1. Introduction
Proton exchange membrane fuel cells (PEMFCs) have emerged as a competitive energy conversion technology in automotive and stationary power systems due to their high efficiency, compactness, and low environmental impact [
1,
2,
3,
4]. Within PEMFC stacks, elastomeric seals are critical components that prevent leakage of hydrogen, oxygen, and coolant while maintaining electrical isolation and mechanical compression. These seals operate under complex, aggressive conditions—elevated temperatures (typically 60–90 °C), high relative humidity (50–100%), and prolonged exposure to mildly acidic aqueous environments due to the formation of sulfuric acid and fluoride-containing condensates [
2,
5,
6].
Among commercially used elastomers, ethylene propylene diene monomer (EPDM) rubber remains a leading material for fuel cell sealing applications due to its thermal stability, flexibility, and inherent resistance to ozone, oxidation, and polar media [
2,
7,
8]. Compared to other sealing materials such as silicone rubber, fluorinated elastomers, and thermoplastic vulcanizates (TPVs), EPDM has demonstrated lower compression set, better chemical resistance, and greater long-term sealing performance [
2,
9]. However, extended operation in PEMFC conditions can still lead to chemical and mechanical degradation. These include oxidative chain scission, de-crosslinking, surface cracking, and leaching of ionic species—processes that compromise mechanical integrity and dimensional stability [
3,
5,
6].
Recent studies have focused on the development of more sustainable EPDM formulations by incorporating alternative fillers such as circular carbon black (CCB) and recycled carbon black (RCB), derived from end-of-life tires through pyrolysis and other post-treatment processes [
10,
11]. While circular carbon black can offer particle morphology and performance characteristics comparable to furnace black, recycled carbon black often shows higher variability and may affect crosslinking density and surface reactivity [
7,
11]. These material differences can have pronounced effects on aging behavior, especially under acidic and thermal stress typical of PEMFC operation [
3,
12,
13,
14].
The degradation behavior of elastomeric compounds in simulated fuel cell environments has been investigated using various immersion aging protocols. Foltuț et al. [
12] subjected EPDM, TPV, and ECO TPV specimens to acidic environments of 0.001 M, 0.1 M, and 1 M H₂SO₄ at 90 °C for 1000 hours. EPDM showed the least dimensional change, with a volume increase of only +1.64% in 1 M H₂SO₄, compared to +4.87% in TPV and +6.50% in ECO TPV. Weight gain trends mirrored this behavior. Surface degradation phenomena such as microcracking were observed in ECO TPV and, to a lesser degree, in TPV, while EPDM largely preserved its surface morphology [
2,
12].
Mechanical testing further supported EPDM’s relative stability: tensile strength retention after acidic aging remained high (from 18.84 MPa in fresh state to 16.7–18.1 MPa), and elongation at break showed only minor reductions (187% to 163–183%) across all acid concentrations [
15]. In contrast, ECO TPV exhibited pronounced mechanical degradation, particularly in high-acid conditions, with up to 15% tensile strength loss and increased surface embrittlement. Complementary DSC analysis revealed minor shifts in thermal transitions in aged EPDM, while ECO TPV displayed marked peak broadening and displacement, indicating structural destabilization. [
16]
Aside from mechanical and dimensional changes, ionic leaching and chemical stability are key considerations for seal materials in PEMFCs. A hot water extraction test performed by Foltuț et al. [
12] showed that EPDM remained below the 5 μS/cm conductivity threshold for over 100 hours of immersion, while ECO TPV rapidly exceeded this value, indicating more aggressive ion release. SEM and EDS analysis further confirmed the migration of polar fillers and additives in TPV and ECO TPV, whereas EPDM retained a more chemically inert surface [
2,
3].
In view of these findings, a more detailed evaluation of next-generation EPDM systems filled with circular or recycled carbon black is necessary to understand their long-term mechanical and chemical stability under conditions simulating the PEMFC environment. While baseline EPDM has proven to be robust, the implications of replacing traditional filler systems with sustainable alternatives must be thoroughly characterized.
The present study investigates the mechanical degradation behavior of two EPDM elastomers—one containing circular carbon black (CCB EPDM) and one containing recycled carbon black (RCB EPDM)—after prolonged exposure to acidic aqueous environments. Through a combination of mass and volume change measurements, mechanical property evaluation (tensile, Shore A hardness, microhardness), and surface analysis (SEM), the goal is to determine the material-specific trends in performance retention and failure mechanisms. This work builds upon previous investigations by incorporating sustainable filler systems while maintaining a testing framework aligned with real PEMFC operational stressors.
2. Materials and Methods
2.1. Materials
The materials investigated in this study were two EPDM compounds, referred to hereafter as CCB EPDM and RCB EPDM, respectively. Both materials are based on a peroxide-cured EPDM matrix but differ in the type of carbon black filler used. CCB EPDM incorporates circular carbon black, obtained through advanced pyrolysis and refinement of end-of-life tires, whereas RCB EPDM contains recycled carbon black, which is also recovered from waste tires but typically involves less controlled processing, resulting in broader particle size distributions and increased variability.
The two materials were supplied as vulcanized sheets with identical base EPDM polymers and crosslinking systems to isolate the influence of filler type on aging and mechanical performance. Basic mechanical and structural characteristics of the fresh materials are summarized in
Table 1.
2.2. Aging Protocols
The aging process was designed to replicate the ambient chemical and thermal conditions encountered in fuel cell systems, with the objective of evaluating the robustness and degradation behavior of the EPDM materials under long-term exposure. Two separate aging procedures were employed: chemical aging in acidic aqueous solutions and thermal aging in dry heat.
