Submitted:
13 April 2025
Posted:
14 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
Weight of dried materials
2.2. Polyphenol Identification
2.3. UV-Vis Analysis of Rutin
2.3.1. Preparation of the Standard Solution and Standard Curve
2.3.2. Total Polyphenol Content in the Rambutan Peel Extract
g
2.4. Analysis of Extracts Using an FTIR
2.5. Data Analysis
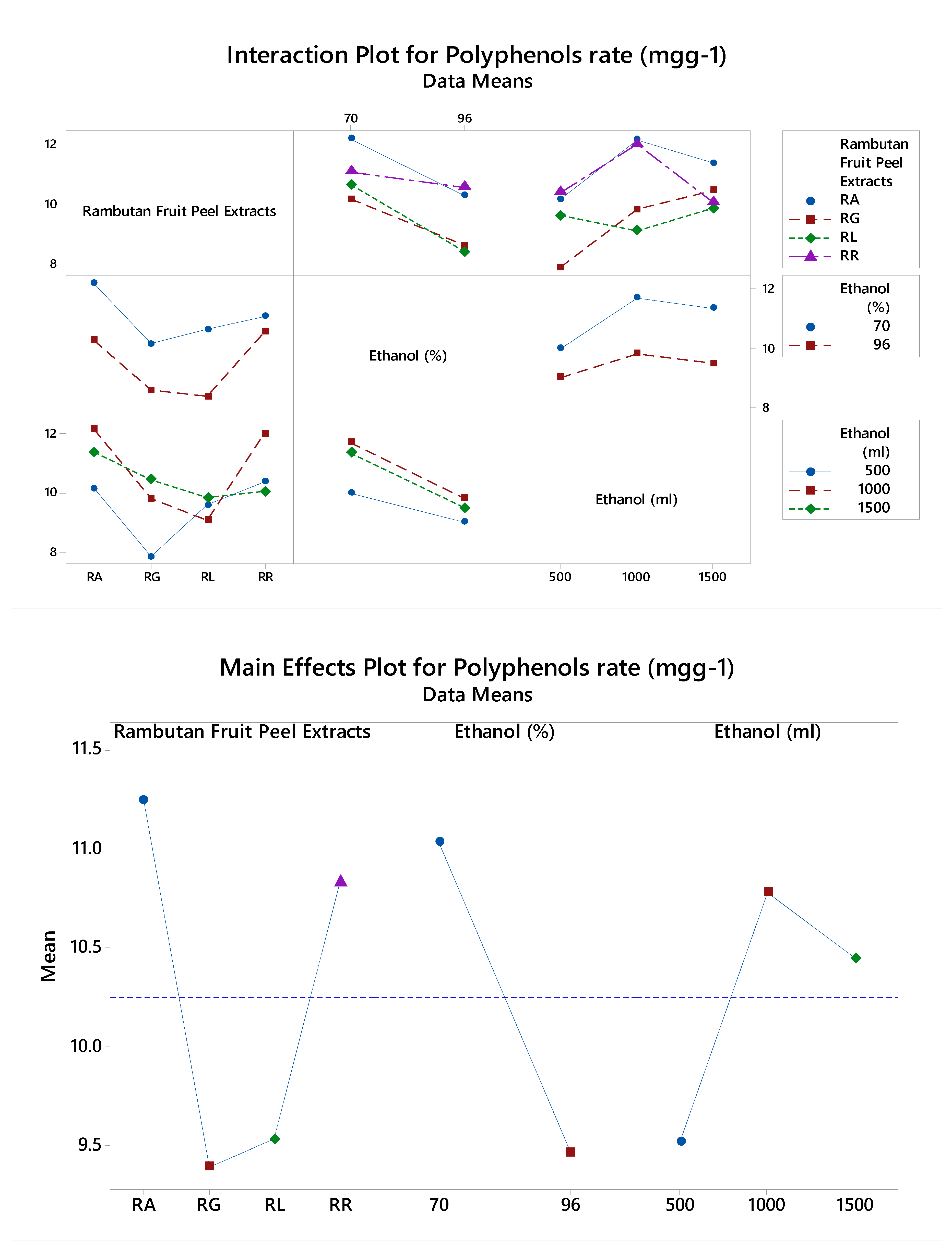
3. Results
| Rambutan fruit peel extracts | Standar compound | Ethanol (%) | Ethanol (ml) |
Absorbance (a.u) |
SD |
Mean |
% CV | Polyphenols Total (mgg-1) |
Polyphenols Rate (mgg-1) |
| RA | Rutin | 70 | 500 | 0.4492 | 0.0014 | 0.4506 | 0.31 | 11.893 | 11.888 |
| 0.4506 | 11.881 | ||||||||
| 0.4520 | 11.891 | ||||||||
| 1000 | 0.6429 | 0.0004 | 0.6429 | 0.06 | 12.475 | 12.474 | |||
| 0.6433 | 12.483 | ||||||||
| 0.6425 | 12.466 | ||||||||
| 1500 | 0.6319 | 0.0001 | 0.6319 | 0.02 | 12.245 | 12.245 | |||
| 0.6320 | 12.247 | ||||||||
| 0.6318 | 12.243 | ||||||||
| RR | Rutin | 70 | 500 | 0.4998 | 0.0001 | 0.4998 | 0.01 | 9.493 | 9.493 |
| 0.4999 | 9.495 | ||||||||
| 0.4998 | 9.493 | ||||||||
| 1000 | 0.5810 | 0.0001 | 0.5809 | 0.01 | 12.854 | 12.850 | |||
| 0.5809 | 12.848 | ||||||||
| 0.5810 | 12.848 | ||||||||
| 1500 | 0.5687 | 0.5686 | 0.0003 | 0.06 | 10.929 | 10.928 | |||
| 0.5690 | 10.935 | ||||||||
| 0.5683 | 10.921 | ||||||||
| RG | Rutin | 70 | 500 | 0.4555 | 0.0001 | 0.4554 | 0.01 | 8.571 | 8.569 |
| 0.4554 | 8.568 | ||||||||
| 0.4554 | 8.568 | ||||||||
| 1000 | 0.5363 | 0.0001 | 0.5363 | 0.02 | 10.254 | 10.252 | |||
| 0.5363 | 10.252 | ||||||||
| 0.5362 | 10.252 | ||||||||
| 1500 | 0.6065 | 0.0001 | 0.6064 | 0.02 | 11.716 | 11.713 | |||
| 0.6063 | 11.712 | ||||||||
| 0.6063 | 11.712 | ||||||||
| RL | Rutin | 70 | 500 | 0.4838 | 0.0001 | 0.4837 | 0.02 | 10.081 | 10.079 |
| 0.4836 | 10.079 | ||||||||
| 0.4836 | 10.079 | ||||||||
| 1000 | 0.3769 | 0.0000 | 0.3769 | 0.01 | 11.295 | 11.289 | |||
| 0.3768 | 11.285 | ||||||||
| 0.3768 | 11.287 | ||||||||
| 1500 | 0.4809 | 0.0001 | 0.4808 | 0.02 | 10.621 | 10.621 | |||
| 0.4807 | 10.621 | ||||||||
| 0.4808 | 10.621 | ||||||||
| RA | Rutin | 96 | 500 | 0.6150 | 0.0003 | 0.6148 | 0.05 | 8.439 | 8.468 |
| 0.6144 | 8.468 | ||||||||
| 0.6149 | 8.497 | ||||||||
| 1000 | 0.6136 | 0.0001 | 0.6135 | 0.01 | 11.864 | 11.863 | |||
| 0.6135 | 11.862 | ||||||||
| 0.6136 | 11.864 | ||||||||
| 1500 | 0.5502 | 0.0002 | 0.5502 | 0.03 | 10.543 | 10.543 | |||
