1. Introduction
Psoriasis is a chronic immune-mediated skin disorder characterized by keratinocyte hyperproliferation and inflammatory cell infiltration, manifesting clinically as erythematous plaques with distinctive silvery scales[
1]. The hallmark histopathological features include acanthosis, parakeratosis, attenuation of the granular layer, dilated dermal capillaries, and neutrophilic infiltration, reflecting underlying dysregulation of keratinocyte death processes and immune responses. Historically, psoriasis has been considered as a hyperproliferative disorder of keratinocytes and studies demonstrate that psoriatic keratinocytes exhibit enhanced resistance to apoptotic induction compared to normal skin cells[
2]. Quantitative analyses reveal differential apoptotic indices: 0.12% in normal epidermis, 0.035% in established psoriatic lesions, and 0.31% in regressing psoriatic lesions[
3]. Thus, the understanding of how cells, particularly keratinocytes, die in psoriasis with epidermal remodeling is important in the natural course and treatment of psoriasis.
Recent advances in cell death research have revealed multiple distinct pathways through which cells undergo programmed or regulated death, including autophagy, apoptosis, anoikis, necroptosis, pyroptosis, PANoptosis, parthanatos, ferroptosis, cuproptosis and NETosis. Each of these pathways exhibits unique molecular signatures and contributes distinctively to the inflammatory cascade associated with psoriasis[
4,
5,
6,
7,
8,
9,
10]. Understanding the intricate interplay between these cell death mechanisms and their contributions to psoriatic pathology is fundamental for developing targeted therapeutic interventions. This comprehensive review investigates the diverse modes of cellular death in psoriatic lesions, particularly focusing on keratinocytes, and their implications for disease progression and possible treatment strategies. Although psoriasis is characterized by aberrant cornification, which process was recognized as a mode of cell death by the previous classification of Nomenclature Committee on Cell Death (NCCD)[
11], herein we analyze keratinocyte cell death patterns in psoriasis, excluding cornification, which is now classified as terminal differentiation rather than cell death following the latest NCCD recommendations[
12].
2. Methods
2.1. The Approach: Bibliographic Research
We performed a literature search in electronic databases (PubMed, Embase, Google Scholar) for relevant articles from inception to 31 Jan, 2025 utilizing the keywords: (cell death or autophagy or autophagic cell death or apoptosis or anoikis or necroptosis or pyroptosis or PANoptosis or parthanatos or ferroptosis or cuproptosis or NETosis) AND (psoriasis or interleukin 17 or interleukin 23 or tumor necrosis factor or methotrexate or retinoid or mTOR inhibitor or calcitriol or calcipotriol or ultraviolet or dimethyl fumarate or nicotinamide or glutathione or selenium or coenzyme Q10 or deferoxamine or myeloperoxidase inhibitor or protein arginine deiminase 4 inhibitor ). Titles and abstracts were inspected to include articles concerning psoriasis and its treatment or pathogenesis for review. Articles of both human and animal models were included. Only articles available in English were included.
2.2. Tools for Manuscript Preparation and Visualization
The Claude artificial intelligence platform was employed to assist in enhancing the quality and fluency of academic text, and all figures were generated using BioRender software.
3. Results
3.1.1. Autophagic Cell Death and Psoriasis
Autophagy, a cellular self-degradation mechanism, is an essential biological process that maintains cellular homeostasis through lysosome-mediated degradation of various cellular components, including nucleic acids, proteins, lipids, and organelles[
13]. This fundamental process plays vital roles in cellular differentiation, development, and survival. In the context of psoriasis pathogenesis, autophagic cell death is particularly significant, modulating inflammatory cascades crucial to disease development[
14]. The intricate interplay between autophagic cell death and inflammatory pathways is evidenced by multiple studies: Toll-like receptor (TLR)2/6 or TLR4 stimulation activates the autophagic cell death in human keratinocytes[
15], while psoriasis-associated cytokines such as TNF-α and IL-17A impair autophagy in these cells[
16,
17]. Several key inflammatory mediators modulate autophagic function in psoriatic conditions. IL-17A induces autophagic dysfunction in keratinocytes through the PI3K/AKT/mTOR pathway, resulting in elevated pro-inflammatory cytokine production, including IL-6 and IL-8[
18]. Similarly, IL-33 inhibits autophagy via STAT3 phosphorylation, thereby exacerbating the inflammatory response in psoriatic mice model[
19]. The aryl hydrocarbon receptor (AhR) activation suppresses autophagy and promotes skin inflammation through the NF-Kappa B (NF-κB)/p38 mitogen-activated protein kinase (MAPK) signaling pathway[
20], leading to inflammatory cytokine secretion (IL-1β, IL-6, and TNF-α) in keratinocytes[
18]. Furthermore, inactivation of the MAPK family decreases keratinocyte autophagy, which correlates positively with psoriatic severity in both patients and mouse models, underscoring the critical role of autophagy in keratinocyte proliferation and differentiation through mechanisms including cell cycle regulation and mitochondrial reactive oxygen species production[
21]. These findings suggest that autophagy dysfunction is integrally associated with inflammatory processes and the pathogenesis of psoriasis.
3.1.2. Therapeutic Modulation of Autophagy in Psoriasis
Current therapeutic approaches targeting autophagy pathways show promising results in psoriasis treatment. Retinoids, widely used in patients with psoriasis, normalize keratinization[
22] and promote autophagosome maturation and autophagy induction[
23], suggesting a potential mechanism through which keratinization may be regulated.
Ultraviolet B (UVB) therapy represents another treatment option for patients with psoriasis, demonstrating dual benefits of both pruritus relief and immunomodulatory effects[
24]. Moreover, studies suggest that UVB stimulates epidermal cell autophagy through the GSK3β/MAPK signaling pathway[
25]. Importantly, therapeutic targeting of these pathways shows promise, as evidenced by rapamycin, an mTOR inhibitor widely used in clinical practice, which has demonstrated therapeutic potential in both cellular and animal models of psoriasis through its inhibition of the mTOR pathway[
26,
27]. In addition, a case report also showed the combination of mTOR inhibitors including everolimus and tacrolimus could treat recalcitrant psoriasis[
28]. Metformin, a well-established therapeutic agent for type II diabetes mellitus, has been demonstrated its efficacy in the treatment of psoriasis[
29]. Metformin is also a mTOR inhibitor and promotes the conversion of T helper 17 (Th17) cells to regulatory T (Treg) cells through enhanced autophagic processes[
30], suggesting its potential therapeutic application in psoriasis management through modulation of type 17-mediated inflammation. Consequently, therapeutic interventions targeting IL-17-mediated signaling cascades may represent a promising strategic approach for psoriasis treatment, operating through the restoration of physiological autophagic function and related mechanisms[
31].
3.2.1. Apoptosis and Psoriasis
Apoptosis, the prototype of programmed cell death (RCD), is orchestrated through the activation of caspase family proteases[
32]. This process manifests through two principal pathways: intrinsic and extrinsic apoptosis. The intrinsic pathway can be triggered by diverse stimuli, including DNA damage, ribotoxic stress response, endoplasmic reticulum stress, oxidative stress, growth factor deprivation, and microtubular alterations[
32,
33]. A pivotal step in intrinsic apoptosis involves the activation of pro-apoptotic BCL2 family effectors—specifically BAX, BAK, and potentially BOK—which facilitate apoptosome formation and sequential activation of caspase 9 and executioner caspases 3 and 7[
34]. The extrinsic pathway initiates through death receptor-ligand interactions (such as FASL-FAS, TNF-TNFR), forming the death-inducing signaling complex (DISC), which activate caspase 8. Subsequently, caspase 8 triggers cell death either directly via caspase 3/7 or through BH3-interacting domain death agonist-mediated mitochondrial outer membrane permeabilization[
34].
Psoriatic skin lesions exhibit complex apoptotic regulation involving multiple protein families and signaling cascades. The Bcl-2 family proteins, comprising both pro-apoptotic (Bax, Bak, Bad) and anti-apoptotic (Bcl-2, Bcl-xL) members, are integral to this regulation. Previous study has demonstrated elevated Bcl-xL expression in psoriatic epidermis compared to normal tissue[
35]. TNF-α signaling demonstrates a complex dual role by upregulating both pro- and anti-apoptotic factors[
36], ultimately conferring hyperproliferation of keratinocytes[
37]. Furthermore, psoriatic lesions exhibit decreased apoptotic activity compared to normal epidermis[
3], with inflammatory mediators IL-15[
38] and TGF-α[
39] serving as potent inhibitors of keratinocyte apoptosis observed in psoriatic tissue.
3.2.2. Therapeutic Modulation of Apoptosis in Psoriasis
Current therapeutic interventions demonstrate differential effects on apoptotic pathways. Notably, enhanced apoptotic activity has been observed following the administration of psoralen plus ultraviolet A (PUVA) therapy to psoriatic lesions[
3]. Narrowband ultraviolet B (nbUVB) phototherapy has emerged as a widely adopted treatment modality for inflammatory skin conditions. Previous studies have demonstrated that nbUVB induces apoptosis not only in keratinocytes[
40,
41] but also in T cells[
42].
Methotrexate (MTX) is one of most widely used systemic medication for the treatment of psoriasis. MTX-induced epidermal necrosis is well-documented in psoriasis[
43], but rarely reported in other inflammatory diseases such as atopic dermatitis[
44]. This disparity largely stems from methotrexate's mechanism of inducing apoptosis in proliferating keratinocytes[
45], coupled with the significantly higher keratinocyte turnover rate in psoriasis compared to atopic dermatitis[
46]. Methotrexate intoxication demonstrated a significant elevation in the gene expression of apoptotic biomarker, namely Bax, and a significant reduction in antiapoptotic marker Bcl2 respectively, as compared to the control value[
47]. Furthermore, MTX induces apoptosis through oxidative stress pathways by reducing nitric oxide and increasing caspase-3 levels, partially explaining its therapeutic efficacy in treating psoriatic acanthosis[
48]. Contemporary molecular research has unveiled microRNA regulation as a pivotal mediator of MTX's therapeutic action. MTX administration markedly suppresses miR-155 expression in psoriatic lesions, with mechanistic studies in HaCaT keratinocytes establishing miR-155's role as a critical determinant of cellular fate. The overexpression of miR-155 significantly modulates cell cycle progression, manifesting in G0/G1 phase arrest and inhibition of apoptotic processes[
49].
Vitamin D demonstrates a concentration-dependent biphasic effect on keratinocyte survival: physiological levels elicit protection against multiple apoptotic triggers, including ceramide, UV radiation, and TNF-α, whereas elevated concentrations promote programmed cell death[
50]. Furthermore, vitamin D exerts its antiapoptotic effects through multiple molecular cascades, encompassing the modulation of the pro-survival to pro-death protein balance, specifically enhancing the ratio of Bcl-2 to Bad and Bax[
51]. Calcipotriol, a vitamin D3 analogue widely used in topical psoriasis treatment, has been demonstrated to enhance apoptosis in human psoriatic keratinocytes[
52]. Retinoids have been extensively validated as an effective systemic therapeutic approach for psoriasis[
53]. They serve crucial roles in vital biological processes, including fetal morphogenesis, cellular differentiation, and apoptosis[
54]. In vitro studies utilizing cultured keratinocytes have demonstrated that retinoids induce apoptotic processes[
55]. The therapeutic efficacy of retinoids in psoriasis treatment may be attributed to their ability to induce keratinocyte apoptosis.
