1. Introduction
Meibomian gland dysfunction (MGD) constitutes the primary etiology of dry eye disease in clinical practice [
1]. This condition, characterized as an evaporative form of dry eye, results from terminal duct obstruction of the Meibomian glands, leading to alterations in the lipid layer and lid margin integrity. Patients with MGD experience diminished meibum secretion with increased viscosity, and lid margin keratinization. The consequent tear film instability compromises ocular surface wetting, precipitating evaporative dry eye syndrome and triggering inflammatory processes that further exacerbate patient discomfort and visual disturbance [
2,
3]. Understanding these pathophysiological mechanisms is essential for the development of targeted therapeutic interventions which will address both the mechanical obstruction and inflammatory components of this prevalent condition.
Changes in meibum secretion can lead to increased colonization of micro-organisms along the lid margin, which may in turn contribute to infections [
4]. The initial approach to treatment usually involves conservative measures such as applying warm compresses, performing eyelid massage, and maintaining eyelid hygiene with cleansers that include tea extract combined with the use of artificial tears [
5,
6]. However, it remains a subject of ongoing discussion whether the underlying cause of MGD is an infection at the lid margin or if the infection develops as a consequence of MGD [
7].
Antibiotics play an important role in the treatment of both blepharitis and MGD because bacteria produce pro-inflammatory substances that can worsen the condition [
8]. Tetracycline antibiotics (such as minocycline and doxycycline) are frequently used, as they not only inhibit bacterial growth but also reduce inflammation by suppressing bacterial lipase activity and controlling pro-inflammatory mediator production [
9,
10]. Azithromycin, a macrolide antibiotic, works in a similar way by limiting bacterial growth, reducing the release of lipase, and modulating pro-inflammatory molecules. It also helps to regulate the function and secretion of the Meibomian glands. Azithromycin can be administered both topically and orally [
11,
12].
Intense Pulsed Light (IPL) therapy offers another treatment modality. It utilizes a high-intensity, non-coherent light source (with wavelengths ranging from 500 to 1200 nm) that stimulates collagen production, targets and destroys abnormal blood vessels, and helps reduce the viscosity of meibum through heat. Furthermore, IPL has been shown to be effective against Demodex mites [
13]. A further treatment option is Cyclosporine A, available as a 0.05% topical emulsion, which is a calcineurin inhibitor that was the first drug approved by the FDA for treating dry eye disease [
6].
The aim of this study was to evaluate the effectiveness of various treatment approaches for MGD by assessing clinical parameters, to determine which treatment method is most effective and best enhances patient comfort.
2. Materials and Methods
The study included 92 cases diagnosed with MGD who presented to Kahramanmaraş Necip Fazıl City Hospital in February 2025. All the study procedures complied with the principles of the Helsinki Declaration. Approval for the study was granted by the Ethics Committee of Harran University (decision no: HRÜ/25.03.33). Informed consent was obtained from all the patients.
All the patients underwent a comprehensive ophthalmological assessment, comprising slit-lamp biomicroscopic examination, detailed evaluation of anterior segment structures and fundoscopic examination, determination of best-corrected visual acuity, and measurement of intraocular pressure using applanation tonometry.
Obstructive MGD was diagnosed based on the Japanese MGD diagnostic criteria [
14]. The diagnosis was confirmed by the presence of ocular symptoms together with abnormal findings in the Meibomian gland orifices such as increased vascularity or the detection of meibomian gland orifice obstruction, as evidenced by plugging and reduced meibum secretion with the application of moderate digital pressure.
Exclusion criteria for the study were defined as signs of allergic or infectious conjunctivitis, significant refractive errors, ectatic corneal conditions, the presence of systemic diseases such as diabetes mellitus and hypertension, systemic rheumatological diseases, a history of prior ophthalmic surgery, or current contact lens usage. Care was taken to ensure that none of the patients had received any eye treatment in the last three months.
Following a comprehensive ophthalmologic evaluation, standardized assessments were made including the Ocular Surface Disease Index (OSDI) and the Standard Patient Evaluation for Eye Dryness (SPEED) scores. The Tear Break-Up Time (TBUT) test was applied and detailed lid margin evaluations were performed to assess ocular surface integrity. All patients were subsequently recalled for a one-month follow-up examination, during which the initial assessment protocols were repeated to monitor clinical response and treatment efficacy.
