Submitted:
28 March 2025
Posted:
31 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Cause of High Conductivity in the Lithospheric Mantle of the Cratons
4.2. Cause of High Resistivity in the Lithospheric Mantle of Mozambique Mobile Belts / East African Rifts
5. Conclusions
- (1)
- The experiments revealed that the dunite samples containing Na2CO3 began to melt above 800°C, leading to a rapid increase in electrical conductivity. A small amount of alkali-carbonate melt can increase the electrical conductivity of dunite by 1-2 orders of magnitude. Pure Na2CO3 started to melt above 1175°C, reaching an electrical conductivity of 200-300 S/m. In partially molten carbonated peridotite samples, Na+ is the main charge carrier.
- (2)
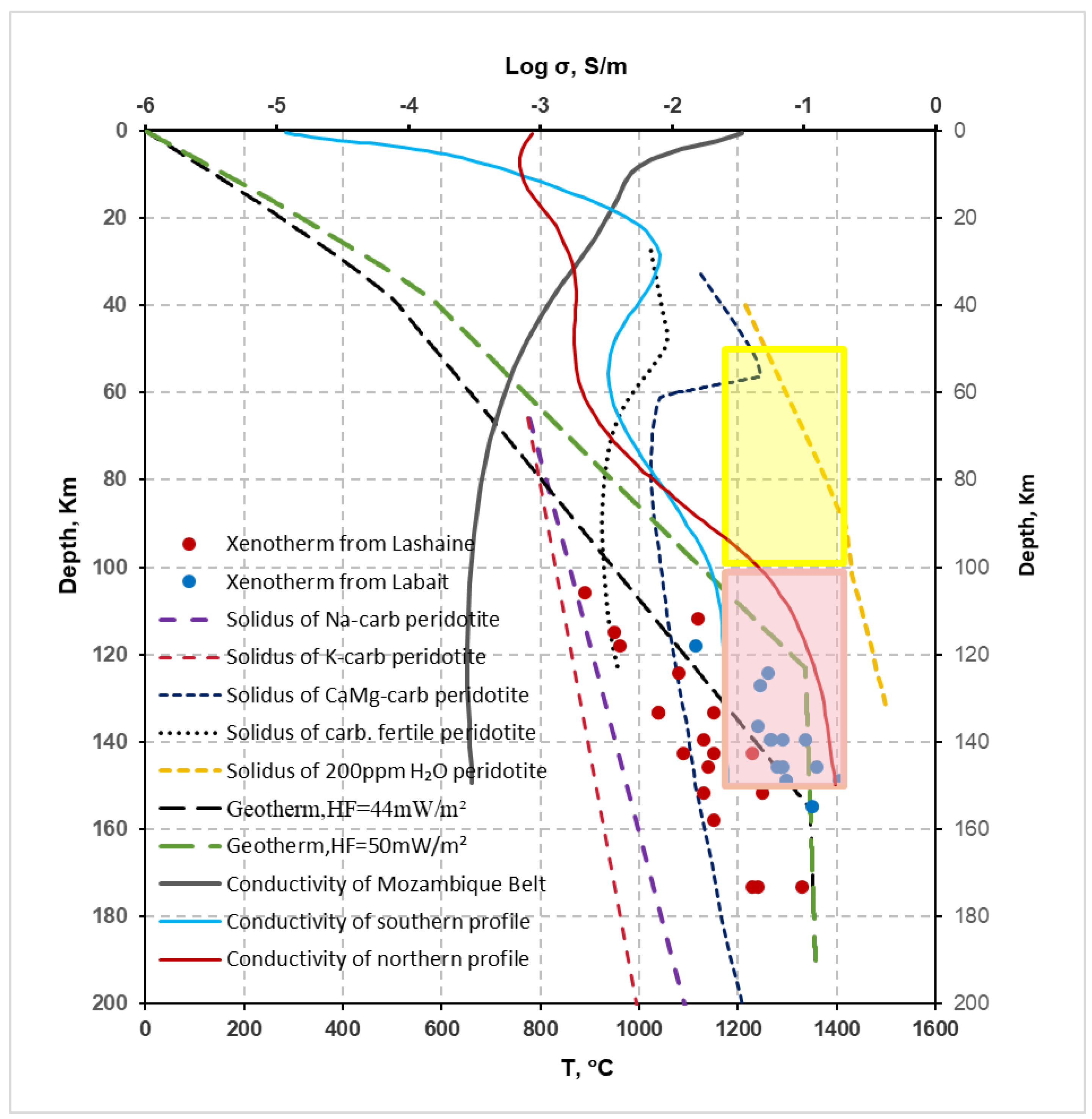
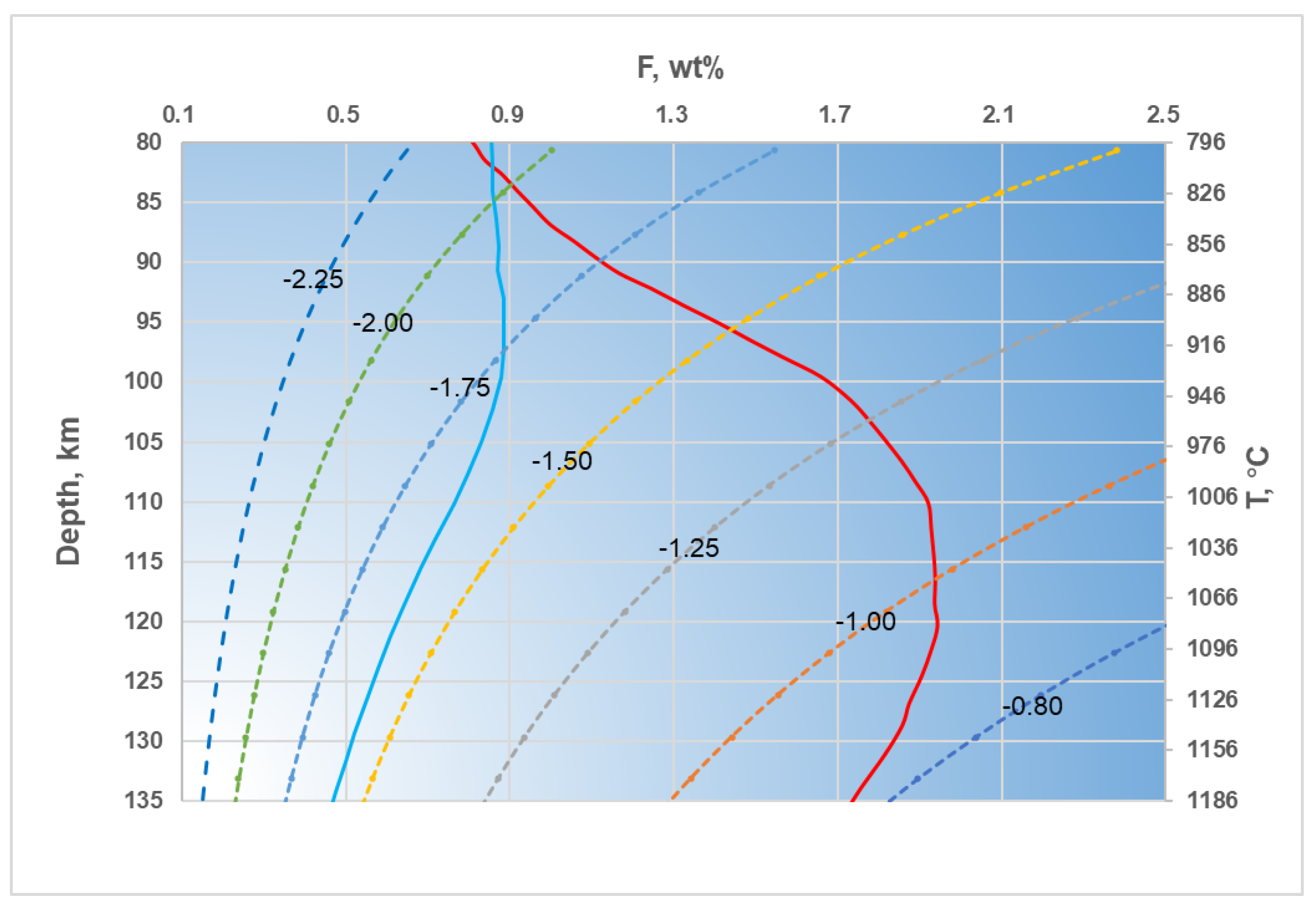
- Based on our experimental results, we estimated that the content of alkali-rich carbonate melt is approximately 1-2 wt% within the high conductivity layer of the Tanzanian craton (80-120 km). Assuming that all carbon is stored in the mantle by the form of carbonate melt, the carbon content is approximately 0.11 - 0.23 wt% in the high conductivity layer. Foley and Fischer estimated that the carbon content enriched at the bottom of the cratonic lithosphere since the formation of the craton is 0.43-0.86 wt%, which is higher than our calculated results[15]. The southern profile of the Tanzanian craton is far from various tectonic activity sites, and the variation in electrical conductivity with depth maybe