1. Introduction
Monofocal lenses are the most frequently implanted intraocular lenses (IOLs) in cataract surgery, because of their relatively low cost, excellent outcomes for distance visual performance, and low values of photic phenomena (glare, halos) compared to multifocal IOLs [
1]. Nevertheless, monofocal IOLs have a very limited depth of field and thus do not provide functional intermediate and near vision required for most daily activities, which nowadays involve tablets, smartphones, and computers. Patients’ expectations of a highly successful cataract surgery outcome with additional presbyopia correction are constantly increasing in terms of visual quality and post-operative functional vision, aiming to achieve a total, or nearly total, spectacle independence [
2].
For these reasons, the number of IOLs with augmented range of vision has significantly increased in recent years. There is a growing interest towards new technologies that may improve the intermediate performance of monofocal IOLs, minimizing the unwanted adverse effects of multifocal IOLs. As a result, Extended Depth of Focus (EDOF) IOLs were introduced to improve intermediate visual acuity while minimizing photic phenomena [
3]. Lately, a new class of monofocal IOLs denominated “enhanced monofocal IOLs” has been developed, providing the same excellent distance vision of a monofocal with improved intermediate vision [
4,
5,
6].
In this increasingly diverse context, classifying IOLs has become progressively more challenging. Recently, a new evidence-based functional classification has been developed [
7,
8]. Two main categories can be identified depending on the defocus curve RoF (Range of field) and shape: PARTIAL-RoF and FULL-RoF. In PARTIAL-RoF, 3 subcategories can be described according to the achieved RoF: narrow, enhance, and extend. On the other hand, (2) FULL-RoF IOLs subcategories depend on how steep is the increase in VA from intermediate to near: continuous, smooth transition, and steep transition.
In this new context, the aim of the current study is to compare visual outcomes, optical quality (including optical bench analysis) and patient satisfaction between eyes implanted with a new enhanced monofocal IOL Evolux and the standard monofocal IOL Tecnis GCB00. Theese IOLs are classified as PARTIAL-RoF, respectively PARTIAL-RoF narrow for Tecnis GCB00 and PARTIAL-RoF enhance for Evolux.
2. Materials and Methods
2.1. Study Design and Patients
A single-center, prospective, non-randomized, comparative clinical trial was conducted at the Eye Clinic, Department of NEUROFARBA, University of Florence, Italy. From June 2023 to July 2024, 100 eyes of 50 study participants were included. Prior to study investigation, local ethics committee approval (protocol number Ethics Committee FA000459, ID study 24175) was obtained. In accordance with the tenets of the Declaration of Helsinki, each study participant received a careful explanation of the intended procedure and signed an informed consent form prior to inclusion.
The inclusion criteria for our analysis were: patients with lens opacification and cataract grade greater than II, according to the Lens Opacities Classification System III (LOCS III); patients with at least 60 years of age. The exclusion criteria comprised an axial length greater than 26.0 mm or less than 21.00 mm, corneal astigmatism higher than ± 0.75 D, angle Kappa > 0.5 mm, post-surgical refractive outcome exceeding ±0.50 D, prior ocular surgeries (including corneal or refractive procedures), and ocular comorbidities such as chronic or recurrent uveitis, external or internal infections, diabetes mellitus with retinal complications, pathological miosis, amblyopia, use of alpha-blockers that could cause floppy-iris syndrome, choroidal hemorrhage, keratoconus, corneal endothelial dystrophy, diabetic retinopathy, uncontrolled glaucoma and/or IOP > 24 mmHg, traumatic cataract, pseudoexfoliation syndrome, pupillary abnormalities including aniridia and/or pupillary diameter in photopic conditions ≤ 2.5 mm and in mesopic conditions ≥ 6 mm, microphthalmia, strabismus, nystagmus, pregnancy or lactation period for female patients, degenerative visual disorders (e.g., macular degeneration, optic nerve atrophy or other retinal disorders).