3.3.1. Anoikis and Psoriasis
Anoikis is a form of apoptotis triggered by detachment from the extracellular matrix (ECM)[
56,
57]. As a caspase-dependent process, anoikis activates caspase family proteinases, initiating a cascade that leads to cell death similar to apoptotic pathways. While sharing features with apoptosis, anoikis demonstrates unique signaling mechanisms following ECM detachment, involving integrin-mediated signaling, PI3K-AkT signaling cascade, and Fas-dependent pathways[
8]. In psoriasis, a hyperproliferative dermatosis, the normal polarized distribution of integrin expression on keratinocytes becomes disrupted[
58].
3.3.2. Therapeutic Modulation of Anoikis in Psoriasis
The integrin-dependent mechanism of anoikis offers a potential therapeutic target for intervention. Integrins mediate ECM signaling and serve as regulatory points in the anoikis pathway. This therapeutic approach has led to the development of integrin-targeting agents, including efalizumab and etaracizumab, evaluated in clinical trials for psoriasis treatment[
59]. However, efalizumab was subsequently withdrawn from the pharmaceutical market in 2009, following its initial regulatory approval, due to an elevated risk of progressive multifocal leukoencephalopathy[
60]. Similarly, the clinical development of etaracizumab was discontinued following the completion of a Phase II randomized, double-blind, placebo-controlled clinical trial investigating its efficacy in psoriasis treatment.
3.4.1. Necroptosis and Psoriasis
Necroptosis, a regulated form of necrotic cell death, is orchestrated through the activation of tumor necrosis factor receptor (TNFR) by TNFα[
61] or FASL/FAS signaling[
62]. These receptors subsequently recruit adapter proteins, including TRADD or FADD which interact with RIPK1 and caspase-8 or -10[
63]. This process involves the molecular interplay of receptor-interacting protein kinase 1 (RIPK1), receptor-interacting protein kinase 3 (RIPK3), and mixed lineage kinase domain-like pseudokinase (MLKL), which has been implicated in various inflammatory conditions. The RIPK1/RIPK3 complex initiates MLKL phosphorylation and activation, leading to oligomerization and necrosome formation[
64]. These structures subsequently traffic to the plasma membrane in association with tight junction proteins, where they accumulate to form micron-sized structures. The MLKL oligomers specifically localize to phosphatidylinositol phosphate (PIP)-rich regions in the plasma membrane, where they form large pores. The formation of these MLKL pores ultimately triggers necroptotic cell death through multiple mechanisms: facilitating ion influx, inducing cell swelling, and promoting membrane lysis[
65]. This cascade of events culminates in the uncontrolled release of intracellular material.
A recent study has demonstrated necroptosis's crucial role in psoriasis pathogenesis, evidenced by significant upregulation of RIPK1 and MLKL throughout all epidermal layers in human psoriatic lesions[
66]. This necroptotic activation has been further validated in imiquimod (IMQ)-induced psoriasiform murine models. Pharmacological inhibition using R-7-Cl-O-Necrostatin-1 (Nec-1s) and necrosulfonamide (NSA), targeting RIPK1 and MLKL respectively, effectively suppressed necroptosis in both HaCaT cells and IMQ mouse models. This intervention simultaneously attenuated IMQ-induced inflammatory responses and significantly reduced the production of key inflammatory mediators, including IL-1β, IL-6, IL-17A, IL-23A, CXCL1, and CCL20[
66]. The suppression of type 17-associated cytokines, particularly IL-17A and IL-23A, through necroptosis inhibition suggests that necroptosis may contribute to psoriasis pathogenesis via type 17 inflammation. These findings present compelling evidence for targeting necroptosis as a potential therapeutic strategy in psoriasis treatment.
3.4.2. Therapeutic Approaches Targeting Necroptosis in Psoriasis
Dimethyl fumarate (DMF), a derivative of fumaric acid esters, has emerged as a significant first-line systemic therapy in some countries for moderate-to-severe plaque psoriasis[
67,
68]. Clinical observations indicate that DMF typically ameliorates skin inflammation within the initial three months of treatment[
69]. However, the precise molecular mechanisms underlying DMF's therapeutic effects in psoriasis remain incompletely understood. Recent evidence suggests that DMF could exert therapeutic effects through the inhibition of the RIPK1-RIPK3-MLKL necroptotic signaling axis, as demonstrated in both mice models and cellular systems[
70]. Additionally, s
aracatinib, a dual Src/Abl kinase inhibitor currently under clinical development for the treatment of Parkinson's disease, psychosis, idiopathic pulmonary fibrosis and fibrodysplasia ossificans progressiva, demonstrated therapeutic potential in an IMQ-induced psoriasis model by suppressing MLKL phosphorylation and subsequent necroptotic cell death[
71]
.
3.5.1. Pyroptosis and Psoriasis
Pyroptosis, a distinctive form of programmed cell death, is primarily mediated through the gasdermin protein family[
72]. The molecular mechanisms of pyroptosis encompass multiple pathways. In the canonical pathway, Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs) initiate intracellular signaling cascades, leading to the assembly of inflammasomes with pro-caspase-1, subsequently activating caspase-1. Activated caspase-1 cleaves both Gasdermin D (GSDMD) and pro-IL-1β/18. The N-terminal fragment of GSDMD (N-GSDMD) forms nonselective pores in the cell membrane, facilitating water influx that culminates in cell lysis and death while enabling IL-1β and IL-18 secretion. In the noncanonical pathway involves cytosolic lipopolysaccharide (LPS) activation of caspase-4/5, triggering pyroptosis through GSDMD cleavage[
73].
GSDMD cleavage induces potassium efflux, which mediates NLRP3 inflammasome assembly and subsequent processing of pro-IL-1β and pro-IL-18. IL-1β facilitates Th17 cell differentiation and activation[
74]
, while IL-18 stimulates both Th17 and γδ T cells, promoting IL-17 secretion[
75]
.
Recent research has elucidated that GSDMD-mediated pyroptosis exhibits significant pro-inflammatory characteristics, characterized by the substantial release of pro-inflammatory mediators, such as IL-1[
76]. In psoriatic conditions, research has demonstrated aberrant expression of N-terminal GSDMD in epidermal lesions of both human patients and imiquimod-induced psoriasis-like dermatitis (IIPLD) mouse models. Enhanced cleavage products of caspase-1, GSDMD, and IL-1β were observed in both IIPLD mouse epidermis and M5 (simulating psoriatic inflammatory challenge)-treated keratinocytes in vitro. The critical role of GSDMD in pyroptosis was further validated through genetic studies, where Gsdmd-/- keratinocytes failed to exhibit pyroptotic morphology under M5 stimulation[
77]. GSDME can be activated through TNF-α and TNF receptor signaling pathways, which contribute to pyroptotis[
78]. Additionally, GSDME has emerged as another key mediator of pyroptosis in psoriatic conditions, with elevated expression observed in keratinocytes. GSDME-mediated pyroptosis of keratinocytes leads to the secretion of inflammatory mediators, including IL-1β and IL-18[
79]. Gsdme-/- mice and caspase-3 inhibitor treatments have demonstrated attenuated skin inflammation and reduced inflammatory cytokine expression[
80]. Significantly higher GSDME expression levels have also been documented in human psoriatic lesions compared to healthy control
s[
81]
. Notably, emerging evidence has revealed potential mechanistic interconnections between RSR and pyroptosis[
33].
3.5.2. Potential Therapeutic Approaches Targeting Pyroptosis in Psoriasis
Disulfiram, a medication historically used in the treatment of alcohol use disorder, addictions, infections, and inflammatory conditions previously[
82]. Clinical studies have demonstrated the superior efficacy of disulfiram compared to placebo in treating nickel dermatitis[
83,
84] although historical literature has presented conflicting evidence regarding the therapeutic potential of disulfiram in psoriasis treatment[
85]. While the precise mechanism of disulfiram's anti-inflammatory action remains incompletely understood, recent investigations have elucidated the efficacy of disulfiram in IMQ-induced psoriasis models[
86]. Research demonstrates that while disulfiram permits the processing of IL-1β and Gasdermin D (GSDMD), it significantly inhibits pore formation, thereby preventing IL-1β release and subsequent pyroptotic cell death[
87]. In addition, recently developed disulfiram-loaded lactoferrin nanoparticles could be used to alleviate inflammatory diseases such as ulcerative colitis in murine model. Furthermore, recently developed disulfiram-loaded lactoferrin nanoparticles have shown promise in alleviating inflammatory diseases, as demonstrated in a murine model of ulcerative colitis[
88]. The newly identified role of disulfiram in GSDMD inhibition, combined with these novel delivery techniques, suggests promising therapeutic applications for this established drug in the management of psoriasis.
3.6. PANoptosis and Psoriasis
PANoptosis represents a novel inflammatory programmed cell death mechanism, characterized by the coordinated interplay of pyroptosis, apoptosis, and necroptosis pathways through specialized PANoptosome complexes[
5]. The molecular architecture of PANoptosomes encompasses three fundamental protein categories: pattern recognition receptors (including ZBP1 and NLRP3) that detect pathogen- or damage-associated molecular patterns, adaptor molecules (such as FADD), and effector proteins (RIPK1, RIPK3, Caspase 1, and Caspase 8)[
89]. While PANoptosome composition varies by stimulus, core regulatory proteins essential for executing the three death pathways remain consistent. This distinctive death modality appears in multiple pathological conditions, including Candida albicans and Aspergillus fumigatus infection[
90], as well as in various disease states such as cancer[
91], organ failure[
92], and inflammatory bowel disease[
93]. Although correlations between psoriasis and various programmed cell death mechanisms have been studied, the role of PANoptosis in psoriasis remains underexplored.
Transcriptomic analyses of psoriatic lesions have revealed enhanced PANoptotic signaling characterized by upregulation of key mediators including Caspase-1, NLRP3, GSDMD, and IL-1β compared to non-lesional skin[
86]. Comparative transcriptomic study between psoriasis patients and healthy controls has demonstrated elevated expression of PANoptotic activators AIM2 and IRF1[
94]
Another analysis of the PANoptosis signatures reveals distinct cellular correlations within the psoriatic microenvironment. Robust positive correlations have been identified with multiple immune and tissue-resident cell populations, including macrophages, dendritic cells, mesenchymal stem cells, Th1 cells, Th2 cells, melanocytes, monocytes, neutrophils, basophils, and keratinocytes. Although conventional research has predominantly focused on lymphocytes, neutrophils, keratinocytes, and dendritic cells as primary mediators in psoriasis pathogenesis, accumulating evidence indicates essential regulatory roles for fibroblasts and mast cells, despite their negative correlation with PANoptotic signatures[
95]. The IL-17 signaling pathway shows significant enrichment within the psoriasis PANoptosis signatures[
95].
3.7.1. Parthanatos and Psoriasis
Parthanatos represents a regulated cell death cascade characterized by sequential molecular events, including poly (ADP-ribose) polymerase 1 (PARP-1) hyperactivation, poly (ADP-ribose) (PAR) polymer accumulation, PAR-mediated recruitment of apoptosis-inducing factor (AIF), mitochondrial AIF release, nuclear translocation of the AIF/macrophage migration inhibitory factor (MIF) complex, and subsequent MIF-dependent large-scale DNA fragmentation[
96]. PARP1-mediated parthanatos has been implicated in psoriasis pathogenesis, wherein PARP1 promotes cutaneous inflammation through the induction of parthanatos-mediated cell death[
9].
