1. Introduction
The global prevalence of diabetes mellitus (DM) is estimated at 9.3%, with projections indicating an increase to 10.2% by 2030 and 10.9% by 2045[
1]. Among patients with type 2 DM, diabetic kidney disease (DKD) affects approximately 27%, with a mortality rate 18.3 times higher than in individuals without DM [
2].
The inflammatory pathway plays the most important role in the pathophysiology of Diabetic Kidney Disease (DKD). Inflammation plays a central role in the pathophysiology of DKD, characterized by the activation of immune cells producing pro-inflammatory cytokines. Key biomarkers such as matrix metalloproteinase 9 (MMP-9) and intercellular adhesion molecule (ICAM-1) are implicated in promoting kidney damage through inflammation and fibrosis [
3,
4,
5]. While these biomarkers are well established in the disease process, their direct correlation with non-invasive imaging biomarkers, particularly in the context of immunotherapy, remains underexplored
Diffusion tensor imaging (DTI) has emerged as a promising non-invasive technique for assessing kidney microstructure, providing insights into tissue integrity through the measurement of fractional anisotropy (FA) and mean diffusivity (MD)[
6]. DTI has been shown to detect kidney inflammation and fibrosis by revealing structural changes in kidney tissue. DTI utilizes water diffusion properties to map tissue microstructure, and one of its metrics, FA, have demonstrated sensitivity to various kidney diseases, including chronic kidney disease (CKD) and glomerulonephritis . FA values correlate closely with renal function and pathological changes, offering a promising method for monitoring CKD progression[
7].
Autologous dendritic cells, derived from the patient’s own immune system, have gained attention as a promising therapeutic option in modulating the immune response in various inflammatory conditions[
8,
9,
10,
11,
12]. These cells can be engineered ex vivo to enhance their immune-regulatory properties and reintroduced into the patient’s body to promote immune tolerance and reduce inflammation [
9]. The therapeutic use of autologous dendritic cells has shown potential in reducing inflammation and fibrosis in various condition, making them a promising candidate for managing DKD.
However, the therapeutic use of autologous dendritic cells to modulate inflammation in DKD, combined with DTI MRI for monitoring, has not been fully investigated. This study aims to fill this gap by evaluating the effects of autologous dendritic cell transfer on renal inflammation and fibrosis, using DTI MRI and inflammatory biomarkers (MMP-9 and ICAM-1) as key measures of treatment efficacy.
2. Materials and Methods
2.1. Study Design
This study employed a quasi-experimental design, specifically a one-group pre-test post-test approach. The research was conducted at the Army Central Hospital, with ethical approval granted by the Ethics Committee of the Gatot Soebroto Army Central Hospital (Ethical Clearance No 110/VIII/KEPK/2024, dated 23 August 2024). Written informed consent was obtained from all participants involved in the study.
2.2. Research Subject
The research subjects were patients with DKD from the internal medicine polyclinic of the Army Hospital. The study employed a nonprobability sampling technique.