2.2. Patient Evaluation
Before surgery, all patients underwent a comprehensive pre-operative ophthalmological examination that included the measurement of the monocular and binocular Uncorrected Distance Visual Acuity (UDVA) (4 m) and Best Corrected Distance Visual acuity (CDVA) under photopic conditions (85 cd/m
2) and 100% contrast with the Early Treatment Diabetic Retinopathy Study (ETDRS) charts, optical biometry (IOL Master 500, Carl Zeiss Meditec AG), subjective and objective refraction, biomicroscopy (Accuref K-900, Shin-Nippon), Goldmann applanation tonometry, corneal topography and anterior segment optical coherence tomography (AS-OCT) (MS-39, CSO, Florence, Italy), macular OCT (DRI OCT Triton 3D, Topcon Medical Systems, Inc.), and dilated fundoscopy. The IOL power and predicted postoperative refraction were based on biometric data (IOL Master 500, Carl Zeiss Meditec, Germany), calculated using the Barret Formula with an A-constant of 118,35. The IOL power was selected to achieve the predicted postoperative refraction closest to emmetropia. Postoperatively, patients were evaluated at 1 day, 1 month, 3 month and 6 months. At the last postoperative visit, in addition to the slit lamp examination and tonometry, subjective and objective refraction were measured and monocular and binocular UDVA, Uncorrected Intermediate Visual Acuity (UIVA) at 66 cm, Uncorrected Near Visual Acuity (UNVA) at 40 cm, BCDVA, Distance-Corrected Intermediate Visual Acuity (DCIVA), and Distance-Corrected Near Visual Acuity (DCNVA) were assessed under photopic conditions; binocular defocus curves were obtained, and contrast sensitivity, ocular scattering, and patients’ satisfaction were assessed. Distance visual acuities (UDVA and CDVA) were measured at 4 m using an ETDRS illumination cabinet at high contrast (96%) with an 85 cd/m
