1. Introduction
Periodontal health is characterized by the presence of a benign polymicrobial community encompassing, e.g.,
Proteobacteria,
Firmicutes phyla, and
Actinobacteria. These bacteria form a symbiotic biofilm and support oral health by releasing antimicrobial compounds and inhibiting pathogenic colonization. Disruption of this host-microbe homeostasis entails proliferation of pathobionts, for instance
Porphyromonas gingivalis or
Tannerella forsythia, which trigger gum inflammation (gingivitis). The presence of the dysbiotic oral microbiome triggers a local host response, which in turn stimulates inflammatory cells, such as neutrophils, lymphocytes, and macrophages. These immune cells release various inflammatory mediators, including tumor necrosis factor-alpha (TNF-α), prostaglandin E2, interleukin (IL)-1, and IL-6. This leads to alveolar bone resorption due to cytokine-mediated activation of osteoclasts and the release of matrix metalloproteinases (MMPs) [
1,
2,
3]. Depending on the individual immune response, the inflammation of the soft tissue may be limited to the gums (gingivitis) or may progress and irreversibly affect the hard and soft tissue (periodontitis). Numerous studies have confirmed a shift in pro-inflammatory biomarkers in saliva as well as in blood serum of diseased patients, underscoring their potential clinical utility for diagnosis, treatment, or treatment control [
4,
5,
6].
Periodontal disease is globally highly prevalent. A recent cross-sectional epidemiological study in seven European countries concluded that the prevalence of oral conditions was higher than reported in previous literature. Gingival inflammation, determined by bleeding on probing (BoP), was observed at a minimum of one site in 87% of the participants. Gingivitis with BoP at more than 10% of the sites was recorded for 66% of the otherwise healthy adults [
7].
Gingival inflammation is attenuated by local periodontal treatment in the course of professional mechanical plaque removal (PMPR). As adjunct treatment, probiotic mouthwashes containing
Lactobacillus species or
Bifidobacteria have been explored [
8]. Although probiotics do not eradicate pathobionts, their colonization in the oral cavity is modulated, supporting the host immune system and reducing the production of inflammatory mediators [
2].
Gingival inflammation is also associated with increased oxidative stress elicited by reactive oxygen species released from, e.g., activated neutrophils [
9]. Patients with periodontitis were shown to have increased levels of free radicals and impaired antioxidant defense mechanisms. Supplementation with antioxidant compounds such as lycopene or vitamin E improved periodontal parameters [
10]. In a double-blind randomized placebo-controlled study, supplementation with capsules containing concentrates of fruits, vegetables and berry juice, BoP and the cumulative plaque score significantly improved in the group receiving fruit-vegetable capsules. Those capsules contained defined amounts of β-carotene, vitamin E, vitamin C and folic acid. The content of polyphenols in the supplement was not analyzed due to varying growth and harvest conditions of the fruits and vegetables [
11]. However, it can be assumed that polyphenols contributed to the effects seen in the study participants. Polyphenols have antioxidant, anti-inflammatory, antimicrobial and immunomodulatory effects [
12] and beneficially interact with gut microbiota [
13]. Polyphenol-containing plant extracts revealed potential for treatment or prevention of periodontal disease in vitro, ex vivo and rodent in vivo studies [
12]. More recently, multiple human trials with bioactive polyphenolic compounds have been reported. These studies applied the polyphenols topically as gels or mouthwashes [
14].
To the best of our knowledge, no clinical trial perorally administering a polyphenol-containing plant extract to human patients with gingival inflammation has been reported. A dietary food supplement, a standardized extract from French maritime pine bark (Pycnogenol
®) containing 65–75% oligomeric procyanidins and various small phenolic compounds such as taxifolin, ferulic acid, catechin, and protocatechuic acid, has been shown to inhibit alveolar bone resorption in a rat periodontitis model [
15]. This extract has been broadly studied in human clinical studies [
16], but not yet in gingival inflammation. Recently, we reported that constituents and a polyphenol metabolite produced by gut bacteria (5-(3,4-dihydroxyphenyl)-γ-valerolactone) were present in human saliva samples after intake of Pycnogenol
® [
17,
18]. The purpose of the present study was to investigate the impact of a dietary supplementation with French maritime pine bark extract (Pycnogenol
®) on gingival inflammation in a randomized placebo-controlled double-blind trial. In this manuscript, we focus on the concentrations of phenolic compounds in saliva and the levels of inflammatory markers in saliva and serum samples. Clinical results will be reported in a separate manuscript.