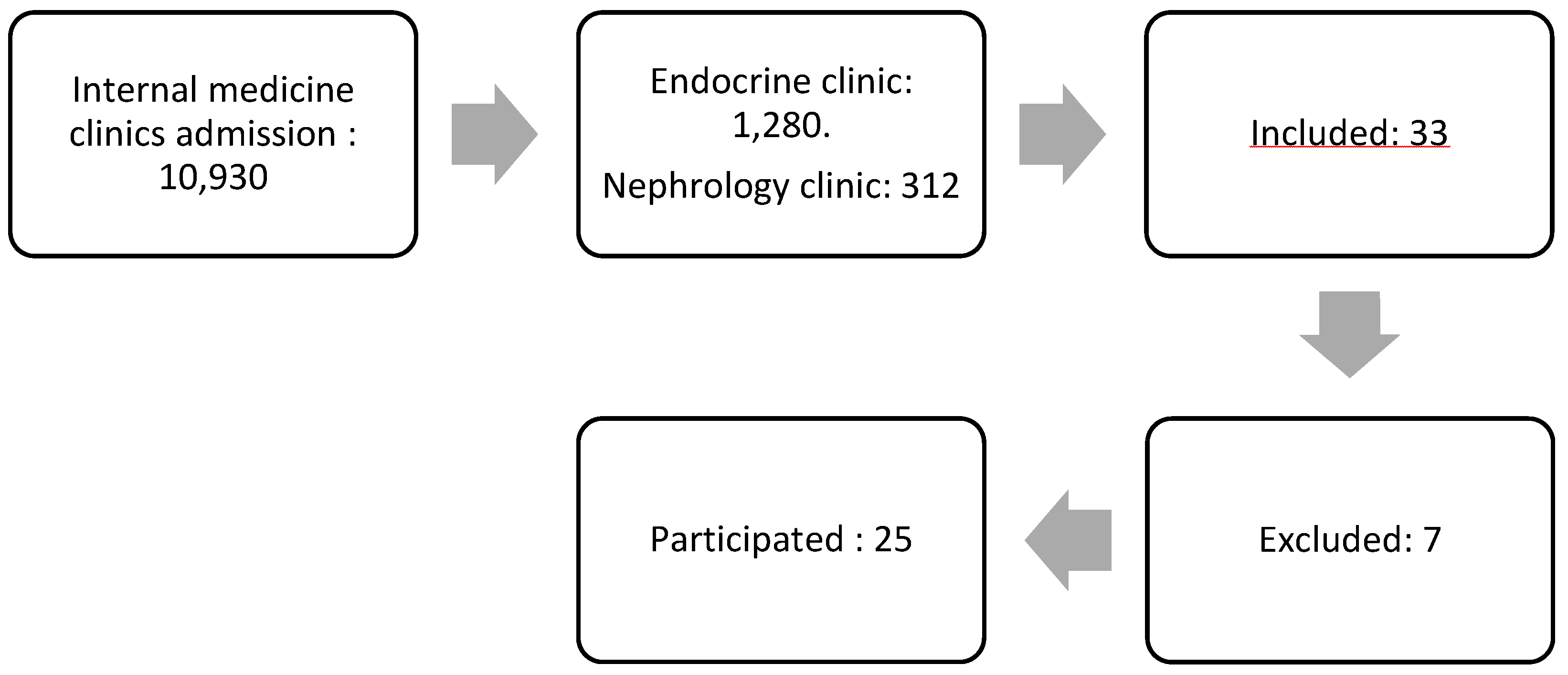
Figure 1 illustrates the flow of subject selection. Initially, the study began with a total of 10,930 patients from the internal medicine polyclinic. After narrowing the selection to 1,280 patients from the Endocrine Clinic and 312 patients from the Kidney Clinic, 33 patients expressed a willingness to participate. However, seven of these patients were excluded, leaving 25 who met the criteria to continue in the study.
Patients were excluded if they had received immunosuppressive treatment in the past four weeks, had other kidney diseases, were diagnosed with conditions causing proteinuria, had other types of DM, were pregnant, required oxygen supplementation, were undergoing cancer hormone therapy, had a history of thromboembolism, had obesity with a BMI > 40 kg/m², or had uncontrolled hypertension (systolic > 180 mmHg or diastolic > 100 mmHg).
The sample size was determined using the G*Power application with a two-tailed t-test approach. The calculation was based on an effect size of 0.8, a significance level (α) of 0.05, and a statistical power of 0.95, resulting in a minimum required sample size of 23 subjects.
2.3. Research Procedure
The study procedure included several key steps. Initially, subjects were prepared for baseline measurements, which involved blood sampling to assess the biomarkers MMP-9 and ICAM-1, as well as the generation of autologous dendritic cells. Following this, subjects underwent Diffusion Tensor Imaging (DTI) MRI examinations of the kidneys.
The MMP-9, ICAM-1, and DTI MRI examinations were performed at two time points: before the administration of autologous dendritic cells and four weeks following the treatment .
2.4. Study Product Generation
Blood samples were collected from the research subjects at baseline. A total of 40 cc of blood was isolated and incubated with GM-CSF (Granulocyte Macrophage Colony Stimulating Factor) media and IL-4 for five days, promoting the formation of dendritic cells. The dendritic cells were then further incubated for an additional two days to induce maturation. Finally, the autologous dendritic cells were injected subcutaneously into the subject’s arm.