3.7.2. Potential Therapeutic Approaches Targeting Pyroptosis in Psoriasis
Nicotinamide (NAM) and its derivative nicotinamide mononucleotide (NMN) function as PARP-1 inhibitors. Previous investigations have demonstrated that NMN exhibits protective effects against IMQ-induced psoriatic inflammation[
97]. Furthermore, clinical research has also established the therapeutic efficacy of topical NAM administration in psoriasis treatment[
98].
3.8.1. Ferroptosis and Psoriasis
Ferroptosis represents an iron-dependent programmed cell death characterized by phospholipid peroxidation. This process integrates multiple cellular metabolic pathways, including redox homeostasis, iron metabolism, mitochondrial function, and the metabolism of amino acids, lipids, and carbohydrates, along with various disease-relevant signaling cascades[
99]. Ferroptotic cell death requires three essential components: transition metal iron, reactive oxygen species (ROS), and phospholipids containing polyunsaturated fatty acid chains (PUFA-PLs)[
100]. The process is intricately regulated by both cellular metabolism, which influences ROS and PUFA levels, and various extracellular factors. A critical regulator of ferroptosis is selenium, an essential micronutrient required for the biosynthesis of ROS-scavenging selenoproteins, particularly glutathione peroxidase 4 (GPX4)[
101]. GPX4 serves as a key inhibitor of phospholipid peroxidation, while cystine uptake through the system Xc⁻ cystine/glutamate antiporter provides additional protection against ferroptosis by supporting GPX activity[
102]. The molecular mechanisms underlying ferroptosis involve multiple interconnected pathways. Central to this process is the inhibition of system Xc⁻, a membrane transport complex composed of SLC7A11 and SLC3A2, which normally facilitates the exchange of extracellular cystine for intracellular glutamate[
103]. Inhibition of this system impairs cellular cystine uptake, leading to reduced glutathione (GSH) synthesis. Concurrently, cellular iron homeostasis plays a crucial role through the uptake of Fe
3⁺ and its subsequent reduction to Fe
2⁺ via the Fenton reaction, generating substantial reactive oxygen species. The ferroptotic cascade is further modulated by GPX4, a GSH-dependent antioxidant enzyme that typically neutralizes toxic lipid peroxides by converting them to their corresponding alcohols[
6]. Either direct inhibition of GPX4 activity or depletion of its essential cofactor GSH results in the accumulation of lethal lipid peroxides. Recent studies have also illuminated the role of p53 in ferroptosis regulation through its transcriptional suppression of SLC7A11, which compromises cellular cystine uptake.
Notably, similar ferroptotic patterns have been observed in both erastin-treated human primary keratinocytes and the imiquimod (IMQ)-induced psoriasis model. Single-cell analysis has revealed a significant correlation between lipid oxidation activity and the Th22/Th17 response in keratinocytes. Furthermore, ferrostatin-1 (Fer-1), a potent inhibitor of lipid peroxidation, has demonstrated therapeutic potential by suppressing ferroptosis-related changes in erastin-treated keratinocytes and alleviating psoriasiform dermatitis in IMQ-induced models. Significantly, Fer-1 exhibited broad anti-inflammatory effects both in vitro and in vivo, reducing the production of multiple inflammatory cytokines, including TNF-α, IL-6, IL-1α, IL-1β, IL-17, IL-22, and IL-23. This sophisticated interplay between iron metabolism, lipid peroxidation, and antioxidant defense systems underscores the complexity of ferroptotic cell death mechanisms and their potential therapeutic implications in inflammatory skin conditions[
104].
3.8.2. Potential Therapeutic Approaches Targeting Ferroptosis in Psoriasis
A published case report demonstrated significant improvement in the Psoriasis Area and Severity Index (PASI) score following intravenous glutathione administration in a patient with scalp psoriasis[
105]. Similarly, another case report documented substantial improvement in PASI scores after dietary supplementation with glutathione-enhancing, nondenatured whey protein isolate[
106]. These clinical observations suggest a potential therapeutic role for glutathione in modulating psoriatic disease activity, warranting further investigation into glutathione-based interventions for psoriasis management. Selenium plays a crucial protective role in modulating oxidative stress, attenuating lesion development, and regulating immune responses in patients with psoriasis[
107]. Recent investigations have elucidated that selenium's anti-ferroptotic properties are intricately linked to the temporal regulation of selenoprotein GPX4 expression, representing a direct and expeditious protective mechanism against lipid peroxidation[
108]. The biological activity of selenium is primarily executed through its incorporation into selenoproteins, which serve as critical mediators in ferroptosis inhibition. Coenzyme Q10, an essential antioxidant that reduces oxidative stress, demonstrates therapeutic efficacy in patients with psoriasis[
109,
110]. Notably, Coenzyme Q10's interaction with ferroptosis suppressor protein 1 has been shown to suppress ferroptotic cell death, suggesting its potential role in ferroptosis inhibition pathways[
111]. Deferoxamine is a chelating agent that binds to iron and facilitates the removal of excess iron from the body. Several studies have demonstrated that iron overload can trigger psoriasisform skin inflammation[
112,
113]. Furthermore, experimental evidence indicates that deferoxamine reduces keratinocyte proliferation and attenuates epidermal thickness in human 3D organotypic skin models[
113]. These findings collectively suggest that deferoxamine may possess therapeutic potential for the treatment of psoriasis.
3.9. Cuproptosis
Cuproptosis, identified by Tsvetkov et al. in 2022, represents a copper-dependent programmed cell death pathway characterized by mitochondrial copper ion accumulation[
114]. This process triggers the aggregation of lipoylated dihydrolipoamide S-acetyltransferase (DLAT), a crucial component of the mitochondrial tricarboxylic acid (TCA) cycle, thereby precipitating proteotoxic stress that ultimately culminates in cell death[
114]. The biological signature of this death pathway is marked by two distinctive features: the specific aggregation of lipoylated mitochondrial enzymes, particularly DLAT, and the concurrent degradation of iron-sulfur cluster (Fe-S)-containing proteins[
115].
Notably, transcriptomic analyses have revealed significant upregulation of cuproptosis-associated genes (MTF1, ATP7B, and SLC31A1) in psoriatic patients[
10], while additional clinical research has demonstrated elevated serum copper levels in individuals with psoriasis[
116]. Moreover, cellular copper uptake is primarily regulated through the IL-17-STEAP4 axis[
117], with elevated STEAP4 expression observed in keratinocytes of patients with generalized pustular psoriasis[
118]. Indeed, serum STEAP4 levels are consistently higher in psoriasis patients compared to healthy controls[
119], suggesting a potential mechanistic link between cuproptosis and the IL-17 inflammatory cascade. Although the precise mechanisms underlying these associations remain largely unelucidated, and studies investigating copper chelation in psoriasis treatment are currently lacking, the established correlation between cuproptosis, elevated copper concentrations, and psoriasis pathogenesis suggests that therapeutic interventions targeting the cuproptosis pathway may represent a promising novel strategy for psoriasis treatment.
3.10.1. NETosis and Psoriasis
Neutrophil extracellular traps (NETs) are released by activated neutrophils in response to various stimuli. While initially discovered in neutrophils, NET formation has subsequently been observed in other innate immune cells, including macrophages, monocytes, mast cells, basophils, dendritic cells, and eosinophils.[
120]. This process, known as NETosis, is mediated by protein-arginine deiminase 4 (PAD4) and involves the release of intracellular granule components that capture and destroy diverse pathogens, including viral, fungal, bacterial, and protozoal organisms[
121]. During NETosis, neutrophils enhance their antimicrobial capabilities through the release of NETs, which comprise extracellular chromatin decorated with histones and various granular proteins[
122]. While NETosis primarily functions as a critical host defense mechanism against pathogens, its implications extend beyond microbial control. Recent evidence has elucidated the complex role of NETosis in psoriasis pathogenesis, particularly through its interaction with the Th17 inflammatory axis[
123]. In psoriatic conditions, NETosis initiates and amplifies immune responses through multiple pathways. The LL37-DNA complexes generated during NETosis stimulate Th17 cells to secrete cytokines, which subsequently promote keratinocyte LL37 production, establishing a positive feedback loop. Furthermore, these LL37-DNA complexes activate plasmacytoid dendritic cells (pDCs) via TLR9 receptors[
124], leading to TNFα production and monocyte activation. The resulting IL-23 secretion further stimulates Th17 cells to produce IL-17A, which reactivates neutrophils and amplifies inflammatory responses. Concurrent IL-8 release recruits additional neutrophils to lesion sites, perpetuating the inflammatory cascade[
125]. Clinical observations support the significance of this mechanism, with NETosis detected in nearly all psoriasis skin specimens, predominantly in the epidermis. Notably, the absence of visible NETosis in two cases correlated with milder disease presentation and reduced peripheral blood NETotic cell counts, suggesting a potential correlation between NETosis intensity and disease severity[
126].
3.10.2. Potential Therapeutic Approaches Targeting NETosis in Psoriasis
Myeloperoxidase inhibition attenuates psoriasis severity in murine models when administered either systemically or topically[
127]. As a result, myeloperoxidase inhibitor could serve as a potential medication for psoriasis. Previous research has shown that protein arginine deiminase 4 (PAD4) inhibitors can effectively prevent NET formation in arthritis mouse models[
128]. Additionally, serum PAD4 levels have been observed to decrease following treatment with anti-IL-17A, anti-TNFα, and methotrexate[
129]. These findings suggest that PAD4 inhibitors may represent a promising therapeutic approach for psoriasis treatment.
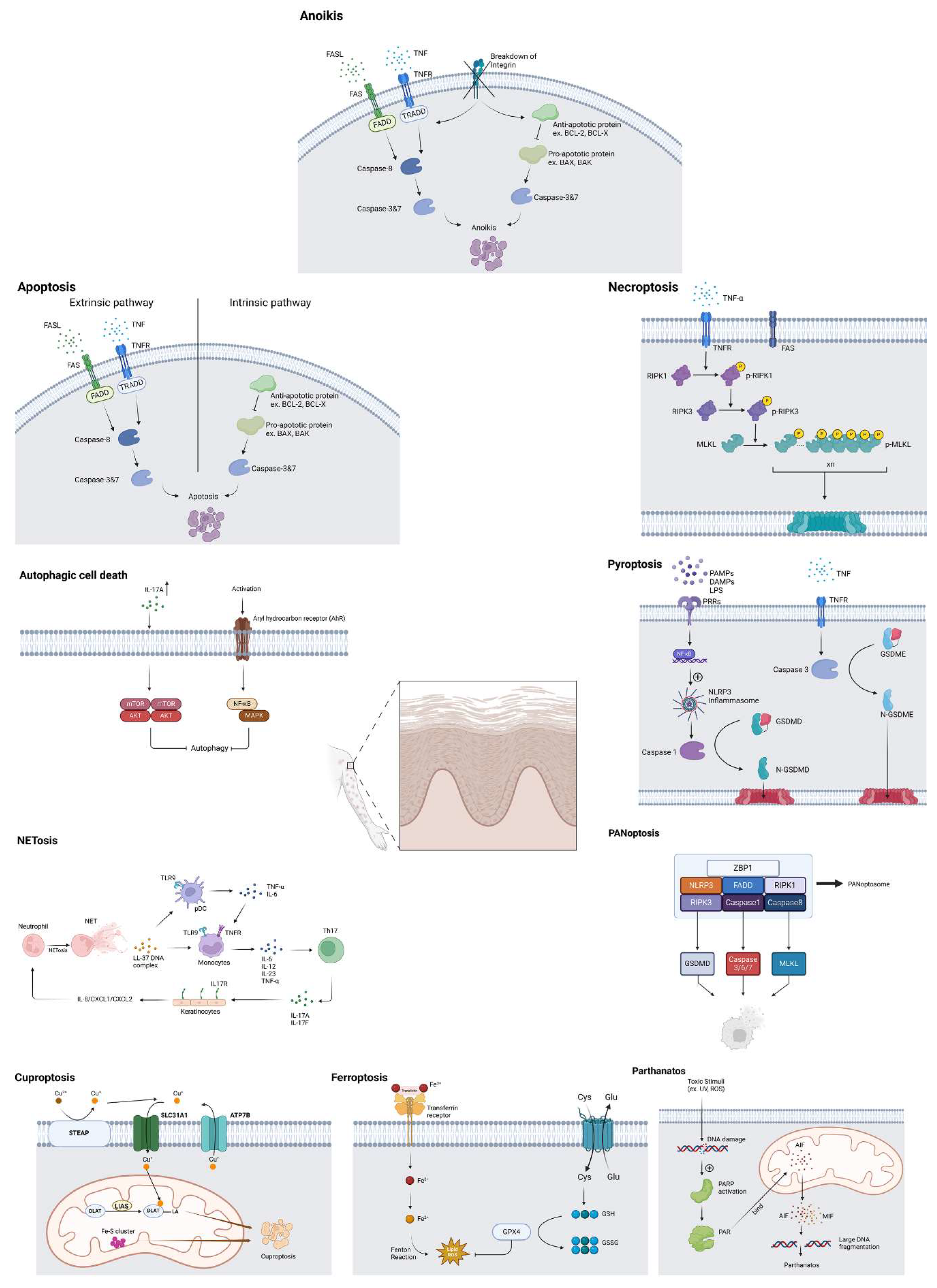
Figure 1.
