Background
Postoperative atrial fibrillation (POAF) is the most common cardiac arrhythmia following cardiac surgery. The incidence ranges from 15% to 50% depending on the type of surgery and patient characteristics [
1,
2,
3,
4]. POAF is associated with increased postoperative morbidity, including stroke and heart failure [
5,
6]. While many episodes of POAF are transient and self-limiting, some patients develop persistent atrial fibrillation, which significantly worsens cardiovascular prognosis [
7]. Preoperative risk prediction tools could aid in personalized prophylactic strategies aiming to reduce the incidence of POAF and associated complications, while limiting overtreatment.
A complex interplay of pathophysiological factors predisposes patients to atrial arrhythmia’s following cardiac surgery. Aging, chronic cardiac conditions, metabolic and neuroendocrine disorders all contribute to POAF susceptibility, for example through atrial stretch and fibrosis, systemic inflammation, endothelial dysfunction, electrolyte imbalances and oxidative stress [
8,
9]. The multifaceted nature of preoperative risk factors makes accurate prediction of POAF challenging. Despite extensive research, existing clinical risk models for POAF remain suboptimal, with moderate predictive performance at best [
2].
The incorporation of predictors that represent the complex and heterogeneous nature of POAF may enhance individualized risk stratification and optimize preventive perioperative strategies, such as early initiation of beta-blockers, anti-inflammatory agents, or tailored electrolyte management. We hypothesize that a multimodal biomarker approach, using pathophysiological biomarkers for POAF, can significantly improve preoperative risk stratification, compared to clinical risk factors alone. This study aims to investigate the added predictive value of a biomarker panel for POAF to the POAF-Score, an established clinical prediction model for atrial fibrillation (AF) after cardiac surgery [
1].
Methods
Study Design and Participants
This was a prospective, observational cohort study conducted at two cardiac surgery centres. Data were derived from the BIGPROMISE cohort, a perioperative biobank designed to investigate the epidemiology and pathophysiology of postoperative complications [
10]. Eligible participants included adult patients who underwent cardiac surgery between Oct 8th 2021 and Sep 9th 2024. Individuals with a documented history of atrial fibrillation were excluded. The study was conducted in accordance with the Declaration of Helsinki and received approval from the institutional review boards of both participating centres [NCT05199025]. Written informed consent was obtained from all participants prior to enrolment. The study adhered to the TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis) guidelines [
11].
Data Collection
Demographic and clinical data were collected preoperatively for all participants in accordance with a prespecified study protocol [
10]. Blood samples were collected after induction of anaesthesia, prior to the start of surgery. In all study patients, blood samples were centrifuged at 1800×g for 5 min and used to analyse a panel of 50 biomarkers [
10]. Biomarker analyses were performed at the local hospital laboratory on Roche Cobas 8000 and Sysmex XN platforms. Results of perioperative biomarker analysis were captured in local laboratory information management systems and uploaded to a central web application for research data (REDCap).
Sample Size
Post-hoc sample size calculation was performed to determine the maximum number of candidate predictors. Sample size estimation was informed by the pmsampsize package in R [
12]. Sample size calculation was repeated with increasing number of candidate predictors using the prevalence calculated from the cohort and an expected discriminative model performance with a c-statistic 0.72. The maximum number of candidate predictors for which the sample size was still sufficient was used for model development.
POAF-Score
The primary objective of this study was to evaluate the added predictive value of a preoperative biomarker panel to the POAF-score - an established and externally validated clinical prediction model for POAF in general cardiac surgery patients, in comparison to the POAF-Score alone. We selected the POAF-Score because it was derived and validated in a general cardiac surgery population and has a superior predictive performance compared to other available models. The POAF-Score is a multivariable risk model incorporating age, history of chronic obstructive pulmonary disease, kidney function, urgency of surgery, preoperative use of an intra-aortic balloon pump, left ventricular ejection fraction, and surgery type [
1].