2.5. Laboratory Testing and MRI DTI
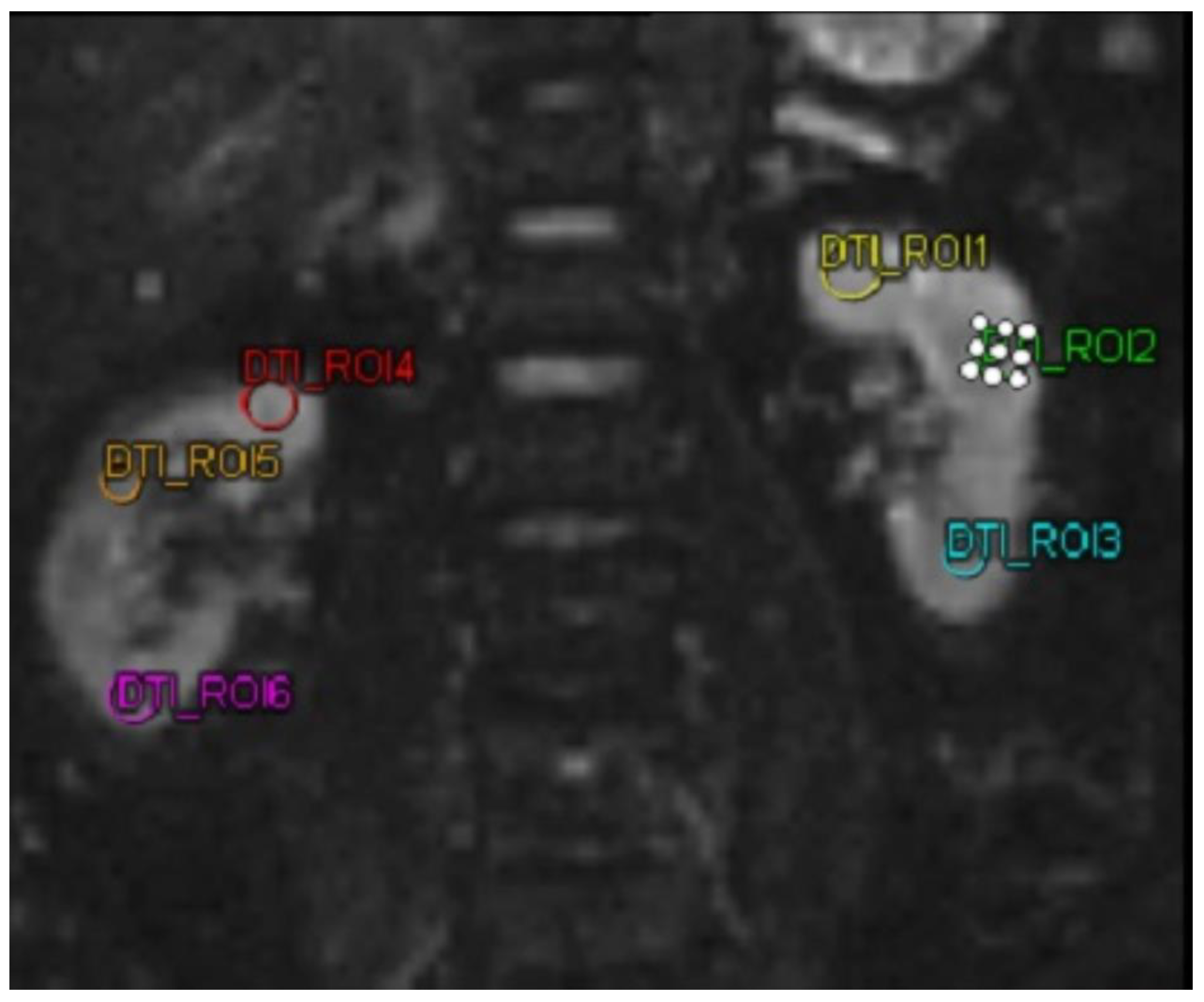
MMP-9 and ICAM-1 biomarkers were examined using a sandwich-ELISA kit (Reed Biotech Ltd). DTI MRI imaging examination in this study used a MAGNETRON Vida MRI scanner (Siemens,Erlangen,Germany). DTI MRI examination was performed on both kidneys of the subject. Then the subject was subjected to 3 ROIs in each kidney. After obtaining the FA value. In this study, FA was taken, with a decrease and increase in FA in patients.