Cellular death mechanisms in psoriasis.
Figure 1.
Cellular death mechanisms in psoriasis.
Table 1.
Cellular Death Mechanisms Potentially Implicated in the Action of Anti-Psoriatic Therapeutic Interventions.
Table 1.
Cellular Death Mechanisms Potentially Implicated in the Action of Anti-Psoriatic Therapeutic Interventions.
| Management |
Possible mechanism |
References |
| Narrowband ultraviolet B (NbUVB) phototherapy |
Autophagy, Apoptosis |
Y. Yang, et al., 2012 [25]
B. M. Aufiero, et al., 2006 [40]
S. C. Weatherhead, et al., 2011 [41]
M. Ozawa, et al., 1999 [42] |
| Rapamycin |
Autophagy |
M. Gao and X. Si, 2018 [26]
C. Bürger, et al., 2017 [27] |
| Everolimus and Tacrolimus |
Autophagy |
K. C. Wei and P. C. Lai, 2015 [28] |
| Retinoids |
Autophagy, Apoptosis |
Y. Rajawat, et al., 2010 [23]
T. C. Islam, et al., 2000 [55] |
| Metformin |
Autophagy |
Z. Huang, et al., 2023 [29] |
| Psoralen plus ultraviolet A (PUVA) |
Apoptosis |
M. Laporte, et al., 2000 [3] |
| Methotrexate (MTX) |
Apoptosis |
T. Elango, et al., 2017 [45] |
| Vitamin D |
Apoptosis |
P. De Haes, et al., 2004 [51] |
| Calcipotriol |
Apoptosis |
R. Tiberio, et al., 2009 [52] |
| Efalizumab and Etaracizumab |
Anoikis |
J. Mei, et al., 2024 [59] |
| R-7-Cl-O-Necrostatin-1 (Nec-1s) and Necrosulfonamide (NSA) |
Necroptosis (Animal model) |
X. Duan, et al., 2020 [66] |
| Dimethyl fumarate (DMF) |
Necroptosis |
M. Burlando, et al., 2023 [67]
M. Corazza, et al., 2021 [68]
F.-l. Shi, et al., 2023 [70] |
| Saracatinib
|
Necroptosis |
J. Li, et al., 2024 [71] |
| Disulfiram |
Pyroptosis |
X.-m. Hu, et al., 2024 [86]
J. J. Hu, et al., 2020 [87] |
| Nicotinamide and nicotinamide mononucleotide |
Parthanatos |
Z. Zhang, et al., 2024 [97]
M. El-Khalawany, et al., 2022 [98] |
| Ferrostatin-1 (Fer-1) |
Ferroptosis (Animal model) |
Y. Shou, et al., 2021 [104] |
| Glutathione |
Ferroptosis |
Nisha Kundu, et al., 2022 [102]
R. Prussick, et al., 2013 [106] |
| Selenium |
Ferroptosis |
M. Nazıroğlu, et al., 2012 [107]
I. G. Chambers and R. R. Ratan, 2024 [108] |
| Coenzyme Q10 |
Ferroptosis |
G. A. Al-Oudah, et al., 2022 [109]
Z. Kharaeva, et al., 2009 [110]
K. Hadian, 2020 [111] |
| Deferoxamine |
Ferroptosis |
E. Abboud, et al., 2024 [113] |
| Myeloperoxidase inhibitor |
NETosis |
S. D. Neu, et al., 2021 [127] |
| Protein arginine deiminase 4 (PAD4) inhibitors |
NETosis (Animal model) |
C. Gajendran, et al., 2023 [128]
J. Czerwińska, et al., 2022 [129] |
4. Discussion
This comprehensive review elucidates the intricate interplay between cell death pathways in psoriasis pathogenesis and the therapeutic implications. A pivotal finding demonstrates the critical role of autophagy dysfunction in psoriasis, wherein IL-17A mediates autophagy suppression via the PI3K/AKT/mTOR pathway, establishing a fundamental link between inflammatory signaling and cellular homeostasis. This mechanism not only substantiates the efficacy of mTOR inhibitors, such as rapamycin, but also provides a theoretical framework for developing targeted interventions that modulate autophagic processes. The observation of attenuated apoptotic activity in psoriatic lesions, coupled with upregulated anti-apoptotic factors including Bcl-xL, suggests that impaired programmed cell death contributes substantially to the characteristic hyperproliferative state, providing mechanistic insight into the therapeutic efficacy of apoptosis-enhancing interventions, notably PUVA therapy, methotrexate, vitamin D and retinoids. Furthermore, anoikis, resulting from integrin disruption, has been implicated in psoriasis pathogenesis. Although integrin-targeting therapeutics have faced limited clinical implementation due to leukoencephalopathy complications, this pathway warrants further investigation for therapeutic development. A significant advancement in the field stems from the identification of enhanced necroptosis signaling through RIPK1 and MLKL in affected tissues, where successful attenuation of inflammation via necroptosis inhibition in experimental models presents promising therapeutic opportunities, particularly through targeted intervention of the RIPK1-RIPK3-MLKL axis. The involvement of pyroptosis, orchestrated by GSDMD and GSDME, introduces additional complexity to the pathogenic cascade, with elevated expression of these proteins, coupled with their association with IL-1β and IL-18 secretion, illuminating novel aspects of the inflammatory pathway and facilitating innovative therapeutic approaches such as the repurposing of disulfiram as a GSDMD inhibitor. The emergence of PANoptosis as a coordinated mechanism represents a paradigm shift in understanding inflammatory cell death. Strong correlations between PANoptotic signatures and various immune cell populations suggest its central role in orchestrating the inflammatory microenvironment of psoriasis. The contribution of ferroptosis, particularly its association with Th22/Th17 responses, provides novel insights into the influence of iron metabolism and lipid peroxidation, substantiating the therapeutic potential of antioxidants and iron chelators in disease management. Cuproptosis and its connection through elevated copper levels and the IL-17-STEAP4 axis presents new intervention possibilities, while the role of NETosis in amplifying immune responses through interaction with the Th17 axis demonstrates the sophisticated interplay between innate and adaptive immunity, with the correlation between NETosis intensity and disease severity suggesting its potential utility as both a biomarker and therapeutic target. These insights collectively indicate that effective therapeutic strategies may require simultaneous modulation of multiple death pathways, as the efficacy of current therapeutic interventions may be attributed to their impact on various cell death mechanisms rather than modification of a single pathway.
Future research priorities should encompass the development of combination therapies targeting multiple death pathways, investigation of the temporal sequence of death mechanisms during disease progression, identification of pathway-specific biomarkers for personalized treatment approaches, and exploration of novel therapeutic agents targeting emerging pathways such as cuproptosis, while acknowledging current methodological limitations including the challenge of distinguishing between primary and secondary effects of pathway activation and the potential oversight of significant synergistic effects due to the predominant focus on individual pathways rather than their interactions.
5. Conclusions
In conclusion, this extensive analysis elucidates the complex interplay of multiple programmed cell death mechanisms in psoriasis pathogenesis. The identification of these distinct death pathways and their molecular signatures provides crucial insights into the multifaceted nature of psoriatic inflammation. The demonstrated involvement of autophagy dysfunction, apoptotic dysregulation, and various forms of regulated cell death, including necroptosis, pyroptosis, and the newly characterized cuproptosis, underscores the complexity of cellular death processes in psoriatic lesions. Understanding these intricate mechanisms has facilitated the development of targeted therapeutic approaches. These findings not only advance our understanding of psoriasis pathophysiology but also establish a foundation for novel therapeutic strategies. Future research should focus on elucidating the temporal dynamics and cross-talk between these death pathways, potentially leading to more effective combination therapies for enhanced clinical outcomes in psoriasis management.
Author Contributions
Conceptualization, T.-F.T.; data acquisition, C.-H.C.; data analysis: T.-F.T. and C.-H.C.; data interpretation: T.-F.T., C.-H.C. and N.-L.W.; writing—original draft preparation, C.-H.C.; writing—review and editing, T.-F.T. and N.-L.W.; visualization, C.-H.C.; Supervision: T.-F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Tsen-Fang Tsai has served as a consultant for Abbvie, AnaptysBio, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli-Lilly, Galderma, GSK, Janssen-Cilag, Leo Pharma, Merck Sharp and Dohme, Novartis International, Pfizer, PharmaEssentia, Sanofi, Sun Pharma and UCB Pharma. The other authors have no conflict of interest to declare.
References
- Greb, Jacqueline E., Ari M. Goldminz, James T. Elder, Mark G. Lebwohl, Dafna D. Gladman, Jashin J. Wu, Nehal N. Mehta, Andrew Y. Finlay, and Alice B. Gottlieb. "Psoriasis." Nature Reviews Disease Primers 2, no. 1 (2016): 16082.
- Wrone-Smith, T., R. S. Mitra, C. B. Thompson, R. Jasty, V. P. Castle, and B. J. Nickoloff. "Keratinocytes Derived from Psoriatic Plaques Are Resistant to Apoptosis Compared with Normal Skin." Am J Pathol 151, no. 5 (1997): 1321-9.
- Laporte, M., P. Galand, D. Fokan, C. de Graef, and M. Heenen. "Apoptosis in Established and Healing Psoriasis." Dermatology 200, no. 4 (2000): 314-6.
- Bedoui, S., M. J. Herold, and A. Strasser. "Emerging Connectivity of Programmed Cell Death Pathways and Its Physiological Implications." Nat Rev Mol Cell Biol 21, no. 11 (2020): 678-95.