represent the electrical distribution of the entire craton. The electrical conductivity of the lithosphere of the Tanzanian craton given by the southern profile is higher than that of other continental lithospheres. We estimate that the content of alkali-rich carbonate melts in the depth range of 80 - 135 km is between 0.89 - 0.45 wt%, corresponding to a carbon content of 0.10 - 0.05 wt%. Aiuppa et al. inferred that the carbon content in the 100 - 150 km depth range of the African Craton is approximately 0.04 - 0.07 wt%, which is close to our lowest value[68]. Taking the average, we believe that the carbon content of the lithospheric mantle of the Tanzanian craton is approximately 0.07 wt%, and the carbon content in the high conductivity layer is approximately 0.20 wt%. The carbon-rich phases at the bottom of the craton, such as alkali-rich carbonates, are more prone to melting due to the thermal influence of the deep mantle plume. Therefore, the presence of alkali-rich carbonate melt is the most likely and suitable mechanism to explain the high conductivity anomalies within the Archean Tanzanian craton.
- (3)
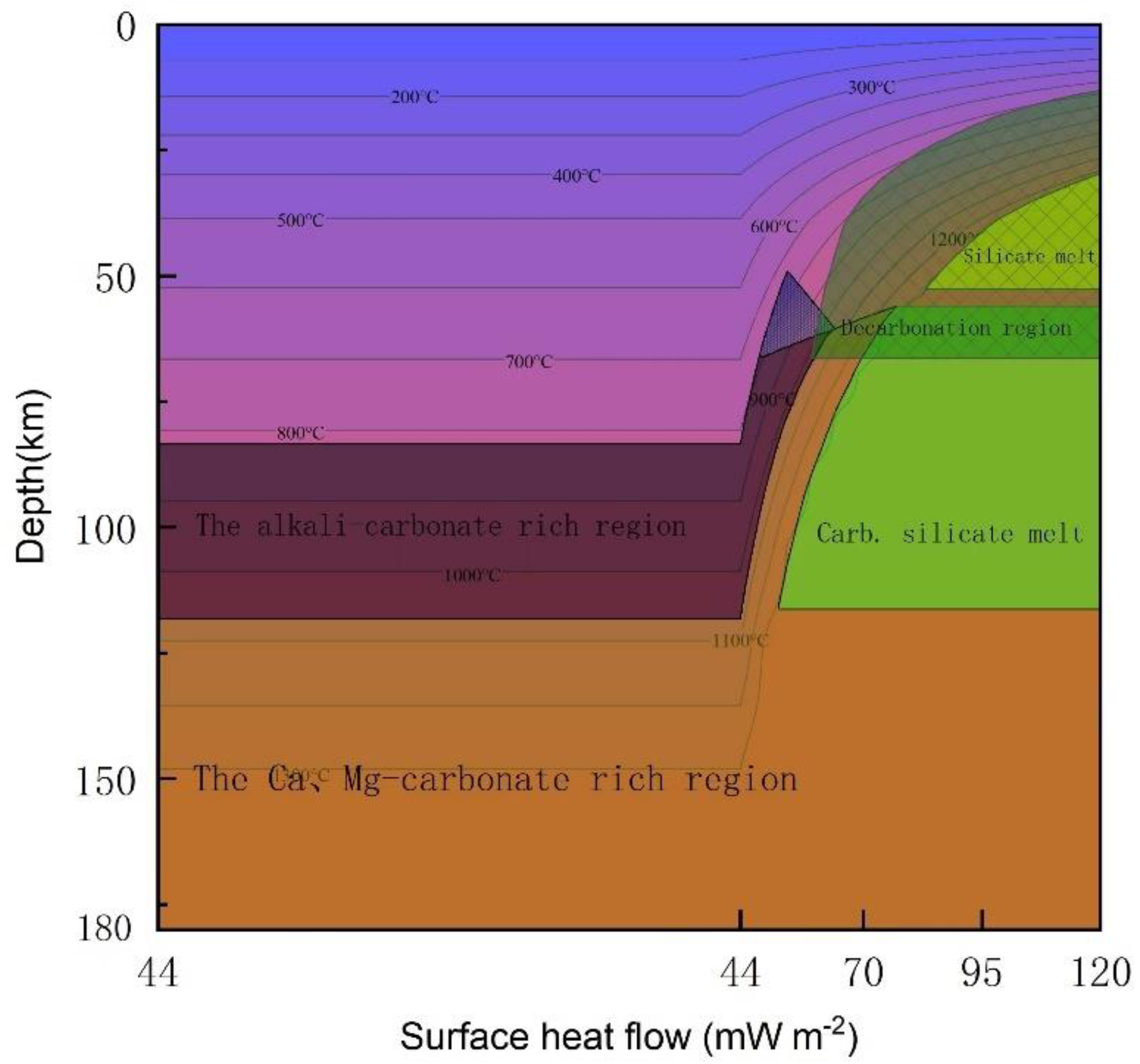
- The permeability barrier of the lithospheric mantle is the main reason why the electrical conductivity of the lithospheric mantle of the Tanzanian craton is 1-2 orders of magnitude higher than that of the lithospheric mantle of the Mozambique mobile belt, and it is also a prerequisite for the existence of a high conductivity layer within the Tanzanian craton.
