Submitted:
15 April 2025
Posted:
16 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of SHED-CM
2.2. Caco-2 Monolayer Assay
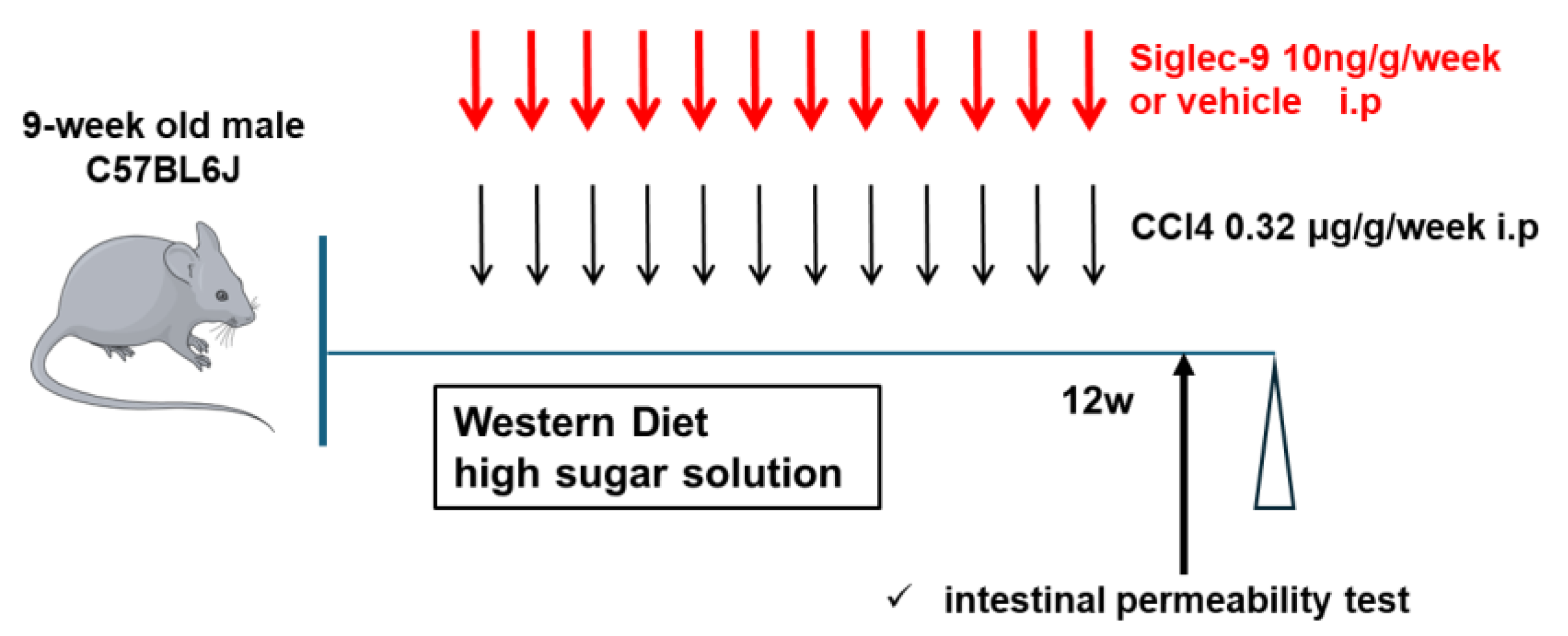
2.3. Murine MASH Model and Treatment with sSiglec-9
2.4. Intestinal Permeability
2.5. Histological Analyses
2.6. RNA Sequencing Analysis
2.7. Analysis of Gut Microbiota
2.8. Statistical Analysis
3. Results
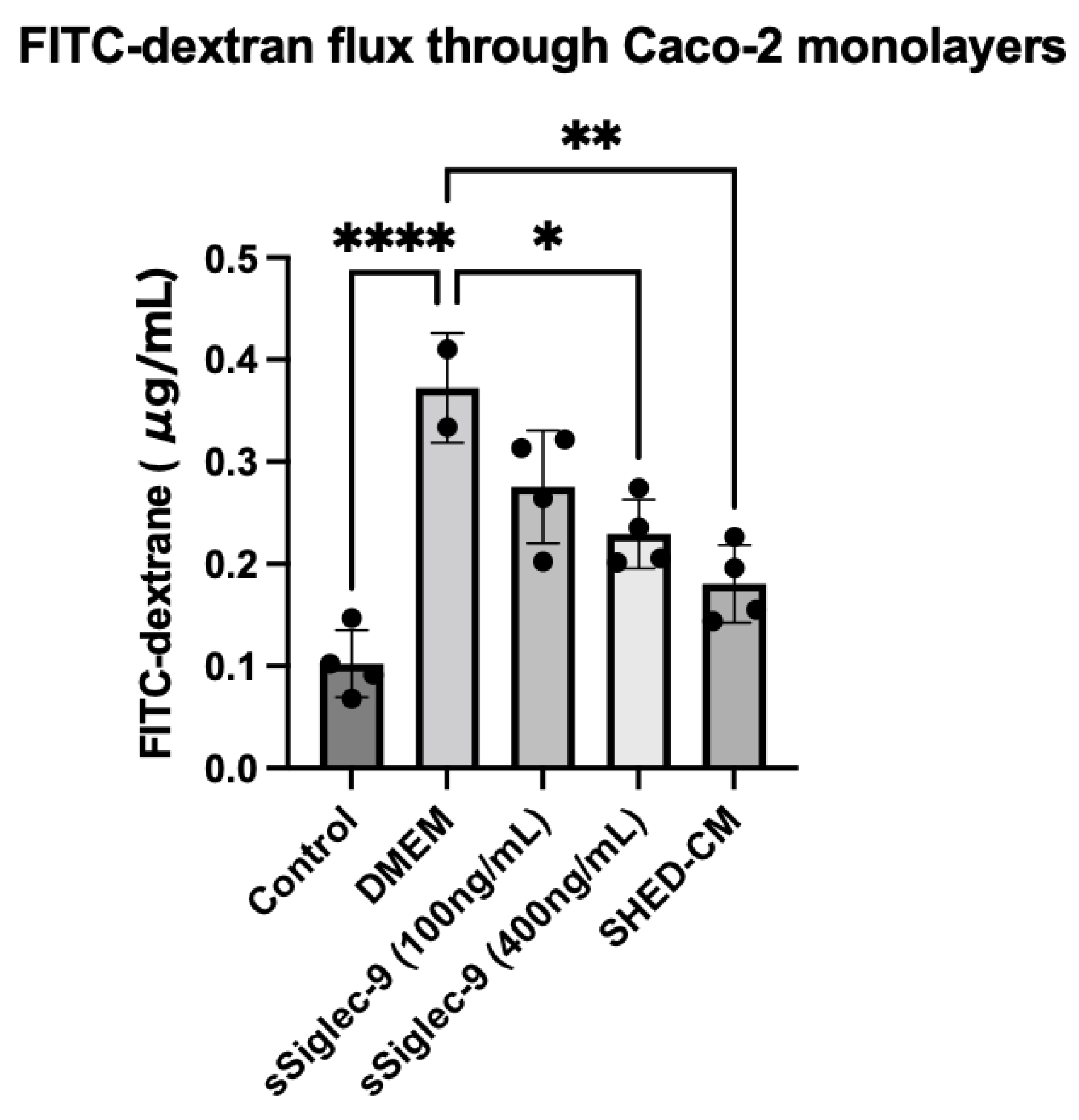
3.1. sSiglec-9 Restores Caco-2 Monolayer Dysfunction In Vitro
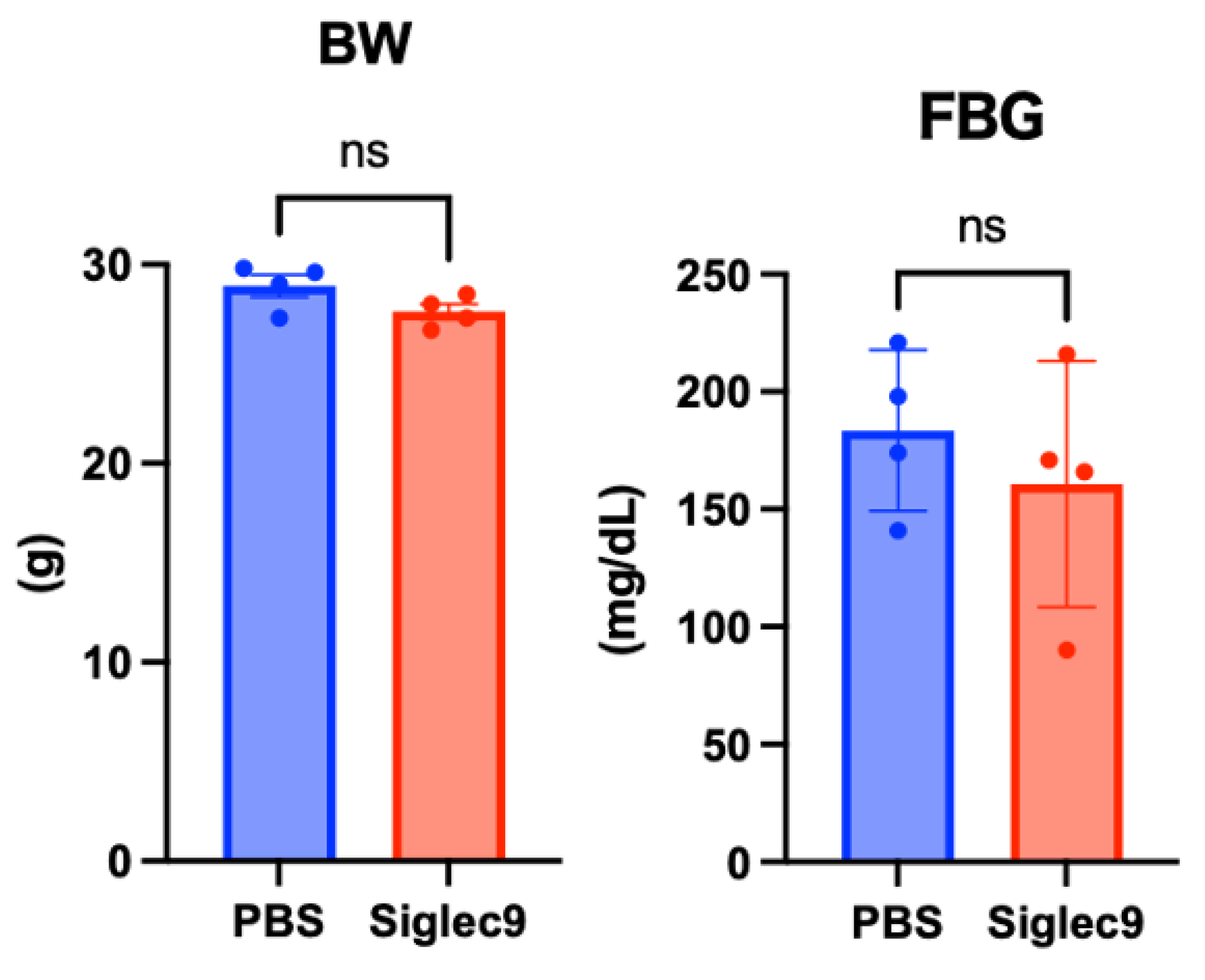
3.2. Establishment of the Murine MASH Model
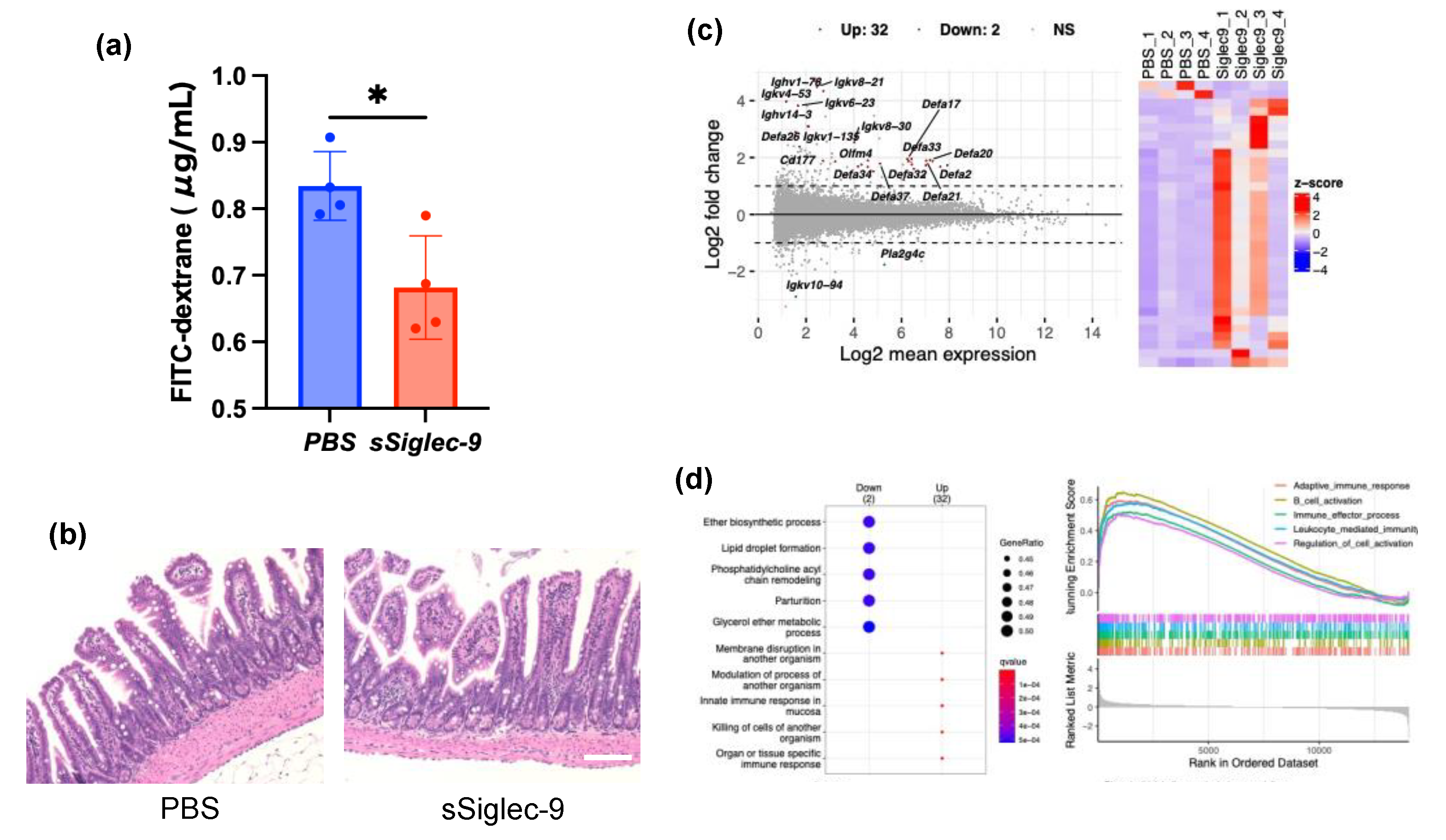
3.3. sSiglec-9 Protects the Intestinal Barrier in the MASH Mouse Model
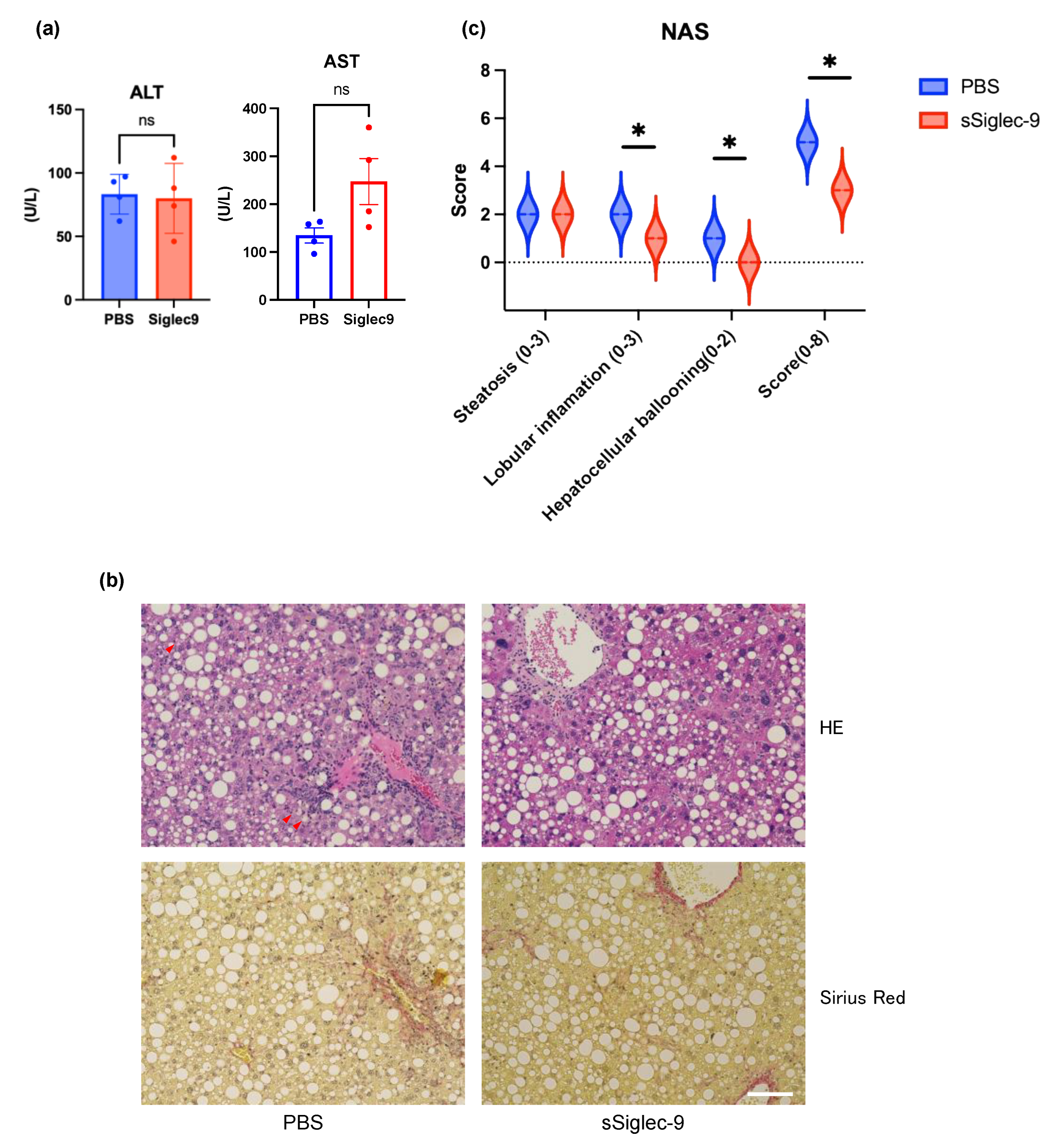
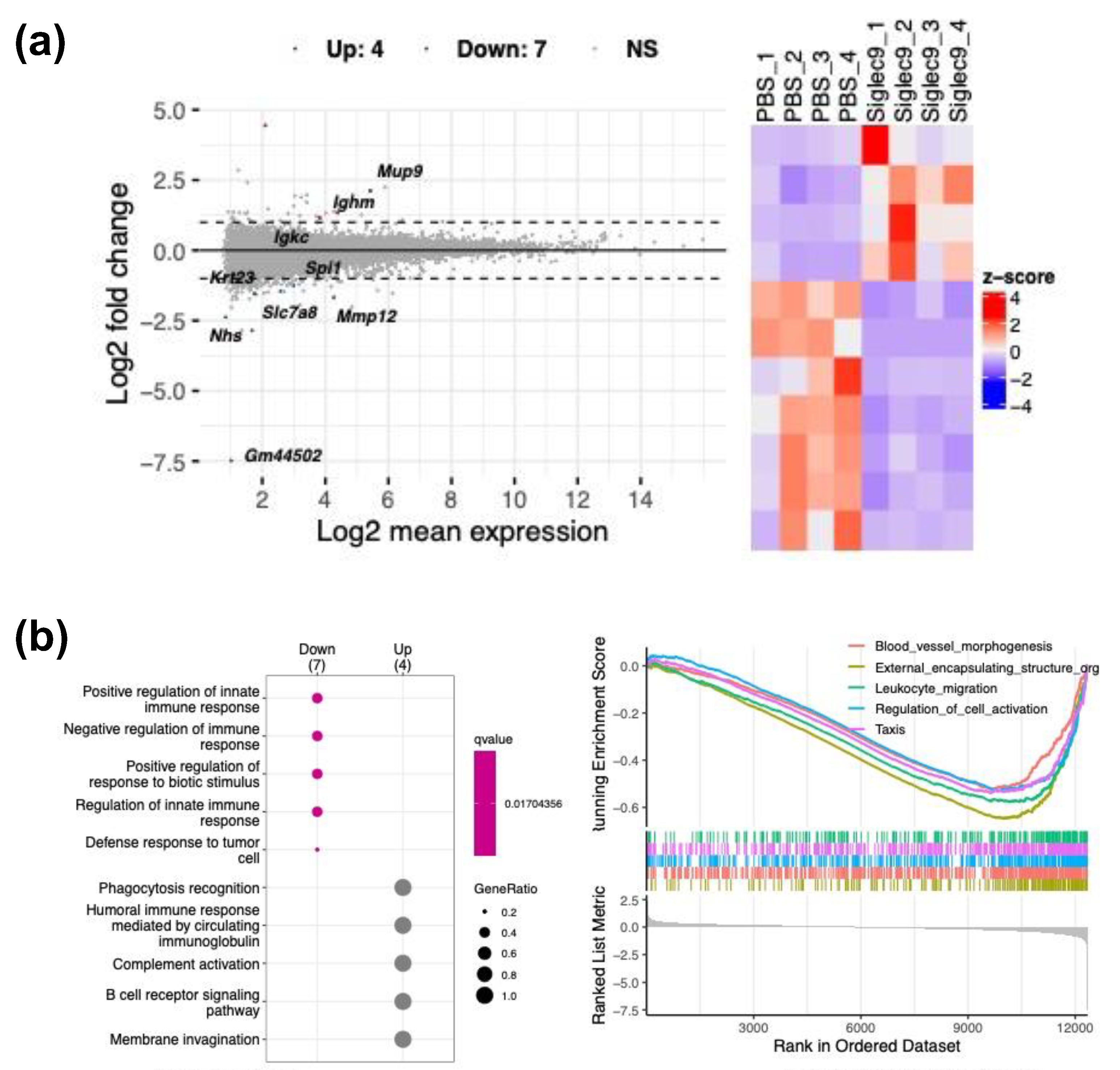
3.4. sSiglec-9 Attenuates Liver Inflammation in the MASH Mouse Model
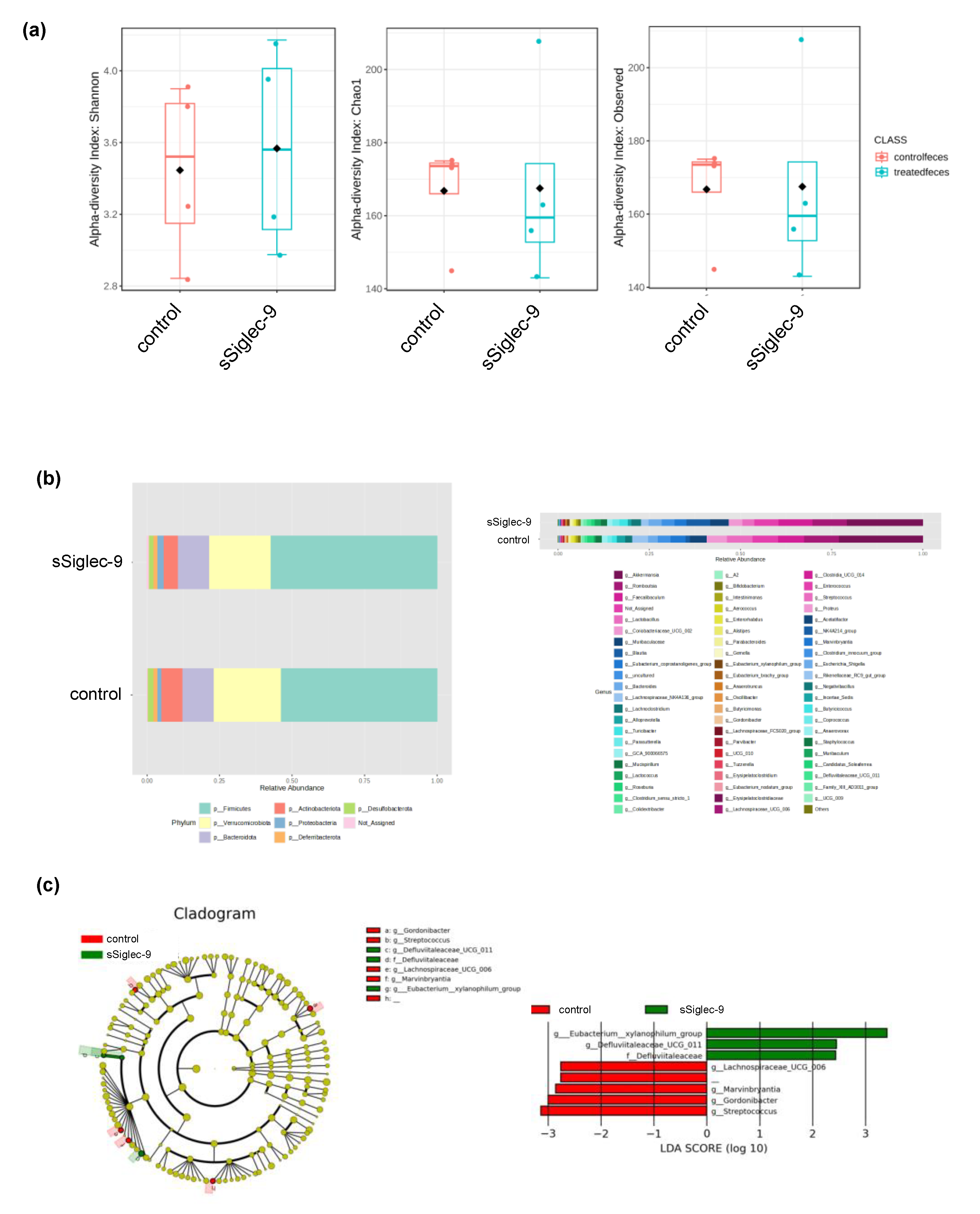
3.5. sSiglec-9 Treatment Does Not Alter Diversity of the Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BW | Body weight |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| DEG | Differentially expressed gene |
| DMEM | Dulbecco's Modified Eagle's Medium |
| FBG | Fasting blood glucose |
| FITC | Fluorescein isothiocyanate |
| GSEA | Gene set enrichment analysis |
| H&E | Hematoxylin–eosin |
| HGF | Hepatocyte growth factor |