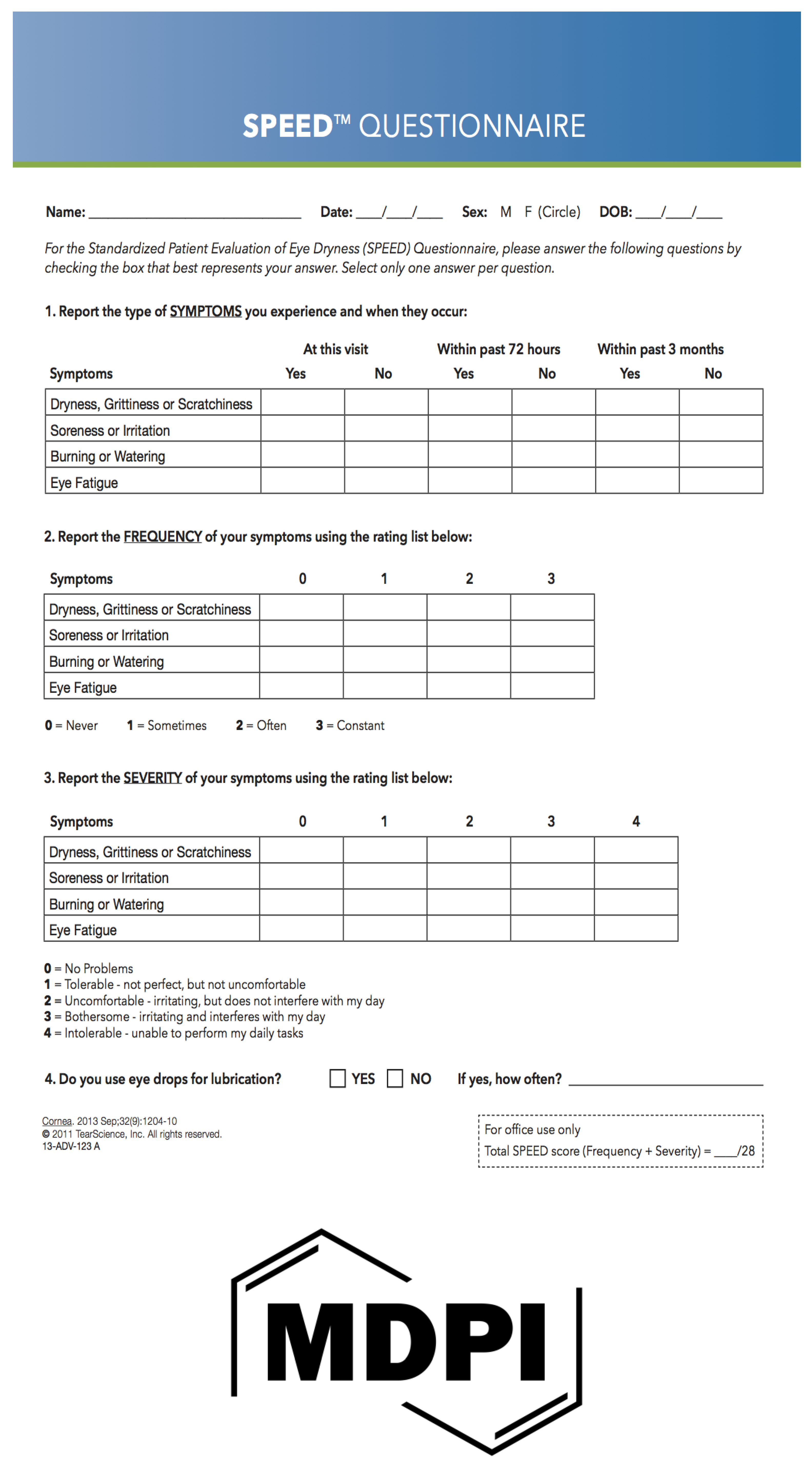
To quantitatively assess the impact of dry eye on quality of life, the 12-item Ocular Surface Disease Index (OSDI), and the 4-item Standard Patient Evaluation for Eye Dryness (SPEED) questionnaire, were administered to all the subjects (see Supplementary Table S1 and Figure 1).
Tear film stability was evaluated using the standardized TBUT assessment. Following instillation of 1% fluorescein dye into the conjunctival sac, the time interval between the last complete blink and the first appearance of a corneal black spot in the stained tear film was measured under cobalt blue illumination. To ensure reliability, three consecutive measurements were obtained for each subject, and the mean value was calculated and recorded as the definitive TBUT
Lid margin findings were assessed with slit-lamp examination using standardized grading scales. Telangiectasia was evaluated on a scale of 0–3, where 0 indicates no findings, 1 represents mild telangiectasia, 2 corresponds to moderate telangiectasia, and 3 denotes severe telangiectasia. The mucocutaneous junction was similarly graded on a scale of 0–3.
