Ceylan, O.; Frederich, M.; Nkengfack, A.E. Disruption of Biofilm Formation and Quorum Sensing in Pathogenic Bacteria by Compounds from Zanthoxylum Gilletti (De Wild) P.G. Waterman. Applied Biochemistry and Biotechnology 2023, 195, 6113–6131. [Google Scholar]
- Omosa, L. K.; and Okemza, E. K. Antiplasmodial activities of the stem bark extract and compounds of Zanthoxylum gilletii (De wild) PG Waterman. Pharmacognosy Communications 2017, 7, 41–46. [Google Scholar]
- Claudio, R. N.; and Lopes, L. M. X. Antiplasmodial natural products (review). Molecules 2011, 16, 2146–2190. [Google Scholar]
- Rao, M.L.N. and Ramakrishna, B.S. Rh-Catalyzed Decarbonylative Addition of Salicylaldehydes with Vinyl Ketones: Synthesis of Taccabulins A–E. European Journal of Organic Chemistry 2019, 7545–7554. [Google Scholar]
- Abouelela, M.E.; Orabi, M.A.A.; Abdelhamid, R.A.; Abdelkader, M.S.A.; Darwish, F.M.M. Chemical and Cytotoxic Investigation of Non-Polar Extract from Ceiba Pentandra (L.) Gaertn.: A Study Supported by Computer Based Screening. Journal of Applied Pharmaceutical Science 2018, 8, 057–064. [Google Scholar]
- Joshi, B.S.; Moore, K.M.; Pelletier, S.W.; Puar, M.S. Alkaloids of Zanthoxylum budrunga Wall.: NMR Assignments of Dihydrochelerythrine, (±)-Evodiamine and Zanthobungeanine. Phytochemical analysis 1991, 2, 20–25. [Google Scholar]
- Dofuor, A.K.; Kwain, S.; Osei, E.; Tetevi, G.M.; Okine, L.K.; Ohashi, M.; Gwira, T.M.; and Kyeremeh, K. N-(Isobutyl)-3,4-methylenedioxy Cinnamoyl Amide. Molbank 2019, M1070, 1–11. [Google Scholar]
- Ali, I.; Li, J.; Cui, L.; Zhao, H.; He, Q.; Wang, D. Efficient extraction and purification of benzo[c]phenanthridine alkaloids from Macleaya cordata (Willd) R. Br. by combination of ultrahigh pressure extraction and pH-zone-refining counter-current chromatography with anti-breast cancer activity in vitro. Phyto. Anal 2021, 32, 423–432. [Google Scholar]
- Eze, F.I.; Siwe-Noundou, X.; Isaac, M.; Patnala, S.; Osadebe, P.O.; Krause, R.W.M. Anti-cancer and anti-trypanosomal properties of alkaloids from the root bark of Zanthoxylum leprieurii Guill and Perr. Tropical Journal of Pharmaceutical Research 2020, 19, 2377–2383. [Google Scholar]
- Matovu, E.; Stewart, M.; Geiser, F.; Brun, R.; Maser, P.; Wallace, L.J.M.; Burchmore, R.J.; Enyaru, J.C.K.; Barrett, M.P.; Kaminsky, R.; Seebeck, T.; and de Koning, H.P. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryotic Cell 2003, 2, 1003–1008. [Google Scholar] [CrossRef]
- Bridges, D.; Gould, M.K.; Nerima, B.; Mäser, P.; Burchmore, R.J.S. and De Koning, H.P. Loss of the High Affinity Pentamidine Transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol 2007, 71, 1098–1108. [Google Scholar]
- Ward, C.P.; Wong, P.E.; Burchmore, R.J.; De Koning, H.P.; and Barrett, M.P. Trypanocidal furamidine analogues: influence of pyridine nitrogens on trypanocidal activity, transport kinetics and resistance patterns. Antimicrob Agents Chemother. 2011, 55, 2352–2361. [Google Scholar]
- Stewart, M.L.; Burchmore, R.J.S.; Clucas, C.; Hertz-Fowler, C.; Brook, K.; Tait, A.; McLeod, A.; Turner, C.M.R.; De Koning, H.P.; Wong, P. E. and Barrett, M.P. Multiple genetic mechanisms lead to the loss of functional TbAT1 expression in drug resistant trypanosomes. Eukaryot Cell 2010, 9, 336–343. [Google Scholar]
- Alghamdi, A.H.S.; 2020. Drug sensitivity and drug resistance in Trypanosoma brucei and Leishmania: the aquaporins. PhD thesis. http://theses.gla.ac.uk/82107/.