Selection of Biomarkers
The initial biomarker selection was guided by existing literature on the pathophysiological mechanisms underlying POAF, including myocardial injury, stress and fibrosis; inflammation; metabolic and neuro-endocrine dysregulation, and hematologic status.[
8,
9] To reduce the number of biomarkers from the initial selection to align with the sample size constraints, univariable associations with POAF were assessed using the Mann–Whitney U test. This non-parametric, rank-based test was chosen to minimize the influence of outliers and accommodate the skewed distributions commonly observed in biomarker data. Biomarkers with the lowest p-values were included for model development.
Outcome
The primary outcome was new-onset POAF, defined as new onset of irregular heart rate in the absence of P waves lasting at least 30 seconds or for the duration of the ECG recording (if less than 30 seconds) [
13]. Patients were monitored for the occurrence of POAF for up to 30 days postoperatively, or until hospital discharge, whichever occurred first. All participants underwent continuous cardiac telemetry for 48 hours after CABG and 72 hours after valve surgery, and standard 12-lead electrocardiograms (ECGs) were obtained at arrival on the intensive care unit, on postoperative days one and three, and on indication.
Missing Data
Missing data were assessed using descriptive statistics. Missing data were addressed using Multiple Imputation by Chained Equations (MICE), creating ten imputation sets with 20 iterations to minimize potential bias associated with complete case analysis. Variables with excessive missingness, defined as greater than 40%, were excluded from the analysis. Imputed datasets were pooled using Rubin’s Rules to derive final estimates.
Statistical Analysis
Baseline characteristics were summarized as mean ± standard deviation (SD) for normally distributed continuous variables or median with interquartile range (IQR) for non-normally distributed variables, as appropriate. Categorical variables were presented as counts with corresponding percentages. Differences between patients with and without POAF were assessed using the Student’s t-test for normally distributed continuous variables, the Mann–Whitney U test for non-normally distributed variables, and the Chi-square test for categorical variables.
Least absolute shrinkage and selection operator (LASSO) regression was used for model development and variable selection. This method shrinks regression coefficients and excludes less informative predictors by setting some coefficients to zero, reducing overfitting of the model. For model development, the POAF-Score was entered as a continuous probability and retained unpenalized to allow assessment of the incremental value of biomarkers. This analysis was repeated with the POAF-Score penalized to evaluate whether biomarkers had a stronger association with the outcome than clinical variables. To meet model assumptions multi-collinearity among candidate predictors was assessed. Correlation matrices were constructed, with a Pearson correlation coefficient (ρ) > 0.5 considered indicative of multi-collinearity. Independent variables were transformed as needed to meet the assumption of linearity with the log-odds of the outcome. LASSO was applied on each of the ten individual imputation sets, creating ten models. Predictors selected in at least five out of ten LASSO models were selected for the final model. LASSO regression was then performed again in all imputation sets using only the final predictor selection, creating the models used for model performance evaluation. For the Decision Curve Analysis (DCA) the mean of each coefficient of the imputation models was used to create a single final model, as to our knowledge no statistical packages are available yet for DCA on multiple imputed datasets.
Model performance was evaluated through discrimination, using the area under the receiver operating characteristic curve (AUROC) with 95% confidence intervals (CI); calibration, using calibration plots; reclassification, using the Net Reclassification Index (NRI) to assess the added value of biomarkers over the clinical model, and clinical benefit, using DCA to quantify the clinical utility of the prediction model according to a decision threshold. For NRI and DCA a threshold probability of 40% was used. To quantify the difference in discriminative performance a DeLong test was performed. Pooling was performed with the pfsmi package [
14]. All statistical analyses were conducted using RStudio, version 4.4.0.
Results
Patient Population and Outcome
The BIGPROMISE study cohort comprised 1,180 adult patients undergoing cardiac surgery. Of these, 221 patients with a history of atrial fibrillation were excluded, resulting in 959 patients included in the final analysis. Preoperative blood samples were missing in 14 patients (1.5%). Follow-up was complete for 100% of the cohort. The median age was 65.0 [59.0 – 70.0] years, and 757 patients (78.9%) were male.