For the Marx line (ML) score, 1% fluorescein drops were instilled on the lower eyelid, and the patient was instructed to blink several times. The lower eyelid was then partitioned into three regions — outer, middle, and inner — and points were assigned based on the extent of fluorescein contact with the Meibomian orifices (MOs). Specifically, an ML score of 0 was assigned when the Marx line courses on the skin side of the MO line without contacting the orifices, 1 when portions of the Marx line touch the MOs, 2 when the Marx line passes directly through the MOs, and 3 when the Marx line courses along the eyelid margin side of the MOs. Lid irregularity, plugging, and foaming were each graded on a scale of 0–2, where 0 signifies no findings, 1 reflects mild findings, and 2 indicates severe findings [
14,
15].
The expressibility of meibum from the central area of both the upper and lower eyelids was evaluated semi-quantitatively using a scale of 0–3: 0 indicated that clear meibum was readily expressed; 1 that cloudy meibum was expressed with mild pressure; 2 that cloudy meibum required more than moderate pressure for expression; and 3 that meibum could not be expressed even under strong pressure [
16,
17].
For the IPL procedure, the Eye-light® device (Topcon) was utilized. Initially, 0.5% proparacaine anesthetic eye drops were instilled in both eyes. The treatment involved administering a series of 20 overlapping light pulses to the targeted periorbital skin, extending from the right temple across the lower eyelid, including the nasal bridge, and continuing to the left temple along the lower eyelid. A 590 nm filter in combination with a 6 mm cylindrical light guide was employed on the handpiece.
The patients diagnosed with MGD were stratified into five distinct groups based on the treatment modalities, all of which were applied for one month.
In Group 1, patients received conservative management only. All the subjects were prescribed preservative-free artificial tears (4 times daily) containing polyvinyl alcohol and povidone (Novaqua, DEVA Holding Inc., Turkey). A comprehensive conservative regimen was recommended, which included the application of warm compresses, performing eyelid massage prior to bedtime, and cleansing the eyelashes with an eyelash shampoo.
In Group 2, patients were administered oral doxycycline 100 mg (Monodox, DEVA Holding Inc., Turkey) as an adjunct to the conservative management regimen. The dosage was structured with twice-daily administration during the initial week, then once daily for the subsequent three weeks, providing a treatment duration of one month.
In Group 3, oral azithromycin 500 mg (Azitro, DEVA Holding Inc., Turkey, 500 mg) was administered as a single daily dose for three consecutive days in conjunction with the conservative treatment regimen.
In Group 4, topical cyclosporine 0.05% ophthalmic emulsion (Ocurin, Bilim Pharmaceuticals, Turkey) was prescribed as an adjunctive therapy to the conservative treatment protocol. The cyclosporine drops were administered twice daily.
Together with the conservative management, the IPL protocol was implemented on days 0, 15, and 30.
Data obtained in the study were analyzed statistically using SPSS version 27 software. The suitability of the variables to normal distribution was examined with Kolmogorov-Smirnov/Shapiro Wilk tests. Descriptive statistics were expressed as mean±standard deviation (SD) or median values for continuous data and as number (n) and percentage (%) for categorical data. For values not showing normal distribution, the Wilcoxon test was applied. A value of p<0.05 was considered statistically significant.
3. Results
The 92 subjects enrolled in this study, comprised 50 (64.2%) females and 40 (35.8%) males with a mean age of 45.16 ± 7.28 years.
Group 1 (conservative treatment only) included 18 subjects (6 males, 12 females) with a mean age of 46.67 years. Group 2 (doxycycline) included 22 subjects (8 males, 14 females) with a mean age of 45.38 years. Group 3 (azithromycin) included 18 subjects (6 males, 12 females) with a mean age of 46.44 years. Group 4 (cyclosporine) included 18 subjects (5 males, 13 females) with a mean age of 47.11 years. Group 5 (IPL) included 16 subjects (5 males, 11 females) with a mean age of 43.88 years. No statistically significant differences were observed in age and gender distributions among the treatment groups (p>0.05).