| 0.5504 | 10.547 | ||||||||
| 0.5500 | 10.539 | ||||||||
| RR | Rutin | 96 | 500 | 0.5875 | 0.0002 | 0.5874 | 0.03 | 11.321 | 11.317 |
| 0.5872 | 11.314 | ||||||||
| 0.5873 | 11.316 | ||||||||
| 1000 | 0.6611 | 0.0002 | 0.6609 | 0.03 | 11.185 | 11.185 | |||
| 0.6608 | 11.183 | ||||||||
| 0.6608 | 11.185 | ||||||||
| 1500 | 0.4854 | 0.0001 | 0.4854 | 0.02 | 9.193 | 9.193 | |||
| 0.4853 | 9.191 | ||||||||
| 0.4855 | 9.195 | ||||||||
| RG | Rutin | 96 | 500 | 0.3880 | 0.0010 | 0.3889 | 0.25 | 7.164 | 7.184 |
| 0.3890 | 7.185 | ||||||||
| 0.3899 | 7.204 | ||||||||
| 1000 | 0.4949 | 0.0001 | 0.4950 | 0.03 | 9.391 | 9.393 | |||
| 0.4951 | 9.395 | ||||||||
| 0.4950 | 9.393 | 9.228 | |||||||
| 1500 | 0.4875 | 0.0005 | 0.4871 | 0.10 | 9.237 | ||||
| 0.4871 | 9.229 | ||||||||
| 0.4866 | 9.218 | ||||||||
| RL | Rutin | 96 | 500 | 0.5280 | 0.0001 | 0.5279 | 0.01 | 9.143 | 9.151 |
| 0.5280 | 9.156 | ||||||||
| 0.5279 | 9.156 | ||||||||
| 1000 | 0.5863 | 0.0002 | 0.5860 | 0.04 | 6.933 | 6.931 | |||
| 0.5858 | 6.931 | ||||||||
| 0.5859 | 6.931 | ||||||||
| 1500 | 0.5539 | 0.0000 | 0.5539 | 0.01 | 9.102 | 9.098 | |||
| 0.5539 | 9.095 | ||||||||
| 0.5539 | 9.097 |
Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tsong, J. L., Goh, L. P. W., Gansau, J. A., & How, S. E. (2021). Review of Nephelium lappaceum and Nephelium ramboutan-ake: a high potential supplement. Molecules, 26(22), 7005. [CrossRef]
- Tingting, Z., Xiuli, Z., Kun, W., Liping, S., & Yongliang, Z. (2022). A review: extraction, phytochemicals, and biological activities of rambutan (Nephelium lappaceum L) peel extract. Heliyon. [CrossRef]
- Jahurul, M. H. A., Azzatul, F. S., Sharifudin, M. S., Norliza, M. J., Hasmadi, M., Lee, J. S., Patricia, M., Jinap, S., George, M. R. A., Khan, M. F., & Zaidul, I. S. M. (2020). Functional and nutritional properties of rambutan (Nephelium lappaceum L.) seed and its industrial application: A review. Trends in Food Science & Technology, 99, 367-374. [CrossRef]
- Sukmandari, N. S., Dash, G. K., Jusof, W. H. W., & Hanafi, M. (2017). A Review on Nephelium lappaceum L. Research Journal of Pharmacy and Technology, 10(8), 2819-2822.
- Mahmood, K., Kamilah, H., Alias, A. K., & Ariffin, F. (2018). Nutritional and therapeutic potentials of rambutan fruit (Nephelium lappaceum L.) and the by-products: a review. Journal of Food Measurement and Characterization, 12, 1556-1571. [CrossRef]