MRI Scanner Specifications
Scanner Type: MAGNETOM Vida
Material Number: 11060815
Serial Number: 176241
Field Strength: 3 Tesla (assumed based on standard MAGNETOM Vida systems)
Modality: Magnetic Resonance Imaging (MR)
Imaging Protocol
Diffusion Tensor Imaging Parameters
Diffusion Mode: Multi-directional diffusion weighting (MDDW)
Diffusion Scheme: Monopolar
Diffusion Directions: 6 directions
-
b-value:
- ○
b = 0 s/mm² (5 averages)
- ○
b = 330 s/mm² (12 averages)
Diffusion Weightings: 2
Slice thickness: 1.8 mm
Echo Time (TE): 71 ms
Repetition Time (TR): 5800 ms
Coil elements used: SP2-4
Post-processing and data analysis
Tensor Fitting: Tensor analysis was conducted to generate maps for Fractional Anisotropy (FA).
FA Maps: Active
Noise Correction: Active.
Dynamic Field Correction: Enabled
Noise Masking: Active
2.6. Statistics
A data normality test was conducted on each variable. Shapiro-Wilk normality test was used for samples below 50, while Kolmogorov-Smirnov test was used for samples above 50. PSV and EDV variables were analysed using paired t-test for normally distributed data, while non-normally distributed data were analysed using Wilcoxon signed ranks test. MMP 9 and TGF β variables used linear regression analysis tests to see the effect before and after administration of autologous dendritic cells.
3. Results
3.1. Subject Characteristics
Table 1 shows the characteristics of the study subjects. This study involved 25 subjects who met the inclusion and exclusion criteria, and followed the research procedures in stages until the end. The average age of the subjects was 63 years old which ranged from 50-78 years old, there were ten men and 15 women, 13 with microalbuminuria and 12 with macroalbuminuria. The majority of comorbidities found were hypertension, which included 24 subjects (96%). Most of the subjects' body mass index was overweight, with 11 subjects (44%). The degree of chronic kidney disease was mostly at degree 3, suffered by 12 (48%).
3.2. MRI Analysis of DTI, MMP-9, and ICAM-1
Table 2 shows the analysis of FA, MMP-9, and ICAM-1. In DTI MRI measurements (FA) before and after administration of autologous dendritic cells (
Figure 2), the median value before administration was 242.57 ± 63.97. After administration of autologous dendritic cells, the median value increased to 305.61 ± 152.32. Statistical tests showed a significant difference with a p-value = 0.042.