Group 1: The TBUT values increased slightly from 9.00±2.67 seconds to 9.94±2.41 seconds. The lid vascularity scores decreased significantly from 1.67±0.84 to 1.06±0.80 (p=0.014). Meibomian gland plugging was determined to have improved slightly from 1.11±0.58 to 0.94±0.41. Lid margin irregularity demonstrated a significant reduction from 0.67±0.48 to 0.33±0.48 (p=0.014). The foaming scores decreased from 0.89±1.56 to 0.44±0.51. Marx line scores showed a significant improvement from 4.28±0.89 to 3.33±1.18 (p=0.003). The meibum grade improved slightly from 1.67±0.48 to 1.50±0.51. Patient-reported outcomes were seen to be significantly improved, with OSDI scores decreasing from 51.67±45.50 to 38.89±8.83 (p<0.01), and SPEED scores reducing from 0.46±0.15 to 0.29±0.12 (p<0.01).
Group 2: The TBUT values increased significantly from 8.15±3.23 to 10.58±3.11 seconds (p<0.01). a statistically significant decrease was determined in the lid vascularity values from 1.46±0.70 to 2.08± 0.93(p<0.01), and in the Meibomian gland plugging values from 1.54±0.76 to 1.12±0.43 (p<0.01). Lid margin irregularity improved from 0.85±0.50 to 0.58±0.50, foaming decreased from 0.85±0.96 to 0.58±0.75, and the Marx line scores improved from 5.46±1.74 to 4.62±1.09. Statistically significant improvements were seen in Meibum grade from 2.00±0.66 to 1.54±0.58 (p<0.01), in OSDI from 46.77±16.22 to 33.77±8.37 (p<0.01) and in SPEED from 0.55±0.14 to 0.34±0.18 (p<0.01).
Group 3: A statistically significant increase was determined in TBUT from 6.83±1.24 to 9.00±1.28 seconds (p<0.01). Lid vascularity decreased from 2.00±0.97 to 1.33±0.68 (p=0.007), and Meibomian gland plugging from 1.44±0.70 to 1.00 (p=0.023). Lid margin irregularity improved from 1.11±0.32 to 0.89±0.58 (p=0.046), foaming decreased from 0.89±0.58 to 0.56±0.51 (p=0.00), and the Marx line score showed a slight improvement from 5.89±1.45 to 5.50±1.65. The Meibum grade improved from 1.89±0.58 to 1.44±0.51. statistically significant improvements were seen in the patient-reported outcomes, with a decrease in OSDI from 41.11±18.69 to 27.33±7.91 (p=0.003) and in SPEED from 0.48±0.21 to 0.32±0.17 (p=0.002).
Group 4: The decrease in TBUT from 11±1.32 to 9.17±0.98 seconds was statistically significant (p<0.01). Lid vascularity showed significant improvement from 1.78±0.94 to 1.28±0.75 (p=0.014). Meibomian gland plugging significantly reduced from 1.11±0.32 to 0.89±0.58 (p=0.046), lid margin irregularity slightly increased from 0.33±0.33 to 0.44±0.51, and foaming remained stable at 0.33±0.48. The Marx line score showed a minimal improvement from 4.00±0.84 to 3.89±1.27. A significant improvement was observed in the Meibum grade from 1.78±0.64 to 1.44±0.70 (p=0.014). Patient-reported outcomes showed significant improvements, with a decrease in OSDI from 52.33±14.66 to 40.33±9.44 (p<0.01) and in SPEED from 0.46±0.15 to 0.29±0.12 (p<0.01).
Group 5: A statistically significant increease was determined in TBUT from 7.20±1.13 seconds to 10.40±0.51 seconds (p=0.004). Lid vascularity scores decreased from 2.00 to 1.33±0.97, and Meibomian gland plugging reduced from 1.40±0.51 to 1.00±0.94 (p=0.046). Pre and post-treatment lid margin irregularity remained stable at 0.80±0.78, and foaming scores were maintained at 0.20±0.42. A statistically significant improvement was observed in the Marx line scores declining from 4.80±1.22 to 3.60±0.51 (p=0.014), and in Meibum grade decreasing from 2.00±0.66 to 1.60±0.84 (p=0.046). Patient-reported outcomes also improved substantially, with a decrease in OSDI scores from 47.20±20.06 to 19.60±7.00 and in SPEED from 0.62±0.13 to 0.34±0.07.
3.1. Figures, Tables and Schemes
Figure 1.
Speed Questionnaire.
Figure 1.
Speed Questionnaire.
Table 1.
Comparisons of Pre-treatment and Post-treatment Clinical Parameters in Patients with Meibomian Gland Dysfunction Across Different Treatments.
Table 1.