In total, 339 (35.3%) patients developed POAF within 30 days following cardiac surgery. Patients who developed POAF were slightly older and underwent coronary artery bypass grafting (CABG) less frequently than those who did not develop POAF (
Table 1). The incidence of POAF was 29.8% among patients undergoing isolated CABG and 36.3 % among those undergoing single valve surgery. Most patient developed POAF on postoperative day 2 (n=129; 38.0%) and 3 (n=94; 27.7%).
Biomarker Selection
An initial panel of 35 biomarkers was selected from the BIGPROMISE biobank. A complete overview of the candidate biomarkers and their respective pathophysiological domains is provided in Supplementary
Table 1. Concentrations of the 13 biomarkers that were most strongly associated with POAF are presented in
Table 2. Among the selected biomarkers, sex hormone-binding globulin (SHBG), along with NT-proBNP, cholesterol, and vitamin D, showed the strongest associations with the development of POAF.
Risk Stratification and Clinical Benefit
At a predicted risk threshold of 40%, the POAF-Score identified 25.7% of patients with POAF as high risk. Incorporation of biomarkers into the model led to correct upward reclassification of 16% of patients who developed POAF. Two percent of patients without POAF were reclassified to a lower risk category, and 13% were incorrectly reclassified as high risk. This resulted in a Net Reclassification Index (NRI) of 0.049 (CI -0.001 – 0.099) (