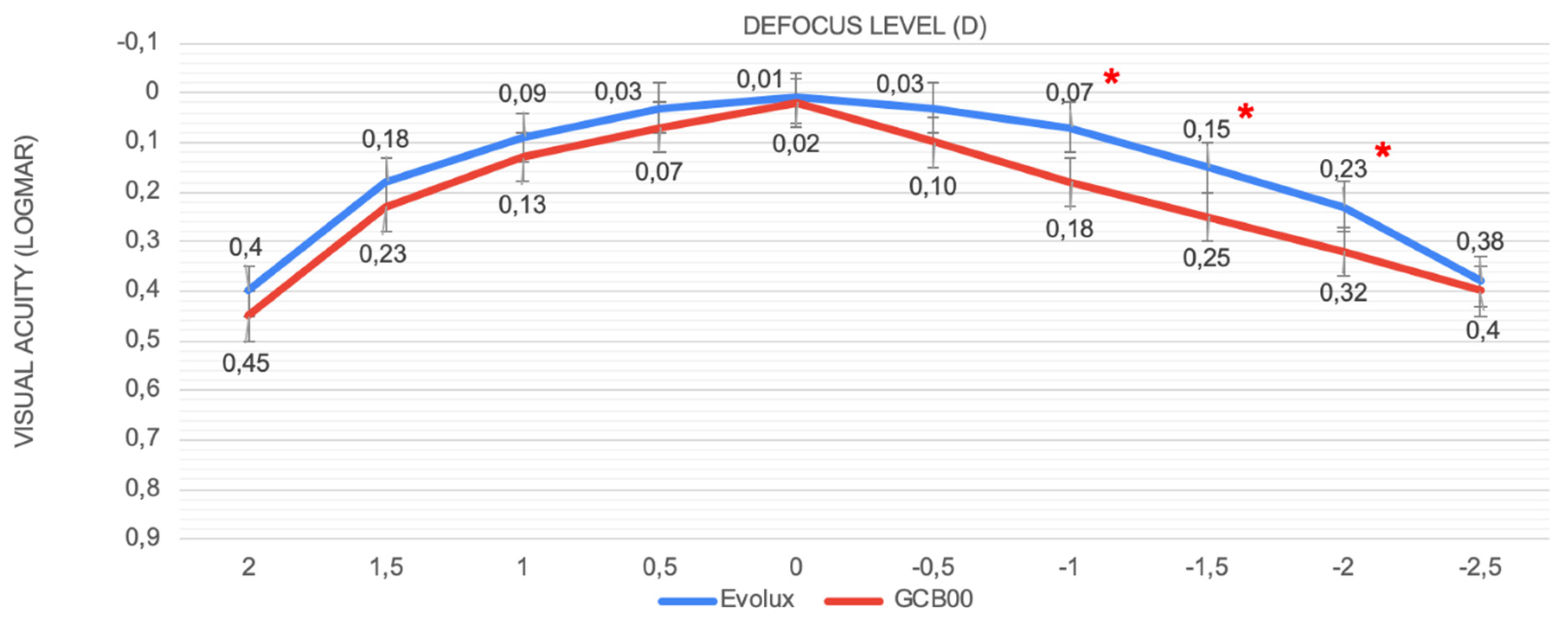
2 lamp filter tube (Precision Vision). Intermediate and near visual acuities were measured using high contrast ETDRS printed charts (Precision Vision); photopic conditions were obtained by adjusting the potentiometer of a halogen lamp according to the room illumination measurements provided by a light meter (ST-1300, STANDARD Instruments Co., Ltd.). To avoid letter set memorization, different charts were presented to the same patient at each visit in a random order. Binocular defocus curves were obtained using best distance correction. To produce defocus, a progression of IOLs in 0.50 D increments was consecutively added (range +1.00 to 2.50 D), after which visual acuity was tested. Visual performance was also evaluated by measuring the binocular contrast sensitivity under photopic conditions (85 cd/m
2) using the Optec 6500 Vision Tester (Stereo Optical Co., Inc). An objective analysis of the ocular optical quality was performed using the AcuTarget HD Analyzer (Visiometrics USA), an Optical Quality Assessment System (OQAS) product based on the double-pass technology [
9,
10]. The device was used at a 4.0 mm pupil to measure the objective scatter index (OSI), the modulation transfer function (MTF) cutoff, and the point spread function (PSF) expressed as the Strehl ratio. At the end of last follow-up visit, halometry was also performed to assess the presence of lens-induced halos [
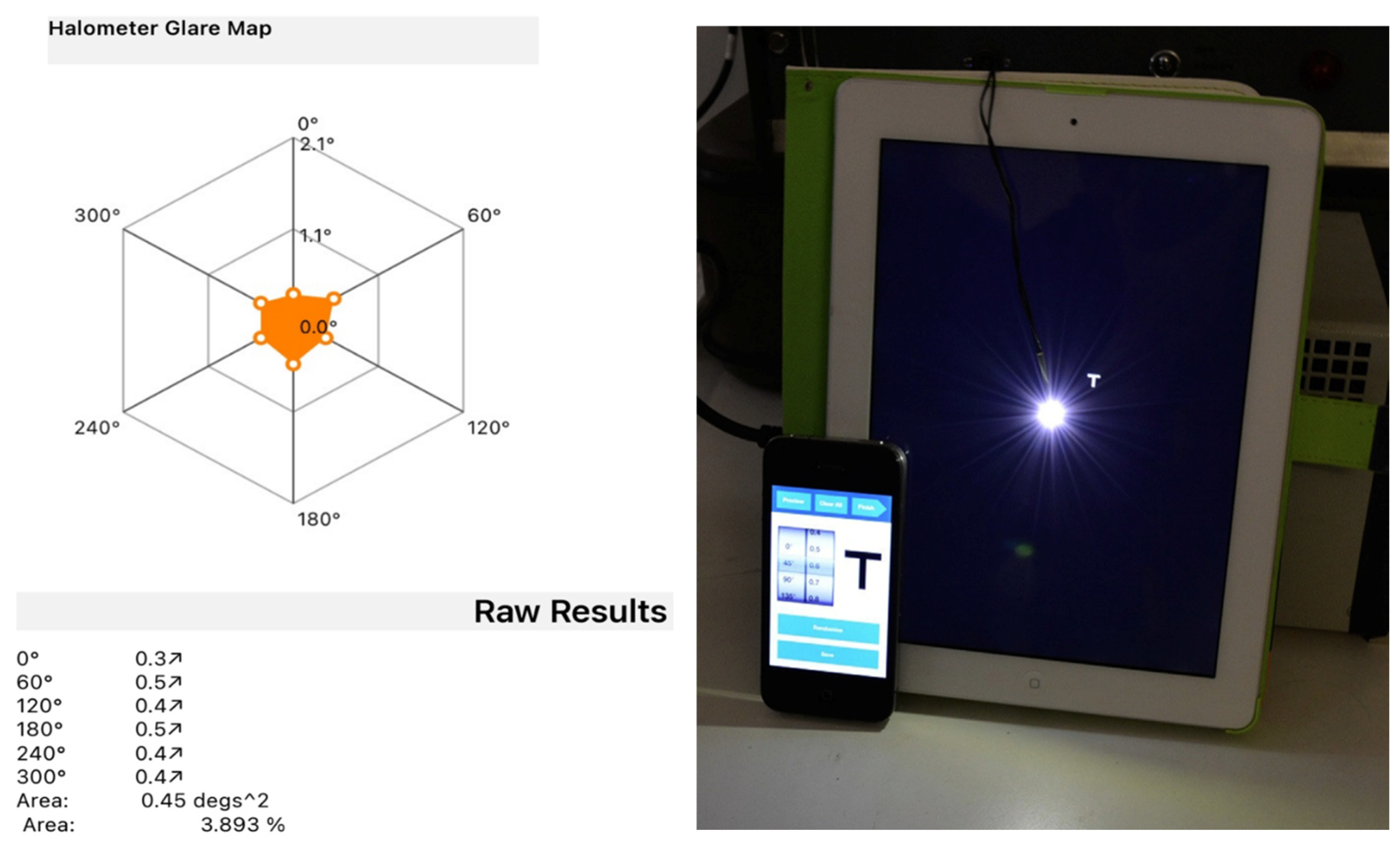
11]. The examination is conducted in a dark room with the patient positioned 2 meters away from an LED device located at the center of an iPad. The operator controls the iPad using an iPhone connected via bluetooth and projects 0.3 LogMAR letters, proceeding from the periphery towards