| LEfSe | Linear discriminant analysis effect size |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Non-alcoholic fatty liver disease |
| NAS | NAFLD activity score |
| PBS | Phosphate-buffered saline |
| SCFA | Short-chain fatty acids |
| SHED | Stem cells from human exfoliated deciduous teeth |
| SHED-CM | Stem cells from human exfoliated deciduous teeth-conditioned media |
| Siglecs | Sialic acid-binding immunoglobulin-like lectins |
| sSiglec-9 | Soluble Siglec-9 |
References
- Tateishi, R.; Uchino, K.; Fujiwara, N.; Takehara, T.; Okanoue, T.; Seike, M.; Yoshiji, H.; Yatsuhashi, H.; Shimizu, M.; Torimura, T.; et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J Gastroenterol 2019, 54, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Akuta, N.; Hikita, H.; Suda, G.; Inoue, J.; Tamaki, N.; Ito, K.; Akahane, T.; Kawaoka, T.; Morishita, A.; et al. Etiological changes of liver cirrhosis and hepatocellular carcinoma-complicated liver cirrhosis in Japan: Updated nationwide survey from 2018 to 2021. Hepatol Res 2024, 54, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023, 77, 1335. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-art review. J Obes Metab Syndr 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Yu, J.; Shen, J.; Sun, T.T.; Zhang, X.; Wong, N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin Cancer Biol 2013, 23, 483–491. