Submitted:
28 March 2025
Posted:
28 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Review
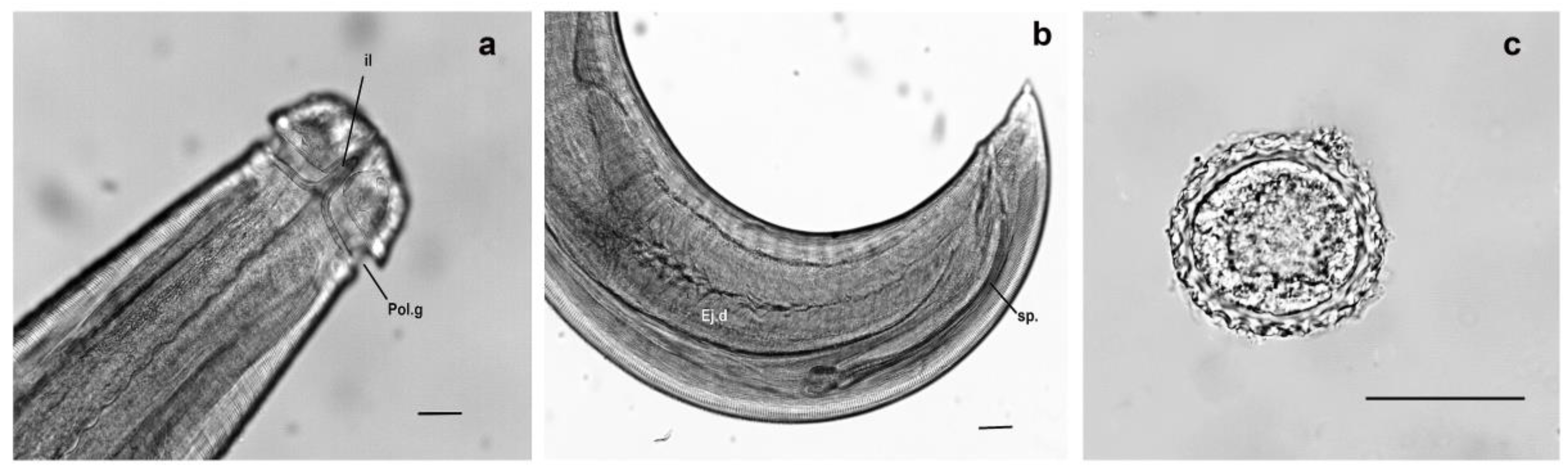
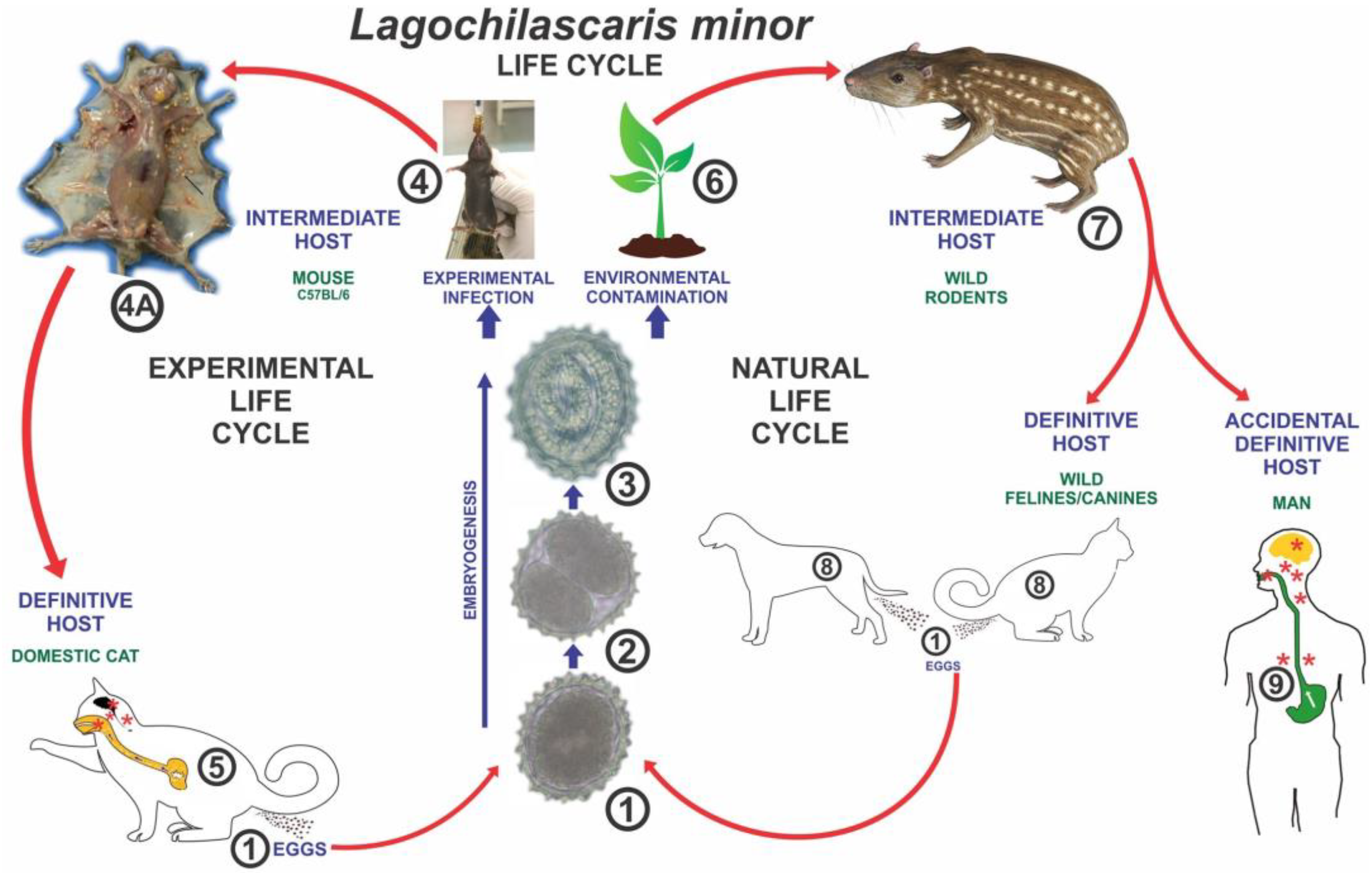
3.1. Overview of Lagochilascariasis
3.2. Epidemiology of Lagochilascariasis
3.3. Role of Wildlife in Disease Transmission
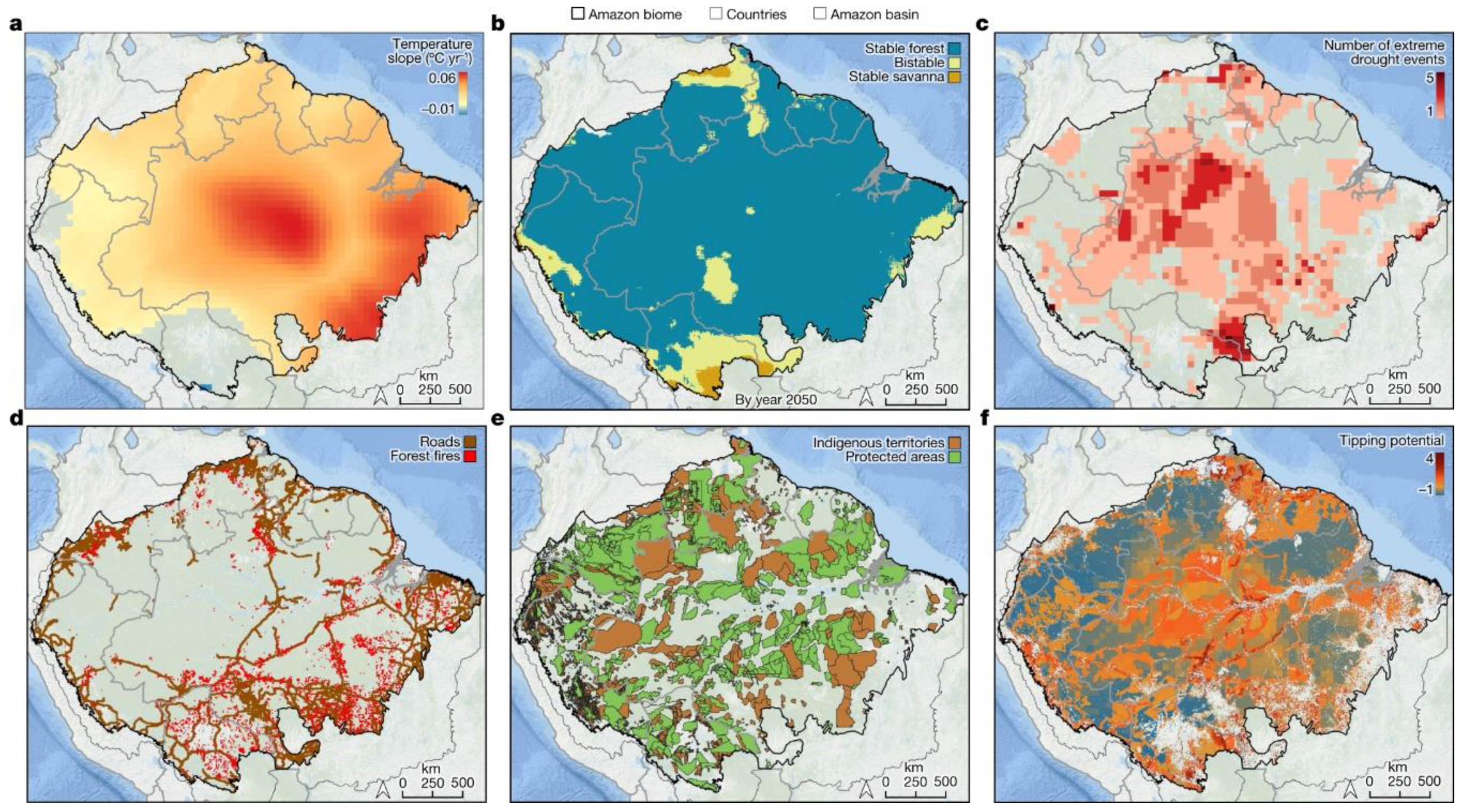
3.4. Impact of Anthropization on Disease Dynamics
3.5. Public Health Implications
3.6. Diagnostic Challenges
3.7. Educational Strategies for Public Awareness
3.8. Research Gaps and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, D.M.B.; Freire Filha, L.G.; Vieira, M.A.; Paçô, J.M.; Maia, M.A. Experimental Life Cycle of Lagochilascaris Minor Leiper, 1909. Rev. Inst. Med. Trop. São Paulo 1992, 34, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Paçô, J.M.; Campos, D.M.B.; de Oliveira, J.A. . Wild Rodents as Experimental Intermediate Hosts of Lagochilascaris minor Leiper, 1909. Mem. Inst. Oswaldo Cruz 1999, 94, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Barreto, L.F.; D'Anunciação, L.; Plisker, P.; Werkema, F.; Berenstein, C.K. Lagochilascariasis: Case Report. J. Bras. Patol. Med. Lab. 2018, 54, 245–248. [Google Scholar] [CrossRef]
- Orihuela, R.; Botto, C.; Delgado, O.; Ortiz, A.; Suarez, J. A.; Arguello, C. Human Lagochilascariasis in Venezuela: Description of a Fatal Case. Rev. Soc. Bras. Med. Trop. 1987, 20, 217–221. [Google Scholar] [CrossRef]
- Salvarani, F.M.; Oliveira, H.G.S.; Correa, L.Y.S.; Soares, A.A.L.; Ferreira, B.C. The Importance of Studying Infectious and Parasitic Diseases of Wild Animals in the Amazon Biome with a Focus on One Health. Vet. Sci. 2025, 12, 100. [Google Scholar] [CrossRef]
- Fraiha Neto, H.; Leão, R.N.Q.; Costa, F.S.A. Lagoquilascaríase humana e dos animais domésticos. Zoonoses - Rev. Int. 1989, 1, 23–33. [Google Scholar]
- Grant, M.J.; Booth, A. A typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Info. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Moncada, L.I.; Alvarez, C.A.; Castellanos, C.; Caceres, E.; Nicholls, S.; Corredor, A. Lagochilascaris minor in a Patient from the Colombian Amazon: A Case Report. Rev. Inst. Med. Trop. São Paulo 1998, 40, 387–389. [Google Scholar] [CrossRef]