the center of the iPad (Aston App Halometer), where the light source is located, until the patient is able to recognize the letter. This evaluation is repeated in each of the 8 or 6 directions (at the operator’s discretion) to determine the objective area of obscuration caused by the patient’s halo. For each patient, a halometer glare map was created to represent the halo area.
Effective lens position (ELP) was also evaluated using AS-OCT imaging in a single-line scan mode. Patients were seated and instructed to fixate on the internal target. Two images were captured at 0 degrees and 90 degrees, and the image with the highest quality from each pair was selected for analysis. No topical cycloplegic agent was administered during the exams and all scans were conducted by the same examiner, who was blinded to the IOL model [
12]. Finally, the Patient-Reported Spectacle Independence Questionnaire (PRSIQ) was conducted to evaluate spectacle independence during daily life. [
13]
2.3. Intraocular Lenses
Evolux (SIFI Spa, Catania, Italy) lens is a novel enhanced monofocal IOL, based on a non-diffractive profile, designed to provide better intermediate vision and equivalent distance vision when compared to a standard monofocal IOL. It is a preloaded hydrophobic acrylic IOL with a 6 mm optic diameter and an overall diameter of 13 mm. It is characterized by innovative biconvex optical design based on positive/negative Spherical Aberration (SA) distributed in central 4.5 mm zone of the anterior surface of the IOL, with an aspheric monofocal periphery. It is available in powers ranging from +5.0 D to 30.0 D, with 0.50 D steps. Evolux was developed on a patented technology platform, creating a single elongated focal point to enhance depth of field in order to improve intermediate vision.