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Stefan, N.; Yki-Järvinen, H.; Neuschwander-Tetri, B.A. Metabolic Dysfunction-Associated Steatotic Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol 2025, 13, 134–148. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Muto, H.; Ito, T.; Tanaka, T.; Yokoyama, S.; Yamamoto, K.; Imai, N.; Ishizu, Y.; Maeda, K.; Honda, T.; Ishikawa, T.; et al. Conditioned medium from stem cells derived from human exfoliated deciduous teeth ameliorates NASH via the Gut-Liver axis. Sci Rep 2021, 11, 18778. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsushita, Y.; Sakai, K.; Kano, F.; Kondo, M.; Noda, M.; Hashimoto, N.; Imagama, S.; Ishiguro, N.; Suzumura, A.; et al. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity. J Neurosci 2015, 35, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishigami, M.; Matsushita, Y.; Hirata, M.; Matsubara, K.; Ishikawa, T.; Hibi, H.; Ueda, M.; Hirooka, Y.; Goto, H.; et al. Secreted ectodomain of SIGLEC-9 and MCP-1 synergistically improve acute liver failure in rats by altering macrophage polarity. Sci Rep 2017, 7, 44043. [Google Scholar] [CrossRef]
- Kang, E.A.; Soh, H.; Park, S.; Lee, H.J.; Im, J.P.; Kim, J.S. Soluble Siglec-9 alleviates intestinal inflammation through inhibition of the NF-κB pathway. Int Immunopharmacol 2020, 86, 106695. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat Rev Immunol 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu Rev Immunol 2012, 30, 357. [Google Scholar] [CrossRef]
- Kukan, E.N.; Fabiano, G.L.; Cobb, B.A. Siglecs as modulators of macrophage phenotype and function. Semin Immunol 2024, 73. [Google Scholar] [CrossRef]
- Wang, J.H.S.; Jiang, N.; Jain, A.; Lim, J. Development of effective Siglec-9 antibodies against cancer. Curr Oncol Rep 2023, 25, 41–49. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, W.; Xie, Y.; Wang, C.; Luo, N.; Chen, Y.; Wang, L.; Cheng, Z.; Gao, Z.; Liu, S. Siglec-9, a putative immune checkpoint marker for cancer progression across multiple cancer types. Front Mol Biosci 2022, 9, 743515. [Google Scholar] [CrossRef]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 2012, 122, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Kamzolas, I.; Harder, L.M.; Oakley, F.; Trautwein, C.; Hatting, M.; Ross, T.; Bernardo, B.; Oldenburger, A.; Hjuler, S.T.; et al. An unbiased ranking of murine dietary models based on their proximity to human metabolic dysfunction-associated steatotic liver disease (MASLD). Nat Metab 2024, 6, 1178–1196. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.