For chemical aging, specimens of both EPDM compounds were fully immersed in aqueous sulfuric acid (H₂SO₄) solutions of three different concentrations: 1 M, 0.1 M, and 0.001 M, representing strongly acidic, moderately acidic, and mildly acidic environments, respectively. The pH values of the prepared solutions were approximately 0.1 for 1 M, 0.84 for 0.1 M, and 2.24 for 0.001 M H₂SO₄. Each specimen was submerged solution within sealed borosilicate glass vessels and aged for 1000 hours at 90 °C in a temperature-controlled oven. This protocol was intended to simulate proton exchange membrane fuel cell (PEMFC) operating environments, where acidic condensate can form and persist over time, leading to potential chemical degradation of sealing components.
In parallel, a separate set of samples was subjected to thermal aging under dry heat conditions. The materials were placed in a forced-air convection oven and exposed to 90 °C for 1000 hours, without immersion in liquid media. This procedure was conducted to isolate and evaluate the effects of thermal exposure alone on the mechanical and microstructural stability of the materials, simulating fuel cell standby or elevated-temperature operating phases without active condensation.
Both chemical and thermal aging conditions were selected to accelerate degradation processes for comparison with earlier studies and to provide insight into the environmental resilience of EPDM compounds containing either circular or recycled carbon black. Following aging, the materials were subjected to mechanical, thermal, and surface characterization to quantify changes in performance and structure.
2.3. Weight and Volume Measurements
The effects of chemical aging on the dimensional and mass stability of the EPDM materials were evaluated through gravimetric and hydrostatic measurements performed before and after immersion. Prior to aging, each sample was weighed in air and then submerged in water using a sinker, allowing for the calculation of its baseline volume through Archimedes’ principle. After aging, the same measurement procedure was repeated under identical conditions to evaluate changes due to fluid absorption, swelling, or degradation.
To determine the percentage mass change, the initial mass of the sample in air
and the final mass after immersion
were used. The mass variation was calculated according to the equation:
This expression quantifies the relative increase or decrease in specimen weight, indicating absorption or leaching behavior during aging.
The percentage change in volume was derived using the hydrostatic method, based on the specimen’s weight difference in air and in water. The volume of the sample was inferred from the buoyant force acting on it, using the formula:
where
and
are the apparent weights of the specimen in water before and after aging, respectively. Because no sinker was used, the expressions account solely for the specimen’s own submerged mass. This approach allowed accurate tracking of swelling or shrinkage behavior because of long-term chemical exposure, without mechanical interference.
2.4. Mechanical Testing
The mechanical performance of the EPDM materials before and after aging was evaluated through tensile testing, Shore A hardness, and microhardness measurements. Tensile properties were assessed in accordance with ISO 37, using Type 2 dumbbell-shaped specimens die-cut from the original vulcanized sheets. Each specimen had a gauge width of 4 mm, and a thickness of 2 mm. Testing was carried out on an Instron universal testing machine, equipped with a video extensometer to accurately track elongation during loading. A crosshead speed of 500 mm/min was applied, and tests were conducted under standard laboratory conditions (23 °C, 50% RH). For each condition, a minimum of three replicates was tested. Stress–strain curves were recorded, and the average tensile strength and elongation at break were calculated for comparison across all aging conditions.
2.5. Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy (SEM-EDS)
Surface morphology and elemental composition of the EPDM materials were investigated using Scanning Electron Microscopy (SEM) combined with Energy-Dispersive X-ray Spectroscopy (EDS) to assess microstructural and chemical changes resulting from thermal and chemical aging. Samples were analyzed in their fresh, heat-aged, and 1 M sulfuric acid-aged states, corresponding to the most representative conditions in the fuel cell-relevant environment.
SEM imaging was performed using a Thermo Fisher Axia ChemiSEM system. Specimens were mounted on aluminum stubs using conductive carbon adhesive and sputter-coated with gold to ensure conductivity. Images were acquired using a CBS detector at an accelerating voltage of 10.00 kV, working distance of 10.2 mm, and magnifications of 500× and 1000×. The horizontal field width (HFW) for 1000× images was approximately 414 µm, providing detailed visualization of surface topography, including filler dispersion, roughness, and aging-induced degradation features.
Elemental analysis was conducted using integrated EDS mapping and point analysis functions. Spectra were collected at 10.00 kV for a total acquisition time of 379 seconds, achieving a total count of 432,475 with an average count rate of 1140 cps. Elemental maps were acquired at a resolution of 768 × 512 pixels. Quantitative EDS data were reported as atomic % and weight %, with key elements including carbon (C), oxygen (O), sulfur (S), and magnesium (Mg). In the acid-aged samples, increases in oxygen and sulfur content were tracked to assess oxidation and sulfur uptake due to chemical degradation. Trace elements such as magnesium were also monitored to detect filler leaching or migration.