Tecnis one-piece GCB00 (Johnson & Johnson Vision Care, Santa Ana, California, CA, US) is a standard monofocal IOL. It is an aspherical acrylic hydrophobic IOL with a 6 mm optic and an overall diameter of 13 mm.
Optical bench characterization of both IOLs is also described below in sections 2.6 and 3.7 (Optical Bench Analysis) of the methods and results.
2.4. Surgery
The surgical interventions were performed by the same highly experienced surgeon (R.M.), all using topical anesthesia. After the creation of a temporal self-sealing corneal incision and a capsulorrhexis of about 5.5 mm, phacoemulsification of the nucleus and irrigation/aspiration were performed (Centurion, Alcon Laboratories Inc.). The IOL was implanted and, at the end of the surgery, intracameral cefuroxime was injected into the anterior chamber. Routine post-operative treatment included topical nonsteroidal anti-inflammatory, steroidal and antibiotic eye drops for 2-4 weeks. No intraoperative or postoperative complications occurred.
2.5. Statistical Analysis
The data analysis for this study was performed using Stata 18.0 (StataCorp, College Station, TX, USA). To summarize the numerical data, descriptive statistics were applied, including the calculation of mean values and standard deviations. Both groups exhibited normally distributed data, with the assumption of normality assessed using the Shapiro-Wilk test. A two-tailed Student’s T-test with 95% confidence intervals was used to compare the clinical characteristics of the two treatment groups. A p-value of <0.05 was set as the threshold for statistical significance.