- Roig, J.L.; Roig-Ocampos Forteza, J.L.; Granato, L.; Serafini, D.P. Otomastoidititis with Right Retroauricular Fistula by Lagochilascaris Minor. Braz. J. Otorhinolaryngol. 2010, 76, 407. [Google Scholar] [CrossRef]
- Bento, R.F.; Mazza, C.C.; Motti, E.F.; Chan, Y.T.; Guimaraes, J. R.; Miniti, A. Human Lagochilascariasis Treated Successfully with Ivermectin: A Case Report. Rev. Inst. Med. Trop. São Paulo 1993, 35, 373–375. [Google Scholar] [CrossRef]
- Aquino, R.T.R.; Magliari, M.E.R.; Vital Filho, J.; Silva, M. A. L. G.; Lima, C. A. C.; Rocha, A. J. Lagochilascariasis Leading to Severe Involvement of Ocular Globes, Ears and Meninges. Rev. Inst. Med. Trop. São Paulo 2008, 50, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Veloso, M.G.; Faria, M.C.; de Freitas, J.D.; Moraes, M.A.; Gorini, D.F.; de Mendonça, J.L. Human Lagochilascariasis: Three Cases Encountered in the Federal District, Brazil. Rev. Inst. Med. Trop. São Paulo 1992, 34, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.A.; Arnaud, M.V.; de Macedo, R.C.; Anglada, A.E. Fatal Pulmonary Infection Caused by Lagochilascaris sp., Probably Lagochilascaris Minor Leiper, 1909. Rev. Inst. Med. Trop. São Paulo 1985, 27, 46–52. [Google Scholar] [CrossRef]
- Rosemberg, S.; Lopes, M.B.S.; Masuda, Z.; Campos, R.; Vieira-Bressan, M.C.R. Fatal encephalopathy due to Lagochilascaris minor infection. Am. J. Trop. Med. Hyg. 1986, 35, 575–578. [Google Scholar] [CrossRef]
- Volcan, G.S.; Medrano, P.C.E.; Payares, G. Experimental heteroxenous cycle of Lagochilascaris minor, 1909 Leiper (Nematoda, Ascarididae) in white mice in cats. Mem. Inst. Oswaldo Cruz 1992, 87, 525–532. [Google Scholar] [CrossRef]
- Semerene, A.R.; Lino Junior, R.S.; Oliveira, J.A.; Magalhães, A.V.; Stefani, M.M.; Barbosa, A.P.; Campos, D.M.B. Experimental lagochilascariosis: histopathological study of inflammatory response to larval migration in the murine model. Mem. Inst. Oswaldo Cruz 2004, 99, 393–398. [Google Scholar] [CrossRef]
- Aguilar-Nascimento, J.E.; Silva, G.M.; Tadano, T.; Valadares Filho, M.; Akiyama, A.M.; Castelo, A. Infection of the soft tissue of the neck due to Lagochilascaris minor. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 198. [Google Scholar] [CrossRef]