- (4)
- The global average carbon content of the upper mantle is approximately 0.035 wt% [68]. In comparison, the Tanzanian craton is more carbon-rich (approximately 0.07 wt%). Carbon plays an important role in the evolution of cratons, as the presence of carbon-rich melts can disrupt the stability of cratons and cause thinning of the cratonic lithosphere. Our explanation for the causes of high conductivity in cratons and high resistivity in active zones also indirectly explains why the lithosphere of the Tanzanian craton is currently only about 135 km thick[22].
Funding
Data Availability Statement
Acknowledgments
Abbreviations
| MT | Magnetotelluric |
| En | Enstatite |
| Mag | Magnesite |
| Fo | Forsterite |
| Dol | Dolomite |
| Di | Diopside |
References
- Evans, R.L.; Jones, A.G.; Garcia, X.; Muller, M.; Hamilton, M.; Evans, S.; Fourie, C.J.S.; Spratt, J.; Webb, S.; Jelsma, H.; et al. Electrical lithosphere beneath the Kaapvaal craton, southern Africa. Journal of Geophysical Research-Solid Earth 2011, 116. [Google Scholar] [CrossRef]
- Miensopust, M.P.; Jones, A.G.; Muller, M.R.; Garcia, X.; Evans, R.L. Lithospheric structures and Precambrian terrane boundaries in northeastern Botswana revealed through magnetotelluric profiling as part of the Southern African Magnetotelluric Experiment. Journal of Geophysical Research-Solid Earth 2011, 116. [Google Scholar] [CrossRef]
- Bologna, M.S.; Dragone, G.N.; Muzio, R.; Peel, E.; Nuñez-Demarco, P.; Ussami, N. Electrical Structure of the Lithosphere From Rio de la Plata Craton to Parana Basin: Amalgamation of Cratonic and Refertilized Lithospheres in SW Gondwanaland. Tectonics 2019, 38, 77–94. [Google Scholar] [CrossRef]
- Azeez, K.K.A.; Veeraswamy, K.; Gupta, A.K.; Babu, N.; Chandrapuri, S.; Harinarayana, T. The electrical resistivity structure of lithosphere across the Dharwar craton nucleus and Coorg block of South Indian shield: Evidence of collision and modified and preserved lithosphere. Journal of Geophysical Research-Solid Earth 2015, 120, 6698–6721. [Google Scholar] [CrossRef]
- Bologna, M.S.; Padilha, A.L.; Vitorello, I.; Pádua, M.B. Signatures of continental collisions and magmatic activity in central Brazil as indicated by a magnetotelluric profile across distinct tectonic provinces. Precambrian Research 2011, 185, 55–64. [Google Scholar] [CrossRef]
- Krueger, H.E.; Gama, I.; Fischer, K.M. Global Patterns in Cratonic Mid-Lithospheric Discontinuities From Sp Receiver Functions. Geochemistry Geophysics Geosystems 2021, 22. [Google Scholar] [CrossRef]
- Selway, K.; Ford, H.; Kelemen, P. The seismic mid-lithosphere discontinuity. Earth and Planetary Science Letters 2015, 414, 45–57. [Google Scholar] [CrossRef]
- Wölbern, I.; Rümpker, G.; Link, K.; Sodoudi, F. Melt infiltration of the lower lithosphere beneath the Tanzania craton and the Albertine rift inferred from S receiver functions. Geochemistry Geophysics Geosystems 2012, 13. [Google Scholar] [CrossRef]
- Bettac, S.P.; Unsworth, M.J.; Pearson, D.G.; Craven, J. New constraints on the structure and composition of the lithospheric mantle beneath the Slave craton, NW Canada from 3-D magnetotelluric data-Origin of the Central Slave Mantle Conductor and possible evidence for lithospheric scale fluid flow. Tectonophysics 2023, 851. [Google Scholar] [CrossRef]
- Jones, A.G.; Lezaeta, P.; Ferguson, I.J.; Chave, A.D.; Evans, R.L.; Garcia, X.; Spratt, J. The electrical structure of the Slave craton. Lithos 2003, 71, 505–527. [Google Scholar] [CrossRef]
- Özaydin, S.; Selway, K.; Foley, S.F.; Ezad, I.S.; Griffin, W.L.; Tarits, P.S.; Hautot, S. Role of Metasomatism in the Development of the East African Rift at the Northern Tanzanian Divergence: Insights From 3D Magnetotelluric Modeling. Geochemistry Geophysics Geosystems 2024, 25. [Google Scholar] [CrossRef]
- Selway, K. Negligible effect of hydrogen content on plate strength in East Africa. Nature Geoscience 2015, 8, 543–546. [Google Scholar] [CrossRef]
- Thiel, S.; Heinson, G. Electrical conductors in Archean mantle—Result of plume interaction? Geophysical Research Letters 2013, 40, 2947–2952. [Google Scholar] [CrossRef]
- Foley, S.F.; Link, K.; Tiberindwa, J.V.; Barifaijo, E. Patterns and origin of igneous activity around the Tanzanian craton. Journal of African Earth Sciences 2012, 62, 1–18. [Google Scholar] [CrossRef]
- Foley, S.F.; Fischer, T.P. An essential role for continental rifts and lithosphere in the deep carbon cycle. Nature Geoscience 2017, 10, 897. [Google Scholar] [CrossRef]
- Dawson, J.B. Quaternary kimberlitic volcanism on the Tanzania craton. Contributions to Mineralogy and Petrology 1994, 116, 473–485. [Google Scholar] [CrossRef]
- Dawson, J.B.; Garson, M.S.; Roberts, B. Altered former alkalic carbonatite lava from Oldoinyo Lengai, Tanzania: Inferences for calcite carbonatite lavas. Geology 1987, 15, 765–768. [Google Scholar] [CrossRef]
- Dawson, J.B. Sodium carbonate lavas from Oldoinyo Lengai. Nature 1962, 195, 1075. [Google Scholar] [CrossRef]
- Selway, K. Electrical Discontinuities in the Continental Lithosphere Imaged with Magnetotellurics. In Lithospheric Discontinuities, Yuan, H., Romanowicz, B., Eds.; Geophysical Monograph Book Series; 2019; Volume 239, pp. 89-109.