- Munday, J.C.; Settimo, L. and De Koning, H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Frontiers Pharmacol. 2015, 6, 32. [Google Scholar]
- Suswam, E.A.; Ross, C.A.; Martin, R.J. Changes in adenosine transport associated with melaminophenyl arsenical (Mel CY) resistance in Trypanosoma evansi: down-regulation and affinity changes of the P2 transporter. Parasitology 2003, 127, 543–9. [Google Scholar] [PubMed]
- Munday, J.C.; Eze, A.A.; Baker, N.; Glover, L.; Clucas, C.; Aguinaga Andrés, D.; Natto, M.J.; Teka, I.A.; McDonald, J.; Lee, R.S.; Graf, F.E.; Ludin, P.; Burchmore, R.J.S.; Turner, C.M.R.; Tait, A.; MacLeod, A.; Mäser, P.; Barrett, M.P.; Horn, D.; De Koning, H.P. Trypanosoma brucei Aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and is the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [Google Scholar] [PubMed]
- Ebiloma, G.U.; Ayuga, T.D.; Balogun, E.O.; Gil, L.A.; Donachie, A.; Kaiser, M.; Herraiz, T.; Inaoka, D.K.; Shiba, T.; Harada, S.; Kita, K.; De Koning, H.P.; Dardonville, C. Inhibition of trypanosome alternative oxidase without its N-terminal mitochondrial targeting signal (ΔMTS-TAO) by cationic and non-cationic 4-hydroxybenzoate and 4-alkoxybenzaldehyde derivatives active against T. brucei and T. congolense. Eur. J. Med. Chem. 2018, 150, 385–402. [Google Scholar]
- Elata, A.; Galon, E.M.; Moumouni, P.F.A.; Ybanez, R.H.D.; Mossaad, E.; Salces, C.B.; Bajenting, G.P.; Ybanez, A.P.; Xuan, X.; Inoue, N.; Suganuma, K. First molecular detection and identification of Trypanosoma evansi in goats from Cebu, Philippines using a PCR-based assay. Vet Parasitol Reg Stud Reports 2020, 21, 100414. [Google Scholar]
- De Koning, H.P.; MacLeod, A.; Barrett, M.P.; Cover, B.; Jarvis, S.M. . Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol Biochem Parasitol. 2000, 106, 181–185. [Google Scholar]
- Dean, S.; Gould, M.K.; Dewar, C.E.; Schnaufer, A.C. Single point mutations in ATP synthase compensate for mitochondrial genome loss in trypanosomes. Proc. Natl. Acad. Sci. USA. 2013, 110, 14741–14746. [Google Scholar]
- Giordani, F.; Khalaf, A.I.; Gillingwater, K.; Munday, J.C.; De Koning, H.P.; Suckling, C.J.; Barrett, M.P.; Scott, F.J. Novel minor groove binders cure animal African trypanosomiasis in an in vivo mouse model. J. Med. Chem. 2019, 62, 3021–3035. [Google Scholar]