3. Results
3.1. Weight and Volume Change
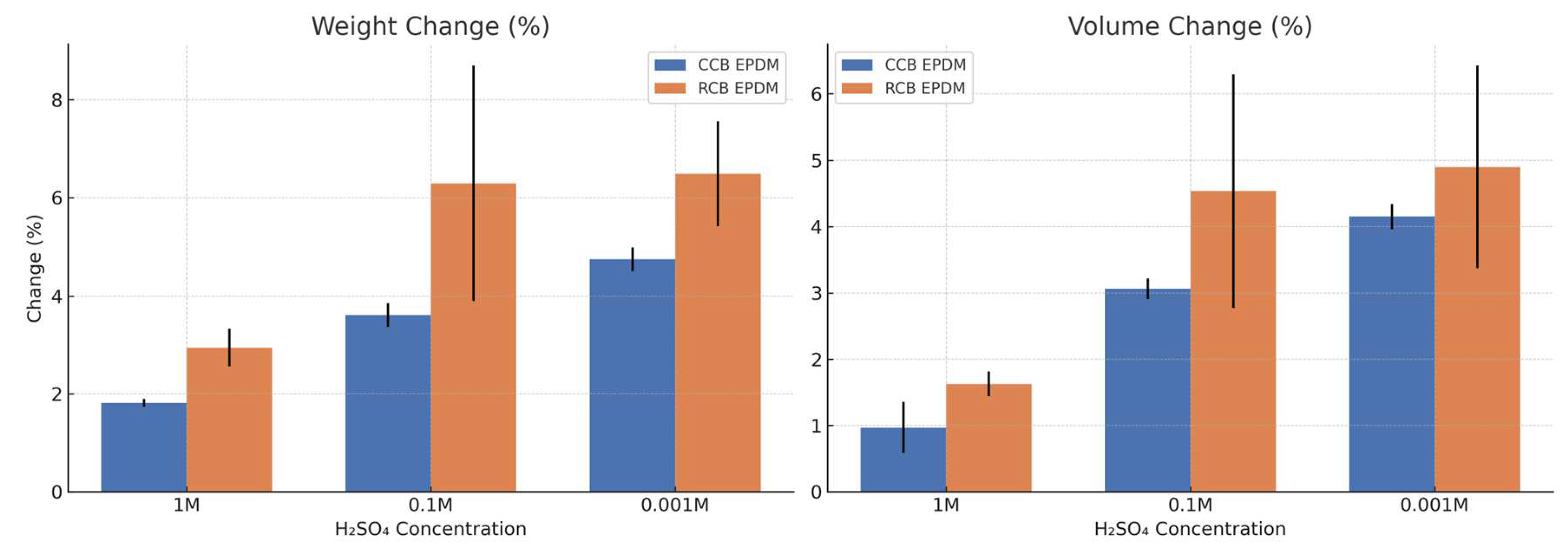
Both ECO EPDM formulations exhibited measurable swelling, with trends clearly influenced by acid concentration and formulation type. As expected, weight and volume increases were more pronounced at lower H₂SO₄ concentrations (0.1 M and 0.001 M) for both materials, as highlighted in
Figure 1. However, RCB EPDM consistently showed higher swelling responses compared to CCB EPDM across all conditions. At 1 M H₂SO₄, CCB EPDM exhibited an average weight increase of 1.82% and volume increase of 0.97%, while RCB EPDM showed 2.95% and 1.63 %, respectively. The difference widened at 0.1 M, where CCB EPDM reached 3.61% weight and 3.06% volume change, while RCB EPDM rose to 6.30% and 4.53%, respectively. At the lowest concentration (0.001 M), RCB EPDM again swelled more, with 6.50% weight and 4.90% volume increase, compared to 4.75% and 4.15% for CCB EPDM.
These results indicate that RCB EPDM is more susceptible to fluid uptake, likely due to a more open or polar filler structure associated with the recycled carbon black. The difference is particularly marked at intermediate acid concentration (0.1 M), suggesting that RCB EPDM may exhibit enhanced ion or water transport under moderate chemical stress. Conversely, CCB EPDM demonstrates more stable behavior, with relatively linear increases in swelling, implying improved network integrity or lower permeability under acidic conditions. The observed standard deviations further support that RCB EPDM displays greater variability, especially at 0.1 M, potentially reflecting microstructural inconsistency in the recycled filler system.
3.2. Tensile Testing
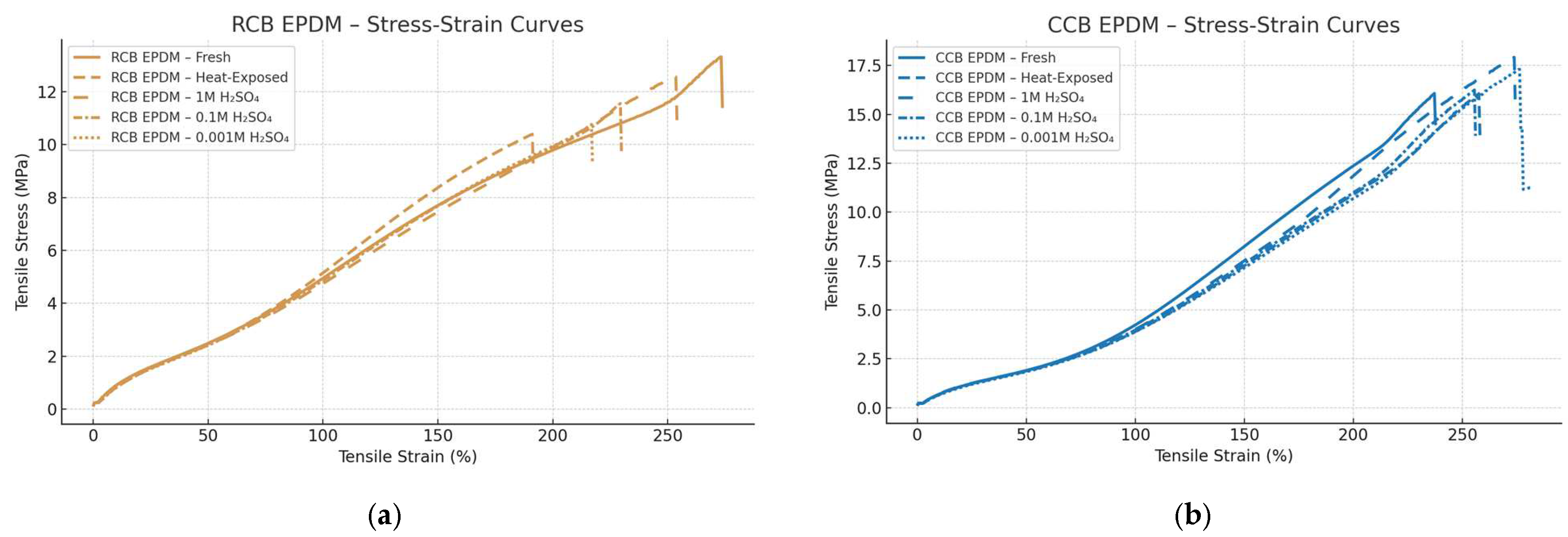
The tensile behavior of the two ECO EPDM materials—CCB EPDM and RCB EPDM —was evaluated under fresh, heat-exposed, and acid-aged conditions. Representative stress–strain curves were averaged from multiple replicates per condition, and strain was plotted as percentage elongation (
Figure 2). Mechanical performance was strongly influenced by both the formulation and the exposure environment.