- O'Donnell, J.P.; Adams, A.; Nyblade, A.A.; Mulibo, G.D.; Tugume, F. The uppermost mantle shear wave velocity structure of eastern Africa from Rayleigh wave tomography: constraints on rift evolution. Geophysical Journal International 2013, 194, 961–978. [Google Scholar] [CrossRef]
- Celli, N.L.; Lebedev, S.; Schaeffer, A.J.; Gaina, C. African cratonic lithosphere carved by mantle plumes. Nature Communications 2020, 11. [Google Scholar] [CrossRef]
- Boyce, A.; Bastow, I.D.; Cottaar, S.; Kounoudis, R.; De Courbeville, J.G.; Caunt, E.; Desai, S. AFRP20: New P-Wavespeed Model for the African Mantle Reveals Two Whole-Mantle Plumes Below East Africa and Neoproterozoic Modification of the Tanzania Craton. Geochemistry Geophysics Geosystems 2021, 22. [Google Scholar] [CrossRef]
- Afonso, J.C.; Ben-Mansour, W.; O'Reilly, S.Y.; Griffin, W.L.; Salajeghegh, F.; Foley, S.; Begg, G.; Selway, K.; Macdonald, A.; Januszczak, N.; et al. Thermochemical structure and evolution of cratonic lithosphere in central and southern Africa. Nature Geoscience 2022, 15, 405. [Google Scholar] [CrossRef]
- Lee, C.-T.; Rudnick, R. Compositionally stratified cratonic lithosphere and Geochemistry of peridototic xenoliths from the Labait volcano Tanzania. The P.H. Nixon volume 1999, 728–735. [Google Scholar]
- Lloyd, F.E.; Bailey, D.K. Light element metasomatism of the continental mantle: The evidence and the consequences. Physics and Chemistry of the Earth 1975, 9, 389–416. [Google Scholar] [CrossRef]
- Foley, S.F.; Jacob, D.E.; O'Neill, H.S.C. Trace element variations in olivine phenocrysts from Ugandan potassic rocks as clues to the chemical characteristics of parental magmas. Contributions to Mineralogy and Petrology 2011, 162, 1–20. [Google Scholar] [CrossRef]
- Rosenthal, A.; Foley, S.F.; Pearson, D.G.; Nowell, G.M.; Tappe, S. Petrogenesis of strongly alkaline primitive volcanic rocks at the propagating tip of the western branch of the East African Rift. Earth and Planetary Science Letters 2009, 284, 236–248. [Google Scholar] [CrossRef]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Tarasov, A.A.; Dymshits, A.M.; Kovaleva, E. Confocal Raman spectroscopic study of melt inclusions in olivine of mantle xenoliths from the Bultfontein kimberlite pipe (Kimberley cluster, South Africa): Evidence for alkali-rich carbonate melt in the mantle beneath Kaapvaal Craton. Journal of Raman Spectroscopy 2022, 53, 508–524. [Google Scholar] [CrossRef]
- Dai, L.D.; Hu, H.Y.; Jiang, J.J.; Sun, W.Q.; Li, H.P.; Wang, M.Q.; Vallianatos, F.; Saltas, V. An Overview of the Experimental Studies on the Electrical Conductivity of Major Minerals in the Upper Mantle and Transition Zone. Materials 2020, 13. [Google Scholar] [CrossRef]
- Yoshino, T.; Laumonier, M.; McIsaac, E.; Katsura, T. Electrical conductivity of basaltic and carbonatite melt-bearing peridotites at high pressures: Implications for melt distribution and melt fraction in the upper mantle. Earth and Planetary Science Letters 2010, 295, 593–602. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Chanyshev, A.D.; Litasov, K.D. The system Na2CO3-MgCO3 at 3GPa. High Pressure Research 2018, 38, 281–292. [Google Scholar] [CrossRef]
- Xu, Y.; Shankland, T.J.; Duba, A.G. Pressure effect on electrical conductivity of mantle olivine. Physics of the Earth and Planetary Interiors 2000, 118, 149–161. [Google Scholar] [CrossRef]
- Yoshino, T.; Gruber, B.; Reinier, C. Effects of pressure and water on electrical conductivity of carbonate melt with implications for conductivity anomaly in continental mantle lithosphere. Physics of the Earth and Planetary Interiors 2018, 281, 8–16. [Google Scholar] [CrossRef]
- Sifré, D.; Hashim, L.; Gaillard, F. Effects of temperature, pressure and chemical compositions on the electrical conductivity of carbonated melts and its relationship with viscosity. Chemical Geology 2015, 418, 189–197. [Google Scholar] [CrossRef]
- Gaillard, F.; Malki, M.; Iacono-Marziano, G.; Pichavant, M.; Scaillet, B. Carbonatite Melts and Electrical Conductivity in the Asthenosphere. Science 2008, 322, 1363–1365. [Google Scholar] [CrossRef]
- Yoshino, T.; McIsaac, E.; Laumonier, M.; Katsura, T. Electrical conductivity of partial molten carbonate peridotite. Physics of the Earth and Planetary Interiors 2012, 194, 1–9. [Google Scholar] [CrossRef]
- Özaydin, S.; Selway, K. MATE: An Analysis Tool for the Interpretation of Magnetotelluric Models of the Mantle. Geochemistry Geophysics Geosystems 2020, 21. [Google Scholar] [CrossRef]
- Rader, E.; Emry, E.; Schmerr, N.; Frost, D.; Cheng, C.; Menard, J.; Yu, C.Q.; Geist, D. Characterization and Petrological Constraints of the Midlithospheric Discontinuity. Geochemistry Geophysics Geosystems 2015, 16, 3484–3504. [Google Scholar] [CrossRef]
- Rudnick, R.; McDonough, W.; Orpin, A. Northern Tanzanian peridotite xenoliths: a comparison with Kaapvaal peridotites and inferences on metasomatic interactions. In Kimberlites, Related Rocks and Mantle Xenoliths, Meyer, H.O.A., Leonardos, O., Eds.; Companhia de Pesquisa de Recursos Minerais: 1994; Volume 1, pp. 336-353.