Comparisons of Pre-treatment and Post-treatment Clinical Parameters in Patients with Meibomian Gland Dysfunction Across Different Treatments.
| |
Group 1- conservative treatment only |
Group 2- conservative treatment + oral doxycycline |
Group 3- conservative treatment + oral azithromycin |
Group 4- conservative treatment + oral cyclosporine |
Group 5- conservative treatment + IPL therapy |
| Clinical Parameters |
Pre-treatment
(mean±sd) |
Post-treatment
(mean±sd) |
Pre- treatment
(mean±sd) |
Post- treatment
(mean±sd) |
Pre- treatment
(mean±sd) |
Post- treatment
(mean±sd) |
Pre- treatment
(mean±sd) |
Post- treatment
(mean±sd) |
Pre- treatment
(mean±sd) |
Post- treatment
(mean±sd) |
| Lid vascularity |
1.67±0.84 |
1.06±0.80 |
1.46±0.70 |
2.08± 0.93
|
2.00±0.97 |
1.33±0.68 |
1.78±0.94 |
1.28±0.75 |
2.00±0.97 |
1.33±0.97 |
| Plugging |
1.11±0.58 |
0.94±0.41
|
1.54±0.76 |
1.12±0.43
|
1.44±0.70 |
1.00±0.00 |
1.11±0.32 |
0.89±0.58 |
1.40±0.51 |
1.00±0.94 |
| Lid margin irregularity |
0.67±0.48 |
0.33±0.48
|
0.85±0.50 |
0.58±0.50 |
1.11±0.32 |
0.89±0.58
|
0.33±0.33 |
0.44±0.51 |
0.80±0.78 |
0.80±0.78 |
| Foaming |
0.89±1.56 |
0.44±0.51 |
0.85±0.96 |
0.58±0.75 |
0.89±0.58 |
0.56±0.51 |
0.33±0.48 |
0.33±0.48 |
0.20±0.42 |
0.20±0.42 |
| Marx line score |
4.28±0.89 |
3.33±1.18
|
5.46±1.74 |
4.62±1.09
|
5.89±1.45 |
5.50±1.65
|
4.00±0.84 |
3.89±1.27
|
4.80±1.22 |
3.60±0.51 |
| Meibum grade |
1.67±0.48 |
1.50±0.51
|
2.38±0.46 |
1.54±0.58
|
1.89±0.58 |
1.44±0.51
|
1.78±0.64 |
1.44±0.70
|
2.00±0.66 |
1.60±0.84 |
| OSDI |
51.67±45.50 |
38.89±8.83
|
46.77±16.22
|
33.77±8.37 |
41.11±18.69 |
27.33±7.91
|
52.33±14.66 |
40.33±9.44
|
47.20±20.06 |
19.60±7 |
| Speed |
0.46±0.15 |
0.29±0.12
|
0.55±0.14 |
0.34±0.18
|
0.48±0.21 |
0.32±0.17
|
0.54±0.15 |
0.37±0.11 |
0.62±0.13 |
0.34±0.07 |
| TBUT |
9.00±2.67 |
9.94±2.41 |
8.15±3.23 |
10.58±3.11
|
6.83±1.24 |
9.00±1.28 |
7.11±1.32 |
9.17±0.98
|
7.20±1.13 |
10.40±0.51 |
Table 2.
Grading scales for the evaluation criteria of Meibomian gland dysfunction.
Table 2.
Grading scales for the evaluation criteria of Meibomian gland dysfunction.
Lid vascularity
|
|
| |
|
0 |
None |
| 1 |
Redness of the palpebral conjunctiva with no vascularity around the gland orifices |
| 2 |
Redness of the palpebral conjunctiva with vascularity around the gland orifices affecting<50% of the full length of the lid margin |
| 3 |
Redness of the palpebral conjunctiva with vascularity around the gland orifices affecting≥50% of the full length of the lid margin |
Lid margin irregularity
|
|
| 0 |
None |
| 1 |
Fewer than three lid margin irregularities with shallow notching
|
| 2 |
Three or more lid margin irregularities or deep notching |
Foaming
|
|
| 0 |
None |
| 1 |
Mild findings |
| 2 |
Severe findings |
Lid plugging
|
|
| 0 |
None |
| 1 |
Plugging of <3 gland orifices |
| 2 |
Plugging of ≥3 gland orifices affecting<50% of the full length of the lid margin |
| 3 |
Plugging of ≥3 gland orifices affecting≥50% of the full length of the lid margin |
Marx line score
|
|
| 0 |
Marx line running entirely along the conjunctival side of the gland orifices |
| 1 |
Part of the Marx line in contact with the gland orifices |
| 2 |
Marx line running through the gland orifices |
| 3 |
Marx line running along the eyelid margin on the side of the gland orifices |
Meibum grade
|
|
| 0 |
Clear meibum is easily expressed |
| 1 |
Cloudy meibum is expressed with mild pressure |
| 2 |
Cloudy meibum is expressed with more than moderate pressure |
| 3 |
Meibum cannot be expressed even with the hard pressure |
4. Discussion
MGD is an increasingly prevalent ocular surface disorder, characterized by terminal duct obstruction and/or qualitative/quantitative changes in glandular secretion. This results in altered tear film lipid layers, evaporative dry eye, and chronic ocular surface inflammation. The multifactorial nature of MGD calls for equally diverse treatment approaches [
20,
21]. It was observed in the literature that while several studies have compared oral treatment modalities for MGD, no comparative studies have specifically evaluated the effectiveness of cyclosporine versus IPL therapy.