Table 3), reflecting a very small improvement in risk classification.
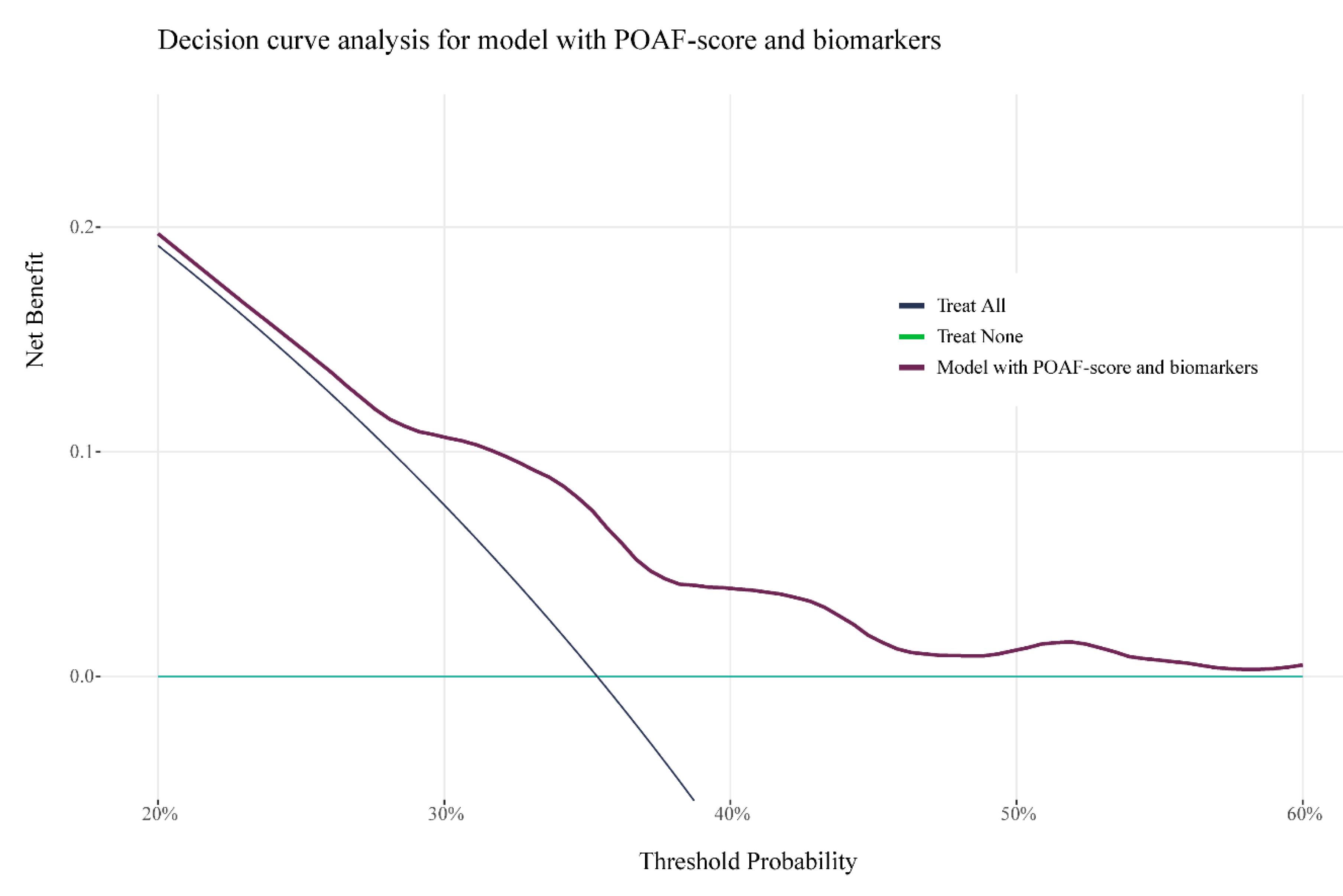
Figure 2 presents the results of DCA, illustrating the net clinical benefit of the biomarker-enhanced model (blue line) for prophylactic treatment of POAF. Compared to a treat-all strategy, the biomarker enhanced model would avoid unnecessary treatment in 18% of patients, while maintaining treatment coverage for those who develop POAF. In comparison, the POAF-Score alone would avoid unnecessary treatment in 17% of patients. When directly comparing the two models, the biomarker-enhanced model results in 2 fewer unnecessary treatments per 100 patients.
Table 1.
Baseline characteristics.
Table 1.
Baseline characteristics.
| |
No POAF |
POAF |
p-value |
| |
N=620 |
N=339 |
|
| Female, n (%) |
126 (20.3) |
76 (22.4) |
0.498 |
| Age, y (IQR) |
64 [59 - 69] |
66 [62 -72] |
<0.001 |
| BMI |
27 [25 - 30] |
27 [25 - 29] |
0.009 |
| Surgery type, n (%) |
|
|
<0.001 |
| AVR |
88 (14.2) |
54 ( 15.9) |
|
| Bentall |
10 ( 1.6) |
13 ( 3.8) |
|
| CABG |
393 (63.4) |
167 ( 49.3) |
|
| CABG + AVR |
51 ( 8.2) |
48 ( 14.2) |
|
| MVR |
42 ( 6.8) |
20 ( 5.9) |
|
| Other |
36 (5.8) |
34 (10.0) |
|
| Urgent surgery |
134 (21.6) |
66 ( 19.5) |
0.485 |
| Diabetes, n (%) |
154 (24.8) |
70 (20.6) |
0.541 |
| COPD, n (%) |
|
|
0.504 |