- Hasterok, D.; Chapman, D.S. Heat production and geotherms for the continental lithosphere. Earth and Planetary Science Letters 2011, 307, 59–70. [Google Scholar] [CrossRef]
- Bekhtenova, A.; Shatskiy, A.; Podborodnikov, I.V.; Are, A.V.; Litasov, K.D. Phase relations in carbonate component of carbonatized eclogite and peridotite along subduction and continental geotherms. Gondwana Research 2021, 94, 186–200. [Google Scholar] [CrossRef]
- Dasgupta, R.; Mallik, A.; Tsuno, K.; Withers, A.C.; Hirth, G.; Hirschmann, M.M. Carbon-dioxide-rich silicate melt in the Earth's upper mantle. Nature 2013, 493, 211–U222. [Google Scholar] [CrossRef] [PubMed]
- O'Leary, J.A.; Gaetani, G.A.; Hauri, E.H. The effect of tetrahedral Al3+ on the partitioning of water between clinopyroxene and silicate melt. Earth and Planetary Science Letters 2010, 297, 111–120. [Google Scholar] [CrossRef]
- Falloon, T.J.; Green, D.H. The solidus of carbonated, fertile peridotite. Earth and Planetary Science Letters 1989, 94, 364–370. [Google Scholar] [CrossRef]
- Rooney, T.O. The Cenozoic magmatism of East Africa: Part III - Rifting of the craton. Lithos 2020, 360. [Google Scholar] [CrossRef]
- Selway, K.; Yi, J.; Karato, S.I. Water content of the Tanzanian lithosphere from magnetotelluric data: Implications for cratonic growth and stability. Earth and Planetary Science Letters 2014, 388, 175–186. [Google Scholar] [CrossRef]
- Minarik, W.G.; Watson, E.B. Interconnectivity of carbonate melt at low melt fraction. Earth and Planetary Science Letters 1995, 133, 423–437. [Google Scholar] [CrossRef]
- Dalton, J.A.; Presnall, D.C. Carbonatitic melts along the solidus of model lherzolite in the system CaO-MgO-Al2O3-SiO2-CO2 from 3 to 7 GPa. Contributions to Mineralogy and Petrology 1998, 131, 123–135. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.L. Influence of mantle CO2 in the generation of carbonatites and kimberlites. Nature 1975, 257, 297–299. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H. Experimental reconstruction of sodic dolomitic carbonatite melts from metasomatised lithosphere. Contributions to Mineralogy and Petrology 1996, 124, 359–369. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Brey, G.P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: implications for petrogenesis of carbonatites. Contributions to Mineralogy and Petrology 2004, 146, 606–619. [Google Scholar] [CrossRef]
- Kono, Y.; Kenney-Benson, C.; Hummer, D.; Ohfuji, H.; Park, C.; Shen, G.Y.; Wang, Y.B.; Kavner, A.; Manning, C.E. Ultralow viscosity of carbonate melts at high pressures. Nature Communications 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.A.; Wood, B.J. The partitioning of Fe and Mg between olivine and carbonate and the stability of carbonate under mantle conditions. Contributions to Mineralogy and Petrology 1993, 114, 501–509. [Google Scholar] [CrossRef]
- Holtzman, B.K. Questions on the existence, persistence, and mechanical effects of a very small melt fraction in the asthenosphere. Geochemistry Geophysics Geosystems 2016, 17, 470–484. [Google Scholar] [CrossRef]
- Heaman, L.M.; Kjarsgaard, B.A.; Creaser, R.A. The timing of kimberlite magmatism in North America: implications for global kimberlite genesis and diamond exploration. Lithos 2003, 71, 153–184. [Google Scholar] [CrossRef]
- Weiss, Y.; Czas, J.; Navon, O. Fluid Inclusions in Fibrous Diamonds. Reviews in Mineralogy and Geochemistry 2022, 88, 475–532. [Google Scholar] [CrossRef]
- Aulbach, S. Cratonic Lithosphere Discontinuities: Dynamics of Small-Volume Melting, Metacratonization, and a Possible Role for Brines. In Lithospheric Discontinuities, Yuan, H., Romanowicz, B., Eds.; Geophysical Monograph Book Series; 2019; Volume 239, pp. 177-203.