2. Materials and Methods
2.1. Study Design and Participants
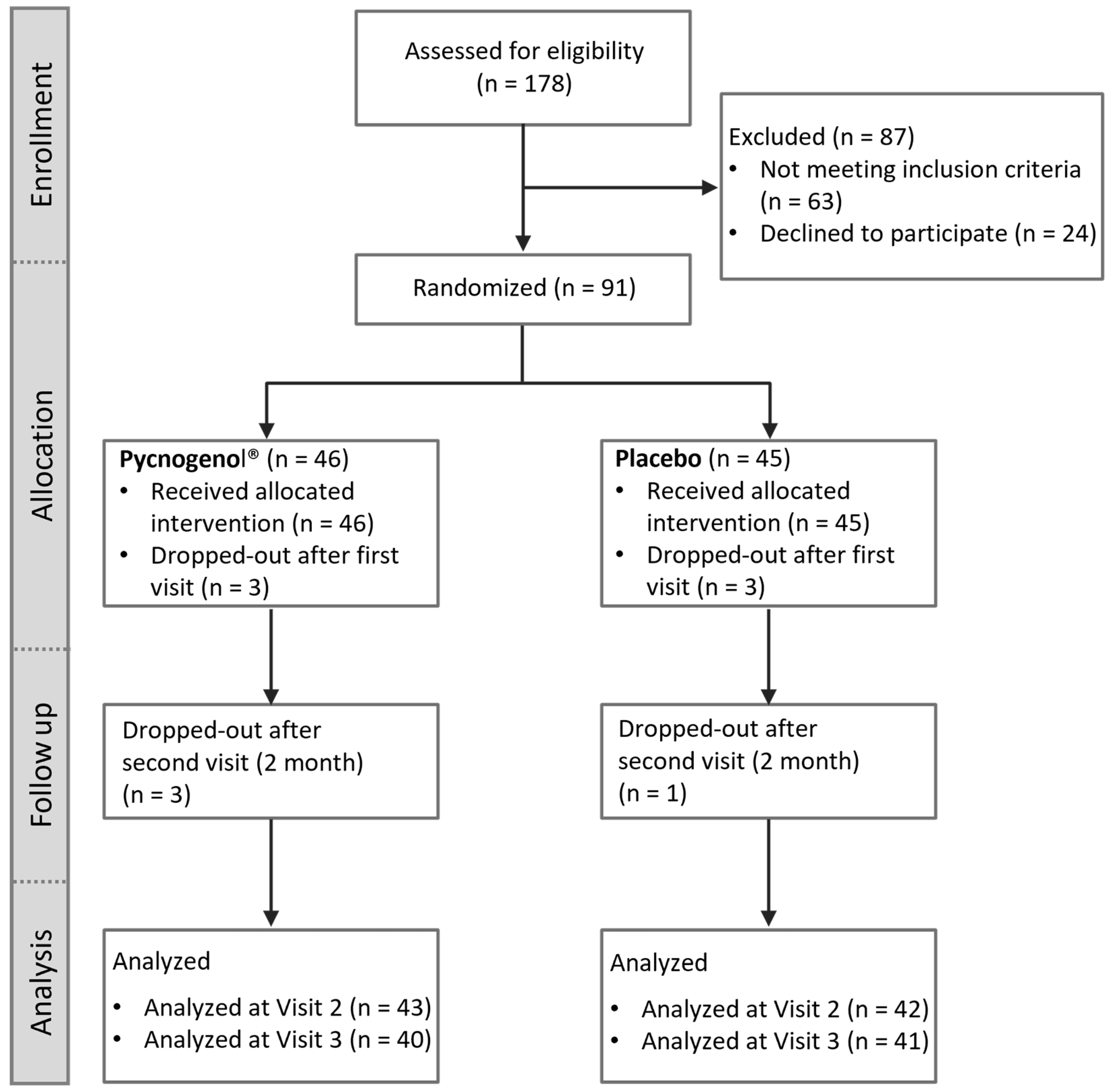
This study was a randomized, double-blind, placebo-controlled clinical trial with a two-arm parallel-group design, registered in the ClinicalTrials.gov database (NCT05786820). It was conducted in accordance with the Declaration of Helsinki for medical research involving human subjects and received approval from the Ethics Committee of the University of Würzburg (260/19-me). A total of 91 subjects were enrolled within the Department of Periodontology at University Hospital of Julius-Maximilians-University and participated in the study between March 2022 and June 2024. All participants were provided with comprehensive information about the study and gave written consent prior to their involvement.
2.2. Intervention and Monitoring
The intervention in this study consisted of the administration of Pycnogenol®, a standardized extract of French maritime pine bark (Pinus pinaster Aiton) that meets the quality standards of the United States Pharmacopeia for dietary supplements, or a placebo. Participants were randomly assigned to one of the two groups in a 1:1 ratio. Randomization was performed using block randomization in Microsoft Excel (Version 2302, Microsoft Corporation, Redmond, USA) with blocks of 10 participants. Random numbers were generated, ranked, and participants were assigned to either the Pycnogenol® and placebo group accordingly. The study followed a double-blind design, ensuring that both participants and the involved dentists (Y.J-S., N.P., J.H.) were blinded to group allocation. The blinding list was securely stored by the study evaluators (J.B., P.H.). All participants underwent professional mechanical plaque removal (PMPR) at the beginning of the study, followed by daily intake of either Pycnogenol® capsules (100 mg twice daily) or identically looking placebo capsules for three months.
During the study period, participants attended four scheduled visits. At the first visit (baseline), clinical, microbiological and cardiovascular data were collected, participants were randomized and the intervention started after the scheduled PMPR. At the second visit, two months after the start of the study, the clinical and microbiological parameters were collected again, as well as at the third visit. The third visit, which took place three months after the first assessment, marked the end of the intervention phase, and the fourth visit was three months after the end of supplementation to assess the long-term outcomes.
The sample size was calculated to achieve a statistical power of 80% and a significance level of 5%. Based on a standard deviation of SD = ± 8.0% and a minimum detectable difference of ∆ = 5.0% of bleeding on probing (BoP), which was the primary objective of the study, the required sample size was determined to be 40 participants per group. To account for potential dropouts, a group size of 45 participants was pursued, resulting in a total of 90 participants.