- Coustou, V.; Guegan, F.; Plazolles, N.; Baltz, T. Complete in vitro life cycle of Trypanosoma congolense: Development of genetic tools. PLoS Negl. Trop. Dis. 2010, 4, e618. [Google Scholar]
- Al-Salabi, M.I.; De Koning, H.P. Purine nucleobase transport in amastigotes of Leishmania mexicana: involvement in allopurinol uptake. Antimicrob Agents Chemother. 2005, 49, 3682–3689. [Google Scholar]
- Al-Salabi, M.I.; Wallace, L.J.M.; and De Koning, H.P. A Leishmania major nucleobase transporter responsible for allopurinol uptake is a functional homologue of the Trypanosoma brucei H2 transporter. Mol Pharmacol. 2003, 63, 814–820. [Google Scholar] [PubMed]
- Ebiloma, G.U.; Igoli, J.O.; Katsoulis, E.; Donachie, A.M.; Eze, A.; Gray, A.I.; De Koning, H.P. Bioassay-guided isolation of active principles from Nigerian medicinal plants identifies new trypanocides with low toxicity and no cross-resistance to diamidines and arsenicals. J. Ethnopharmacol. 2017, 20, 256–264. [Google Scholar]
- Gould, M.K.; Vu, X.L.; Seebeck, T.; and De Koning, H.P. Propidium iodide-based methods for monitoring drug action in the kinetoplastidae: comparison with the Alamar Blue assay. Anal Biochem. 2008, 382, 87–93. [Google Scholar] [PubMed]
- Ene, A.C.; Atawodi, S.E.; Apeh, Y.E.O. In vitro and In vivo antitrypanosomal effects of petroleum ether, chloroform and methanol extracts of Artemisia maritime Linn. Brit. J. Pharm. Res. 2014, 4, 751–58. [Google Scholar]
- Ebiloma, G.U.; Katsoulis, E.; Igoli, J.O.; Gray, A.I.; De Koning, H.P. Multi-target mode of action of a Clerodane-type diterpenoid from Polyalthia longifolia targeting African trypanosomes. Sci. Rep. 2018, 8, 4613. [Google Scholar]
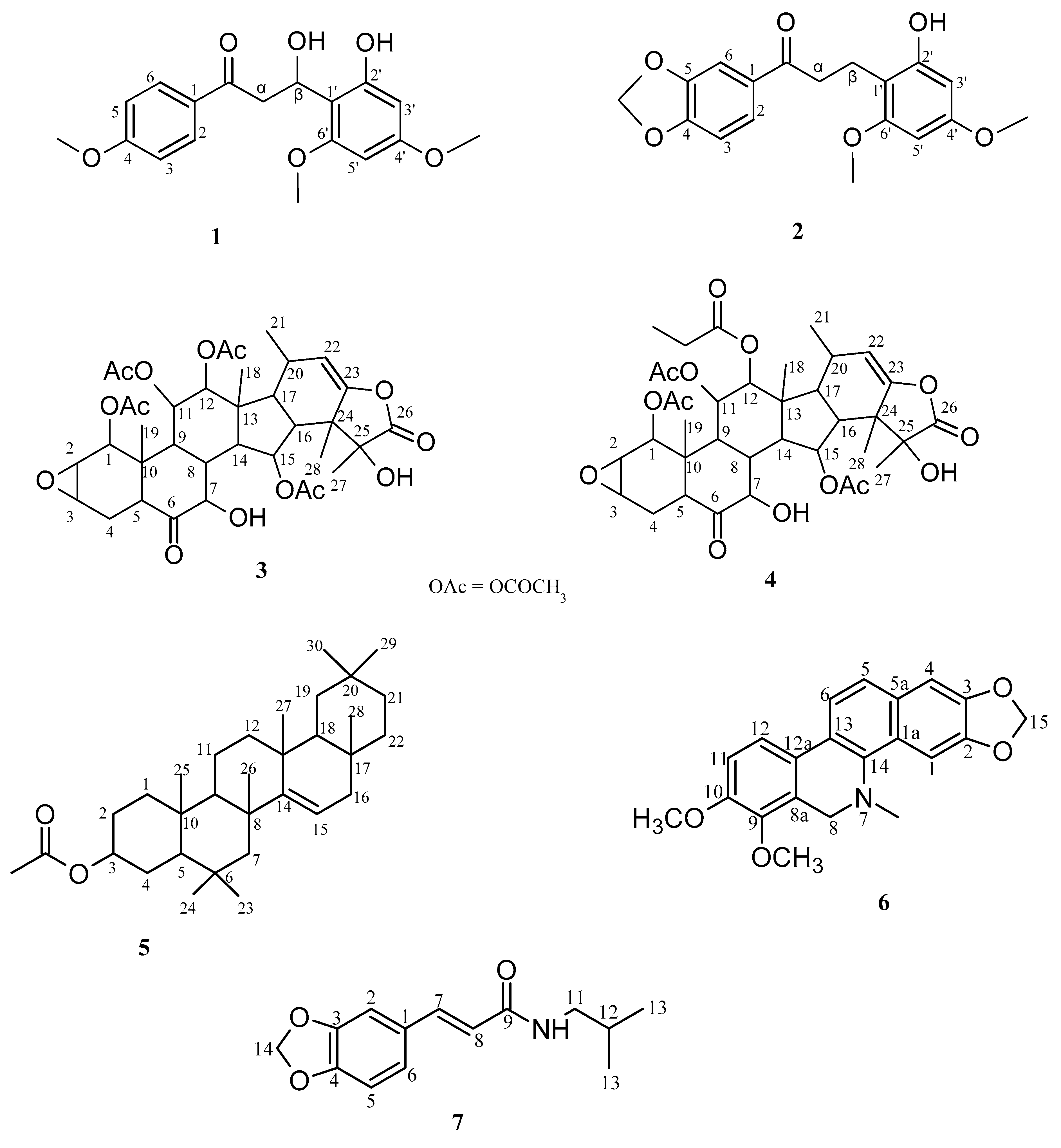
| Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| 1H (ẟ ppm, m, J (Hz)) | 13C (m) | 1H (ẟ ppm, m, J (Hz)) | 13C (m) | |
| 1 | - | 125.9 (C) | - | 131.4 (C) |
| 2 | 8.09 (d, 8.9) | 131.5 (CH) | 7.63 (dd, 8.3, 1.8) | 125.0 (CH) |
| 3 | 7.04 (d, 9.0) | 114.0 (CH) | 6.84 (d, 8.3) | 107.9 (CH) |
| 4 | - | 164.4 (C) | - | 152.3 (C) |
| 5 | 7.04 (d, 9.0) | 114.0 (CH) | - | 147.7 (C) |
| 6 | 8.09 (d, 8.9) | 131.5 (CH) | 7.47 (d, 1.8) | 108.1 (CH) |
| 1′ | - | 105.1 (C) | - | 108.7 (C) |
| 2′ | - | 157.7 (C) | - | 156.3 (C) |
| 3′ | 6.23 (d, 2.3) | 94.8 (CH) | 6.20 (d, 2.4) | 94.7 (CH) |