| None |
571 (92.1) |
314 ( 92.6) |
|
| GOLD I |
3 ( 0.5) |
4 ( 1.2) |
|
| GOLD II |
12 ( 1.9) |
8 ( 2.4) |
|
| GOLD III |
6 ( 1.0) |
4 ( 1.2) |
|
| Unknown |
28 (4.6) |
9 (2.7) |
|
| Hypertension, n (%) |
344 (55.7) |
187 ( 55.2) |
0.935 |
| Heart failure, n (%) |
43 ( 7.0) |
26 ( 7.7) |
0.787 |
| History of ischemic heart disease, n (%) |
418 (67.6) |
214 ( 63.3) |
0.201 |
| Previous myocardial infarction, n (%) |
194 (31.3) |
90 ( 26.5) |
0.139 |
| Myocardial infarction within 90 days before surgery, n (%) |
128 (20.7) |
58 ( 17.1) |
0.207 |
| Peripheral artery disease, n (%) |
91 (14.7) |
64 ( 18.9) |
0.115 |
| Pulmonary hypertension, n (%) |
|
|
0.332 |
| No |
614 (99.0) |
335 ( 99.1) |
|
| Moderate |
6 ( 1.0) |
2 ( 0.6) |
|
| Severe |
0 ( 0.0) |
1 ( 0.3) |
|
| LVEF, n (%) |
|
|
0.204 |
| >50 |
460 (74.2) |
260 ( 77.2) |
|
| 31-50 |
116 (18.7) |
62 ( 18.4) |
|
| 21-30 |
14 ( 2.3) |
8 ( 2.4) |
|
| <20 |
7 ( 1.1) |
0 ( 0.0) |
|
| Unknown |
23 ( 3.7) |
7 ( 2.1) |
|
| NYHA, n (%) |
|
|
0.737 |
| Class 1 |
132 (21.3) |
82 ( 24.2) |
|
| Class 2 |
270 (43.6) |
150 ( 44.2) |
|
| Class 3 |
66 (10.7) |
29 ( 8.6) |
|
| Class 4 |
11 ( 1.8) |
6 ( 1.8) |
|
| Unknown |
140 (22.6) |
72 ( 21.2) |
|
| CCS IV, n (%) |
|
|
0.256 |
| No |
509 (82.2) |
287 ( 84.7) |
|
| Yes |
55 ( 8.9) |
20 ( 5.9) |
|
| Unknown |
55 ( 8.9) |
32 ( 9.4) |
|
| Previous cardiac surgery, n (%) |
75 (12.1) |
34 ( 10.0) |