2.6. Optical Bench Analysis
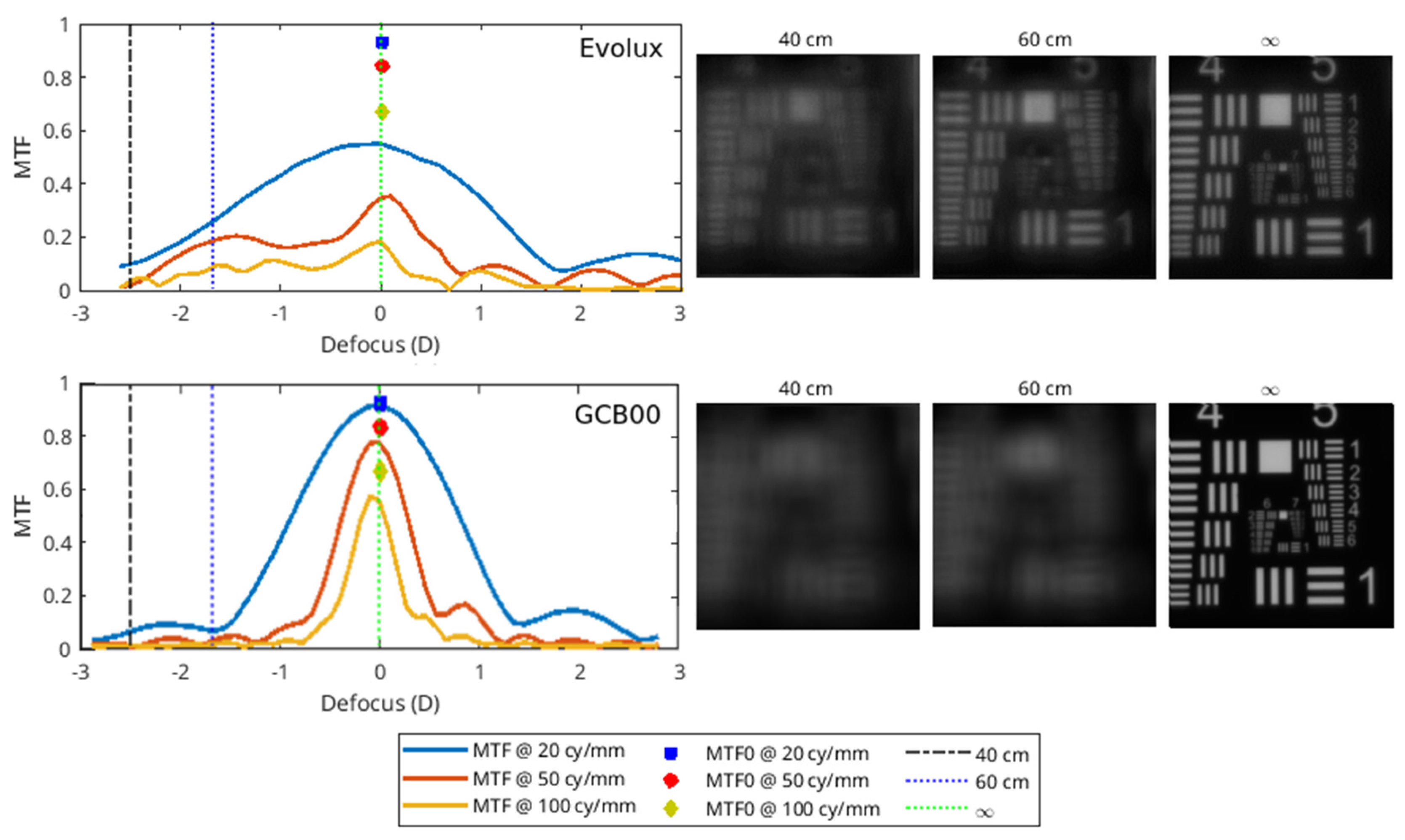
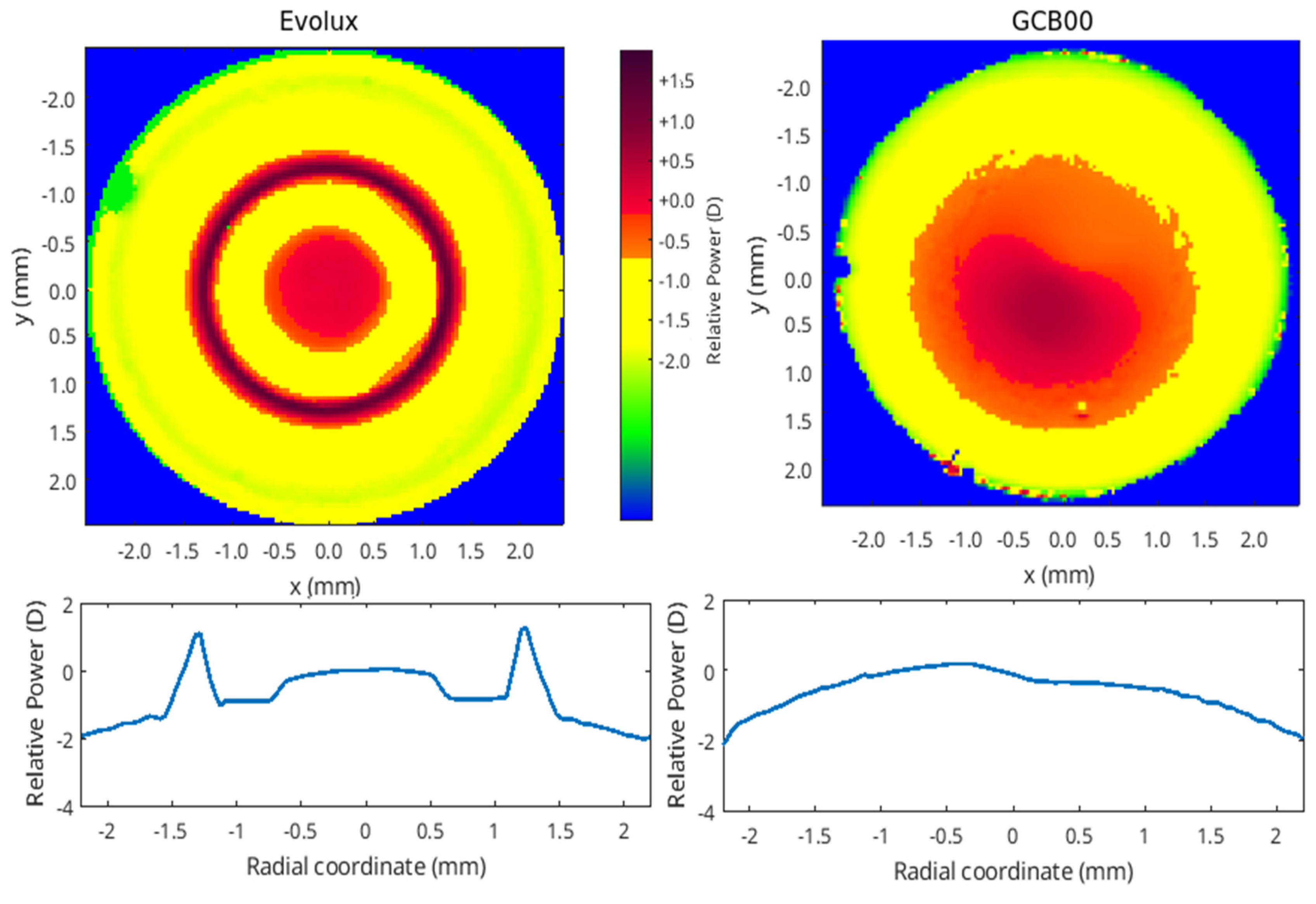
The optical characterization of the IOLs were performed with the optical benches PMTF (LambdaX), and NIMO (Lambda X), and then analyzed with our software written in Matlab programming language. We measured the modulation transfer function (MTF) of the IOLs and the images of the USAF (United States Air Force) resolution target as a function of the defocus using the PMTF in through focus mode. We also measured the power map using the NIMO optical bench, based on phase-shifting and Schlieren techniques. The MTF measures the degradation of contrast in an image compared to the contrast of the original object, quantifying the image quality of an optical system. USAF is a standard image system used as an evaluation tool to test the resolution. Moreover, the power map represents the spatially resolved power of the IOL.
4. Discussion
In the contemporary era of cataract surgery, patients not only expect improved visual acuity but also seek greater spectacle independence, aiming at a significant overall improvement in their quality of life. This shift in patients’ expectations has driven the medical device industry to explore and develop innovative solutions, leading to significant advancements in IOL technology. Presently, a wide variety of IOLs, including bifocal, trifocal, EDOF and enhanced monofocal lenses, are available on the market as alternatives to conventional monofocal IOLs.
Despite the ability of multifocal IOLs to provide uncorrected visual acuity for near, intermediate, and far distances, multiple clinical studies have demonstrated their negative impact on contrast sensitivity. Additionally, the quality of vision with multifocal IOLs can be influenced by illumination levels, and the presence of photic phenomena remains one of the most common causes of patient complaints during both the early and long-term postoperative periods [
14,
15,
16]. These characteristics of multifocal IOLs underscore the importance of a thorough and meticulous patient selection process for implantation. Furthermore, the high cost of multifocal IOLs represents another critical barrier to their widespread use, as it often limits patient access to this technology, even when they meet the appropriate selection criteria.
These challenges have prompted leading companies and ophthalmologists to pursue the development of IOLs that provide an extended depth of field while minimizing the typical side effects associated with multifocal technologies. Within this broader category, partial range of field IOLs [
7,
8], including enhanced and extended depth of field lenses, have emerged. The recently introduced Evolux IOL is an example of an enhanced lens, designed to extend the depth of field. It achieves this by incorporating a 4.5 mm central zone with both positive and negative spherical aberrations, offering a depth of field that is suitable for both distance and intermediate vision.