Monitoring included adherence assessment by counting returned capsules and the documentation of intake in the participants diaries. The results of the salivary polyphenol levels and various inflammation-related biomarkers, analyzed at baseline, as well as two and three months after the start of the intervention, are reported here. Additional clinical parameters (e.g., bleeding on probing, gingival index) will be detailed in a separate manuscript.
2.3. Selection Criteria: Inclusion/Exclusion Criteria
The selection criteria for participants in the study included specific inclusion and exclusion criteria. Adults between 35 and 85 years of age with a body mass index (BMI) between 20 and 30 kg/m2 and at least ten natural teeth were eligible to participate in the study. They had to have clinical signs of gingivitis, defined as at least 10% bleeding on probing (BoP) and a gingival index (GI) between 0 and 2 on at least three teeth. Exclusion criteria were the presence of inflammatory oral mucosal diseases other than gingivitis, a salivary flow rate below 0.1 ml/min, the inability to maintain regular oral hygiene or physical or mental impairments that prevented compliance with the study protocol. Participants were excluded if they had a history of malignant disease, chemotherapy or radiotherapy in the past five years, were pregnant or breastfeeding, had acute infections such as HIV, or had metabolic bone disease. Patients were also excluded if they had taken antibiotics or anti-inflammatory drugs in the four weeks before screening, if they were taking drugs known to affect gingival inflammation (such as corticosteroids), or if they were undergoing active orthodontic treatment.
2.4. Biomarkers and Salivary Polyphenol Levels
Saliva and blood samples were collected to analyze specific biomarkers (IL-1β, IL-6, MMP-8, MMP-9) and to quantify the concentration of seven polyphenols (taxifolin, ferulic acid, caffeic acid, gallic acid, trans para-coumaric acid, protocatechuic acid, M1 (5-(3,4-dihydroxyphenyl)-γ-valerolactone)) in saliva. Saliva was collected using the SalivaBio Oral Swab (Biozol Diagnostica GmbH, Eching, Germany) by placing the swab under the tongue for approximately 5 minutes. Following collection, the swab was immediately stored at 4°C before being centrifuged at 4000 x g for 10 min at 4°C. The collected saliva samples were aliquoted, rapidly frozen in liquid nitrogen, and stored at - 80°C until analysis. Blood samples were collected using the S-Monovette
® Serum Gel CAT (SARSTED AG & Co. KG, Nümbrecht, Germany) and stored at 4°C immediately before centrifugation at 2500 x g for 10 min to separate serum, which was aliquoted, frozen in liquid nitrogen, and stored at - 80°C until further analysis. To quantify the concentrations of the inflammatory biomarkers, enzyme-linked immunosorbent assay (ELISA) kits (IL-1 Beta EIA Kit, Salimetrics; Human MMP-9 ELISA Kit, Biorbyt; Human MMP-8 ELISA Kit, Biorbyt; Interleukin 6 ELISA Kit, Biorbyt; all procured from Biozol Diagnostica GmbH, Eching, Germany) were utilized, whereas a previously validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method was employed to quantify the polyphenols [
18].
2.5. Statistical Analysis
A range of statistical tests were employed to analyse the differences between the groups. All calculations were performed using GraphPad Prism Version 6.07 for Windows (Graphpad Software, La Jolla, California, USA). Prior to the statistical tests, a two-tailed outlier test was conducted using the ROUT method in GraphPad Prism to identify and remove any outliers from the dataset. The differences in demographic characteristics were determined using t-test (for continuous variables) and Fisher’s exact test (for categorical variables). Polyphenol and biomarker concentrations within groups were analyzed using the Wilcoxon matched pairs signed rank test. The Mann-Whitney test and the repeated-measured ANOVA were used for the comparison between the groups. Furthermore, Fisher’s exact test was utilized to compare the number of participants for whom a particular polyphenol could be quantified. Correlation analyses were performed using Spearman’s rank correlation test. Significance was assumed at an α-level of < 0.05.
4. Discussion
In the present study we investigated for the first time the impact of a procyanidine-rich dietary supplementation with French maritime pine bark (Pycnogenol®) on selected salivary and serum inflammatory biomarkers in a randomized placebo-controlled double-blind trial including 91 participants undergoing non-surgical periodontal therapy.
The French maritime pine bark extract Pycnogenol
® had been previously reported to beneficially influence inflammatory processes in various disease contexts, such as osteoarthritis [
16]. These effects have been attributed to the ability of its constituents and/or metabolites to inhibit activation of the key inflammatory switch NF-κB and activity of cyclooxygenase (COX) enzymes, as well as to decrease the release of matrix metalloproteinases (MMPs), which play an essential role in tissue degradation and inflammation [
16,
19]. However, the impact of Pycnogenol
® on inflammatory biomarkers in oral inflammation had not been studied in a controlled human trial before.