0.386 |
| Previous CVA or TIA, n (%) |
70 (11.3) |
39 ( 11.5) |
1.000 |
| Kidney function, n (%) |
|
|
0.299 |
| CC >85 |
290 (46.8) |
154 ( 45.4) |
|
| CC 50-85 |
298 (48.1) |
160 ( 47.2) |
|
| CC <50 |
30 ( 4.8) |
25 ( 7.4) |
|
| Dialysis |
2 ( 0.3) |
0 ( 0.0) |
|
Table 2.
Baseline biomarker concentrations.
Table 2.
Baseline biomarker concentrations.
| |
No POAF |
POAF |
p-value |
| |
N=620 |
N=339 |
|
| SHBG (nmol/l) |
32.00[23.7 - 42.0] |
35.8 [27.8 - 47.1] |
<0.001 |
| NT-proBNP (pg/ml) |
176.8 [76.1 - 430.2] |
228.3 [96.0 - 508.2] |
0.012 |
| Cholesterol (mmol/l) |
3.6 [3.0 - 4.2] |
3.7 [3.2- 4.5] |
0.016 |
| Vitamin D (nmol/l) |
45.0 [31.5 - 60.9] |
49.0 [34.9 - 63.8] |
0.024 |
| Thrombocytes (x 109/l) |
205.0 [173.0 -237.0] |
198.0 [169.0 - 226.0] |
0.038 |
| IGF-1 (nmol/l) |
15.4 [12.0 - 19.1] |
14.5 [11.7 - 18.0] |
0.044 |
| Glucose (mmol/l) |
5.9 [5.5 - 6.8] |
5.81 [5.40, 6.40] |
0.044 |
| IL-6 (pg/ml) |
3.1 [1.9 - 4.1] |
2.9 [1.9 - 3.6] |
0.328 |
| Red cell distribution width (%) |
12.9 [12.4 - 13.4] |
13.0 [12.5 - 13.5] |
0.070 |
| Reticulocytes (x109/l) |
61.0 [49.0 - 74.0] |
58.3 [48.5 - 72.0] |
0.088 |
| Potassium (mmol/l) |
3.9 [3.7 - 4.1] |
3.9 [3.7 - 4.1] |
0.181 |
| Sodium (mmol/l) |
139.5 [138.0 - 141.0] |
139.9 [138.0 -141.0] |
0.200 |
| GDF-15 (pg/ml) |
1076.5 [779.5-1660.0] |
1164.0 [858.5- 1626.0] |
0.186 |
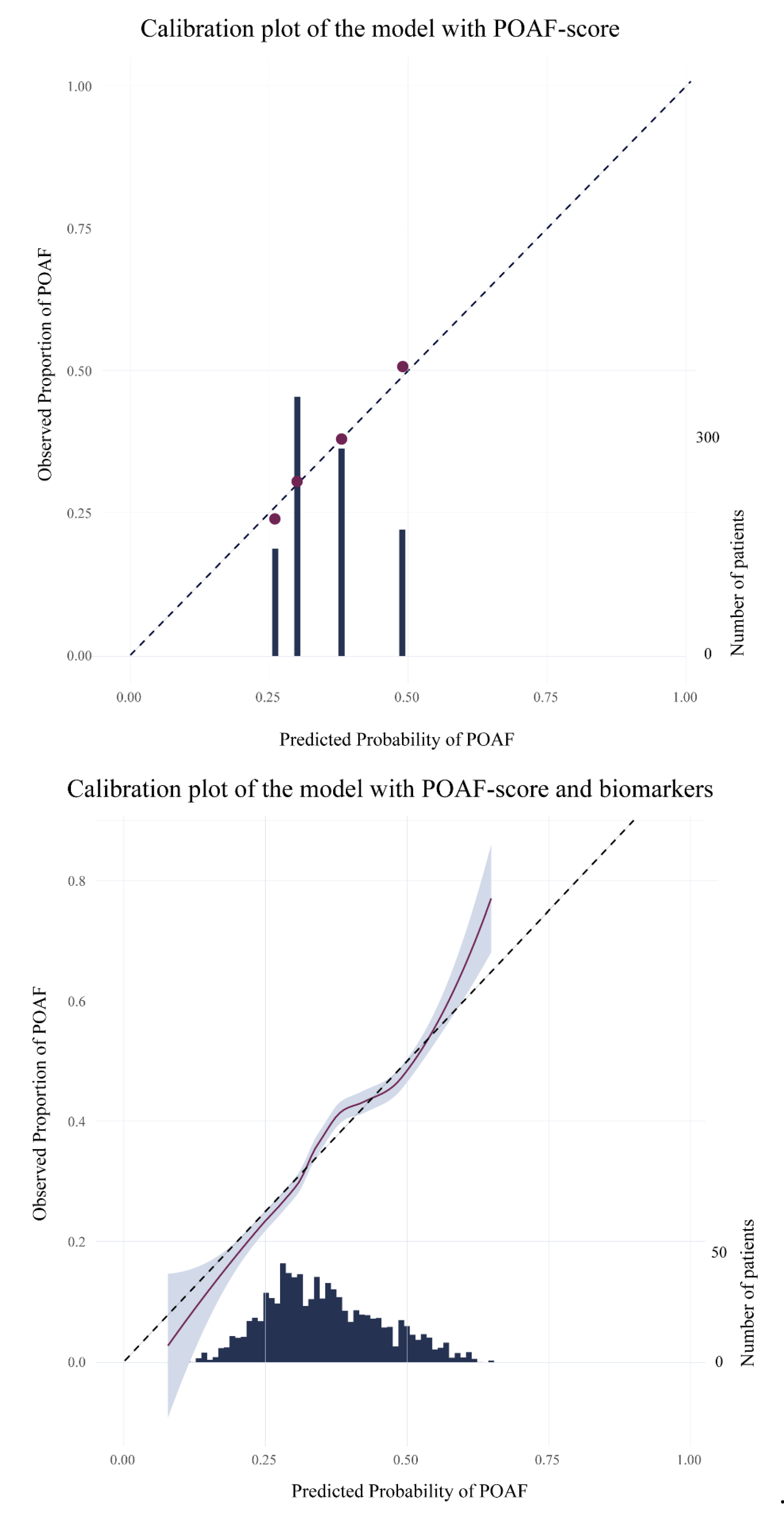
Figure 1.