Conservative management (preservative-free artificial tears, warm compresses, eyelid massage, and eyelash cleansing) forms the foundation of MGD treatment, providing relief for mild cases by improving meibum properties and tear film stability. However, this approach alone often fails to address underlying glandular abnormalities in moderate-to-severe cases or those with persistent inflammation, necessitating adjunctive therapies to target the complex pathophysiology of MGD.
In Groups 2 and 3, adjunctive oral antibiotic therapy was evaluated. Doxycycline, a tetracycline antibiotic, exerts anti-inflammatory effects by inhibiting matrix metalloproteinases and modifying the lipid composition of meibum, which together reduce inflammation and enhance glandular function. In contrast, oral azithromycin provides the advantage of a shorter treatment course, improved patient compliance, and a lower incidence of adverse effects. The clinical distinction between these antibiotics is significant, as effective adjunctive antibiotic therapy hinges on both antimicrobial activity and the modulation of inflammatory pathways in MGD. The current study findings support previous studies that have reported that both treatments lead to improved TBUT, reduced meibomian gland plugging, and better subjective patient outcomes. There was also observed to be a greater improvement in eyelid symptoms with azithromycin than with doxycycline. Kashkouli et al. and Bukhari et al. compared oral azithromycin and doxycycline treatments, and reported that azithromycin was more effective on conjunctival redness and corneal staining [
22,
23]. In another study comparing topical azithromycin and oral doxycycline treatment, oral doxycycline treatment caused a greater prolongation of tear break-up time, whereas topical azithromycin treatment had a greater effect on symptoms [
24].
Topical cyclosporine was incorporated in the treatment of Group 4 in this study, reflecting an alternative therapeutic approach that leverages immunomodulation. Topical cyclosporine is known to suppress T-cell activity and reduce ocular surface inflammation [
6,
25]. Although primarily used in the management of chronic dry eye syndrome, its role in MGD is gaining recognition, particularly in cases where inflammation is a dominant feature. The statistically significant improvement in lid vascularity and meibum quality observed in this group suggests that localized immunomodulatory therapy can be effectively integrated into a comprehensive treatment regimen for MGD. These results are encouraging, especially for patients who might be contraindicated for systemic therapy or who experience adverse effects related to systemic antibiotics. Jeon et al. found that patients treated with a combination of cyclosporine and IPL showed reduced inflammatory markers and improved eyelid condition. These results hint at a possible synergistic effect between the two treatments, tackling inflammation and enhancing clinical signs in a complementary manner [
26]. Iaccheri et al. applied only artificial tear drop treatment to one group of patients with MGD-related dry eye and artificial tear drop treatment together with cyclosporine drops to the other group, and observed a significant improvement in meibum expression and quality from the first months in the group in which cyclosporine treatment was added [
27].
In Group 5 of the current study, which received adjunctive IPL therapy, significant clinical improvements were observed one month post-treatment. The thermal effects of IPL appear to liquefy meibum and reduce abnormal telangiectatic vessels, thereby modulating inflammatory mediators and enhancing the ocular surface microenvironment. In this study, IPL treatment was associated with decreased meibomian gland plugging, improved Marx line scores and meibum grade, and a longer TBUT. These results align with previous findings that support the efficacy of IPL in reducing MGD symptoms and improving both eyelid and gland function [
21].