In addition to professional mechanical plaque removal (PMPR), nutritional supplements have been investigated as adjunct approach in periodontal therapy. Several studies suggest that such dietary interventions may positively impact inflammatory processes [
12,
20,
21,
22]. Unlike traditional antimicrobials, which come with concerns regarding bacterial resistance, probiotics and dietary supplements may offer a safer alternative for reducing inflammation and restoring a healthy microbial balance [
23,
24]. Sugimoto et al. [
15] demonstrated that Pycnogenol
® reduced alveolar bone resorption in a rat periodontitis model by inhibiting bacterial adhesion and osteoclastogenesis, suggesting potential benefits for restoring periodontal health. The present study is the first human study addressing the question of whether oral supplementation with Pycnogenol
® can positively influence inflammatory biomarkers after professional mechanical plaque removal.
Our results indicate that Pycnogenol
® supplementation led to significant reductions in key inflammatory biomarkers and increased concentrations of specific salivary polyphenols. These findings align with previous research on the systemic anti-inflammatory effects of Pycnogenol
® [
16].
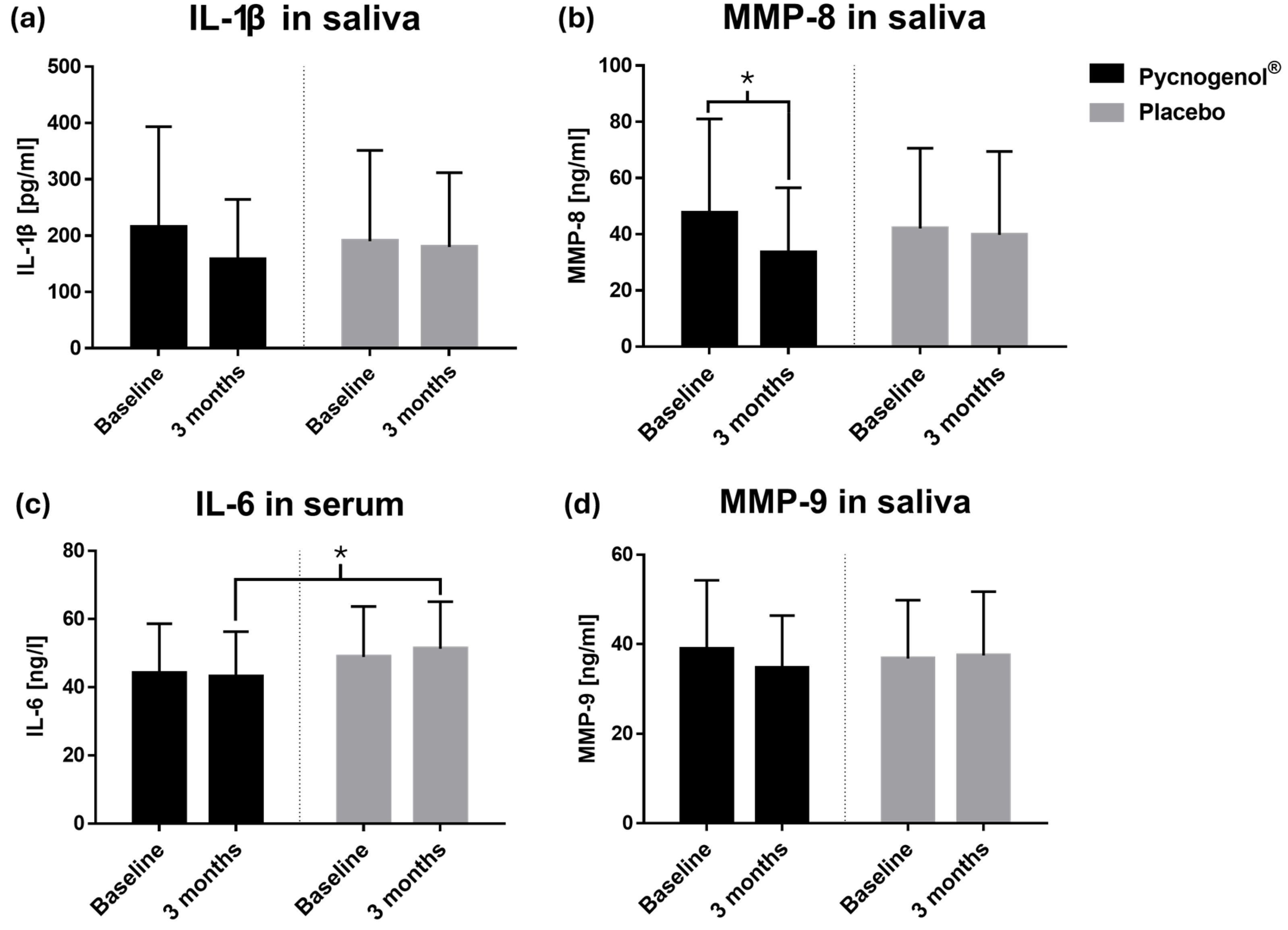
MMP-8, a collagenase involved in periodontal tissue degradation, was significantly reduced in the Pycnogenol
® group compared to placebo. Given that elevated MMP-8 levels are associated with periodontal disease progression [
25], its reduction suggests a potential protective effect of Pycnogenol
® on periodontal tissues. This finding is consistent with in vitro studies demonstrating that Pycnogenol
® inhibits matrix metalloproteinases [
19]. A non-significant trend towards lower IL-1β levels in saliva was also observed, while IL-6 concentrations in serum were significantly reduced in the Pycnogenol
® group, further supporting its anti-inflammatory potential.