- Barbosa, C.A.; Campos, D.M. Assessment of ivermectin therapeutic efficacy on fourth-stage larvae of Lagochilascaris minor in experimentally infected cats. Rev. Soc. Bras. Med. Trop. 2001, 34, 373–376. [Google Scholar] [CrossRef]
- Campos, D.M.B.; Barbosa, A.P.; Oliveira, J.A.; Barbosa, C.A.L.; Lobo, T.C.; Silva, L.G.; et al. Evaluation of the therapeutic efficacy of levamisole hydrochloride on third-stage larvae of Lagochilascaris minor in experimentally infected mice. Rev. Inst. Med. Trop. São Paulo 2016, 58, 1–5. [Google Scholar] [CrossRef]
- Moura, M.Q.; Jeske, S.; Gallina, T.; Borsuk, S.; Berne, M.E.A.; Villela, M.M. First report of Lagochilascaris (Nematoda: Ascarididae) eggs in a public park in Southern Brazil. Vet. Parasitol. 2012, 184, 359–361. [Google Scholar] [CrossRef]
- Sudré, A.P.; Uchôa, F.; Brener, B. Lagochilascariasis in a housecat and the potential risk for human disease. Braz. J. Infect. Dis. 2012, 16, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Telles, F.; Salvador, G. Nodular human Lagochilascariasis lesion in hunter, Brazil. Emerg. Infect. Dis. 2019, 25, 2331–2332. [Google Scholar] [CrossRef] [PubMed]
- Solano-Barquero, A.; Estrada, A.; Medaglia, A.; Montenegro, V.M.; Rojas, A. Emerging Lagochilascaris minor infections in domestic cats from Costa Rica: A zoonotic threat for the region. Vet. Parasitol. Reg. Stud. Rep. 2022, 36, 100797. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.R.; Lignon, J.S.; Martins, N.S.; Dos Santos, T.S.; Martins, K.R.; Cunha, R.C.; de Oliveira, R.; Valente, C.L.; Scheid, H.V.; Pappen, F.G.; Pinto, D.M. Atypical Case of Recurrent Otitis with Polyp Formation in the Ear Canal Associated with Lagochilascariasis in a Domestic Feline in Brazil. Vet. Parasitol. Reg. Stud. Rep. 2025, 57, 101177. [Google Scholar] [CrossRef]
- Lignon, J.S.; Pinto, D.M.; dos Santos, T.S.; et al. Survey of Parasitic Fauna Data from Wild Animals through Coproparasitological Diagnosis in Southern Brazil. BMC Vet. Res. 2025, 21, 7. [Google Scholar] [CrossRef]
- Fagundes-Moreira, R.; Schwartz, C.I.; de Sousa, F.A.B.; et al. Zoonotic Lagochilascaris minor and Nine Other Parasites in a Cat. Parasitol. Res. 2024, 123, 392. [Google Scholar] [CrossRef]
- Flores, B.M. , Montoya, E., Sakschewski, B. et al. Critical transitions in the Amazon forest system. Nature, 2024, 626, 555–564. [Google Scholar] [CrossRef]