- Guo, H.H.; Keppler, H. Electrical Conductivity of NaCl-Bearing Aqueous Fluids to 900°C and 5GPa. Journal of Geophysical Research-Solid Earth 2019, 124, 1397–1411. [Google Scholar] [CrossRef]
- Glover, P.W.J. A generalized Archie's law for n phases. Geophysics 2010, 75, E247–E265. [Google Scholar] [CrossRef]
- Newton, R.C.; Sharp, W.E. Stability of forsterite + CO2 and its bearing on the role of CO2 in the mantle. Earth and Planetary Science Letters 1975, 26, 239–244. [Google Scholar] [CrossRef]
- Hirschmann, M.M. Mantle solidus: Experimental constraints and the effects of peridotite composition. Geochemistry Geophysics Geosystems 2000, 1. [Google Scholar] [CrossRef]
- Russell, J.K.; Porritt, L.A.; Lavallée, Y.; Dingwell, D.B. Kimberlite ascent by assimilation-fuelled buoyancy. Nature 2012, 481, 352–U133. [Google Scholar] [CrossRef]
- Stamm, N.; Schmidt, M.W. Asthenospheric kimberlites: Volatile contents and bulk compositions at 7 GPa. Earth and Planetary Science Letters 2017, 474, 309–321. [Google Scholar] [CrossRef]