Based on the pre- and post-treatment score reductions, slight improvements were seen in Group 1, which served as the baseline. In the comparisons, greater reductions in parameters related to tear film stability and gland obstruction were observed in Groups 2 and 3, reflecting the benefits of systemic anti-inflammatory and lipid-modulating effects. Significant improvements in inflammatory indicators and lid vascularity were determined in Group 4, highlighting the advantage of localized immunomodulation, and inflammatory markers without systemic effects, making this treatment suitable for patients with contraindications to oral antibiotics. The most significant overall score reductions especially in meibomian gland plugging, Marx line scores, and TBUT were obtained in Group 5, indicating that IPL therapy was more effective on both the obstructive and inflammatory components of MGD. While conservative management provides the basis of MGD treatment, adjunctive therapies can significantly enhance outcomes. Systemic antibiotics and topical cyclosporine were seen to improve tear film stability and reduce inflammation, whereas IPL therapy emerged as the most effective non-invasive modality for comprehensive management due to its superior impact on both obstructive and inflammatory components.
Limitations
As topical azithromycin is not available in Türkiye, this treatment modality could not be included, so no comparison could be made with oral azithromycin. A further limitation could be said to be that the one-month follow-up period may not have been sufficient to fully capture the long-term benefits of treatments such as topical cyclosporine, which has been shown to be more effective with extended use. Variations in follow-up periods in the literature also pose challenges for direct comparison of the current study findings with those of previous studies.
5. Conclusions
The results of this study demonstrate that while conservative management provides a baseline therapeutic effect in MGD, adjunctive therapies significantly enhance clinical outcomes. Oral antibiotics (doxycycline and azithromycin) improved tear film stability and reduced glandular obstruction, while topical cyclosporine effectively targeted ocular surface inflammation. Notably, IPL therapy emerged as the most efficacious adjunctive modality, providing greater improvements in both objective clinical parameters and subjective patient-reported outcomes. These findings support a personalized, multimodal approach to MGD management, with treatment selection guided by individual patient characteristics and disease severity. Further research with extended follow-up periods is warranted to establish long-term efficacy profiles of these interventions
Author Contributions
Each author meets all four of the ICMJE’s authorship criteria 2024. “Conceptualization, M.B. and A.H.R.; methodology, M.B.; software, M.B.; validation, A.H.R., G.Y. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
No financial support was received from any source for this study.
Institutional Review Board Statement
Ethical approval for this study was granted by the Harran University Clinical Research Ethics Committee (approval number HRÜ/25.03.33).
Acknowledgments
There is no sponsorship for this study.
Conflicts of Interest
The authors declare that they have no financial interests or potential conflict of interests related to the subject matter discussed in this article.
Appendix A
Supplementary Table 1
Table S1.
This is a table caption. Supplementary Table 1. OSDI.
Table S1.
This is a table caption. Supplementary Table 1. OSDI.
| Have you experienced any of the following during the last week: |
| |
All of the time |
Most of the time |
Half of the time |
Some of the time |
None of the time |
|
| 1. Eyes that are sensitive to light |
4 |
3 |
2 |
1 |
0 |
|
| 2. Eyes that feel gritty? |
4 |
3 |
2 |
1 |
0 |
|
| 3. Painful or sore eyes? |
4 |
3 |
2 |
1 |
0 |
|
| 4. Blurred vision? |
4 |
3 |
2 |
1 |
0 |
|
| 5. Poor vision? |
4 |
3 |
2 |
1 |
0 |
|
| Have problems with your eyes limited you in performing any of the following during the last week: |
| |
All of the time |
Most of the time |
Half of the time |
Some of the time |
None of the time |
|
| 6. Reading? |
4 |
3 |
2 |
1 |
0 |
N/A |
| 7. Driving at night? |
4 |
3 |
2 |
1 |
0 |
N/A |
| 8. Working with a computer or a bank machine(ATM)? |
4 |
3 |
2 |
1 |
0 |
N/A |
| 9. Watching TV? |
4 |
3 |
2 |
1 |
0 |
N/A |
| Have your eyes felt uncomfortable in any of the following situations during the last week: |
| |
All of the time |
Most of the time |
Half of the time |
Some of the time |
None of the time |
|
| 10. Windy conditions? |
4 |
3 |
2 |
1 |
0 |
N/A |
| 11. Places or areas with low humidity (very dry)? |
4 |
3 |
2 |
1 |
0 |
N/A |
| 12. Areas that are air conditioned? |
4 |
3 |
2 |
1 |
0 |
N/A |
References
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283. [CrossRef]
- Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929. Published 2011 Mar 30. [CrossRef]
- Suzuki T. Inflamed Obstructive Meibomian Gland Dysfunction Causes Ocular Surface Inflammation. Invest Ophthalmol Vis Sci. 2018;59(14):DES94-DES101. [CrossRef]
- Wladis EJ, Bradley EA, Bilyk JR, Yen MT, Mawn LA. Oral Antibiotics for Meibomian Gland-Related Ocular Surface Disease: A Report by the American Academy of Ophthalmology. Ophthalmology. 2016;123(3):492-496. [CrossRef]
- Romero JM, Biser SA, Perry HD, et al. Conservative treatment of meibomian gland dysfunction. Eye Contact Lens. 2004;30(1):14–19. [CrossRef]
- Geerling G, Tauber J, Baudouin C, et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Management and Treatment of Meibomian Gland Dysfunction. Investig Opthalmology Vis Sci. 2011;52(4):2050-2064. [CrossRef]
- Yan X, Qiao X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797-1803. [CrossRef]
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938-1978. [CrossRef]
- Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32(11):2970-2975.