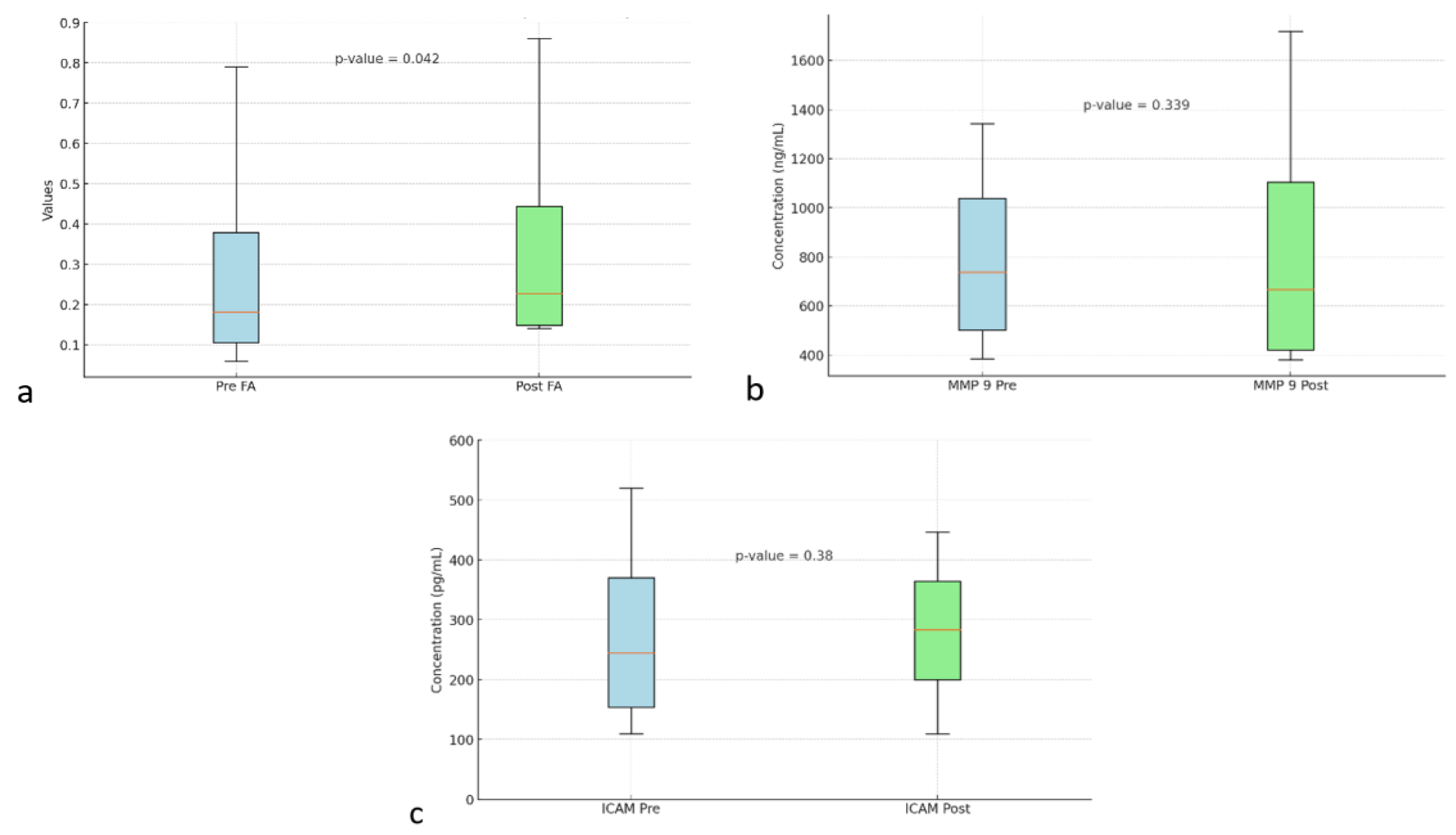
Figure 3a shows box and whisker FA on DTI MRI.
Figure 3b shows the box and whisker MMP-9. MMP-9 biomarker, the mean value before intervention was 873.68 ± 306.32. After the intervention, the mean value increased to 921.97 ± 333.33. However, no statistically significant difference was found with a p value = 0.339.
Figure 3c shows box and whisker ICAM-1. ICAM-1 biomarker, the mean before intervention was 321.74 ± 84.83 with a median of 320.2 ± 109.9. After administration of autologous dendritic cells, the mean increased to 331.8 ± 61.05. However, no statistically significant difference was found with a p value = 0.38.
Table 3 shows the relationship between the variables in the study. There was a significant negative relationship between FA and MMP-9, with an r value of -0.347 and a p value of 0.016. This indicates that an increase in FA is associated with a decrease in MMP-9. Meanwhile, the relationship between FA and ICAM-1 showed no significance, with a p value of 0.402. The relationship between MMP-9 and ICAM-1 was not significant with a p value of 0.409.