- Hernández-Hernández, C., Aguilar, C. N., Rodríguez-Herrera, R., Flores-Gallegos, A. C., Morlett-Chávez, J., Govea-Salas, M., & Ascacio-Valdés, J. A. (2019). Rambutan (Nephelium lappaceum L.): Nutritional and functional properties. Trends in food science & technology, 85, 201-210. [CrossRef]
- Chaiwarit, T., Kantrong, N., Sommano, S. R., Rachtanapun, P., Junmahasathien, T., Kumpugdee-Vollrath, M., & Jantrawut, P. (2021). Extraction of tropical fruit peels and development of hpmc film containing the extracts as an active antibacterial packaging material. Molecules, 26(8), 2265. [CrossRef]
- Sun, L., Zhang, H., & Zhuang, Y. (2012). Preparation of free, soluble conjugate, and insoluble-bound phenolic compounds from peels of rambutan (Nephelium lappaceum) and evaluation of antioxidant activities in vitro. Journal of Food Science, 77(2), C198-C204. [CrossRef]
- Nguyen, N. M. P., Le, T. T., Vissenaekens, H., Gonzales, G. B., Van Camp, J., Smagghe, G., & Raes, K. (2019). In vitro antioxidant activity and phenolic profiles of tropical fruit by-products. International Journal of Food Science & Technology, 54(4), 1169-1178. [CrossRef]
- Phuong, N. N. M., Le, T. T., Van Camp, J., & Raes, K. (2020). Evaluation of antimicrobial activity of rambutan (Nephelium lappaceum L.) peel extracts. International journal of food microbiology, 321, 108539. [CrossRef]
- Xu, H., Li, Y., Tang, H. W., Liu, C. M., & Wu, Q. S. (2010). Determination of rutin with UV-Vis spectrophotometric and laser-induced fluorimetric detections using a non-scanning spectrometer. Analytical letters, 43(6), 893-904. [CrossRef]
- Zhu, H., Wang, Y., Liu, Y., Xia, Y., & Tang, T. (2010). Analysis of flavonoids in Portulaca oleracea L. by UV–vis spectrophotometry with comparative study on different extraction technologies. Food Analytical Methods, 3, 90-97. [CrossRef]
- Tejamukti, E. P., Setyaningsih, W., Irnawati, Yasir, B., Alam, G., & Rohman, A. (2020). Application of FTIR spectroscopy and HPLC combined with multivariate calibration for analysis of xanthones in mangosteen extracts. Scientia Pharmaceutica, 88(3), 35. [CrossRef]
- Yasir, B., Astuti, A. D., Raihan, M., Natzir, R., Subehan, S., Rohman, A., & Alam, G. (2022). Optimization of Pagoda (Clerodendrum paniculatum L.) Extraction Based By Analytical Factorial Design Approach, Its Phytochemical Compound, and Cytotoxicity Activity. Egyptian Journal of Chemistry, 65(9), 421-430.