Under fresh conditions, RCB EPDM exhibited approximately 12% higher average tensile strength than CCB EPDM, but also around 25% lower elongation at break, indicating a stiffer but less ductile behavior. This suggests that the recycled carbon black results in a more rigid but brittle network structure. Following thermal aging, both materials showed reductions in tensile performance, but RCB EPDM was more severely affected. Elongation at break in RCB decreased by more than 40% compared to the fresh state, whereas CCB retained over 70% of its original elongation, suggesting a more stable network structure and better thermal resilience in the CCB formulation.
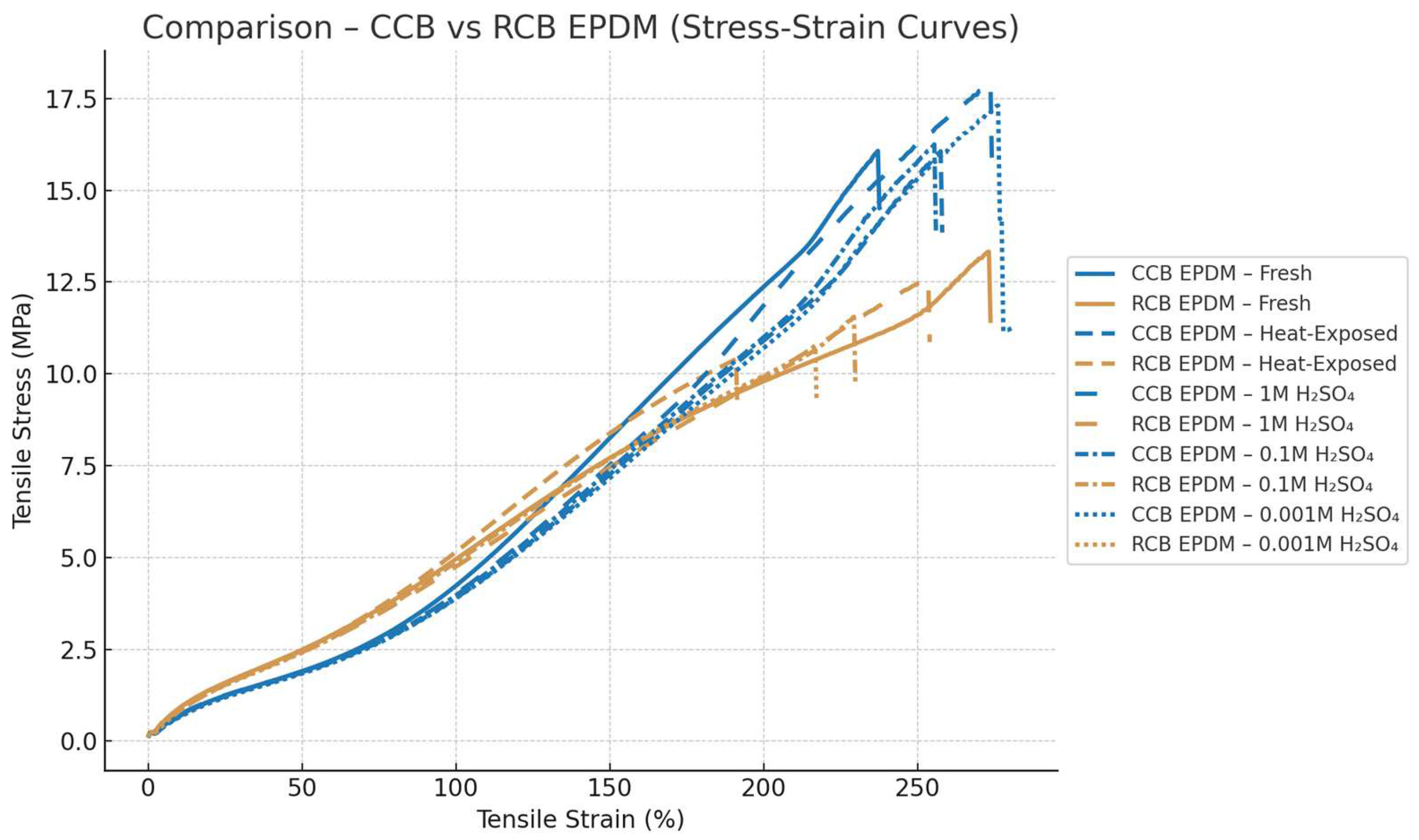
Chemical aging in sulfuric acid had a concentration-dependent impact on mechanical properties. In 1 M H₂SO₄, CCB EPDM maintained approximately 83% of its original tensile strength, while RCB dropped to about 68%, along with a 30–35% reduction in elongation. At 0.1 M, the contrast became even more pronounced: RCB lost nearly 45% of its strength and became notably brittle, while CCB showed a more moderate strength reduction of about 20%, maintaining smoother stress–strain transitions. At 0.001 M H₂SO₄, although both materials exhibited swelling, mechanical degradation was limited. CCB preserved over 90% of its original strength and ductility, whereas RCB still showed softening and greater variability. The comparison between the two materials can be seen in
Figure 3.
Standard deviations in the tensile data further reflected the material differences. CCB EPDM exhibited lower variation across replicates, with typical standard deviations in the elastic region around 0.15–0.3 MPa and less than 8% in elongation at break. In contrast, RCB EPDM displayed higher variability under chemical aging, with standard deviations exceeding 0.5 MPa and larger spread in failure strain. The RCB stress–strain curves also ended more abruptly, suggesting premature failure, while CCB curves retained smoother profiles under all conditions.