- Wang, Lian, Yanghui Zhu, Lu Zhang, Linghong Guo, Xiaoyun Wang, Zhaoping Pan, Xian Jiang, Fengbo Wu, and Gu He. "Mechanisms of Panoptosis and Relevant Small-Molecule Compounds for Fighting Diseases." Cell Death & Disease 14, no. 12 (2023): 851.
- Li, Jie, Feng Cao, He-liang Yin, Zi-jian Huang, Zhi-tao Lin, Ning Mao, Bei Sun, and Gang Wang. "Ferroptosis: Past, Present and Future." Cell Death & Disease 11, no. 2 (2020): 88.
- Vorobjeva, N. V. , and B. V. Chernyak. "Netosis: Molecular Mechanisms, Role in Physiology and Pathology." Biochemistry (Mosc) 85, no. 10 (2020): 1178-90.
- Han, Ying-Hao, Yuan Wang, Seung-Jae Lee, Mei-Hua Jin, Hu-Nan Sun, and Taeho Kwon. "Regulation of Anoikis by Extrinsic Death Receptor Pathways." Cell Communication and Signaling 21, no. 1 (2023): 227.
- Martínez-Morcillo, F. J., J. Cantón-Sandoval, F. J. Martínez-Navarro, I. Cabas, I. Martínez-Vicente, J. Armistead, J. Hatzold, A. López-Muñoz, T. Martínez-Menchón, R. Corbalán-Vélez, J. Lacal, M. Hammerschmidt, J. C. García-Borrón, A. García-Ayala, M. L. Cayuela, A. B. Pérez-Oliva, D. García-Moreno, and V. Mulero. "Nampt-Derived Nad+ Fuels Parp1 to Promote Skin Inflammation through Parthanatos Cell Death." PLoS Biol 19, no. 11 (2021): e3001455.
- Lin, Q., J. Zhu, J. Chen, S. Jia, and S. Nie. "Significance of Cuproptosis- Related Genes in the Diagnosis and Classification of Psoriasis." Front Mol Biosci 10 (2023): 1115091.
- Kroemer, G., L. Galluzzi, P. Vandenabeele, J. Abrams, E. S. Alnemri, E. H. Baehrecke, M. V. Blagosklonny, W. S. El-Deiry, P. Golstein, D. R. Green, M. Hengartner, R. A. Knight, S. Kumar, S. A. Lipton, W. Malorni, G. Nuñez, M. E. Peter, J. Tschopp, J. Yuan, M. Piacentini, B. Zhivotovsky, and G. Melino. "Classification of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2009." Cell Death & Differentiation 16, no. 1 (2009): 3-11.
- Galluzzi, L., I. Vitale, S. A. Aaronson, J. M. Abrams, D. Adam, P. Agostinis, E. S. Alnemri, L. Altucci, I. Amelio, D. W. Andrews, M. Annicchiarico-Petruzzelli, A. V. Antonov, E. Arama, E. H. Baehrecke, N. A. Barlev, N. G. Bazan, F. Bernassola, M. J. M. Bertrand, K. Bianchi, M. V. Blagosklonny, K. Blomgren, C. Borner, P. Boya, C. Brenner, M. Campanella, E. Candi, D. Carmona-Gutierrez, F. Cecconi, F. K. Chan, N. S. Chandel, E. H. Cheng, J. E. Chipuk, J. A. Cidlowski, A. Ciechanover, G. M. Cohen, M. Conrad, J. R. Cubillos-Ruiz, P. E. Czabotar, V. D'Angiolella, T. M. Dawson, V. L. Dawson, V. De Laurenzi, R. De Maria, K. M. Debatin, R. J. DeBerardinis, M. Deshmukh, N. Di Daniele, F. Di Virgilio, V. M. Dixit, S. J. Dixon, C. S. Duckett, B. D. Dynlacht, W. S. El-Deiry, J. W. Elrod, G. M. Fimia, S. Fulda, A. J. García-Sáez, A. D. Garg, C. Garrido, E. Gavathiotis, P. Golstein, E. Gottlieb, D. R. Green, L. A. Greene, H. Gronemeyer, A. Gross, G. Hajnoczky, J. M. Hardwick, I. S. Harris, M. O. Hengartner, C. Hetz, H. Ichijo, M. Jäättelä, B. Joseph, P. J. Jost, P. P. Juin, W. J. Kaiser, M. Karin, T. Kaufmann, O. Kepp, A. Kimchi, R. N. Kitsis, D. J. Klionsky, R. A. Knight, S. Kumar, S. W. Lee, J. J. Lemasters, B. Levine, A. Linkermann, S. A. Lipton, R. A. Lockshin, C. López-Otín, S. W. Lowe, T. Luedde, E. Lugli, M. MacFarlane, F. Madeo, M. Malewicz, W. Malorni, G. Manic, J. C. Marine, S. J. Martin, J. C. Martinou, J. P. Medema, P. Mehlen, P. Meier, S. Melino, E. A. Miao, J. D. Molkentin, U. M. Moll, C. Muñoz-Pinedo, S. Nagata, G. Nuñez, A. Oberst, M. Oren, M. Overholtzer, M. Pagano, T. Panaretakis, M. Pasparakis, J. M. Penninger, D. M. Pereira, S. Pervaiz, M. E. Peter, M. Piacentini, P. Pinton, J. H. M. Prehn, H. Puthalakath, G. A. Rabinovich, M. Rehm, R. Rizzuto, C. M. P. Rodrigues, D. C. Rubinsztein, T. Rudel, K. M. Ryan, E. Sayan, L. Scorrano, F. Shao, Y. Shi, J. Silke, H. U. Simon, A. Sistigu, B. R. Stockwell, A. Strasser, G. Szabadkai, S. W. G. Tait, D. Tang, N. Tavernarakis, A. Thorburn, Y. Tsujimoto, B. Turk, T. Vanden Berghe, P. Vandenabeele, M. G. Vander Heiden, A. Villunger, H. W. Virgin, K. H. Vousden, D. Vucic, E. F. Wagner, H. Walczak, D. Wallach, Y. Wang, J. A. Wells, W. Wood, J. Yuan, Z. Zakeri, B. Zhivotovsky, L. Zitvogel, G. Melino, and G. Kroemer. "Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018." Cell Death Differ 25, no. 3 (2018): 486-541.
- Khandia, R., M. Dadar, A. Munjal, K. Dhama, K. Karthik, R. Tiwari, M. I. Yatoo, H. M. N. Iqbal, K. P. Singh, S. K. Joshi, and W. Chaicumpa. "A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy." Cells 8, no. 7 (2019) : 674.
- Aman, Yahyah, Tomas Schmauck-Medina, Malene Hansen, Richard I. Morimoto, Anna Katharina Simon, Ivana Bjedov, Konstantinos Palikaras, Anne Simonsen, Terje Johansen, Nektarios Tavernarakis, David C. Rubinsztein, Linda Partridge, Guido Kroemer, John Labbadia, and Evandro F. Fang. "Autophagy in Healthy Aging and Disease." Nature Aging 1, no. 8 (2021): 634-50.
- Lee, H. M., D. M. Shin, J. M. Yuk, G. Shi, D. K. Choi, S. H. Lee, S. M. Huang, J. M. Kim, C. D. Kim, J. H. Lee, and E. K. Jo. "Autophagy Negatively Regulates Keratinocyte Inflammatory Responses Via Scaffolding Protein P62/Sqstm1." J Immunol 186, no. 2 (2011): 1248-58.
- Varshney, P. , and N. Saini. "Pi3k/Akt/Mtor Activation and Autophagy Inhibition Plays a Key Role in Increased Cholesterol During Il-17a Mediated Inflammatory Response in Psoriasis." Biochim Biophys Acta Mol Basis Dis 1864, no. 5 Pt A (2018): 1795-803.
- Klapan, K., D. Simon, A. Karaulov, M. Gomzikova, A. Rizvanov, S. Yousefi, and H. U. Simon. "Autophagy and Skin Diseases." Front Pharmacol 13 (2022): 844756.
- Wu, Xinxin, Jiankun Song, Ying Zhang, Le Kuai, Changya Liu, Xin Ma, Bin Li, Zhan Zhang, and Ying Luo. "Exploring the Role of Autophagy in Psoriasis Pathogenesis: Insights into Sustained Inflammation and Dysfunctional Keratinocyte Differentiation." International Immunopharmacology 135 (2024): 112244.
- Duan, Y., Y. Dong, H. Hu, Q. Wang, S. Guo, D. Fu, X. Song, D. V. Kalvakolanu, and Z. Tian. "Il-33 Contributes to Disease Severity in Psoriasis-Like Models of Mouse." Cytokine 119 (2019): 159-67.
- Kim, H. R., S. Y. Kang, H. O. Kim, C. W. Park, and B. Y. Chung. "Role of Aryl Hydrocarbon Receptor Activation and Autophagy in Psoriasis-Related Inflammation." Int J Mol Sci 21, no. 6 (2020) : 2195.
- Wang, Z., H. Zhou, H. Zheng, X. Zhou, G. Shen, X. Teng, X. Liu, J. Zhang, X. Wei, Z. Hu, F. Zeng, Y. Hu, J. Hu, X. Wang, S. Chen, J. Cheng, C. Zhang, Y. Gui, S. Zou, Y. Hao, Q. Zhao, W. Wu, Y. Zhou, K. Cui, N. Huang, Y. Wei, W. Li, and J. Li. "Autophagy-Based Unconventional Secretion of Hmgb1 by Keratinocytes Plays a Pivotal Role in Psoriatic Skin Inflammation." Autophagy 17, no. 2 (2021): 529-52.
- Törmä, H. "Regulation of Keratin Expression by Retinoids." Dermatoendocrinol 3, no. 3 (2011): 136-40.
- Rajawat, Y., Z. Hilioti, and I. Bossis. "Autophagy: A Target for Retinoic Acids." Autophagy 6, no. 8 (2010): 1224-6.
- Wong, T., L. Hsu, and W. Liao. "Phototherapy in Psoriasis: A Review of Mechanisms of Action." J Cutan Med Surg 17, no. 1 (2013): 6-12.
- Yang, Y., H. Wang, S. Wang, M. Xu, M. Liu, M. Liao, J. A. Frank, S. Adhikari, K. A. Bower, X. Shi, C. Ma, and J. Luo. "Gsk3β Signaling Is Involved in Ultraviolet B-Induced Activation of Autophagy in Epidermal Cells." Int J Oncol 41, no. 5 (2012): 1782-8.
- Gao, M. , and X. Si. "Rapamycin Ameliorates Psoriasis by Regulating the Expression and Methylation Levels of Tropomyosin Via Erk1/2 and Mtor Pathways in Vitro and in Vivo." Exp Dermatol 27, no. 10 (2018): 1112-19.
- Bürger, C., N. Shirsath, V. Lang, S. Diehl, R. Kaufmann, A. Weigert, Y. Y. Han, C. Ringel, and P. Wolf. "Blocking Mtor Signalling with Rapamycin Ameliorates Imiquimod-Induced Psoriasis in Mice." Acta Derm Venereol 97, no. 9 (2017): 1087-94.
- Wei, K. C. , and P. C. Lai. "Combination of Everolimus and Tacrolimus: A Potentially Effective Regimen for Recalcitrant Psoriasis." Dermatol Ther 28, no. 1 (2015): 25-7.
- Huang, Z., J. Li, H. Chen, D. Yu, and S. Sun. "The Efficacy of Metformin for the Treatment of Psoriasis: A Meta-Analysis Study." Postepy Dermatol Alergol 40, no. 5 (2023): 606-10.