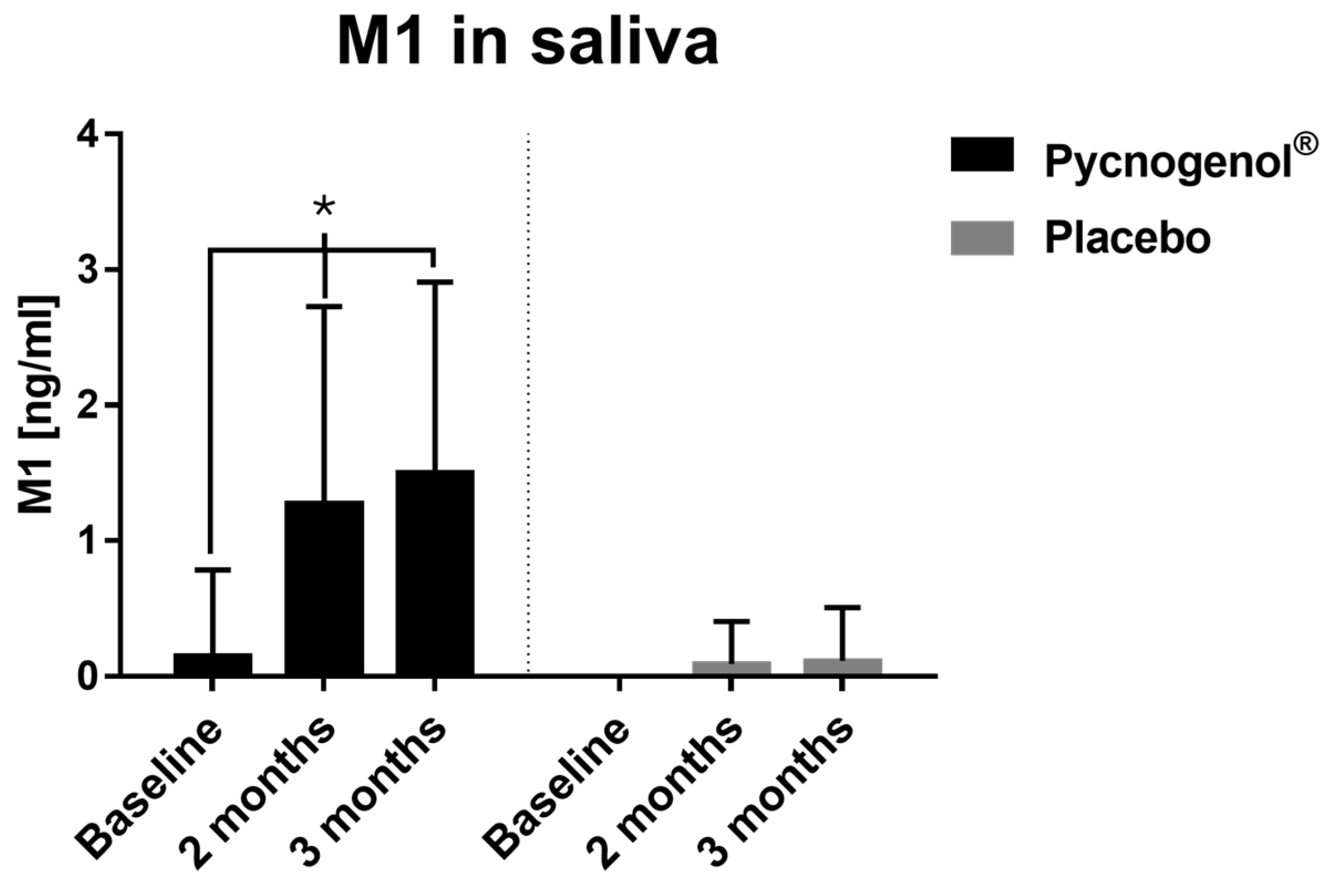
Salivary polyphenol analysis revealed that Pycnogenol
® supplementation increased levels of several polyphenols, including ferulic acid and caffeic acid. Notably, M1 (5-(3,4-dihydroxyphenyl)-γ-valerolactone), a gut-derived metabolite of Pycnogenol
® constituents, was significantly elevated in the Pycnogenol
® group compared to the placebo group. This increase not only confirms patient adherence to the supplementation protocol but also highlights M1 as a potential biomarker for compliance in future studies. Beyond its role in monitoring adherence, M1 may also function as a bioactive effector, contributing to the anti-inflammatory and antioxidant effects associated with Pycnogenol
® [
16].