- Uribe, M.; Brabec, J.; Chaparro-Gutiérrez, J.J.; Hermosilla, C. Neglected Zoonotic Helminthiases in Wild Canids: New Insights from South America. Front. Vet. Sci. 2023, 10, 1235182. [Google Scholar] [CrossRef]
- Saldanha, B.M.; Chame, M.; Nunes, G.K.M.; Sianto, L.; Leles, D. Parasites of the Brazilian Rock Cavy, Kerodon rupestris: Revealing Their History in the Brazilian Semiarid Region. J. Parasitol. 2022, 108, 395–402. [Google Scholar] [CrossRef]
- Assy, J.G.P.L.; Esper, H.R.; Quiroga, M.M.M.; Brandão, A.D.S.; Said, R.D.C.; Pinheiro, O.C.; Ribeiro, A.P.D.S.; Santo, M.C.C.D.E.; França, F.O.S. Unusual Case of Lagochilascariasis with Breast Involvement: The First Case Report in Pregnancy. Rev. Inst. Med. Trop. São Paulo 2020, 62, e86. [Google Scholar] [CrossRef]
- Campos, D.M.B.; Barbosa, A.P.; Oliveira, J.A.; Tavares, G.G.; Cravo, P.V.L.; Ostermayer, A.L. Human Lagochilascariasis—A Rare Helminthic Disease. PLoS Negl. Trop. Dis. 2017, 11, e0005510. [Google Scholar] [CrossRef] [PubMed]
- Trindade, M.A.C.; Macedo, M.R.P.; Drehmer, C.J.; Muller, G. First Record of Lagochilascaris minor (Nematoda: Ascarididae) in Leopardus geoffroyi (Carnivora: Felidae) in Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Vizcaychipi, K.A.; Rinas, M.; Irazu, L.; Miyagi, A.; Argüelles, C.F.; DeMatteo, K.E. Neotropical Zoonotic Parasites in Bush Dogs (Speothos venaticus) from Upper Paraná Atlantic Forests in Misiones, Argentina. Vector Borne Zoonotic Dis. 2016, 16, 664–672. [Google Scholar] [CrossRef] [PubMed]
- de Moura, M.Q.; Jeske, S.; Gallina, T.; Borsuk, S.; Berne, M.E.; Villela, M.M. First Report of Lagochilascaris (Nematoda: Ascarididae) Eggs in a Public Park in Southern Brazil. Vet. Parasitol. 2012, 184, 359–361. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Yeager, J.; Vasconez-Gonzalez, J.; Culqui-Sánchez, M.; Izquierdo-Condoy, J.S. Integrating Environmental Conservation and Public Health Strategies to Combat Zoonotic Disease Emergence: A Call to Action from the Amazon Rainforest. Front. Cel.l Infect. Microbiol. 2024, 14, 1405472. [Google Scholar] [CrossRef]
- Silva Junior, C.H.L.; Pessôa, A.C.M.; Carvalho, N.S.; Reis, J.B.C.; Anderson, L.O.; Aragão, L.E.O.C. The Brazilian Amazon Deforestation Rate in 2020 Is the Greatest of the Decade. Nat. Ecol. Evol. 2021, 5, 144–145. [Google Scholar] [CrossRef]