- Suhendi, A., Hanwar, D., Santoso, B., Wicaksono, A. N., & Widiana, L. (2018). Antioxidant activity of non polar and semipolar fractions of ethanol extract of zingiber zerumbet smith leaves by spectrophotometer and ELISA reader. Journal of Pharmaceutical Sciences and Research, 10(3), 439-441.
- Zu, C., Zhao, X., & Du, X. (2014). Enhanced water-solubility of Licorice extract microparticle prepared by antisolvent precipitation process. Advanced Powder Technology, 25(2), 787-794. [CrossRef]
- Nguyen, N. M. P., Le, T. T., Vissenaekens, H., Gonzales, G. B., Van Camp, J., Smagghe, G., & Raes, K. (2019). In vitro antioxidant activity and phenolic profiles of tropical fruit by-products. International Journal of Food Science & Technology, 54(4), 1169-1178. [CrossRef]
- Mirgorod, Y. A., Borodina, V. G., & Borsch, N. A. (2013). Investigation of interaction between silver ions and rutin in water by physical methods. Biophysics, 58, 743-747. [CrossRef]
- Lohvina, H., Sándor, M., & Wink, M. (2021). Effect of Ethanol Solvents on Total Phenolic Content and Antioxidant Properties of Seed Extracts of Fenugreek (Trigonella foenum-graecum L.) varieties and determination of phenolic Composition by HPLC-ESI-MS. Diversity, 14(1), 7. [CrossRef]
- Ngo, T. V., Scarlett, C. J., Bowyer, M. C., Ngo, P. D., & Vuong, Q. V. (2017). Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. Journal of Food Quality, 2017. [CrossRef]
- Sekar, M. (2020). Rambutan fruits extract in aging skin. In Aging (pp. 303-307). Academic Press. [CrossRef]
- Crump, M. L. (1981). Variation in propagule size as a function of environmental uncertainty for tree frogs. The American Naturalist, 117(5), 724-737. [CrossRef]
- Mendez-Flores, A., Hérnandez-Almanza, A., Sáenz-Galindo, A., Morlett-Chávez, J., Aguilar, C. N., & Ascacio-Valdés, J. (2018). Ultrasound-assisted extraction of antioxidant polyphenolic compounds from Nephelium lappaceum L.(Mexican variety) husk. Asian Pacific journal of tropical medicine, 11(12), 676-681.