- Sparks, D.W.; Parmentier, E.M. Melt extraction from the mantle beneath spreading centers. Earth and Planetary Science Letters 1991, 105, 368–377. [Google Scholar] [CrossRef]
- Muirhead, J.D.; Fischer, T.P.; Oliva, S.J.; Laizer, A.; van Wijk, J.; Currie, C.A.; Lee, H.; Judd, E.J.; Kazimoto, E.; Sano, Y.; et al. Displaced cratonic mantle concentrates deep carbon during continental rifting. Nature 2020, 582, 67. [Google Scholar] [CrossRef]
- Hammouda, T.; Laporte, D. Ultrafast mantle impregnation by carbonatite melts. Geology 2000, 28, 283–285. [Google Scholar] [CrossRef]
- Aiuppa, A.; Casetta, F.; Coltorti, M.; Stagno, V.; Tamburello, G. Carbon concentration increases with depth of melting in Earth's upper mantle. Nature Geoscience 2021, 14, 697. [Google Scholar] [CrossRef]
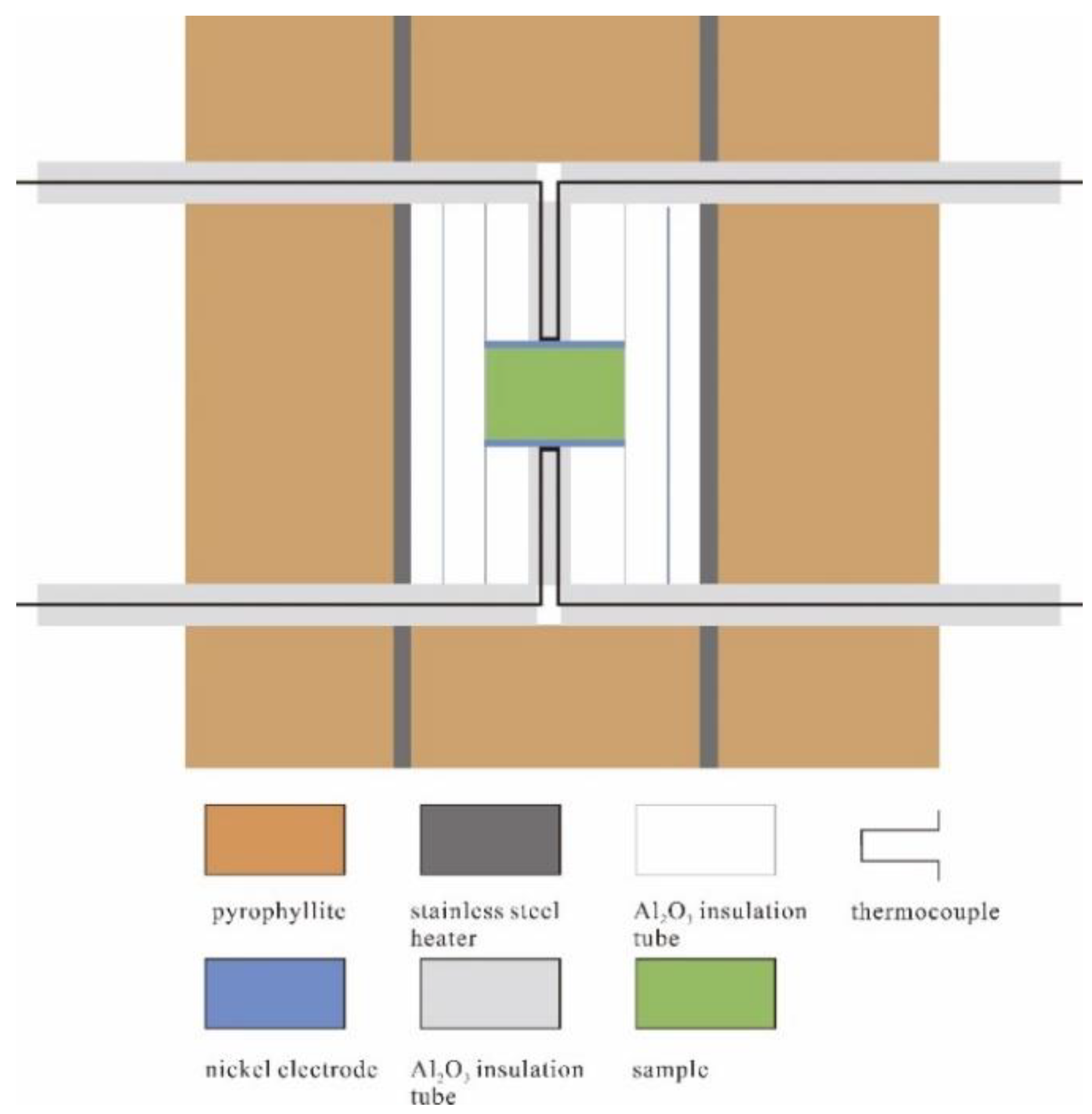
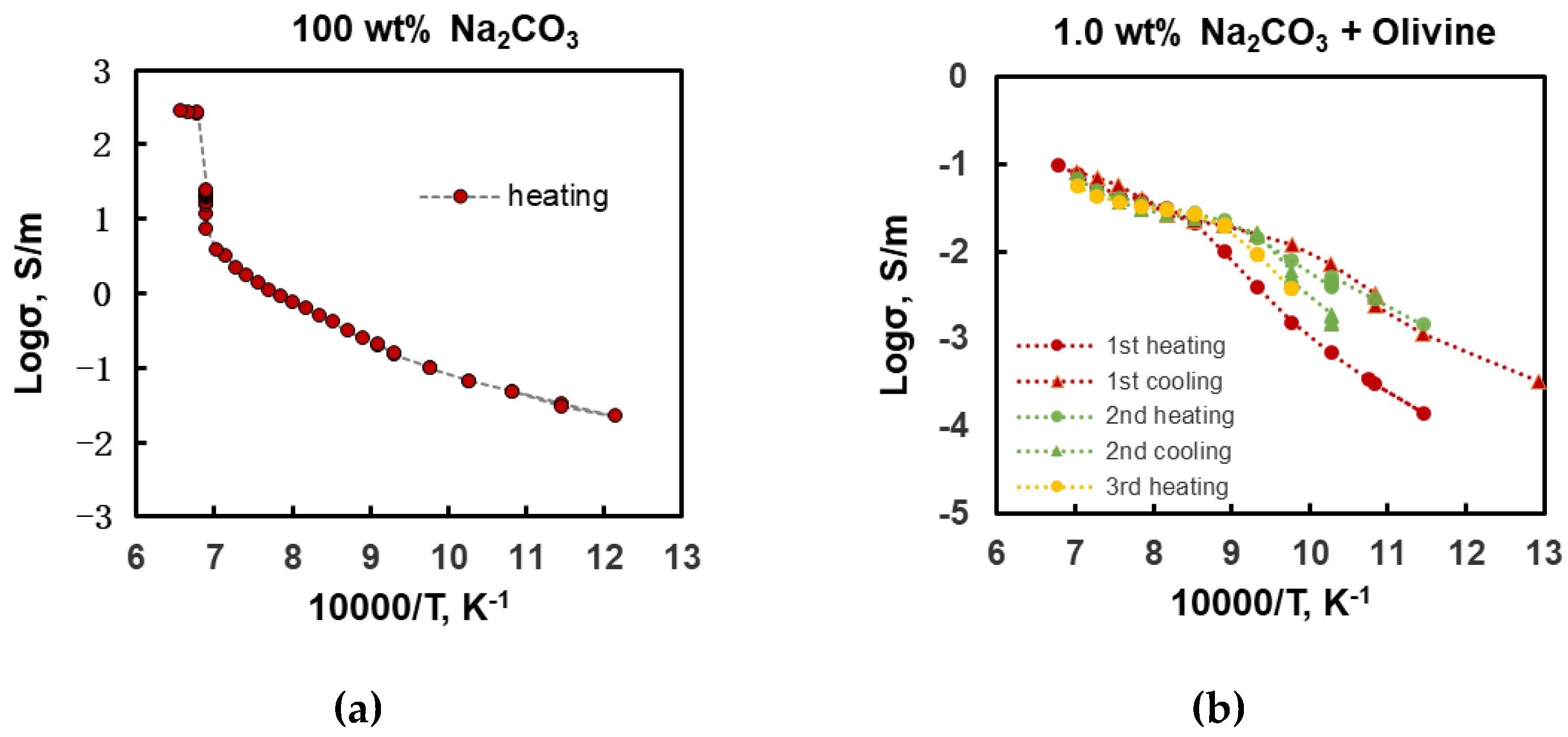