- Martinez-Hernandez, F.; Prado-Calleros, H.M.; Ramirez-Hinojosa, J.P.; Figueroa-Angel, V.; Lopez-Reynoso, M.T.; Jimenez-Andrade, M.D.C.; Estrada-Moscoso, I.; Rivas, N.; Escobedo-Ortegon, J.; Flisser, A.; Romero-Valdovinos, M.G.; Maravilla, P. An Unexpected Case of Lagochilascariasis: Interdisciplinary Management and Use of 12S and 18S rDNA Analysis. Am. J. Med. Sci. 2020, 359, 235–241. [Google Scholar] [CrossRef]
- Scioscia, N.P.; Olmos, L.; Gorosábel, A.; Bernad, L.; Pedrana, J.; Denegri, G.M. Natural Infection in Pampas Fox (Lycalopex gymnocercus) by Lagochilascaris major Leiper, 1910 (Nematoda: Ascarididae) in Buenos Aires, Argentina. Parasitol. Res. 2018, 117, 3023–3027. [Google Scholar] [CrossRef]
- Castro, M.C.; Baeza, A.; Codeço, C.T.; Cucunubá, Z.M.; Dal’Asta, A.P.; Leo, G.A.D.; Dobson, A.P.; Carrasco-Escobar, G.; Lana, R.M.; Lowe, R.; et al. Development, Environmental Degradation, and Disease Spread in the Brazilian Amazon. PLOS Biol. 2019, 17, e3000526. [Google Scholar] [CrossRef]
- Antunes, A.P.; Fewster, R.M.; Venticinque, E.M.; Peres, C.A.; Levi, T.; Rohe, F.; Shepard, G.H. Empty Forest or Empty Rivers? A Century of Commercial Hunting in Amazonia. Sci. Adv. 2016, 2, e1600936. [Google Scholar] [CrossRef]
- Marchi, S.; Guarducci, G.; Marotta, M.G.; Peccetti, B.; Viviani, S.; Messina, G.; Montomoli, E.; Martella, V.; Camero, M.; Trombetta, C.M. Improving the One Health Approach: A Lesson from SARS-CoV-2 Pandemic. J. Prev. Med. Hyg. 2024, 65, E312–E322. [Google Scholar] [CrossRef] [PubMed]
- Horefti, E. The Importance of the One Health Concept in Combating Zoonoses. Pathogens 2023, 12, 977. [Google Scholar] [CrossRef]
- Ferrante, L.; Fearnside, P.M. Brazil Threatens Indigenous Lands. Science 2020, 368, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Winck, G.R.; Raimundo, R.L.G.; Fernandes-Ferreira, H.; Bueno, M.G.; D’Andrea, P.S.; Rocha, F.L.; Cruz, G.L.T.; Vilar, E.M.; Brandão, M.; Cordeiro, J.L.P.; et al. Socioecological Vulnerability and the Risk of Zoonotic Disease Emergence in Brazil. Sci. Adv. 2022, 8, eabo5774. [Google Scholar] [CrossRef]