-I.; Hoshida, Y.; et al. A simple diet-and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol 2018, 69, 385–395. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Wu, H.; Li, H.; Liu, L.; Guo, J.; Li, C.; Shih, D.Q.; Zhang, X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol 2012, 12, 57. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Prieto, C.; Barrios, D. RaNA-Seq: interactive RNA-Seq analysis from FASTQ files to functional analysis. Bioinformatics 2020, 36, 1955–1956. [Google Scholar] [CrossRef]
- Etoh, K.; Nakao, M. A web-based integrative transcriptome analysis, RNAseqChef, uncovers the cell/tissue type-dependent action of sulforaphane. J Biol Chem 2023, 299, 104810. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Maeda, K.; Ohashi, A.; Urano, T.; Nariai, Y.; Kamino, H.; Nakamura, M.; Yamamura, T.; Sawada, T.; Ishikawa, E.; et al. Monoclonal antibodies against mature interleukin-18 ameliorate colitis and repair goblet cell function. Dig Dis Sci 2024, 69, 2573–2585. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019, 37, 852. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol 2011, 12, R60. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res 2023, 51, W310–W318. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishigami, M.; Zou, B.; Tanaka, T.; Takahashi, H.; Kurosaki, M.; Maeda, M.; Thin, K.N.; Tanaka, K.; Takahashi, Y.; et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995–2040. Hepatol Int 2021, 15, 366–379. [Google Scholar] [CrossRef]