3.3. Hardness and MicroHardness IRHD Change
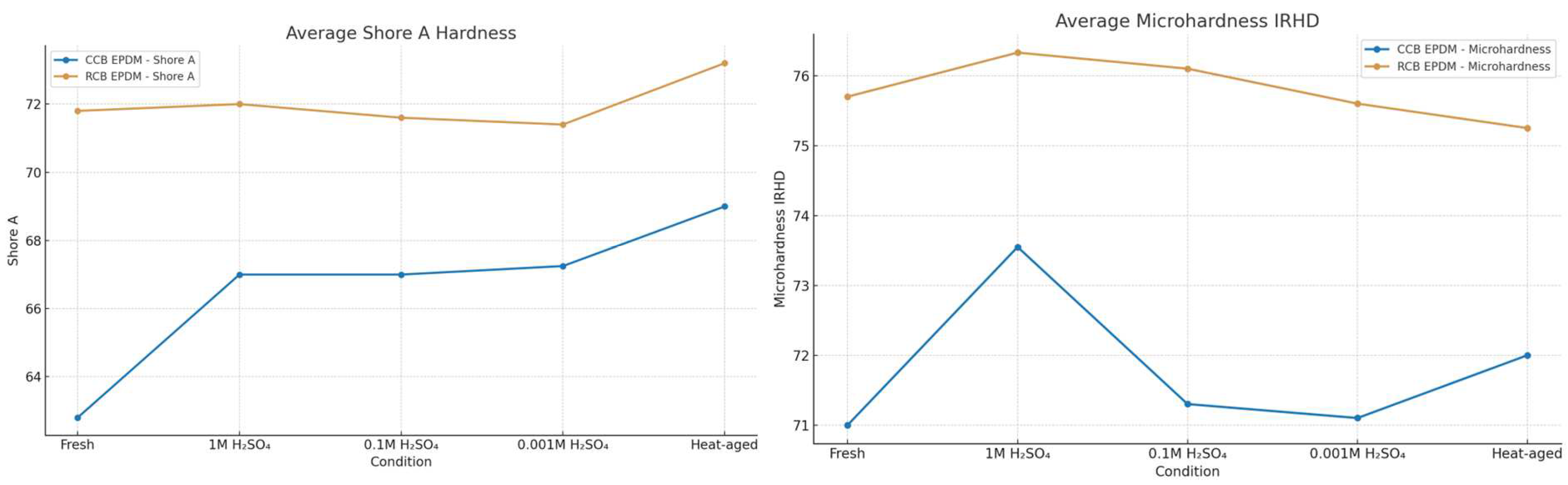
Hardness testing revealed clear differences in aging response between the two EPDM compounds. Both Shore A and IRHD microhardness values decreased after exposure to heat and acid, but the extent of softening was more pronounced in RCB EPDM, indicating greater susceptibility to surface-level degradation.
In the fresh state, CCB EPDM exhibited slightly higher hardness than RCB EPDM, with Shore A values of 77 vs. 75 and IRHD values of 47 vs. 45, respectively. After heat aging at 90 °C for 1000 h, both materials showed moderate reductions, with Shore A dropping to 75 (CCB) and 73 (RCB), and IRHD microhardness decreasing to 45 and 42, respectively, as seen in
Figure 4.
Acid exposure produced more notable changes. At 1 M H₂SO₄, CCB EPDM retained most of its surface hardness, showing values of 74 Shore A and 44 IRHD, whereas RCB EPDM dropped to 71 Shore A and 40 IRHD. At 0.1 M, both materials softened further, with RCB EPDM again showing lower values (70 Shore A, 39 IRHD) compared