- Park, M. J., S. Y. Lee, S. J. Moon, H. J. Son, S. H. Lee, E. K. Kim, J. K. Byun, D. Y. Shin, S. H. Park, C. W. Yang, and M. L. Cho. "Metformin Attenuates Graft-Versus-Host Disease Via Restricting Mammalian Target of Rapamycin/Signal Transducer and Activator of Transcription 3 and Promoting Adenosine Monophosphate-Activated Protein Kinase-Autophagy for the Balance between T Helper 17 and Tregs." Transl Res 173 (2016): 115-30.
- Chang, J. E. , and M. S. Choi. "A Molecular Perspective on the Potential Benefits of Metformin for the Treatment of Inflammatory Skin Disorders." Int J Mol Sci 21, no. 23 (2020) : 8960.
- Elmore, S. "Apoptosis: A Review of Programmed Cell Death." Toxicol Pathol 35, no. 4 (2007): 495-516.
- Vind, A. C., Z. Wu, M. J. Firdaus, G. Snieckute, G. A. Toh, M. Jessen, J. F. Martínez, P. Haahr, T. L. Andersen, M. Blasius, L. F. Koh, N. L. Maartensson, J. E. A. Common, M. Gyrd-Hansen, F. L. Zhong, and S. Bekker-Jensen. "The Ribotoxic Stress Response Drives Acute Inflammation, Cell Death, and Epidermal Thickening in Uv-Irradiated Skin In vivo." Mol Cell 84, no. 24 (2024): 4774-89.e9.
- Jan, R. , and G. E. Chaudhry. "Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics." Adv Pharm Bull 9, no. 2 (2019): 205-18.
- Takahashi, H., A. Manabe, A. Ishida-Yamamoto, Y. Hashimoto, and H. Iizuka. "Aberrant Expression of Apoptosis-Related Molecules in Psoriatic Epidermis." J Dermatol Sci 28, no. 3 (2002): 187-97.
- Krawczyk, A., J. Miśkiewicz, K. Strzelec, D. Wcisło-Dziadecka, and B. Strzalka-Mrozik. "Apoptosis in Autoimmunological Diseases, with Particular Consideration of Molecular Aspects of Psoriasis." Med Sci Monit 26 (2020): e922035.
- Victor, F. C. , and A. B. Gottlieb. "Tnf-Alpha and Apoptosis: Implications for the Pathogenesis and Treatment of Psoriasis." J Drugs Dermatol 1, no. 3 (2002): 264-75.
- Rückert, R., K. Asadullah, M. Seifert, V. M. Budagian, R. Arnold, C. Trombotto, R. Paus, and S. Bulfone-Paus. "Inhibition of Keratinocyte Apoptosis by Il-15: A New Parameter in the Pathogenesis of Psoriasis?" J Immunol 165, no. 4 (2000): 2240-50.
- Stoll, S. W., M. Benedict, R. Mitra, A. Hiniker, J. T. Elder, and G. Nuñez. "Egf Receptor Signaling Inhibits Keratinocyte Apoptosis: Evidence for Mediation by Bcl-Xl." Oncogene 16, no. 11 (1998): 1493-9.
- Aufiero, Barbara M., Harvinder Talwar, Chen Young, Murali Krishnan, James S. Hatfield, Hae Kyung Lee, Henry K. Wong, Iltefat Hamzavi, and George J. Murakawa. "Narrow-Band Uvb Induces Apoptosis in Human Keratinocytes." Journal of Photochemistry and Photobiology B: Biology 82, no. 2 (2006): 132-39.
- Weatherhead, S. C., P. M. Farr, D. Jamieson, J. S. Hallinan, J. J. Lloyd, A. Wipat, and N. J. Reynolds. "Keratinocyte Apoptosis in Epidermal Remodeling and Clearance of Psoriasis Induced by Uv Radiation." J Invest Dermatol 131, no. 9 (2011): 1916-26.
- Ozawa, M., K. Ferenczi, T. Kikuchi, I. Cardinale, L. M. Austin, T. R. Coven, L. H. Burack, and J. G. Krueger. "312-Nanometer Ultraviolet B Light (Narrow-Band Uvb) Induces Apoptosis of T Cells within Psoriatic Lesions." J Exp Med 189, no. 4 (1999): 711-8.
- Chen, Ting-Jui, Wen-Hung Chung, Chun-Bing Chen, Rosaline Chung-Yee Hui, Yu-Huei Huang, Yueh-Tsung Lu, Chang-Wei Wang, Kuo-Hsien Wang, Li-Cheng Yang, and Shuen-Iu Hung. "Methotrexate-Induced Epidermal Necrosis: A Case Series of 24 Patients." Journal of the American Academy of Dermatology 77, no. 2 (2017): 247-55.e2.
- Ladha, M. A., B. Edgerton, J. Levy, M. N. Mahmood, A. R. Devani, P. S. Grewal, and V. H. Prajapati. "Methotrexate-Induced Cutaneous Ulceration and Necrosis in Chronic Atopic Dermatitis." JAAD Case Rep 6, no. 9 (2020): 864-67.
- Elango, T., A. Thirupathi, S. Subramanian, P. Ethiraj, H. Dayalan, and P. Gnanaraj. "Methotrexate Treatment Provokes Apoptosis of Proliferating Keratinocyte in Psoriasis Patients." Clin Exp Med 17, no. 3 (2017): 371-81.
- Halprin, K. M. "Epidermal "Turnover Time"--a Re-Examination." Br J Dermatol 86, no. 1 (1972): 14-9.
- Kadry, Mai O., Naglaa M. Ammar, Heba A. Hassan, and Rehab M. Abdel Megeed. "Insights on Attenuating Autophagy Cellular and Molecular Pathways Versus Methotrexate-Induced Toxicity Via Liposomal Turmeric Therapy." Journal of Genetic Engineering and Biotechnology 20, no. 1 (2022): 147.
- Elango, T., H. Dayalan, P. Gnanaraj, H. Malligarjunan, and S. Subramanian. "Impact of Methotrexate on Oxidative Stress and Apoptosis Markers in Psoriatic Patients." Clin Exp Med 14, no. 4 (2014): 431-7.
- Soonthornchai, W., P. Tangtanatakul, J. Meephansan, K. Ruchusatsawat, R. Reantragoon, N. Hirankarn, and J. Wongpiyabovorn. "Down-Regulation of Mir-155 after Treatment with Narrow-Band Uvb and Methotrexate Associates with Apoptosis of Keratinocytes in Psoriasis." Asian Pac J Allergy Immunol 39, no. 3 (2021): 206-13.
- Manggau, M., D. S. Kim, L. Ruwisch, R. Vogler, H. C. Korting, M. Schäfer-Korting, and B. Kleuser. "1alpha,25-Dihydroxyvitamin D3 Protects Human Keratinocytes from Apoptosis by the Formation of Sphingosine-1-Phosphate." J Invest Dermatol 117, no. 5 (2001): 1241-9.
- De Haes, P., M. Garmyn, G. Carmeliet, H. Degreef, K. Vantieghem, R. Bouillon, and S. Segaert. "Molecular Pathways Involved in the Anti-Apoptotic Effect of 1,25-Dihydroxyvitamin D3 in Primary Human Keratinocytes." J Cell Biochem 93, no. 5 (2004): 951-67.
- Tiberio, R., C. Bozzo, G. Pertusi, F. Graziola, M. Gattoni, P. Griffanti, P. Boggio, E. Colombo, and G. Leigheb. "Calcipotriol Induces Apoptosis in Psoriatic Keratinocytes." Clin Exp Dermatol 34, no. 8 (2009): e972-4.
- Saurat, J. H. "Retinoids and Psoriasis: Novel Issues in Retinoid Pharmacology and Implications for Psoriasis Treatment." Journal of the American Academy of Dermatology 41, no. 3, Supplement (1999): S2-S6.
- Kerr, P. E. , and J. J. DiGiovanna. "From Vitamin to Vesanoid: Systemic Retinoids for the New Millennium." Med Health R I 84, no. 7 (2001): 228-31.
- Islam, T. C., T. Skarin, S. Sumitran, and R. Toftgård. "Retinoids Induce Apoptosis in Cultured Keratinocytes." Br J Dermatol 143, no. 4 (2000): 709-19.
- Kakavandi, E., R. Shahbahrami, H. Goudarzi, G. Eslami, and E. Faghihloo. "Anoikis Resistance and Oncoviruses." J Cell Biochem 119, no. 3 (2018): 2484-91.
- Taddei, M. L., E. Giannoni, T. Fiaschi, and P. Chiarugi. "Anoikis: An Emerging Hallmark in Health and Diseases." J Pathol 226, no. 2 (2012): 380-93.
- De Luca, M., G. Pellegrini, G. Zambruno, and P. C. Marchisio. "Role of Integrins in Cell Adhesion and Polarity in Normal Keratinocytes and Human Skin Pathologies." J Dermatol 21, no. 11 (1994): 821-8.
- Mei, J., X. Y. Jiang, H. X. Tian, D. C. Rong, J. N. Song, L. Wang, Y. S. Chen, R. C. B. Wong, C. X. Guo, L. S. Wang, L. Y. Wang, P. Y. Wang, and J. Y. Yin. "Anoikis in Cell Fate, Physiopathology, and Therapeutic Interventions." MedComm (2020) 5, no. 10 (2024): e718.
- Kothary, N., I. L. Diak, A. Brinker, S. Bezabeh, M. Avigan, and G. Dal Pan. "Progressive Multifocal Leukoencephalopathy Associated with Efalizumab Use in Psoriasis Patients." J Am Acad Dermatol 65, no. 3 (2011): 546-51.
- Sun, L., H. Wang, Z. Wang, S. He, S. Chen, D. Liao, L. Wang, J. Yan, W. Liu, X. Lei, and X. Wang. "Mixed Lineage Kinase Domain-Like Protein Mediates Necrosis Signaling Downstream of Rip3 Kinase." Cell 148, no. 1-2 (2012): 213-27.
- Estlack, L. E., C. C. Roth, G. L. Thompson, 3rd, W. A. Lambert, 3rd, and B. L. Ibey. "Nanosecond Pulsed Electric Fields Modulate the Expression of Fas/Cd95 Death Receptor Pathway Regulators in U937 and Jurkat Cells." Apoptosis 19, no. 12 (2014): 1755-68.
- Feoktistova, M., P. Geserick, B. Kellert, D. P. Dimitrova, C. Langlais, M. Hupe, K. Cain, M. MacFarlane, G. Häcker, and M. Leverkus. "Ciaps Block Ripoptosome Formation, a Rip1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by Cflip Isoforms." Mol Cell 43, no. 3 (2011): 449-63.
- Murphy, J. M., P. E. Czabotar, J. M. Hildebrand, I. S. Lucet, J. G. Zhang, S. Alvarez-Diaz, R. Lewis, N. Lalaoui, D. Metcalf, A. I. Webb, S. N. Young, L. N. Varghese, G. M. Tannahill, E. C. Hatchell, I. J. Majewski, T. Okamoto, R. C. Dobson, D. J. Hilton, J. J. Babon, N. A. Nicola, A. Strasser, J. Silke, and W. S. Alexander. "The Pseudokinase Mlkl Mediates Necroptosis Via a Molecular Switch Mechanism." Immunity 39, no. 3 (2013): 443-53.