- Tincopa, M.A.; Anstee, Q.M.; Loomba, R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab 2024, 36, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Moreno-Navarrete, J.M. The relevance of intestinal barrier dysfunction, antimicrobial proteins and bacterial endotoxin in metabolic dysfunction-associated steatotic liver disease. Eur J Clin Invest 2024, 54. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat Rev Immunol 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Kukan, E.N.; Fabiano, G.L.; Cobb, B.A. Siglecs as modulators of macrophage phenotype and function. Semin Immunol 2024, 73, 101887. [Google Scholar] [CrossRef]
- Crouch, L.I.; Rodrigues, C.S.; Bakshani, C.R.; Tavares-Gomes, L.; Gaifem, J.; Pinho, S.S. The role of glycans in health and disease: regulators of the interaction between gut microbiota and host immune system. Semin Immunol 2024, 73, 101891. [Google Scholar] [CrossRef]
- Avril, T.; Floyd, H.; Lopez, F.; Vivier, E.; Crocker, P.R. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and-9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol 2004, 173, 6841–6849. [Google Scholar] [CrossRef]
- Favier, B. Regulation of neutrophil functions through inhibitory receptors: an emerging paradigm in health and disease. Immunol Rev 2016, 273, 140–155. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and regulation of defensins in host defense. Signal Transduct Target Ther 2023, 8, 1–30. [Google Scholar] [CrossRef]
- Nakamura, S.; Nakamura, K.; Yokoi, Y.; Shimizu, Y.; Ohira, S.; Hagiwara, M.; Song, Z.; Gan, L.; Aizawa, T.; Hashimoto, D.; et al. Decreased Paneth cell α-defensins promote fibrosis in a choline-deficient L-amino acid-defined high-fat diet-induced mouse model of nonalcoholic steatohepatitis via disrupting intestinal microbiota. Sci Rep 2023, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simms, L.A.; Doecke, J.D.; Walsh, M.D.; Huang, N.; Fowler, E. V.; Radford-Smith, G.L. Reduced α-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn's disease. Gut 2008, 57, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Itoh, K.; Hirohata, S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol 1995, 154, 4341–4350. [Google Scholar] [CrossRef]
- Paul, G.; Khare, V.; Gasche, C. Inflamed gut mucosa: downstream of interleukin-10. Eur J Clin Invest 2012, 42, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Huang, H.S.; Lin, Y.E.; Panyod, S.; Chen, R.A.; Lin, Y.C.; Chai, L.M.X.; Hsu, C.C.; Wu, W.K.; Lu, K.H.; Huang, Y.J.; et