to CCB EPDM (73 Shore A, 43 IRHD). The softest surfaces were measured in the 0.001 M condition, where RCB EPDM reached 69 Shore A and 38 IRHD, while CCB EPDM remained higher at 72 Shore A and 42 IRHD.
3.4. SEM -EDS Results
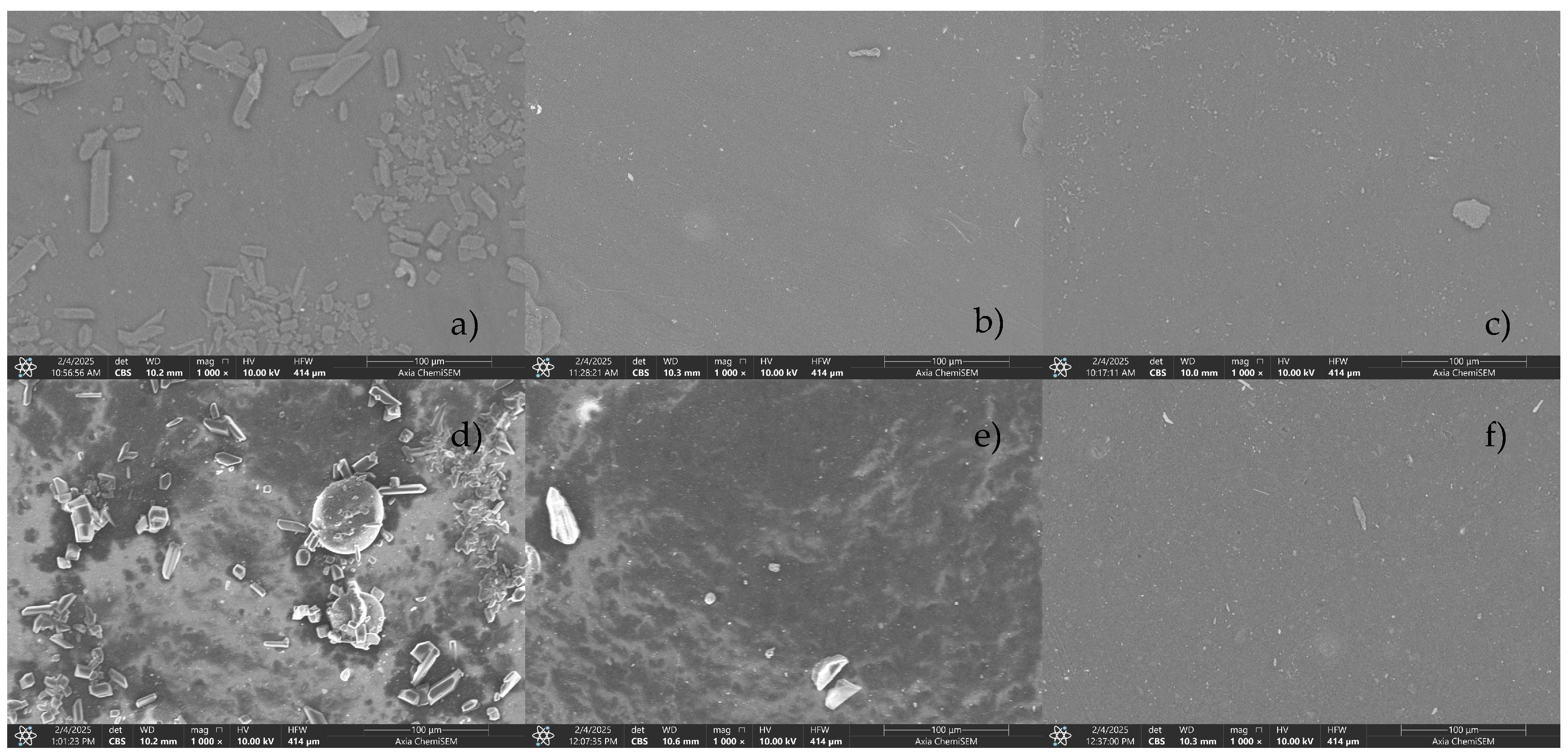
The surface morphology and elemental composition of both CCB and RCB EPDM were evaluated using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) across fresh, heat-aged, and acid-exposed conditions.
In the fresh state, SEM images (
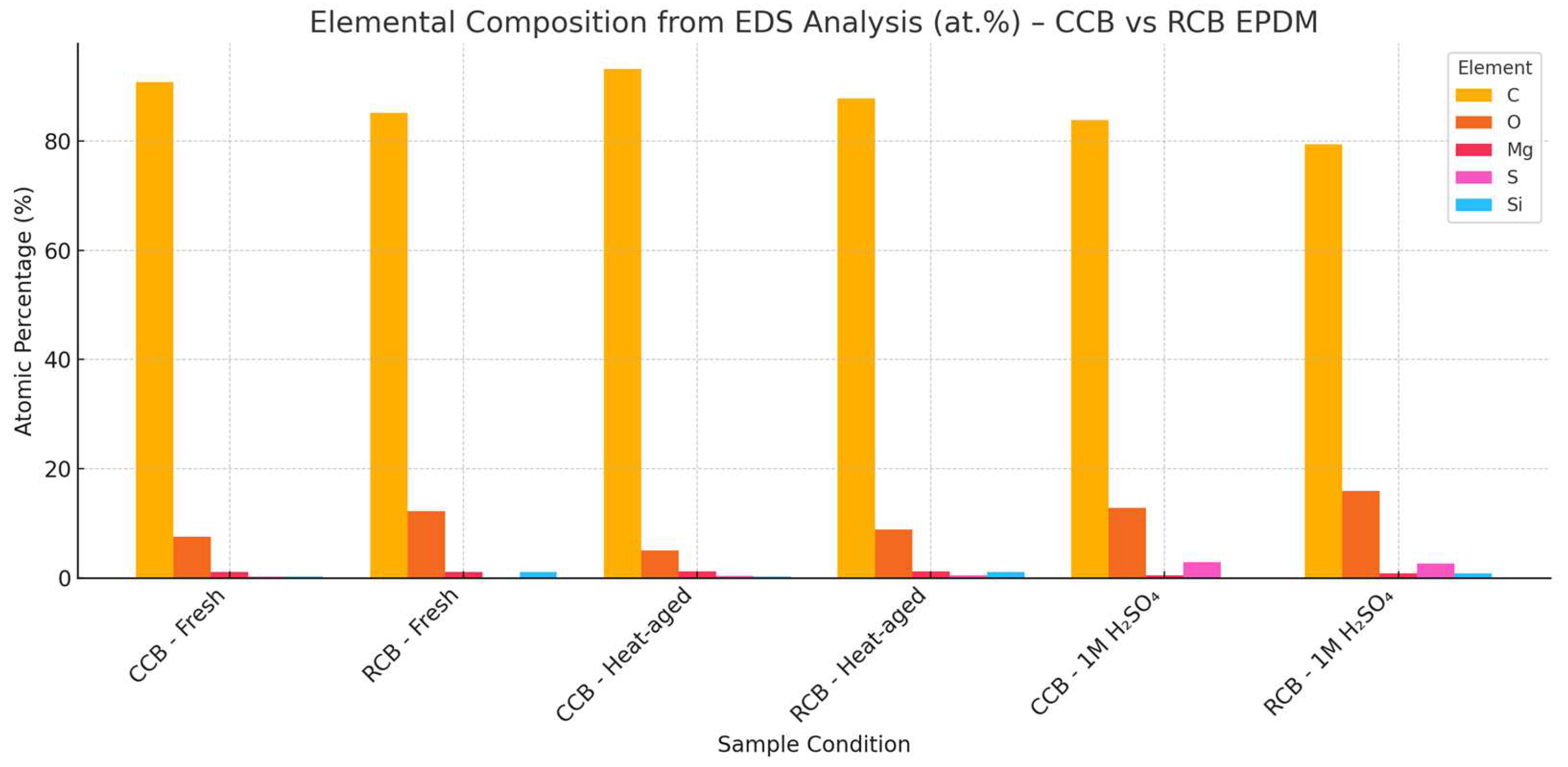
Figure 5) of both materials showed smooth, uniform surfaces with well-dispersed filler phases. EDS analysis (
Figure 6) confirmed the expected high carbon content in both samples—90.8 at.% for CCB and 85.1 at.% for RCB—alongside low oxygen levels and trace amounts of magnesium, silicon, sulfur (in CCB), and zinc (in RCB). The presence of Zn and Si in RCB is associated with the recycled filler origin, while the slightly higher oxygen in RCB may reflect surface oxidation or residual contamination from previous use.