Table 1. PSV by sex, age, and UACR before and after autologous dendritic cell administration
4. Discussion
In this study, it was found that most of the comorbidities suffered by the research subjects were hypertension. Hypertension plays a role in exacerbating DKD by increasing intraglomerular pressure. DM and hypertension cause synergistic effects that increase endoplasmic reticulum stress and mitochondrial dysfunction in glomerular cells[
13]. DKD associated with hypertension results from a chronic inflammatory reaction, which causes glomerular capillary injury and decreased renal function. This chronic inflammation will release pro-inflammatory cytokines, contributing to increased blood pressure and fibrosis in the kidney[
14].
Based on the research results presented above, it can be seen that therapy using autologous dendritic cells has different effects on the various parameters measured. On MRI DTI FA, there was a significant improvement after therapy with a p-value of 0.042, indicating a positive change in tissue structure or function measured through MRI DTI FA imaging. This indicates that the therapy may have contributed to the improvement. Fractional Anisotropy (FA) in the kidney is related to the use of DTI sequences in renal imaging, which aims to assess the diffusion of water molecules in kidney tissue. FA is one of the important parameters in DTI that measures the direction and extent of water diffusion anisotropy, which is related to kidney structure and function. The use of DTI MRI in the kidney has shown that FA can be used as an indicator of microstructural damage, such as fibrosis or changes in the renal tubules. In the context of Diabetic Kidney Disease, FA measurement could be an important diagnostic tool to detect microstructural changes that cannot be seen through conventional imaging, enabling earlier detection and assessment of response to therapy[
15]. FA values are negatively associated with serum creatinine and cystatin C, and positively associated with eGFR[
16].
This study also found a significant correlation between Fractional Anisotropy (FA) and inflammatory biomarker MMP-9 (p = 0.025). This is in line with the research of Seah et al. which states that there is a relationship between FA and inflammatory biomarkers[
17]. In the early stages of DKD, MMP-9 increases as an initial response to hyperglycemia and oxidative stress. Increased MMP-9 is involved in tubular basement membrane degradation and inflammatory cell recruitment, leading to damage to the kidneys[
18]. This study shows that an increase in FA is associated with a decrease in MMP-9. A decrease in MMP-9 can improve kidney function in DKD. Decreased MMP-9 can modulate podocyte function and inhibit dedifferentiation. Suggesting that lower MMP-9 may contribute to improved renal function by maintaining podocyte integrity and function, which is important in maintaining glomerular filtration[
19]. MMP-9 is also positively associated with albuminuria, so a decrease in MMP-9 indicates a decrease in albuminuria[
19].
In this study, there were changes in MMP-9 values that were not significant. Changes in MMP-9 are likely due to the longer follow-up time required in this study and the administration of autologous dendritic cells more than once. This is in accordance with research conducted by Miyazaki et al. The study examined changes in MMP-9 which decreased significantly within 12 months after the administration of ACEi or ARB during the same period[
20].
In this study, there were no significant changes in ICAM-1 values. Future studies are expected to assess ICAM-1 once a week for one month. Research conducted by Shukla et al. assessed ICAM-1 seven days after the intervention[
21]. Further research is expected to assess ICAM-1 once a week for seven days to assess changes in ICAM-1.
In this study, there were significant changes in FA on DTI MRI, while the biomarkers MMP-9 and ICAM-1 showed no significant changes. This may be because there are other inflammatory biomarkers, which can affect the value of FA. Research conducted by Tripathi et al. showed a significant correlation of FA with IL-1β and TNF α[
22]. In research conducted by Rodrigue et al., there is a significant relationship between FA with IL 8 and TNF α[
23].
5. Conclusions
This study found a significant increase in FA (Fraction Anisotropy) on DTI (Diffuse Tensor Imaging) MRI overall and found a significant negative correlation between FA and MMP-9, indicating that an increase in FA is associated with a decrease in MMP-9.
Author Contributions
E.K designed the study, supervised data collection, performed data analysis, and wrote the manuscript.. A.P.L collected data and managed administrative work. Y.R provided senior supervision and reviewed the manuscript B.A.H provided review and editing of the manuscript. J. supervised data collection and reviewed the manuscript. C.N.L provided senior supervision and reviewed the manuscript. T.A.P provided senior supervision and reviewed the manuscript.
Funding
This research APC was funded by PT. JES Kasih Nusantar Sejahtera.