Calibration plots. 1.1 calibration_plot_POAF score; 1.2 calibration_plot biomarker model.
Figure 1.
Calibration plots. 1.1 calibration_plot_POAF score; 1.2 calibration_plot biomarker model.
Table 3.
Reclassification of cases at a risk threshold of 40%.
Table 3.
Reclassification of cases at a risk threshold of 40%.
| |
Biomarker enhanced model |
| POAF-score |
<0.4 |
>= 0.4 |
| < 0.4 |
196 |
56 |
| ≥ 0.4 |
0 |
87 |
Table 4.
Reclassification of controls at a risk threshold of 40%.
Table 4.
Reclassification of controls at a risk threshold of 40%.
| |
Biomarker enhanced model |
| POAF-score |
<0.4 |
>= 0.4 |
| < 0.4 |
454 |
82 |
| ≥ 0.4 |
9 |
75 |
Figure 2.
Decision curve analysis of biomarker enhanced model.
Figure 2.
Decision curve analysis of biomarker enhanced model.
Discussion
This study aimed to improve the preoperative prediction of POAF after cardiac surgery by adding a panel of pathophysiological biomarkers to an established clinical risk model. While the POAF-Score is the best preoperative tool specifically developed for this purpose, its discriminative ability was poor in our cohort, which is consistent with prior external validations [
2]. We demonstrated that the addition of pathophysiological biomarkers modestly but significantly improves the predictive performance of POAF following cardiac surgery.
Although the overall improvement in discrimination of our biomarker enhanced model was statistically significant, the incremental clinical value was limited. Given the poor predictive performance of the POAF-Score alone (C-index 0.600), we anticipated that adding biomarkers would yield a more substantial improvement rather than a limited increase to 0.633. To further explore this, we repeated the analysis with the POAF-Score penalized, allowing us to assess whether biomarkers had a stronger association with the outcome than clinical variables. Interestingly, even in this setting, the clinical predictors remained part of the final model, underscoring their robust predictive value despite the availability of a wide range of biomarker data. One might question the utility of a clinical prediction model with moderate performance. However, when used for risk stratification of prophylactic treatment for POAF our model could prevent unnecessary treatment in 18% of patients compared to a treat-all strategy. Moreover, no clearly superior predictive models are currently available.
The limited added value of preoperative biomarkers in our model may be explained by the timing and context in which they were measured. Although biologically plausible, a single preoperative measurement may not adequately capture the dynamic, multifactorial processes that leads to POAF. Inflammatory and cardiac stress responses evolve rapidly during and after surgery, and preoperative biomarkers may not fully reflect the critical changes that precede the onset of POAF. Surgical trauma, extracorporeal circulation, fluid shifts, and temperature fluctuations likely contribute substantially to POAF by provoking inflammatory and autonomic responses [
8,
9]. Similarly, postoperative variables including blood loss, systemic inflammation, and haemodynamic instability are not captured in preoperative models, yet may carry significant predictive weight. This is supported by our finding that POAF development was most common on postoperative days 2 and 3. Incorporating postoperative biomarker measurements may therefore offer greater predictive value and enable earlier, more individualized clinical decision-making.
It is important to acknowledge several limitations. First, our biomarker enhanced model was developed within a single study cohort without external validation. Second, biomarkers were collected after induction of anaesthesia, which may restrict generalisation to earlier preoperative settings. Third, dynamic intra- and postoperative factors were not included. Nevertheless, the strengths of this study include its prospective, multicentre design, systematic biomarker selection based on POAF pathophysiology and use of penalised regression techniques to reduce overfitting.
In conclusion, our findings demonstrate that adding pathophysiological biomarkers modestly improve preoperative risk stratification for POAF after cardiac surgery, with limited clinical benefit.