- Wegner, G.I.; Murray, K.A.; Springmann, M.; Muller, A.; Sokolow, S.H.; Saylors, K.; Morens, D.M. Averting Wildlife-Borne Infectious Disease Epidemics Requires a Focus on Socio-Ecological Drivers and a Redesign of the Global Food System. EClinicalMedicine 2022, 47, 101386. [Google Scholar] [CrossRef]
- Fagre, A.C.; Cohen, L.E.; Eskew, E.A.; Farrell, M.; Glennon, E.; Joseph, M.B.; Frank, H.K.; Ryan, S.J.; Carlson, C.J.; Albery, G.F. Assessing the Risk of Human-to-Wildlife Pathogen Transmission for Conservation and Public Health. Ecol. Lett. 2022, 25, 1534–1549. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond Diversity Loss and Climate Change: Impacts of Amazon on Infectious Diseases and Public Health. Ann. Acad. Bras. Cienc. 2020, 92, e20191375. [Google Scholar] [CrossRef]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Marco, M.D.; Breit, N.; Olival, K.J.; Daszak, P. Global Hotspots and Correlates of Emerging Zoonotic Diseases. Nat. Commun. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Suárez, J.A.; Franco-Paredes, C.; Vilcarromero, S.; Mattar, S.; Gómez-Marín, J.E.; Villamil-Gómez, W.E.; Ruíz-Sáenz, J.; Cardona-Ospina, J.A.; Idarraga-Bedoya, S.E.; et al. Brazil Burning! What is the Potential Impact of the Amazon Wildfires on Vector-Borne and Zoonotic Emerging Diseases?—A Statement from an International Experts Meeting. Travel Med. Infect. Dis. 2019, 31, 101474. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Chomel, B.B.; Martínez-López, B. Climate Change and Zoonoses: A Review of the Current Status, Knowledge Gaps, and Future Trends. Acta Trop. 2022, 226, 106225. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A. Changing Patterns of Emerging Zoonotic Diseases in Wildlife, Domestic Animals, and Humans Linked to Biodiversity Loss and Globalization. ILAR J. 2017, 58, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.; Kordick, L. Bartonella Infection in Animals: Carriership, Reservoir Potential, Pathogenicity, and Zoonotic Potential for Human Infection. Clin. Microbiol. Rev. 2000, 13, 428–438. [Google Scholar] [CrossRef] [PubMed]
- WHO. New Report Highlights the Impact of Changes in Environment on One Health; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int (accessed on 27 March 2025).
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Ruaro, R.; Ferrante, L.; Fearnside, P.M. Brazil’s Doomed Environmental Licensing. Science 2021, 372, 1049–1050. [Google Scholar] [CrossRef]