Few studies have been published on the Evolux IOL [
17,
18]. Available literature suggests that the Evolux IOL offers good visual performance in both corrected and uncorrected visual acuity, without significantly increasing photic phenomena or reducing contrast sensitivity. Our retrospective, comparative study further supports these findings, confirming the positive outcomes of the Evolux IOL in a group of selected patients. It demonstrates statistically superior results compared to the standard monofocal GCB00 IOL, particularly in terms of UIVA, DCIVA and UNVA. In fact, in our selected group of Evolux patients UNVA was surprisingly superior compared to the GCB00 group, which requires a higher spherical addition for near vision. The results obtained for near vision should be validated and, if necessary, confirmed in a larger group of patients, as suggested by recent literature. [
19]
Regarding the defocus curve, our findings revealed that patients consistently achieved a visual acuity superior to 0.2 logMAR for defocus levels ranging from +1.00 D to -2.00 D. These results highlight both the outstanding defocus tolerance and the capacity of the Evolux IOL to maintain high visual quality across a broad spectrum of distances, showing a smoother curve in the intermediate defocus range (−1 to −2 D). This is consistent with superior performance at intermediate distances compared to the GCB00 IOL group. In fact, PRSIQ showed positive results in the Evolux group of patients, with 70% of them being able to perform intermediate tasks without spectacles.
Moreover, Evolux IOL shows a favorable aberrometric profile, high optical quality and a contrast sensitivity curve comparable to that of the GCB00 IOL. Our study provides valuable additional insights to the overall assessment, including the use of halometry, a tool that enabled objective confirmation that patients with the Evolux IOL experience no greater halo formation compared to those with our reference monofocal lens. To the best of our knowledge, this is the first study to objectively evaluate the quality of vision in relation to post-operative halo presence.
Optical bench analysis confirmed from a physical point of view these clinical results, showing a larger depth of field for Evolux IOL, providing better performance for the near-intermediate distance. In addition, the power map revealed an alternate variation of power in the central part of Evolux IOL, reflecting the optical design based on positive/negative SA in the central 4.5 mm zone. This larger range of power enables to achieve a larger depth of field compared to the GCB00.
This new IOL demonstrated excellent refractive predictability, with all patients implanted with the Evolux IOL achieving a post-operative spherical equivalent (SE) within ±0.50 D. Specifically, the Evolux group achieved a SE of -0.14 ± 0.31, comparable to the GCB00 group. Similarly, the ELP assessed at 1, 3, and 6 months remained stable over time. The well-known Evolux IOL design with C-loops, combined with hydrophobic acrylic material, ensures this optimal refractive and structural stability, with no significant differences from the monofocal group.
However, we acknowledge several limitations in the current study. Firstly, the patient sample is relatively small and highly selective. Moreover, the non-randomized design may increase the potential for bias. Therefore, a larger, more diverse patient population is necessary to validate these findings. Moreover, we excluded patients with pupillary diameter in photopic conditions ≤ 2.5 mm and in mesopic conditions ≥ 6 mm. We know that pupillary dynamics might play a role in enabling the optical properties of enhanced or EDOF IOLs, especially in a lens like Evolux, which concentrates its main aberrometric characteristics within the central 4.5 mm zone. This relationship between pupil dynamics and Evolux performance should be addressed and clarified in future studies.
In conclusion, Evolux IOL demonstrated superior performance compared to the monofocal IOL in intermediate vision, providing a promising extension of the depth of field. This makes it a suitable option for patients seeking some degree of versatility in their vision while still prioritizing distance clarity. The unique refractive design of the Evolux IOL, which lacks steps or rings, presents a compelling option for patients who prioritize a seamless visual transition between different distances, particularly for tasks requiring clear vision at closer ranges. Further research will be necessary to validate our results and to compare the optical properties and clinical outcomes of Evolux lens with other new PARTIAL-RoF-Enhanced IOLs.