Author Contributions
P.G. Noordzij: This author helped with study design, data interpretation, drafting the paper, final approval. M.S.Y. Thio : This author helped with study design, data acquisition, data analysis and interpretation, final approval; T. Reniers: This author helped with study design, data acquisition, critical revision, final approval; I. Dijkstra: This author helped with data acquisition, critical revision, final approval; G. Mondelli: This author helped with data acquisition, critical revision, final approval; M. Langelaan: This author helped with data acquisition, critical revision, final approval; H.J.T. Ruven: This author helped with critical revision, final approval; T.C.D. Rettig: This author helped with critical revision, final approval
Funding
This study uses data from BIGPROMISE, a perioperative biobank study, which was funded by a research grant from Roche Diagnostics.
Data availability
The dataset supporting the findings of this study is available from the corresponding author upon reasonable request. Access to the data may be subject to institutional and ethical approvals, in accordance with applicable data sharing policies.
Conflicts of Interest
P.G. Noordzij is a member of the advisory board of Roche Diagnostics on the perioperative use of biomarkers and receives funding from Roche Diagnostics for an investigator initiated perioperative biomarker study; T.C. Rettig receives funding from Roche Diagnostics for an investigator initiated perioperative biomarker study.
References
- Mariscalco G, Biancari F, Zanobini M, et al. Bedside Tool for Predicting the Risk of Postoperative Atrial Fibrillation After Cardiac Surgery: The POAF Score. J Am Heart Assoc. 2014;3(1):e000752. [CrossRef]
- Cameron MJ, Tran DTT, Abboud J, et al. Prospective External Validation of Three Preoperative Risk Scores for Prediction of New Onset Atrial Fibrillation After Cardiac Surgery. Anesth Analg. 2018;126(1):33–38. [CrossRef]
- Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001 Dec 18;135(12):1061-73. [CrossRef] [PubMed]
- Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004 Apr 14;291(14):1720-9. [CrossRef] [PubMed]
- Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, Lopez JA, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004 Mar 3;43(5):742-8. [CrossRef] [PubMed]
- Lahtinen J, Biancari F, Salmela E, Mosorin M, Satta J, Rainio P, Rimpiläinen J, Lepojärvi M, Juvonen T. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004 Apr;77(4):1241-4. [CrossRef] [PubMed]
- Ambrosetti M, Tramarin R, Griffo R, De Feo S, Fattirolli F, Vestri A, Riccio C, Temporelli PL; ISYDE and ICAROS Investigators of the Italian Society for Cardiovascular Prevention, Rehabilitation and Epidemiology (IACPR-GICR). Late postoperative atrial fibrillation after cardiac surgery: a national survey within the cardiac rehabilitation setting. J Cardiovasc Med (Hagerstown). 2011 Jun;12(6):390-5. [CrossRef] [PubMed]
- Gaudino M, Di Franco A, Rong LQ, Piccini J, Mack M. Postoperative atrial fibrillation: from mechanisms to treatment. Eur Heart J. 2023;44(12):1020–1039. [CrossRef]
- Echahidi N, Pibarot P, O'Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51(8):793–801. [CrossRef]
- Noordzij PG, Ruven HJT, Reniers T, Idema RN, Thio MS, Cremer OL, et al. Cohort profile of BIGPROMISE: a perioperative biobank of a high-risk surgical population. BMJ Open. 2024;14(6):e078307. [CrossRef]
- Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Ann Intern Med. 2015;162(1):55–63. [CrossRef]
- Riley RD, Snell KIE, Ensor J, Burke DL, Harrell FE Jr, Moons KGM, Collins GS. Minimum sample size for developing a multivariable prediction model: Part II – binary and time-to-event outcomes. Stat Med. 2019;38(7):1276–1296. [CrossRef]
- Beattie WS, Lalu M, Bocock M, et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: cardiovascular outcomes. Br J Anaesth. 2021;126(1):56–66. [CrossRef]
- Heymans MW (2023). psfmi: Prediction Model Pooling, Selection and Performance Evaluation Across Multiply Imputed Datasets. R package version 1.4.0.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).