- Cai, Zhenyu, Siriporn Jitkaew, Jie Zhao, Hsueh-Cheng Chiang, Swati Choksi, Jie Liu, Yvona Ward, Ling-gang Wu, and Zheng-Gang Liu. "Plasma Membrane Translocation of Trimerized Mlkl Protein Is Required for Tnf-Induced Necroptosis." Nature Cell Biology 16, no. 1 (2014): 55-65.
- Duan, X., X. Liu, N. Liu, Y. Huang, Z. Jin, S. Zhang, Z. Ming, and H. Chen. "Inhibition of Keratinocyte Necroptosis Mediated by Ripk1/Ripk3/Mlkl Provides a Protective Effect against Psoriatic Inflammation." Cell Death Dis 11, no. 2 (2020): 134.
- Burlando, M., E. Campione, A. Cuccia, G. Malara, L. Naldi, F. Prignano, and L. Zichichi. "Real-World Use of Dimethyl Fumarate in Patients with Plaque Psoriasis: A Delphi-Based Expert Consensus." Dermatol Reports 15, no. 2 (2023): 9613.
- Corazza, M., G. Odorici, A. Conti, V. Di Lernia, A. Motolese, F. Bardazzi, S. Di Nuzzo, A. Monti, F. Arginelli, F. Filippi, G. Valpiani, C. Morotti, and A. Borghi. "Dimethyl Fumarate Treatment for Psoriasis in a Real-Life Setting: A Multicentric Retrospective Study." Dermatol Ther 34, no. 5 (2021): e15066.
- Morrison, P.J., I. Suhrkamp, S. Gerdes, and U. Mrowietz. "Oral Dimethyl Fumarate Induces Changes within the Peripheral Neutrophil Compartment of Patients with Psoriasis That Are Linked with Skin Improvement*." British Journal of Dermatology 185, no. 3 (2021): 605-15.
- Shi, Fu-li, Li-sha Yuan, Tak-sui Wong, Qing Li, Ya-ping Li, Rong Xu, Yi-ping You, Tao Yuan, Hong-rui zhang, Zi-jian Shi, Qing-bing Zha, Bo Hu, Xian-hui He, and Dong-yun Ouyang. "Dimethyl Fumarate Inhibits Necroptosis and Alleviates Systemic Inflammatory Response Syndrome by Blocking the Ripk1-Ripk3-Mlkl Axis." Pharmacological Research 189 (2023): 106697.
- Li, Jingyi, Xingfeng Liu, Yuanyuan Liu, Fangmin Huang, Jiankun Liang, Yingying Lin, Fen Hu, Jianting Feng, Zeteng Han, Yushi Chen, Xuan Chen, Qiaofa Lin, Lanqin Wu, and Lisheng Li. "Saracatinib Inhibits Necroptosis and Ameliorates Psoriatic Inflammation by Targeting Mlkl." Cell Death & Disease 15, no. 2 (2024): 122.
- Lu, Liqing, Ye Zhang, Xuemei Tan, Yulia Merkher, Sergey Leonov, Li Zhu, Yalan Deng, Huajun zhang, Dandan Zhu, Yuying Tan, Ying Fu, Ting Liu, and Yongheng Chen. "Emerging Mechanisms of Pyroptosis and Its Therapeutic Strategy in Cancer." Cell Death Discovery 8, no. 1 (2022): 338.
- Yu, Pian, Xu Zhang, Nian Liu, Ling Tang, Cong Peng, and Xiang Chen. "Pyroptosis: Mechanisms and Diseases." Signal Transduction and Targeted Therapy 6, no. 1 (2021): 128.
- Liu, Xianhong, Jing Chen, Shaoyu Yue, Cheng Zhang, Jian Song, Hu Liang, Chaozhao Liang, and Xianguo Chen. "Nlrp3-Mediated Il-1β in Regulating the Imbalance between Th17 and Treg in Experimental Autoimmune Prostatitis." Scientific Reports 14, no. 1 (2024): 18829.
- Lalor, S. J., L. S. Dungan, C. E. Sutton, S. A. Basdeo, J. M. Fletcher, and K. H. Mills. "Caspase-1-Processed Cytokines Il-1beta and Il-18 Promote Il-17 Production by Gammadelta and Cd4 T Cells That Mediate Autoimmunity." J Immunol 186, no. 10 (2011): 5738-48.
- Lieberman, J., H. Wu, and J. C. Kagan. "Gasdermin D Activity in Inflammation and Host Defense." Sci Immunol 4, no. 39 (2019): eaav1447.
- Lian, Ni, Yujie Chen, Sihan Chen, Ying Zhang, Hao Chen, Yong Yang, Heng Gu, Qing Chen, Min Li, and Xu Chen. "Gasdermin D-Mediated Keratinocyte Pyroptosis as a Key Step in Psoriasis Pathogenesis." Cell Death & Disease 14, no. 9 (2023): 595.
- Wu, Jingying, Siming Lin, Weixiao Chen, Guili Lian, Weibin Wu, Ai Chen, Mohammad Ismail Hajary Sagor, Li Luo, Huajun Wang, and Liangdi Xie. "Tnf-A Contributes to Sarcopenia through Caspase-8/Caspase-3/Gsdme-Mediated Pyroptosis." Cell Death Discovery 9, no. 1 (2023): 76.
- Hu, Yixiang, Ya Liu, Lijuan Zong, Wenyou Zhang, Renzhu Liu, Qichang Xing, Zheng Liu, Qingzi Yan, Wencan Li, Haibo Lei, and Xiang Liu. "The Multifaceted Roles of Gsdme-Mediated Pyroptosis in Cancer: Therapeutic Strategies and Persisting Obstacles." Cell Death & Disease 14, no. 12 (2023): 836.
- Li, Y., Y. He, F. Yang, R. Liang, W. Xu, Y. Li, J. Cheng, B. Liang, M. Tang, X. Shi, J. Zhuang, M. Luo, L. Li, R. Zhang, H. Liu, H. Jie, X. Li, X. Han, E. Sun, and Z. Zhai. "Gasdermin E-Mediated Keratinocyte Pyroptosis Participates in the Pathogenesis of Psoriasis by Promoting Skin Inflammation." Br J Dermatol 191, no. 3 (2024): 385-96.
- Nowowiejska, J., A. Baran, A. Pryczynicz, J. M. Hermanowicz, B. Sieklucka, D. Pawlak, and I. Flisiak. "Gasdermin E (Gsdme)-a New Potential Marker of Psoriasis and Its Metabolic Complications: The First Combined Study on Human Serum, Urine and Tissue." Cells 12, no. 17 (2023): 2149.
- Lanz, J., N. Biniaz-Harris, M. Kuvaldina, S. Jain, K. Lewis, and B. A. Fallon. "Disulfiram: Mechanisms, Applications, and Challenges." Antibiotics (Basel) 12, no. 3 (2023): 524.
- Kaaber, K., T. Menné, N. Veien, and P. Hougaard. "Treatment of Nickel Dermatitis with Antabuse; a Double Blind Study." Contact Dermatitis 9, no. 4 (1983): 297-9.
- Kaaber, K., T. Menne, J. C. Tjell, and N. Veien. "Antabuse Treatment of Nickel Dermatitis. Chelation--a New Principle in the Treatment of Nickel Dermatitis." Contact Dermatitis 5, no. 4 (1979): 221-8.
- Jewell, E. William. "Does Disulfiram Cure Psoriasis?" JAMA 237, no. 20 (1977): 2189-89.
- Hu, X. M., S. Zheng, Q. Zhang, X. Wan, J. Li, R. Mao, R. Yang, and K. Xiong. "Panoptosis Signaling Enables Broad Immune Response in Psoriasis: From Pathogenesis to New Therapeutic Strategies." Comput Struct Biotechnol J 23 (2024): 64-76.
- Hu, Jun Jacob, Xing Liu, Shiyu Xia, Zhibin Zhang, Ying Zhang, Jingxia Zhao, Jianbin Ruan, Xuemei Luo, Xiwen Lou, Yang Bai, Junhong Wang, L. Robert Hollingsworth, Venkat Giri Magupalli, Li Zhao, Hongbo R. Luo, Justin Kim, Judy Lieberman, and Hao Wu. "Fda-Approved Disulfiram Inhibits Pyroptosis by Blocking Gasdermin D Pore Formation." Nature Immunology 21, no. 7 (2020): 736-45.
- Ou, An-te, Jia-xin Zhang, Yue-fei Fang, Rong Wang, Xue-ping Tang, Peng-fei Zhao, Yu-ge Zhao, Meng Zhang, and Yong-zhuo Huang. "Disulfiram-Loaded Lactoferrin Nanoparticles for Treating Inflammatory Diseases." Acta Pharmacologica Sinica 42, no. 11 (2021): 1913-20.
- Samir, P., R. K. S. Malireddi, and T. D. Kanneganti. "The Panoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (Panoptosis)." Front Cell Infect Microbiol 10 (2020): 238.
- Banoth, B., S. Tuladhar, R. Karki, B. R. Sharma, B. Briard, S. Kesavardhana, A. Burton, and T. D. Kanneganti. "Zbp1 Promotes Fungi-Induced Inflammasome Activation and Pyroptosis, Apoptosis, and Necroptosis (Panoptosis)." J Biol Chem 295, no. 52 (2020): 18276-83.
- Gao, Jie, Anying Xiong, Jiliu Liu, Xiaolan Li, Junyi Wang, Lei Zhang, Yao Liu, Ying Xiong, Guoping Li, and Xiang He. "Panoptosis: Bridging Apoptosis, Pyroptosis, and Necroptosis in Cancer Progression and Treatment." Cancer Gene Therapy 31, no. 7 (2024): 970-83.
- Oh, S. , and S. Lee. "Recent Advances in Zbp1-Derived Panoptosis against Viral Infections." Front Immunol 14 (2023): 1148727.
- Yang, Yang, Alphonse Houssou Hounye, Yiqian Chen, Zhuqing Liu, Guanzhong Shi, and Ying Xiao. "Characterization of Panoptosis-Related Genes in Crohn’s Disease by Integrated Bioinformatics, Machine Learning and Experiments." Scientific Reports 14, no. 1 (2024): 11731.
- Lu, Lingling, Buxin Zhang, Meiling Shi, and Aimin Liu. "Identification of Panoptosis-Related Biomarkers and Immune Infiltration Characteristics in Psoriasis." Medicine 102, no. 42 (2023): e35627.
- Wu, Li, Xin-long Jiao, Ming Jing, Sheng-xiao Zhang, Yang Wang, Chen-long Li, Gao-xiang Shi, Zhuo-yang Li, Ge-liang Liu, Kai Yan, Li-xuan Yan, Qi Wang, Pei-feng He, and Qi Yu. "Discovery of Panoptosis-Related Signatures Correlates with Immune Cell Infiltration in Psoriasis." PLOS ONE 19, no. 10 (2024): e0310362.
- Liu, Libo, Jiaxiang Li, Yueshuang Ke, Xianlu Zeng, Jinmin Gao, Xueqing Ba, and Ruoxi Wang. "The Key Players of Parthanatos: Opportunities for Targeting Multiple Levels in the Therapy of Parthanatos-Based Pathogenesis." Cellular and Molecular Life Sciences 79, no. 1 (2022): 60.
- Zhang, Z., B. Cheng, W. Du, M. Zeng, K. He, T. Yin, S. Shang, T. Su, D. Han, X. Gan, Z. Wang, M. Liu, M. Wang, J. Liu, and Y. Zheng. "The Role of Nicotinamide Mononucleotide Supplementation in Psoriasis Treatment." Antioxidants (Basel) 13, no. 2 (2024): 186.