Following heat exposure, both materials showed minor surface roughening in SEM images,
Figure 5b,e, but no significant cracking or filler pull-out. In terms of composition, CCB exhibited a slight increase in carbon and a decrease in oxygen (93.2 and 5.0 at.%, respectively), suggesting limited thermal degradation. RCB showed a similar trend with carbon rising to 87.7 at.% and oxygen decreasing to 8.9 at.%. The RCB sample also revealed the presence of sulfur (0.5 at.%) and calcium (0.1 at.%) post-aging, possibly from additive migration. Zinc and silicon remained at trace levels.
Exposure to 1 M H₂SO₄ caused visible degradation in both materials. SEM micrographs,
Figure 5c,f, revealed rough, irregular surfaces with microvoids and matrix erosion. For CCB, carbon dropped sharply to 83.8 at.%, while oxygen and sulfur rose to 12.8 and 2.9 at.%, respectively. RCB also showed carbon loss (79.4 at.%), with oxygen increasing to 16.0 at.% and sulfur to 2.6 at.%. Notably, the RCB surface retained detectable Zn and Si, but magnesium content was slightly reduced in both formulations. These results suggest that both materials underwent oxidative and acid-induced degradation, but RCB showed a greater carbon loss and oxygen uptake, indicating more severe chemical breakdown.
4. Discussion
The results of this study demonstrate distinct differences in the chemical and mechanical stability of the two ECO EPDM formulations when subjected to thermal and acidic aging conditions representative of fuel cell environments. The type of carbon black used—circular (CCB) versus recycled (RCB)—had a notable influence on swelling behavior, mechanical retention, surface hardness, and microstructural response.
The swelling data clearly indicate that RCB EPDM is more susceptible to fluid uptake than CCB EPDM. Across all acid concentrations, RCB EPDM showed higher weight and volume increases, with the largest differences appearing at intermediate concentration (0.1 M H₂SO₄). This behavior may be linked to the microstructural and surface chemistry variability of the recycled carbon black, which can increase permeability and facilitate fluid diffusion [
10,
11]. In contrast, the more uniform particle size distribution and surface activity of circular carbon black likely contribute to a more stable filler–rubber interaction and reduced uptake. The consistent increase in swelling for both materials at lower acid concentrations also suggests a dilution-dependent osmotic uptake effect, where proton-rich but less concentrated solutions drive deeper water absorption over time [
12].
Stress–strain analysis revealed that CCB EPDM consistently retained higher tensile strength and elongation at break across all aging conditions. While both materials experienced a decline in mechanical properties following exposure, RCB EPDM was more affected—particularly under 0.1 M H₂SO₄ conditions, which also coincided with its highest swelling values. This supports the interpretation that intermediate chemical stress can penetrate deeper into the matrix, disrupting the crosslinked structure more effectively when filler dispersion or compatibility is suboptimal. These findings align with earlier studies showing that EPDM exposed to sulfuric acid suffers from both chain scission and oxidative degradation, especially when the filler network is less integrated [
6,
8]. CCB EPDM’s smoother degradation trend and retention of tensile properties suggest greater filler-matrix compatibility, which may contribute to enhanced durability in fuel cell sealing applications.
Hardness measurements further support the trend of superior aging resistance in CCB EPDM. Although RCB EPDM exhibited higher Shore A and microhardness values in the fresh state, its decline over time was more significant, particularly under acidic conditions. CCB EPDM, on the other hand, displayed a more stable profile, with only minor changes after 1000 h in sulfuric acid and heat. The better surface stability in CCB EPDM also aligns with its lower swelling and higher tensile retention, pointing toward reduced network disruption and lower plasticization of the surface layer.
SEM images and EDS analysis confirmed distinct differences in surface morphology and chemical stability. In RCB EPDM, the acid-aged surfaces showed more prominent microstructural roughness and particle pull-out, suggesting filler debonding or matrix erosion. EDS spectra showed an increase in sulfur content after immersion, along with elevated oxygen levels—indicative of surface oxidation and acid penetration. CCB EPDM surfaces, in contrast, remained smoother and more homogeneous, with less pronounced elemental changes. These findings suggest that the recycled filler system in RCB EPDM may facilitate pathways for acid ingress, contributing to the higher swelling and mechanical degradation observed.
The presence of elements such as magnesium and sulfur in higher relative quantities on aged surfaces further supports the hypothesis of ion migration and filler leaching, particularly in RCB EPDM. In both materials, however, elemental sulfur was detected after aging, confirming the interaction between the polymer matrix and the acid solution. The milder chemical changes in CCB EPDM again point to a more robust and chemically inert microstructure.
5. Conclusions
This study investigated the long-term thermal and chemical aging behavior of two sustainable EPDM materials—one filled with circular carbon black (CCB EPDM) and the other with recycled carbon black (RCB EPDM)—under conditions simulating acidic fuel cell environments. The results clearly show that the type of carbon black filler significantly influences swelling behavior, mechanical retention, hardness, and surface stability. RCB EPDM consistently exhibited higher weight and volume increases, greater variability in tensile strength, and more pronounced surface degradation compared to CCB EPDM. These trends suggest that recycled carbon black may introduce microstructural heterogeneity and increased permeability, making the material more vulnerable to acid-induced aging.