- Chicco, D., Warrens, M. J., & Jurman, G. (2021). The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Computer Science, 7, e623. [CrossRef]
- Valbuena, R., Hernando, A., Manzanera, J. A., Görgens, E. B., Almeida, D. R., Silva, C. A., & García-Abril, A. (2019). Evaluating observed versus predicted forest biomass: R-squared, index of agreement or maximal information coefficient?. European Journal of Remote Sensing, 52(1), 345-358. [CrossRef]
- Xu, S. (2017). Predicted residual error sum of squares of mixed models: an application for genomic prediction. G3: Genes, Genomes, Genetics, 7(3), 895-909. [CrossRef]

| Sample code | Rambutan fruit peel extracts | Ethanol Concentration (%) |
Ethanol Volume (ml) | Dry simplicia (g) |
Extract obtained (g) | Yield extracts (%) |
Polyphenol content |
| 1 | RA | 70 | 500 | 100 | 10.04 | 10.04 | + |
| 2 | RA | 70 | 1000 | 100 | 11.26 | 11.26 | + |
| 3 | RA | 70 | 1500 | 100 | 12.99 | 12.99 | + |
| 4 | RA | 96 | 500 | 100 | 11.18 | 11.18 | + |
| 5 | RA | 96 | 1000 | 100 | 13.28 | 13.28 | + |
| 6 | RA | 96 | 1500 | 100 | 14.02 | 14.02 | + |
| 7 | RR | 70 | 500 | 100 | 12.24 | 12.24 | + |
| 8 | RR | 70 | 1000 | 100 | 13,04 | 13.04 | + |
| 9 | RR | 70 | 1500 | 100 | 14.13 | 14.13 | + |
| 10 | RR | 96 | 500 | 100 | 13.15 | 13.15 | + |
| 11 | RR | 96 | 1000 | 100 | 14.17 | 14.17 | + |
| 12 | RR | 96 | 1500 | 100 | 14.37 | 14.37 | + |
| 13 | RG | 70 | 500 | 100 | 10.51 | 9.510 | + |
| 14 | RG | 70 | 1000 | 100 | 11.17 | 11.17 | + |
| 15 | RG | 70 | 1500 | 100 | 11.75 | 11.75 | + |
| 16 | RG | 96 | 500 | 100 | 14.68 | 14.68 | + |
| 17 | RG | 96 | 1000 | 100 | 15.39 | 15.39 | + |
| 18 | RG | 96 | 1500 | 100 | 15.68 | 15.68 | + |
| 19 | RL | 70 | 500 | 100 | 12.34 | 12.34 | + |
| 20 | RL | 70 | 1000 | 100 | 13.47 | 13.47 | + |
| 21 | RL | 70 | 1500 | 100 | 14.62 | 14.62 | + |
| 22 | RL | 96 | 500 | 100 | 13.44 | 13.44 | + |
| 23 | RL | 96 | 1000 | 100 | 14.12 | 14.12 | + |
| 24 | RL | 96 | 1500 | 100 | 15.29 | 15.29 | + |