- Anderson, B.D.; Barnes, A.N.; Umar, S.; Guo, X.; Thongthum, T.; Gray, G.C. Reverse Zoonotic Transmission (Zooanthroponosis): An Increasing Threat to Animal Health. In Zoonoses: Infections Affecting Humans and Animals; Sing, A., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Keatts, L.O.; Robards, M.; Olson, S.H.; Hueffer, K.; Insley, S.J.; Joly, D.O.; Kutz, S.; Lee, D.S.; Chetkiewicz, C.B.; Lair, S.; et al. Implications of Zoonoses from Hunting and Use of Wildlife in North American Arctic and Boreal Biomes: Pandemic Potential, Monitoring, and Mitigation. Front. Public Health 2021, 9, 627654. [Google Scholar] [CrossRef]
- Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of Environmental Change on Emerging Parasitic Diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef]
- Bezerra-Santos, M.A.; Ramos, R.A.N.; Campos, A.K. Didelphis spp. Opossums and Their Parasites in the Americas: A One Health Perspective. Parasitol. Res. 2021, 120, 4091–4111. [Google Scholar] [CrossRef]
- Morand, S.; Lajaunie, C. Outbreaks of Vector-Borne and Zoonotic Diseases Are Associated with Changes in Forest Cover and Oil Palm Expansion at Global Scale. Front. Vet. Sci. 2021, 8, 661063. [Google Scholar] [CrossRef]
- Otranto, D.; Deplazes, P. Zoonotic Nematodes of Wild Carnivores. Int. J. Parasitol. Parasitol. Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Confalonieri, U.E.; Margonari, C.; Quintão, A.F. Environmental Change and the Dynamics of Parasitic Diseases in the Amazon. Acta Trop. 2014, 129, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.P.A.; Sánchez-Arcila, J.C.; Peres, L.; Sousa, P.S.F.; Santos Alvarenga, M.A.; Castro-Alves, J.; Ferreira-da-Cruz, M.F.; Maia-Herzog, M.; Oliveira-Ferreira, J. Malarial and Intestinal Parasitic Co-infections in Indigenous Populations of the Brazilian Amazon Rainforest. J. Infect. Public Health 2023, 16, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.F.S.; Ramos, R.A.N.; Giannelli, A.; Schettino, S.C.; Galina, A.B.; Oliveira, J.C.P.; Meira-Santos, P.O.; Alves, L.C. Zoonotic Parasites in Wild Animals such as Carnivores and Primates That are Traded Illegally in Brazil. Braz. J. Vet. Med. 2021, 43, e113720. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E. How Globalization and Climate Change Could Affect Foodborne Parasites. Exp. Parasitol. 2020, 208, 107807. [Google Scholar] [CrossRef]