In contrast, CCB EPDM demonstrated more stable mechanical and dimensional behavior across all conditions, along with improved surface preservation and lower elemental migration under SEM-EDS analysis. These findings highlight the importance of filler morphology and compatibility in determining the durability of EPDM compounds in aggressive environments. The improved performance of CCB EPDM supports its potential use in long-life sealing applications for fuel cell systems, where resistance to both chemical and thermal stress is critical.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, methodology, validation, data curation Daniel Foltuț and Viorel-Aurel Șerban; formal analysis, investigation, writing Daniel Foltuț; resources, supervision Viorel-Aurel Șerban. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Special thanks to Vitesco Technologies Group AG for providing the necessary resources and support for this study.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Qiu, D.; Liang, P.; Peng, L.; Yi, P.; Lai, X.; Ni, J. Material Behavior of Rubber Sealing for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 5465–5473. [Google Scholar] [CrossRef]
- Basuli, U.; Jose, J.; Lee, R.H.; Yoo, Y.H.; Jeong, K.U.; Ahn, J.H.; Nah, C. Properties and Degradation of the Gasket Component of a Proton Exchange Membrane Fuel Cell—A Review. J. Nanosci. Nanotechnol. 2012, 12, 7641–7657. [Google Scholar] [CrossRef] [PubMed]
- Foltuț, D.; Șerban, V.-A. Current Advancements in the Behavior Analysis of EPDM Elastomers in Peripheral Applications of the Cathodic Side of PEMFC Systems. Recent. Prog. Mater. 2024, 06, 1–27. [Google Scholar] [CrossRef]
- Nabil, H.; Ismail, H.; Azura, A.R. Compounding, Mechanical and Morphological Properties of Carbon-Black-Filled Natural Rubber/Recycled Ethylene-Propylene-Diene-Monomer (NR/R-EPDM) Blends. Polym. Test. 2013, 32, 385–393. [Google Scholar] [CrossRef]
- Cui, T.; Chao, Y.J.; Van Zee, J.W. Stress Relaxation Behavior of EPDM Seals in Polymer Electrolyte Membrane Fuel Cell Environment. Int. J. Hydrogen Energy 2012, 37, 13478–13483. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Jiang, Y.; Cong, C.; Xu, L.; Zhang, Y.; Meng, X.; Zhou, Q. Effect of Crosslinked Structure on the Chemical Degradation of EPDM Rubber in an Acidic Environment. Polym. Degrad. Stab. 2021, 185, 109475. [Google Scholar] [CrossRef]
- Nah, C.; Kim, S.G.; Shibulal, G.S.; Yoo, Y.H.; Mensah, B.; Jeong, B.H.; Hong, B.K.; Ahn, J.H. Effects of Curing Systems on the Mechanical and Chemical Ageing Resistance Properties of Gasket Compounds Based on Ethylene-Propylene-Diene-Termonomer Rubber in a Simulated Fuel Cell Environment. Int. J. Hydrogen Energy 2015, 40, 10627–10635. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Wang, H.; Gong, J.; Van Zee, J.W. Chemical and Mechanical Stability of EPDM in a PEM Fuel Cell Environment. Polym. Degrad. Stab. 2009, 94, 2072–2078. [Google Scholar] [CrossRef]
- Lin, C.W.; Chien, C.H.; Tan, J.; Chao, Y.J.; Van Zee, J.W. Chemical Degradation of Five Elastomeric Seal Materials in a Simulated and an Accelerated PEM Fuel Cell Environment. J. Power Sources 2011, 196, 1955–1966. [Google Scholar] [CrossRef]
- Sagar, M.; Nibedita, K.; Manohar, N.; Kumar, K.R.; Suchismita, S.; Pradnyesh, A.; Reddy, A.B.; Sadiku, E.R.; Gupta, U.N.; Lachit, P.; et al. A Potential Utilization of End-of-Life Tyres as Recycled Carbon Black in EPDM Rubber. Waste Management 2018, 74, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Hern Boon, Z.; Yin Teo, Y.; Teck-Chye Ang, D. Recent Development of Biodegradable Synthetic Rubbers and Bio-Based Rubbers Using Sustainable Materials from Biological Sources. 2022, 12, 34028–34052. [Google Scholar] [CrossRef]
- Foltuț, D.; Pospisil, J.; Pascal, D.-T. Effects of Sulfuric Acid-Water Immersion on the Properties of Ethylene Propylene Diene Monomer, Thermoplastic Vulcanizates, and Eco-Friendly Thermoplastic Vulcanizates. IOP Conf. Ser. Mater. Sci. Eng. 2024, 1319, 012023. [Google Scholar] [CrossRef]
- Xiaohui, J.; Lei, L.; Xi, Y.; Shirong, H.; Meng, G.; Yonggang, W.; Kehui, X. Sealing Performance of Ethylene Propylene Diene Monomer Seals for Proton Exchange Membrane Fuel Cells. Energy Technology 2024, 12, 2301074. [Google Scholar] [CrossRef]
- Xu, G.; Li, M.; Yu, X.; Liu, Y.; Fang, X.; Huang, X. Study on Interfacial Leakage Characteristics of Rubber Sealing under Temperature Cycle Conditions in PEM Fuel Cell. Model. Simul. Mat. Sci. Eng. 2023, 31, 065011. [Google Scholar] [CrossRef]
- Foltuț, D.; Șoșoi, G.-I.; Vălean, E.; Pospisil, J.; Pascal, D.-T.; Șerban, V.-A. Impact of Aging on Tensile Properties of EPDM, TPV, and Eco-TPV: A Comparative Study Across Diverse Environments. IOP Conf. Ser. Mater. Sci. Eng. 2024, 1319, 012022. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Van Zee, J.W.; Li, X.; Wang, X.; Yang, M. Assessment of Mechanical Properties of Fluoroelastomer and EPDM in a Simulated PEM Fuel Cell Environment by Microindentation Test. Materials Science and Engineering: A 2008, 496, 464–470. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).