- Duerden JM, Tiffany JM. Lipid synthesis in vitro by rabbit meibomian gland tissue and its inhibition by tetracycline. Biochim Biophys Acta - Lipids Lipid Metab. 1990;1042(1):13-18. [CrossRef]
- Foulks GN, Borchman D, Yappert M, Kakar S. Topical Azithromycin and Oral Doxycycline Therapy of Meibomian Gland Dysfunction: A Comparative Clinical and Spectroscopic Pilot Study. Cornea. 2013;32(1):44-53. [CrossRef]
- Zhang L, Su Z, Zhang Z, Lin J, Li D-Q, Pflugfelder SC. Effects of Azithromycin on Gene Expression Profiles of Proinflammatory and Anti-inflammatory Mediators in the Eyelid Margin and Conjunctiva of Patients With Meibomian Gland Disease. JAMA Ophthalmol. 2015;133(10):1117-1123. [CrossRef]
- Goldberg DJ. Current trends in intense pulsed light. J Clin Aesthet Dermatol. 2012;5(6):45-53.
- Amano S, MGD Working Group. Definition and diagnostic criteria for meibomian gland dysfunction. J Eye. 2010;27:627–31. (in Japanese).
- Van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969;82(1):10-14. [CrossRef]
- Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology. 1998;105(8):1485-1488. [CrossRef]
- Arita R, Minoura I, Morishige N, et al. Development of Definitive and Reliable Grading Scales for Meibomian Gland Dysfunction. Am J Ophthalmol. 2016;169:125-137. [CrossRef]
- Benitez-Del-Castillo JM, López-Pérez MD, Cano-Ortiz A, et al. Efficacy and safety of intense pulsed light of upper and lower eyelids in Meibomian gland dysfunction: A prospective multicentric study. Eur J Ophthalmol. 2024;34(3):700-707. [CrossRef]
- Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul Surf. 2019;17(1):104-110. [CrossRef]
- Sabeti S, Kheirkhah A, Yin J, Dana R. Management of meibomian gland dysfunction: a review. Surv Ophthalmol. 2020;65(2):205-217. [CrossRef]
- Jiang X, Lv H, Song H, et al. Evaluation of the Safety and Effectiveness of Intense Pulsed Light in the Treatment of Meibomian Gland Dysfunction. J Ophthalmol. 2016;2016:1910694. [CrossRef]
- Kashkouli MB, Fazel AJ, Kiavash V, Nojomi M, Ghiasian L. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015;99(2):199–204. [CrossRef]
- De Benedetti G, Vaiano A. Oral azithromycin and oral doxycycline for the treatment of Meibomian gland dysfunction: a 9-month comparative case series. Indian J Ophthalmol. 2019;67(4):464. [CrossRef]
- Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32(1):44-53. [CrossRef]
- Perry HD, Doshi-Carnevale S, Donnenfeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006;25(2):171-175. [CrossRef]
- Jeon YY, Bae S, Chung HS, Kim JY, Lee H. Effects of combined intense pulsed light and cyclosporine 0.05% eyedrops in ocular surface matrix metalloproteinase-9 levels in patients with moderate-to-severe MGD. Lasers Med Sci. 2024 Aug 1;39(1):203. [CrossRef] [PubMed]
- Iaccheri B, Torroni G, Cerquaglia A, et al. Evaluation of warm compresses and topical cyclosporine treatment in meibomian gland dysfunction by confocal scanning laser microscopy. Eur J Ophthalmol. Published online October 22, 2022. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).