Institutional Review Board Statement
Ethical approval for the study was granted by the Ethics Committee of the Army Hospital (Ethical Clearance No 110/VIII/KEPK/2024, dated 23 August 2024).
Informed Consent Statement
All participants provided written informed consent prior to their inclusion in the study.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DM |
Diabetes Mellitus |
| DKD |
Diabetic Kidney Disease |
| MMP-9 |
Matrix Metalloproteinase 9 |
| ICAM-1 |
Intercellular Adhesion Molecule 1 |
| DTI |
Diffusion Tensor Imaging |
| FA |
Fractional Anisotropy |
| MD |
Mean Diffusivity |
| CKD |
Chronic Kidney Disease |
| GM-CSF |
Granulocyte Macrophage Colony Stimulating Factor |
| IL-4 |
Interleukin 4 |
| MRI |
Magnetic Resonance Imaging |
| UACR |
Urinary Albumin-Creatinine Ratio |
| ACEi |
Angiotensin Converting Enzyme Inhibitor |
| ARB |
Angiotensin Receptor Blocker |
| SGLT2-i |
Sodium-glucose Cotransporter-2 Inhibitor |
| GLP-1 |
Glucagon-Like Peptide-1 |
| DPP4-i |
Dipeptidyl Peptidase-4 Inhibitor |
| CCB |
Calcium Channel Blocker |
| HCT |
Hydrochlorothiazide |
| DHP |
Dihydropyridine |
| Non-DHP |
Non-Dihydropyridine |
| b-blocker |
Beta-blocker |
| Diuretic |
Diuretic |
| PSV |
Peak Systolic Velocity |
| EDV |
End-Diastolic Velocity |
| TNF α |
Tumor Necrosis Factor Alpha |
| IL-1β |
Interleukin 1 Beta |
| IL-8 |
Interleukin 8 |
References
- Daniel, S.; George, S.; Dongre, D.; Prajapat, P. Development and Evaluation of Information Booklet on Knowledge Regarding Type 3 Diabetes among Type 2 Diabetes Mallitus Patients. International Journal For Multidisciplinary Research 2024, 6, 1–5. [Google Scholar]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front Endocrinol (Lausanne) 2021, 12, 672350. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, H.; Liu, F.; Ma, Q. Up-regulation of matrix metalloproteinases-9 in the kidneys of diabetic rats and the association with neutrophil gelatinase-associated lipocalin. BMC Nephrol 2021, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Seman, N.A.; Falhammar, H.; Brismar, K.; Gu, H.F. Genetic and Biological Effects of ICAM-1 E469K Polymorphism in Diabetic Kidney Disease. J Diabetes Res 2020, 2020, 8305460. [Google Scholar] [CrossRef] [PubMed]
- Avraham, S.; Virgincar, R.S.; Webster, J.; Brightbill, H.D.; Korin, B.; Shaw, A.S.; et al. Evaluating Renal Fibrosis Using Diffusion Tensor Imaging (DTI)-MRI. Journal of the American Society of Nephrology 2022, 33, 575. [Google Scholar] [CrossRef]
- Maharjan, S.; Chen, J.; Gaughan, A.; Chen, N.X.; Wang, N. Diffusion tractography of kidney by high angular resolution diffusion imaging. Magnetic Resonance Letters 2024, 4, 200117. [Google Scholar] [CrossRef]
- Jonny Sitepu, E.C.; Hernowo, B.A.; Chiuman, L.; Lister, I.N.E.; Putranto, T.A. Open-Label Clinical Trial on the Impact of Autologous Dendritic Cell Therapy on Albuminuria and Inflammatory Biomarkers (Interleukin-6, Interleukin-10, Tumor Necrosis Factor α) in Diabetic Kidney Disease (DKD). Curr Issues Mol Biol 2024, 46, 13662–13674. [Google Scholar] [CrossRef]
- Jonny, J.; Sitepu, E.C.; Lister, I.N.E.; Chiuman, L.; Putranto, T.A. The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus. Vaccines (Basel) 2024, 12, 972. [Google Scholar] [CrossRef]
- Dillman, R.O.; Nistor, G.I.; Jonny, J.; Yana, M.L.; Langford, J.L.; Putranto, T.A.; et al. Prevention of Symptomatic Covid-19 Infection by Personal Dendritic Cell Vaccine. Journal of Vaccines, Immunology and Immunopathology 2023, 8, 189. [Google Scholar] [CrossRef]