| Rambutan fruit peel extracts | Ethanol (ml) | Ethanol (%) |
Extracts wavenumber (cm-1) |
Rutin wavenumber (cm-1) |
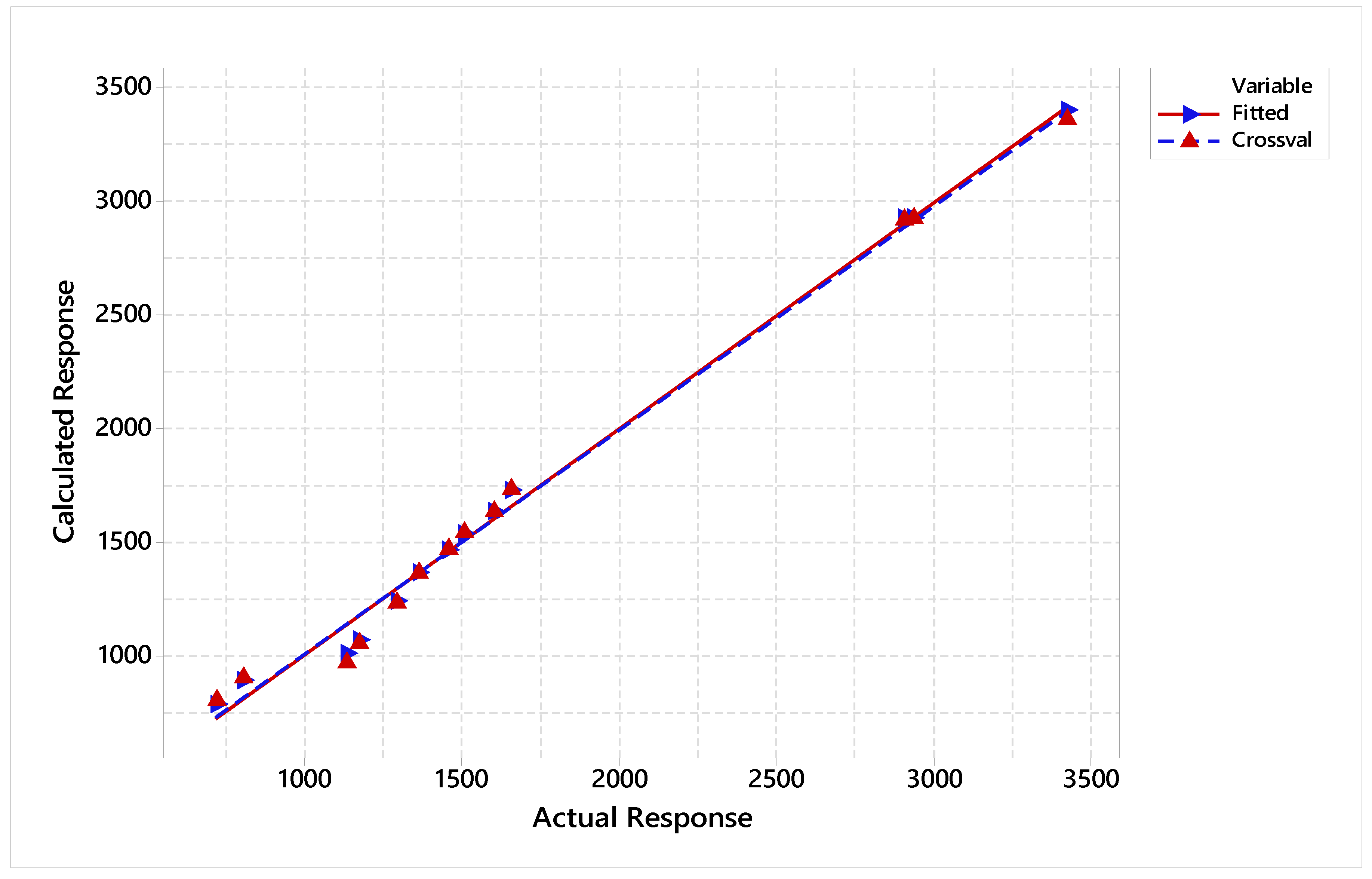
Model selection and validation for Rutin | |||
| X variance | R-Sq | PRESS | R-Sq (pred) | |||||
| RA | 500 | 70 | 1049.28-759.95 | 1170.79-717.52 | 0.971787 | 0.901956 | 62178 | 0.600937 |
| RR | 1000 | 1213.23-759.95 | 1294.24-717.52 | 0.989160 | 0.885387 | 89450 | 0.638176 | |
| RG | 1500 | 1340.53-759.95 | 1361.74-717.52 | 0.994415 | 0.883783 | 90686 | 0.735130 | |
| RL | 1448.54-759.95 | 1456.26-717.52 | 0.996537 | 0.903870 | 84844 | 0.817003 | ||
| 1519.91-759.95 | 1504.48-717.52 | 0.997518 | 0.917515 | 86928 | 0.851104 | |||
| 1616.35-759.95 | 1600.92-717.52 | 0.998159 | 0.933134 | 86125 | 0.883797 | |||
| 1714.72-759.95 | 1654.92-717.52 | 0.998586 | 0.941857 | 90331 | 0.900298 | |||
| 2856.58-759.95 | 2906.73-717.52 | 0.999515 | 0.987438 | 110009 | 0.967084 | |||
| 2926.01-759.95 | 2933.73-717.52 | 0.999666 | 0.991040 | 107098 | 0.980337 | |||
| 3520.09-759.95 | 3425.58-717.52 | 0.999788 | 0.994053 | 99569 | 0.988571 | |||
| RA | 500 | 96 | 1051.20-759.95 | 1170.79-717.52 | 0.997504 | 0.933426 | 33638 | 0.784108 |
| RR | 1000 | 1219.01-759.95 | 1294.24-717.52 | 0.998986 | 0.907671 | 74229 | 0.699744 | |
| RG | 1500 | 1352.10-759.95 | 1361.74-717.52 | 0.999433 | 0.897296 | 82636 | 0.758640 | |
| RL | 1448.54-759.95 | 1456.26-717.52 | 0.999637 | 0.914121 | 74999 | 0.838237 | ||
| 1521.84-759.95 | 1504.48-717.52 | 0.999732 | 0.925542 | 77459 | 0.867324 | |||
| 1618.28-759.95 | 1600.92-717.52 | 0.999798 | 0.939353 | 77078 | 0.896003 | |||
| 1712.79-759.95 | 1654.92-717.52 | 0.999838 | 0.946882 | 81671 | 0.909857 | |||
| 2856.58-759.95 | 2906.73-717.52 | 1.000000 | 0.995588 | 66051 | 0.980237 | |||
| 2926.01-759.95 | 2933.73-717.52 | 0.999990 | 0.993319 | 58802 | 0.989204 | |||
| 3437.15-759.95 | 3425.58-717.52 | 1.000000 | 0.998340 | 38890 | 0.995536 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