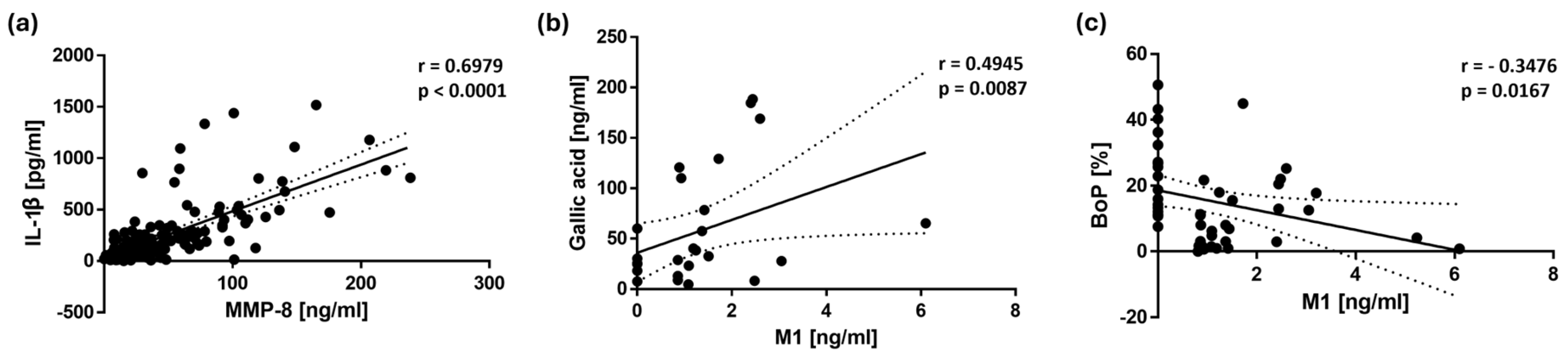
Correlation analyses further support these findings, revealing a significant inverse correlation between BoP and M1 (r = -0.3476,
p = 0.0167), suggesting that M1 may play a role in reducing gingival inflammation. In addition, BoP showed a significant positive correlation with MMP-8 (r = 0.1906,
p = 0.0203) and IL-1β (r = 0.2380,
p = 0.0038), indicating that higher levels of gingival bleeding were associated with increased inflammatory activity. Furthermore, the strong correlation between MMP-8 and IL-1β underscores the relevance of these biomarkers in periodontal disease progression. IL-1β is a key pro-inflammatory cytokine that promotes the activation of matrix metalloproteinases, including MMP-8, leading to extracellular matrix degradation and tissue destruction [
26]. The observed association between these two markers reinforces the role of IL-1β in promoting periodontal inflammation by inducing MMP-8-mediated tissue breakdown, highlighting the potential benefits of Pycnogenol
® in modulating these inflammatory pathways.
Our findings are in line with previous research on dietary interventions for periodontal health in animal models [
15,
27]. For instance, Laky et al. [
27] demonstrated that quercetin, a polyphenol found in various foods, mitigated disease progression in experimental periodontitis.
This study represents the first controlled human trial assessing the effects of Pycnogenol® on gingival inflammation, providing novel insights into its potential as an adjunctive therapy for periodontitis. The observed reductions of inflammation-related biomarkers and the correlation between BoP and M1 suggest that Pycnogenol® may influence periodontal health through anti-inflammatory mechanisms.
However, the three-month administration of Pycnogenol® may not fully reflect its long-term effects. Future research should investigate whether these effects persist over extended periods and whether Pycnogenol® can contribute to sustained periodontal stability.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow chart illustrating the participant flow through the study, including enrollment, allocation to intervention groups (Pycnogenol® and placebo), follow-up, and analysis.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow chart illustrating the participant flow through the study, including enrollment, allocation to intervention groups (Pycnogenol® and placebo), follow-up, and analysis.
Figure 2.
Saliva concentrations of M1 in the Pycnogenol® and placebo group at three time points (baseline, after 2 months and after 3 months). Data are presented as mean with standard deviation and standardized to 13 participants, as 13 was the maximum number of participants in the Pycnogenol® group with the metabolite M1 in saliva. In the placebo group, M1 was detectable in only 2 participants. Significant differences are indicated by asterisks (*), with a significance level of α < 0.05.
Figure 2.
Saliva concentrations of M1 in the Pycnogenol® and placebo group at three time points (baseline, after 2 months and after 3 months). Data are presented as mean with standard deviation and standardized to 13 participants, as 13 was the maximum number of participants in the Pycnogenol® group with the metabolite M1 in saliva. In the placebo group, M1 was detectable in only 2 participants. Significant differences are indicated by asterisks (*), with a significance level of α < 0.05.
Figure 3.
Concentrations at two time points (baseline and after 3 months) of (a) IL-1β in saliva, n(Pycnogenol®) = 35, n(Placebo) = 38; (b) MMP-8 in saliva, n(Pycnogenol®) = 37, n(Placebo) = 37; (c) IL-6 in serum, n(Pycnogenol®) = 38, n(Placebo) = 40; (d) MMP-9 in saliva, n(Pycnogenol®) = 37, n(Placebo) = 38. Data are presented as mean with standard deviation. Significant differences are indicated by asterisks (*), with a significance level of α < 0.05.
Figure 3.
Concentrations at two time points (baseline and after 3 months) of (a) IL-1β in saliva, n(Pycnogenol®) = 35, n(Placebo) = 38; (b) MMP-8 in saliva, n(Pycnogenol®) = 37, n(Placebo) = 37; (c) IL-6 in serum, n(Pycnogenol®) = 38, n(Placebo) = 40; (d) MMP-9 in saliva, n(Pycnogenol®) = 37, n(Placebo) = 38. Data are presented as mean with standard deviation. Significant differences are indicated by asterisks (*), with a significance level of α < 0.05.
Figure 4.
Scatter plots illustrating the correlations with 95% confidence intervals between (a) MMP-8 and IL-1β concentrations in saliva, (b) M1 and gallic acid concentrations in saliva, and (c) M1 concentration in saliva and bleeding on probing (BoP). The Spearman’s rank correlation analysis revealed a strong positive association between MMP-8 and IL-1β (r = 0.6979, p < 0.0001, n = 144), as well as between M1 and gallic acid (r = 0.4945, p = 0.0087, n = 27). A significant inverse correlation was observed between M1 and BoP (r = - 0.3476, p = 0.0167, n = 47). Each point represents an individual data pair.