- Deiana, G.; Arghittu, A.; Dettori, M.; Castiglia, P. One World, One Health: Zoonotic Diseases, Parasitic Diseases, and Infectious Diseases. Healthcare 2024, 12, 922. [Google Scholar] [CrossRef]
- Chakraborty, S.; Andrade, F.; Smith, R.L. An Approach to One Health: Course Design, Development, and Delivery. J. Vet. Med. Educ. 2022, 49, 568–574. [Google Scholar] [CrossRef]
- Bruno, A.; Arnoldi, I.; Barzaghi, B.; Boffi, M.; Casiraghi, M.; Colombo, B.; Di Gennaro, P.; Epis, S.; Facciotti, F.; Ferrari, N. The One Health Approach in Urban Ecosystem Rehabilitation: An Evidence-Based Framework for Designing Sustainable Cities. iScience 2024, 27, 110959. [Google Scholar] [CrossRef]
- Plowright, R.K.; Reaser, J.K.; Locke, H.; Woodley, S.J.; Patz, J.A.; Becker, D.J.; Oppler, G.; Hudson, P.J.; Tabor, G.M. Land Use-Induced Spillover: A Call to Action to Safeguard Environmental, Animal, and Human Health. Lancet Planet. Health 2021, 5, e237–e245. [Google Scholar] [CrossRef]
- Tasker, A.; Braam, D. Positioning Zoonotic Disease Research in Forced Migration: A Systematic Literature Review of Theoretical Frameworks and Approaches. PLoS ONE 2021, 16, e0254746. [Google Scholar] [CrossRef]
- Narrod, C.; Zinsstag, J.; Tiongco, M. A One Health Framework for Estimating the Economic Costs of Zoonotic Diseases on Society. EcoHealth 2012, 9, 150–162. [Google Scholar] [CrossRef]
- Oppenheim, B.; Gallivan, M.; Madhav, N.K.; Brown, N.; Serhiyenko, V.; Wolfe, N.D.; Ayscue, P. Assessing Global Preparedness for the Next Pandemic: Development and Application of an Epidemic Preparedness Index. BMJ Glob. Health 2019, 4, e001157. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sandoval, N.; Cifuentes, L.; León, G.; Lecaro, P.; Ortiz-Rico, C.; Cooper, P.; Martín, M. High Rates of Exposures to Waterborne Pathogens in Indigenous Communities in the Amazon Region of Ecuador. Am. J. Trop. Med. Hyg. 2019, 101, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, K.G. The Greening of One Health: Plants, Pathogens and the Environment. Annu. Rev. Phytopathol. 2024, 62, 401–421. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