| 4′ | - | 160.4 (C) | - | 159.9 (C) |
| 5′ | 6.05 (d, 2.4) | 91.3 (CH) | 6.07 (d, 2.5) | 91.3 (CH) |
| 6′ | - | 158.3 (C) | - | 159.3 (C) |
| α | 3.46 (dd, 14.9, 2.9)2.86 (dd, 14.9, 7.6) | 30.5 (CH2) | 3.33 (m) | 38.8 (CH2) |
| Β | 5.18 (dd, 7.6, 2.9) | 75.3 (CH) | 2.95 (dd, 6.6,4.4) | 16.6 (CH2) |
| -C=O | - | 197.9 (C) | - | 201.4 (C) |
| O-CH2-O | - | - | 6.02 (s) | 101.9 (CH2) |
| 4-OCH3 | 3.95 (s) | 55.6 (CH3) | - | - |
| 4′-OCH3 | 3.79 (s) | 55.3 (CH3) | 3.77 (s) | 55.3 (CH3) |
| 6′ -OCH3 | 3.61 (s) | 55.4 (CH3) | 3.81 (s) | 55.5 (CH3) |
| 2′-OH | 6.13 (s, br) | - | 8.80 (s, br) | - |
| Compound | T. b. brucei s427 | T. b. brucei B48 | T. evansi | T. equiperdum | T. congolense | L. mexicana | HEK 293 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (µg/mL) | SI | EC50 (µg/mL) | RF | SI | EC50 (µg/mL) | RF | SI | EC50 (µg/mL) | RF | SI | EC50 (µg/mL) | RF | SI | EC50 (µg/mL) | SI | EC50 (µg/mL) | |
| 1 | 11.8 ± 1.2 |
>8.5 | 16.7 ± 5.0 |
1.4 | >6.0 | 15.3 ± 0.8 | 1.3 | >6.5 | 7.1 ± 1.3 | 0.60 | >14 | 81.7 ± 1.7*** | 6.9 | >1.2 | 193 ± 1*** | 0.52 | >100 |
| 3 | 45.5 ± 14.9 |
>2.2 | 31.9 ± 2.9 |
0.7 | >3.1 | 46.6 ± 6.7 | 1.0 | >2.2 | 39.2 ± 0.0 | 0.86 | >2.6 | 73.9 ± 28.9 | 1.6 | >1.4 | >200 | ND | >100 |
| 4 | 38.2 ± 6.2 |
>2.6 | 53.8 ± 8.8 | 1.4 | >1.9 | 59.3 ± 5.3 | 1.6 | >1.7 | 55.7 ± 7.5 | 1.5 | >1.8 | >100 | >2.8 | ND | >200 | ND | >100 |
| 5 | 64.5 ± 28.5 | >3.1 | 59.8 ± 2.1 | 0.75 | >3.4 | 29.5 ± 0.7 | 0.4 | >6.8 | 9.98 ± 0.12 | 0.13 | >20 | 83.6 ± 6.7 | 1.1 | 2.4 | >200 | ND | >200 |
| 6 | 0.48 ± 0.15 | 68.0 | 0.89 ± 0.10 | 1.8 | 37.0 | 0.38 ± 0.02 | 0.8 | 87.0 | 1.08 ± 0.18 | 2.2 | 30.4 | 2.9 ± 0.2*** | 6.0 | 11.4 | 1.9 ± 0.2** | 18 | 33.0 ± 8.1 |
| 7 | 4.4 ± 1.8 | 20.4 | 2.7 ± 0.1 | 0.6 | 33.8 | 2.7 ± 0.1 | 0.6 | 33.0 | 6.70 ± 0.03 | 1.5 | 13.4 | 8.5 ± 1.4 | 1.9 | 10.6 | 3.3 ± 0.1 | 27 | 90.1 ± 10.8 |
| PMD (µM) |
a0.0066 ± 0.0001 b0.0052 ± 0.0006 |
- |
a0.312 ± 0.0349*** b0.288 ± 0.05*** |
a47.3 b55.4 |
a0.016 ± 0.004** b0.0025 ± 0.0003** |
a0.0081 ± 0.001 b0.0033 ± 0.001 |
ND |
a1.10 ± 0.03*** b0.76 ± 0.05*** |
ND | ||||||||
| DA (µM) |
ND | ND | ND | ND |
a0.51 ± 0.01 b0.46 ± 0.15 |
ND | ND | ||||||||||
| PAO (µM) | ND | ND | ND | ND | ND | ND |
a0.17 ± 0.01 b0.048 ± 0.011 |
||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