Figure 4.
Scatter plots illustrating the correlations with 95% confidence intervals between (a) MMP-8 and IL-1β concentrations in saliva, (b) M1 and gallic acid concentrations in saliva, and (c) M1 concentration in saliva and bleeding on probing (BoP). The Spearman’s rank correlation analysis revealed a strong positive association between MMP-8 and IL-1β (r = 0.6979, p < 0.0001, n = 144), as well as between M1 and gallic acid (r = 0.4945, p = 0.0087, n = 27). A significant inverse correlation was observed between M1 and BoP (r = - 0.3476, p = 0.0167, n = 47). Each point represents an individual data pair.
Table 1.
Demographic and lifestyle characteristics.
Table 1.
Demographic and lifestyle characteristics.
| Variables |
Pycnogenol® Group
(n = 46) |
Placebo Group
(n = 45) |
p-Value 1
|
| Age (years), mean ± SD |
67.83 ± 9.18 |
66.96 ± 8.20 |
0.6348 (T) |
| Gender, n (%) |
|
|
|
| Male |
27 (58.7) |
12 (26.7) |
0.0029* (F) |
| Female |
19 (41.3) |
33 (73.3) |
|
| Vegetarian diet, n (%) |
|
|
|
| Yes |
6 (13.0) |
2 (4.4) |
0.2668 (F) |
| No |
40 (87.0) |
43 (95.6) |
|
| Smoking status, n (%) |
|
|
|
| Yes |
5 (10.9) |
2 (4.4) |
0.4345 (F) |
| No |
41 (89.1) |
43 (95.6) |
|
| Drinking status 2, n (%) |
|
|
|
| Yes |
22 (47.8) |
15 (33.3) |
0.2018 (F) |
| No |
24 (52.2) |
30 (66.7) |
|
Table 2.
Concentrations of specific polyphenols in saliva and biomarker levels in saliva and serum, measured in the Pycnogenol® and placebo group during the study.
Table 2.
Concentrations of specific polyphenols in saliva and biomarker levels in saliva and serum, measured in the Pycnogenol® and placebo group during the study.
| Analyte |
Measurement
Time |
Pycnogenol®
|
Placebo |
p-Value b
|
p-Value c
|
p-Value d
|
| Mean ± SD |
n 1
|
Mean ± SD |
n 1
|
| Taxifolin [ng/ml] |
Baseline (Visit 1) |
3.46 ± 2.14 |
5 |
0.00 |
0 |
0.0357* |
|
0.0257* |
| 2 months (Visit 2) |
5.57 ± 6.12 |
8 |
4.47 ± 2.07 |
4 |
0.5697 |
|
0.2258 |
|
p-Value a
|
0.3750 |
|
0.1250 |
|
|
|
|
| 3 months (Visit 3) |
3.29 ± 2.46 |
5 |
2.89 ± 1.39 |
5 |
0.8413 |
0.1229 |
1.000 |
|
p-Value a
|
0.7695 |
|
0.0625 |
|
|
|
|
| Ferulic acid [ng/ml] |
Baseline (Visit 1) |
10.28 ± 6.76 |
11 |
6.38 ± 2.85 |
9 |
0.3410 |
|
0.6135 |
| 2 months (Visit 2) |
18.47 ± 36.97 |
17 |
7.89 ± 7.53 |
7 |
0.9014 |
|
0.0155* |
|
p-Value a
|
0.2774 |
|
0.4131 |
|
|
|
|
| 3 months (Visit 3) |
12.87 ± 16.23 |
17 |
7.56 ± 4.24 |
5 |
0.8912 |
0.5775 |
0.0027* |
|
p-Value a
|
0.2935 |
|
0.4697 |
|
|
|
|
| Caffeic acid [ng/ml] |
Baseline (Visit 1) |
17.70 ± 21.49 |
19 |
22.90 ± 26.34 |
9 |
0.9912 |
|
0.0201* |
| 2 months (Visit 2) |
28.12 ± 28.84 |
28 |
11.30 ± 15.34 |
22 |
0.0077* |
|
0.1714 |
|
p-Value a
|
0.0002* |
|
0.2726 |
|
|
|
|
| 3 months (Visit 3) |
27.71 ± 30.99 |
21 |
16.38 ± 17.83 |
17 |
0.9364 |
0.0388* |
0.3766 |
|
p-Value a
|
0.1908 |
|
0.3209 |
|
|
|
|
| Gallic acid [ng/ml] |
Baseline (Visit 1) |
45.59 ± 50.04 |
9 |
89.82 ± 66.88 |
3 |
0.4818 |
|
0.0667 |
| 2 months (Visit 2) |
82.27 ± 84.34 |
15 |
68.69 ± 88.27 |
11 |
0.3051 |
|
0.3474 |
|
p-Value a
|
0.0046* |
|
0.0020* |
|
|
|
|
| 3 months (Visit 3) |
45.32 ± 52.92 |
14 |
63.92 ± 66.28 |
8 |