- Lu, W.; Arraes, L.C.; Ferreira, W.T.; Andrieu, J.-M. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med 2004, 10, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Jonny Putranto, T.A.; Purnama, Y.; Djatmiko, R.; Yana, M.L.; Sitepu, E.C.; et al. Significant improvement of systemic lupus erythematosus manifestation in children after autologous dendritic cell transfer: A case report and review of literature. Ther Adv Vaccines Immunother 2023, 11, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; do Carmo, J.M.; da Silva, A.A.; Fu, Y.; Hall, J.E. Mechanisms of Synergistic Interactions of Diabetes and Hypertension in Chronic Kidney Disease: Role of Mitochondrial Dysfunction and ER Stress. Curr Hypertens Rep 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Li, Y. Relevance of the Pyroptosis-Related Inflammasome Pathway in the Pathogenesis of Diabetic Kidney Disease. Front Immunol 2021, 12, 603416. [Google Scholar] [CrossRef]
- Ye, X.J.; Cui, S.H.; Song, J.W.; Liu, K.; Huang, X.Y.; Wang, L.; et al. Using magnetic resonance diffusion tensor imaging to evaluate renal function changes in diabetic patients with early-stage chronic kidney disease. Clin Radiol 2019, 74, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, R.; Zhou, X.; Ni, L.; Zha, D.; Feng, H.; et al. Alterations of Renal Function in Patients with Diabetic Kidney Disease: A BOLD and DTI Study. Comput Intell Neurosci 2022, 2022, 6844102. [Google Scholar] [CrossRef]
- Seah, J.; Botterill, E.; MacIsaac, R.J.; Milne, M.; Ekinci, E.I.; Lim, R.P. Functional MRI in assessment of diabetic kidney disease in people with type 1 diabetes. J Diabetes Complications 2022, 36, 108076. [Google Scholar] [CrossRef]
- Wang, H.; Gao, M.; Li, J.; Sun, J.; Wu, R.; Han, D.; et al. MMP-9-positive neutrophils are essential for establishing profibrotic microenvironment in the obstructed kidney of UUO mice. Acta Physiologica 2019, 227, e13317. [Google Scholar] [CrossRef]
- Li, S.Y.; Huang, P.H.; Yang, A.H.; Tarng, D.C.; Yang, W.C.; Lin, C.C.; et al. Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int 2014, 86, 358–369. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kasai, T.; Miyauchi, K.; Miyazaki, T.; Akimoto, Y.; Takagi, A.; et al. Changes of Matrix Metalloproteinase-9 Level Is Associated With Left Ventricular Remodeling Following Acute Myocardial Infarction Among Patients Treated With Trandolapril, Valsartan or Both. Circulation Journal 2010, 74, 1158–1164. [Google Scholar] [CrossRef]
- Shukla, S.D.; Shastri, M.D.; Vanka, S.K.; Jha, N.K.; Dureja, H.; Gupta, G.; et al. Targeting intercellular adhesion molecule-1 (ICAM-1) to reduce rhinovirus-induced acute exacerbations in chronic respiratory diseases. Inflammopharmacology 2022, 30, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Awasthi, R.; Agarwal, V.; Agrawal, V.; Rathore, R.K.S.; Sharma, K.; et al. Diffusion Tensor and Dynamic Contrast-Enhanced Magnetic Resonance Imaging Correlate with Molecular Markers of Inflammation in the Synovium. Diagnostics 2022, 12, 3041. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, A.L.; Knowles, E.E.M.; Mollon, J.; Mathias, S.R.; Koenis, M.M.G.; Peralta, J.M.; et al. Evidence for genetic correlation between human cerebral white matter microstructure and inflammation. Hum Brain Mapp 2019, 40, 4180–4191. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).