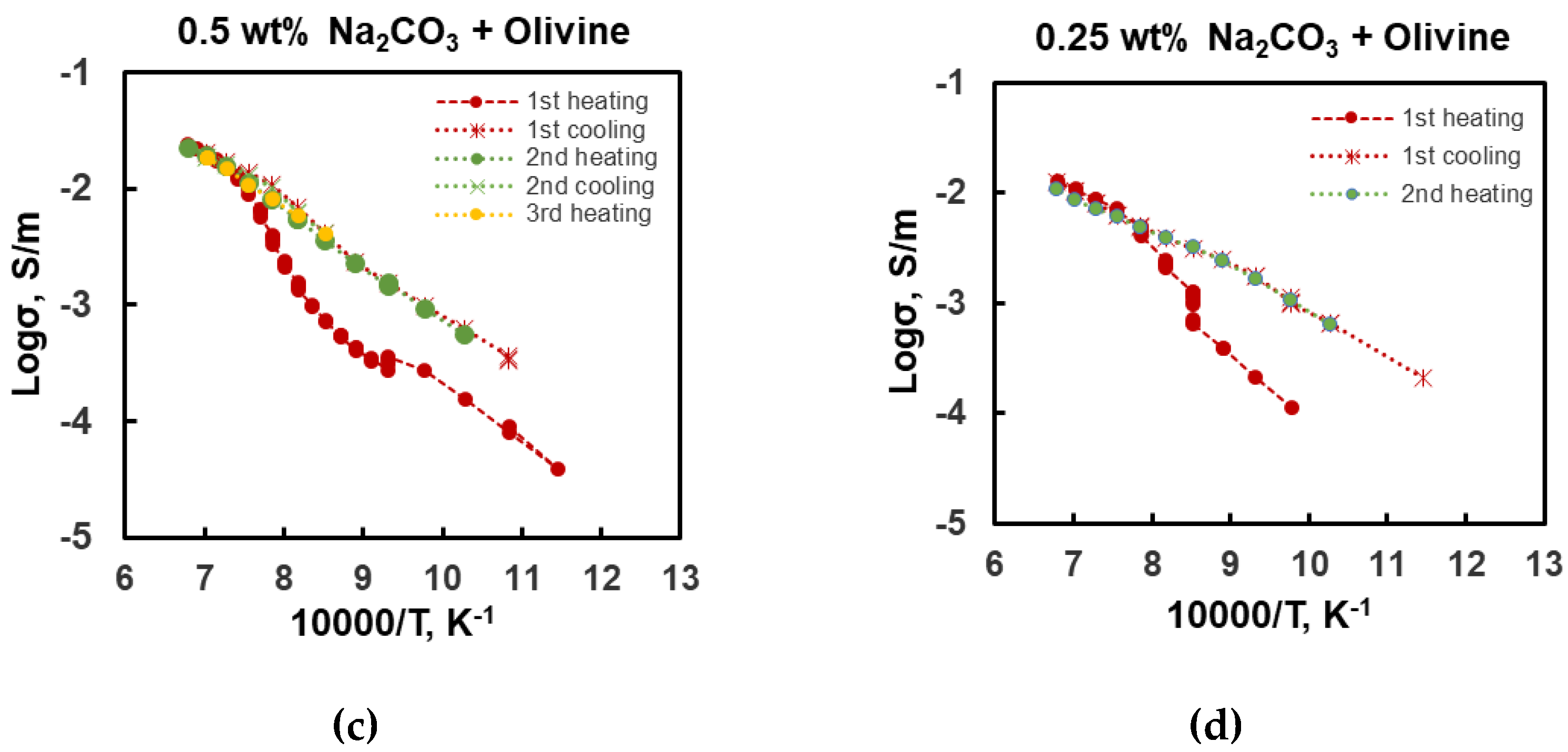
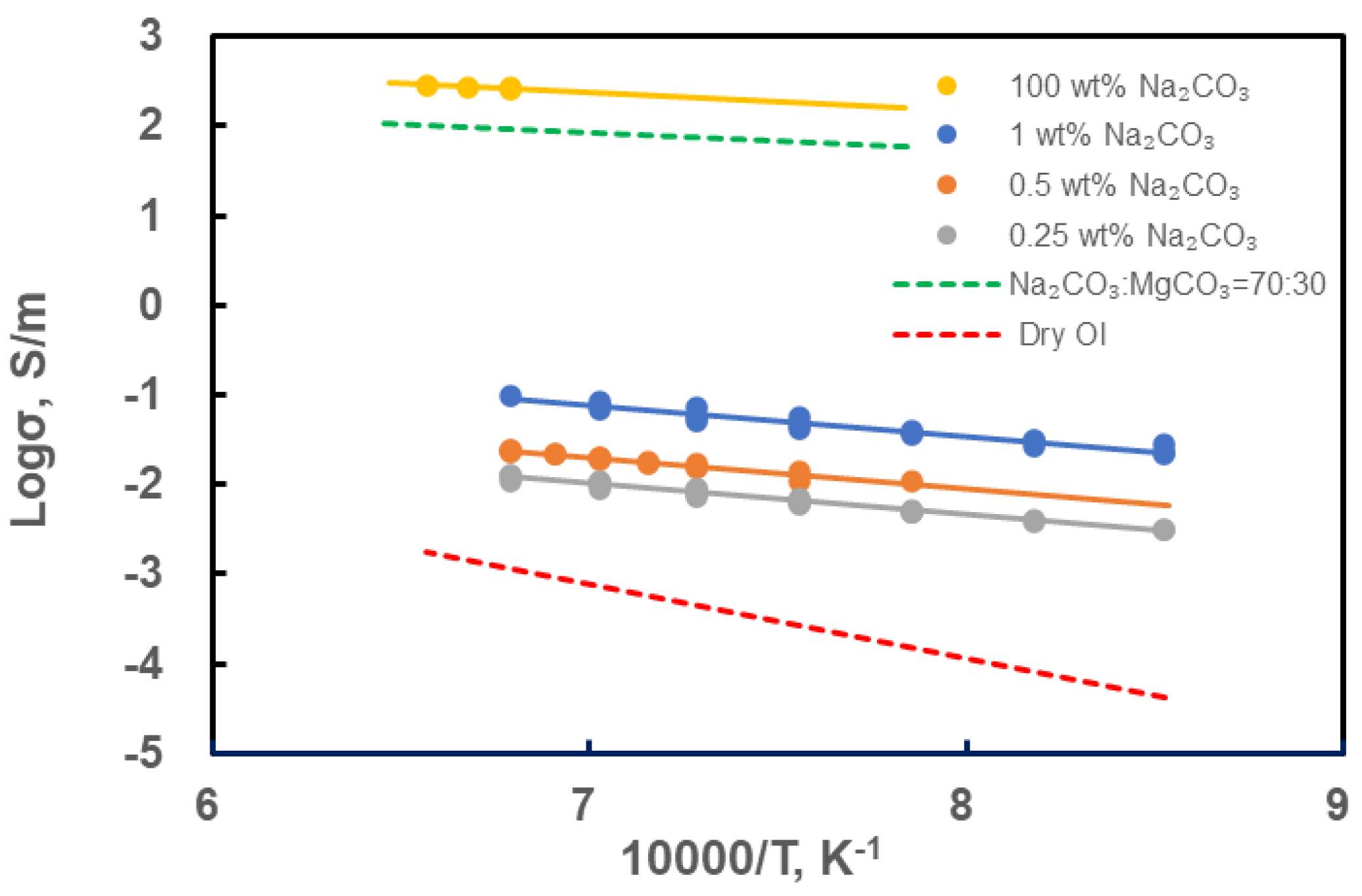
| Sample | P (GPa) | T (°C) | logσ0 (S/m) | H (kJ/mol) | R2 | Ref. |
|---|---|---|---|---|---|---|
| 0.25% wt. Na2CO3 | 3 | 900–1200 | 0.46 | 67.05 | 0.97 | This study |
| 0.5% wt. Na2CO3 | 3 | 1000–1200 | 0.70 | 65.64 | 0.95 | This study |
| 1% wt. Na2CO3 | 3 | 900–1200 | 1.26 | 65.22 | 0.94 | This study |
| 100% wt. Na2CO3 | 3 | 1200–1250 | 3.71 | 36.48 | 0.81 | This study |
| Na2CO3:MgCO3=70:30 | 3.4 | 1000–1427 | 3.20 | 34.73 | [34] | |
| Na2CO3:MgCO3=50:50 | 3 | 1050–1350 | 3.33 | 33.55 | [35] | |
| Dolomite | 3 | 1327–1527 | 3.13 | 38 | [37] | |
| San Carlos olivine | 4 | 1000–1400 | 2.69 | 159 | [33] |
| Conductivities | 75km/760°C | 100km/938°C | 130km/1152°C |
|---|---|---|---|
| σ max=0.10 | 7.03 | 3.00 | 1.43 |
| σ median=0.03 | 2.84 | 1.21 | 0.58 |
| σ min=0.01 | 1.24 | 0.53 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