0.2973 |
0.0102* |
0.1394 |
|
p-Value a
|
0.4037 |
|
0.1641 |
|
|
|
|
| p-Coumaric acid [ng/ml] |
Baseline (Visit 1) |
3.99 ± 2.92 |
22 |
5.09 ± 6.59 |
22 |
0.4678 |
|
1.000 |
| 2 months (Visit 2) |
6.71 ± 8.75 |
25 |
4.26 ± 3.27 |
28 |
0.9472 |
|
0.6445 |
|
p-Value a
|
0.3369 |
|
0.3058 |
|
|
|
|
| 3 months (Visit 3) |
7.93 ± 15.43 |
29 |
4.41 ± 4.42 |
25 |
0.8063 |
0.2630 |
0.3774 |
|
p-Value a
|
0.1827 |
|
0.9911 |
|
|
|
|
| Protocatechuic acid [ng/ml] |
Baseline (Visit 1) |
24.17 ± 16.74 |
17 |
22.84 ± 26.36 |
22 |
0.4617 |
|
0.3766 |
| 2 months (Visit 2) |
48.01 ± 87.84 |
20 |
31.19 ± 36.18 |
24 |
0.5360 |
|
0.5067 |
|
p-Value a
|
0.3596 |
|
0.2746 |
|
|
|
|
| 3 months (Visit 3) |
35.47 ± 36.43 |
18 |
29.43 ± 29.43 |
22 |
0.4228 |
0.0954 |
0.5077 |
|
p-Value a
|
0.2292 |
|
0.9019 |
|
|
|
|
M1
[ng/ml] |
Baseline (Visit 1) |
2.60 |
1 |
0.00 |
0 |
- |
|
0.4938 |
| 2 months (Visit 2) |
1.67 ± 1.45 |
13 |
1.09 |
1 |
0.0929 |
|
0.0003* |
|
p-Value a
|
0.0005* |
|
- |
|
|
|
|
| 3 months (Visit 3) |
1.96 ± 1.28 |
13 |
1.37 |
1 |
0.0643 |
0.0252* |
0.0003* |
|
p-Value a
|
0.0024* |
|
- |
|
|
|
|
IL-1β
[pg/ml] |
Baseline (Visit 1) |
214.69 ± 178.80 |
35 |
190.11 ± 161.39 |
38 |
0.4897 |
|
- |
| 3 months (Visit 3) |
157.22 ± 107.14 |
35 |
179.88 ± 132.07 |
38 |
0.6072 |
0.1145 |
- |
|
p-Value a
|
0.1347 |
|
0.6379 |
|
|
|
|
MMP-8
[ng/ml] |
Baseline (Visit 1) |
47.65 ± 33.39 |
37 |
42.06 ± 28.58 |
37 |
0.5790 |
|
- |
| 3 months (Visit 3) |
33.43 ± 23.15 |
37 |
39.78 ± 29.69 |
37 |
0.5209 |
0.0414* |
- |
|
p-Value a
|
0.0261* |
|
0.6916 |
|
|
|
|
MMP-9
[ng/ml] |
Baseline (Visit 1) |
38.91 ± 15.37 |
37 |
36.75 ± 13.09 |
38 |
0.4435 |
|
- |
| 3 months (Visit 3) |
34.67 ± 11.69 |
37 |
37.48 ± 14.23 |
38 |
0.8187 |
0.1215 |
- |
|
p-Value a
|
0.1633 |
|
0.8075 |
|
|
|
|
IL-6 2
[ng/ml] |
Baseline (Visit 1) |
44.20 ± 14.43 |
38 |
48.88 ± 14.83 |
40 |
0.1793 |
|
- |
| 3 months (Visit 3) |
43.16 ± 13.17 |
38 |
51.27 ± 13.84 |
40 |
0.0409* |
0.2561 |
- |
|
p-Value a
|
0.5326 |
|
0.3193 |
|
|
|
|
Table 3.
Spearman’s rank correlation coefficients (r) and p-values for the associations between the clinical parameter BoP (Bleeding on Probing), inflammation-related biomarkers and salivary polyphenol levels.
Table 3.
Spearman’s rank correlation coefficients (r) and p-values for the associations between the clinical parameter BoP (Bleeding on Probing), inflammation-related biomarkers and salivary polyphenol levels.
| Variable 1 |
Variable 2 |
n1
|
Spearman’s r |
p-Value* |
| BoP |
MMP-8 |
148 |
0.1906 |
0.0203 |
| BoP |
IL-1β |
146 |
0.2380 |
0.0038 |
| MMP-8 |
IL-1β |
144 |
0.6979 |
< 0.0001 |
| MMP-8 |
IL-6 |
146 |
0.1922 |
0.0201 |
| IL-1β |
IL-6 |
144 |
0.1781 |
0.0324 |
| M1 |
Gallic acid |
27 |
0.4945 |
0.0087 |
| Ferulic acid |
Caffeic acid |
62 |
0.5108 |
< 0.0001 |
| Ferulic acid |
p-Coumaric acid |
70 |
0.4420 |
0.0001 |
| Caffeic acid |
p-Coumaric acid |
99 |
0.2946 |
0.0031 |
| Caffeic acid |
Protocatechuic acid |
88 |
0.2608 |
0.0141 |
| Protocatechuic acid |
p-Coumaric acid |
109 |
0.3053 |
0.0012 |
| Protocatechuic acid |
Gallic acid |
54 |
0.3780 |
0.0048 |
| M1 |
BoP |
47 |
- 0.3476 |
0.0167 |