al. Anti-depressive-like and cognitive impairment alleviation effects of Gastrodia elata Blume water extract is related to gut microbiome remodeling in ApoE−/− mice exposed to unpredictable chronic mild stress. J Ethnopharmacol 2023, 302, 115872. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S. Gut microbiota and immune mediation: a Mendelian randomization study on granulomatosis with polyangiitis. Front Immunol 2023, 14, 1296016. [Google Scholar] [CrossRef]
- Cui, G.; Li, S.; Ye, H.; Yang, Y.; Jia, X.; Lin, M.; Chu, Y.; Feng, Y.; Wang, Z.; Shi, Z.; et al. Gut microbiome and frailty: insight from genetic correlation and mendelian randomization. Gut Microbes 2023, 15. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishigami, M.; Honda, T.; Takeyama, T.; Ito, T.; Ishizu, Y.; Kuzuya, T.; Hayashi, K.; Goto, H.; Hirooka, Y. Influence of proton pump inhibitors on microbiota in chronic liver disease patients. Hepatol Int 2019, 13, 234–244. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Ishigami, M.; Matsushita, Y.; Ito, T.; Hattori, H.; Hibi, H.; Goto, H.; Ueda, M.; Yamamoto, A. Multifaceted therapeutic benefits of factors derived from dental pulp stem cells for mouse liver fibrosis. Stem Cells Transl Med 2016, 5, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