- El-Khalawany, M., A. H. Nouh, A. S. Kadah, M. Elsheikh, and M. Said. "Evaluation of Safety and Efficacy of Topical 4% Nicotinamide in Treatment of Psoriasis; among a Representative Sample of Egyptians (an Analytical Observational Study)." Dermatol Ther 35, no. 9 (2022): e15734.
- Jiang, Xuejun, Brent R. Stockwell, and Marcus Conrad. "Ferroptosis: Mechanisms, Biology and Role in Disease." Nature Reviews Molecular Cell Biology 22, no. 4 (2021): 266-82.
- Yan, Hong-fa, Ting Zou, Qing-zhang Tuo, Shuo Xu, Hua Li, Abdel Ali Belaidi, and Peng Lei. "Ferroptosis: Mechanisms and Links with Diseases." Signal Transduction and Targeted Therapy 6, no. 1 (2021): 49.
- Ingold, I., C. Berndt, S. Schmitt, S. Doll, G. Poschmann, K. Buday, A. Roveri, X. Peng, F. Porto Freitas, T. Seibt, L. Mehr, M. Aichler, A. Walch, D. Lamp, M. Jastroch, S. Miyamoto, W. Wurst, F. Ursini, E. S. J. Arnér, N. Fradejas-Villar, U. Schweizer, H. Zischka, J. P. Friedmann Angeli, and M. Conrad. "Selenium Utilization by Gpx4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis." Cell 172, no. 3 (2018): 409-22.e21.
- Li, F. J., H. Z. Long, Z. W. Zhou, H. Y. Luo, S. G. Xu, and L. C. Gao. "System X(C) (-)/Gsh/Gpx4 Axis: An Important Antioxidant System for the Ferroptosis in Drug-Resistant Solid Tumor Therapy." Front Pharmacol 13 (2022): 910292.
- Wang, Weimin, Michael Green, Jae Eun Choi, Miguel Gijón, Paul D. Kennedy, Jeffrey K. Johnson, Peng Liao, Xueting Lang, Ilona Kryczek, Amanda Sell, Houjun Xia, Jiajia Zhou, Gaopeng Li, Jing Li, Wei Li, Shuang Wei, Linda Vatan, Hongjuan Zhang, Wojciech Szeliga, Wei Gu, Rebecca Liu, Theodore S. Lawrence, Candice Lamb, Yuri Tanno, Marcin Cieslik, Everett Stone, George Georgiou, Timothy A. Chan, Arul Chinnaiyan, and Weiping Zou. "Cd8+ T Cells Regulate Tumour Ferroptosis During Cancer Immunotherapy." Nature 569, no. 7755 (2019): 270-74.
- Shou, Yanhong, Lu Yang, Yongsheng Yang, and Jinhua Xu. "Inhibition of Keratinocyte Ferroptosis Suppresses Psoriatic Inflammation." Cell Death & Disease 12, no. 11 (2021): 1009.
- Nisha Kundu, Rohit Kothari and Sunmeet Sandhu. "Intravenous Glutathione Can Improve Scalp Psoriasis: A Serendipity." Biomedical Journal of Scientific & Technical Research 43, no.1 (2022): 34162-64.
- Prussick, R., L. Prussick, and J. Gutman. "Psoriasis Improvement in Patients Using Glutathione-Enhancing, Nondenatured Whey Protein Isolate: A Pilot Study." J Clin Aesthet Dermatol 6, no. 10 (2013): 23-6.
- Nazıroğlu, M., K. Yıldız, B. Tamtürk, İ Erturan, and M. Flores-Arce. "Selenium and Psoriasis." Biol Trace Elem Res 150, no. 1-3 (2012): 3-9.
- Chambers, Ian G., and Rajiv R. Ratan. "Selenium Abandons Selenoproteins to Inhibit Ferroptosis Rapidly." Nature Metabolism 6, no. 2 (2024): 200-02.
- Al-Oudah, G. A., A. S. Sahib, M. K. Al-Hattab, and A. A. Al-Ameedee. "Effect of Coq10 Administration to Psoriatic Iraqi Patients on Biological Therapy Upon Severity Index (Pasi) and Quality of Life Index (Dlqi) before and after Therapy." J Popul Ther Clin Pharmacol 29, no. 2 (2022): e52-e60.
- Kharaeva, Z., E. Gostova, C. De Luca, D. Raskovic, and L. Korkina. "Clinical and Biochemical Effects of Coenzyme Q(10), Vitamin E, and Selenium Supplementation to Psoriasis Patients." Nutrition 25, no. 3 (2009): 295-302.
- Hadian, K. "Ferroptosis Suppressor Protein 1 (Fsp1) and Coenzyme Q(10) Cooperatively Suppress Ferroptosis." Biochemistry 59, no. 5 (2020): 637-38.
- Li, Siying, Xin Luo, Suhan Zhang, Yuwen Su, Min Deng, Yanshan Zhu, Peng Zhang, Ruifang Wu, and Ming Zhao. "Ferroptosis Activation Contributes to the Formation of Skin Lesions in Psoriasis Vulgaris." Antioxidants 12, no. 2 (2023): 310.
- Abboud, Elise, Doha Chrayteh, Nadia Boussetta, Héloise Dalle, Mariangela Malerba, Ting-Di Wu, Morgane Le Gall, Olivier Reelfs, Charareh Pourzand, Mark Mellett, Florence Assan, Hervé Bachelez, Joël Poupon, Selim Aractingi, Sophie Vaulont, Pierre Sohier, Bénédicte Oules, Zoubida Karim, and Carole Peyssonnaux. "Skin Hepcidin Initiates Psoriasiform Skin Inflammation Via Fe-Driven Hyperproliferation and Neutrophil Recruitment." Nature Communications 15, no. 1 (2024): 6718.
- Tsvetkov, P., S. Coy, B. Petrova, M. Dreishpoon, A. Verma, M. Abdusamad, J. Rossen, L. Joesch-Cohen, R. Humeidi, R. D. Spangler, J. K. Eaton, E. Frenkel, M. Kocak, S. M. Corsello, S. Lutsenko, N. Kanarek, S. Santagata, and T. R. Golub. "Copper Induces Cell Death by Targeting Lipoylated Tca Cycle Proteins." Science 375, no. 6586 (2022): 1254-61.
- Tang, Daolin, Xin Chen, and Guido Kroemer. "Cuproptosis: A Copper-Triggered Modality of Mitochondrial Cell Death." Cell Research 32, no. 5 (2022): 417-18.
- Lei, L., J. Su, J. Chen, W. Chen, X. Chen, and C. Peng. "Abnormal Serum Copper and Zinc Levels in Patients with Psoriasis: A Meta-Analysis." Indian J Dermatol 64, no. 3 (2019): 224-30.
- Liao, Y., J. Zhao, K. Bulek, F. Tang, X. Chen, G. Cai, S. Jia, P. L. Fox, E. Huang, T. T. Pizarro, M. F. Kalady, M. W. Jackson, S. Bao, G. C. Sen, G. R. Stark, C. J. Chang, and X. Li. "Inflammation Mobilizes Copper Metabolism to Promote Colon Tumorigenesis Via an Il-17-Steap4-Xiap Axis." Nat Commun 11, no. 1 (2020): 900.
- Liang, Y., X. Xing, M. A. Beamer, W. R. Swindell, M. K. Sarkar, L. W. Roberts, J. J. Voorhees, J. M. Kahlenberg, P. W. Harms, A. Johnston, and J. E. Gudjonsson. "Six-Transmembrane Epithelial Antigens of the Prostate Comprise a Novel Inflammatory Nexus in Patients with Pustular Skin Disorders." J Allergy Clin Immunol 139, no. 4 (2017): 1217-27.
- Kaya Ö, Keskinkaya Z, Şehitoğlu MH, Mermutlu SI, Kılıç SO. "Evaluation of Serum Tumor Necrosis Factor-Alpha-Induced Adipose-Associated Protein (Tiarp/Steap4) Level and Its Association with Disease Activity in Patients with Psoriasis: A Single-Center Prospective Comparative Study." Turk J Dermatol. (2024): 119-24.
- Huang, S. U. , and K. M. O'Sullivan. "The Expanding Role of Extracellular Traps in Inflammation and Autoimmunity: The New Players in Casting Dark Webs." Int J Mol Sci 23, no. 7 (2022): 3793.
- Mutua, V. , and L. J. Gershwin. "A Review of Neutrophil Extracellular Traps (Nets) in Disease: Potential Anti-Nets Therapeutics." Clin Rev Allergy Immunol 61, no. 2 (2021): 194-211.
- Thiam, H. R., S. L. Wong, D. D. Wagner, and C. M. Waterman. "Cellular Mechanisms of Netosis." Annu Rev Cell Dev Biol 36 (2020): 191-218.
- Lambert, S., C. A. Hambro, A. Johnston, P. E. Stuart, L. C. Tsoi, R. P. Nair, and J. T. Elder. "Neutrophil Extracellular Traps Induce Human th17 Cells: Effect of Psoriasis-Associated Traf3ip2 Genotype." J Invest Dermatol 139, no. 6 (2019): 1245-53.
- Lande, R., J. Gregorio, V. Facchinetti, B. Chatterjee, Y. H. Wang, B. Homey, W. Cao, Y. H. Wang, B. Su, F. O. Nestle, T. Zal, I. Mellman, J. M. Schröder, Y. J. Liu, and M. Gilliet. "Plasmacytoid Dendritic Cells Sense Self-DNA Coupled with Antimicrobial Peptide." Nature 449, no. 7162 (2007): 564-9.
- Zhang, J., Y. Feng, and D. Shi. "Netosis of Psoriasis: A Critical Step in Amplifying the Inflammatory Response." Front Immunol 15 (2024): 1374934.
- Hu, Stephen Chu-Sung, Hsin-Su Yu, Feng-Lin Yen, Chi-Ling Lin, Gwo-Shing Chen, and Cheng-Che E. Lan. "Neutrophil Extracellular Trap Formation Is Increased in Psoriasis and Induces Human Β-Defensin-2 Production in Epidermal Keratinocytes." Scientific Reports 6, no. 1 (2016): 31119.
- Neu, S. D., A. Strzepa, D. Martin, M. G. Sorci-Thomas, K. A. Pritchard, Jr., and B. N. Dittel. "Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice." Antioxidants (Basel) 10, no. 9 (2021): 1338.
- Gajendran, Chandru, Shoichi Fukui, Naveen M. Sadhu, Mohammed Zainuddin, Sridharan Rajagopal, Ramachandraiah Gosu, Sarah Gutch, Saeko Fukui, Casey E. Sheehy, Long Chu, Santosh Vishwakarma, D. A. Jeyaraj, Gurulingappa Hallur, Denisa D. Wagner, and Dhanalakshmi Sivanandhan. "Alleviation of Arthritis through Prevention of Neutrophil Extracellular Traps by an Orally Available Inhibitor of Protein Arginine Deiminase 4." Scientific Reports 13, no. 1 (2023): 3189.
- Czerwińska, J., M. Kasprowicz-Furmańczyk, W. Placek, and A. Owczarczyk-Saczonek. "Changes in Tumor Necrosis Factor A (Tnfα) and Peptidyl Arginine Deiminase 4 (Pad-4) Levels in Serum of General Treated Psoriatic Patients." Int J Environ Res Public Health 19, no. 14 (2022): 8723.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).