Submitted:
27 March 2025
Posted:
28 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Approvals
2.2. AlloStim® Formulation
2.3. Study Participants
2.4. Protocol
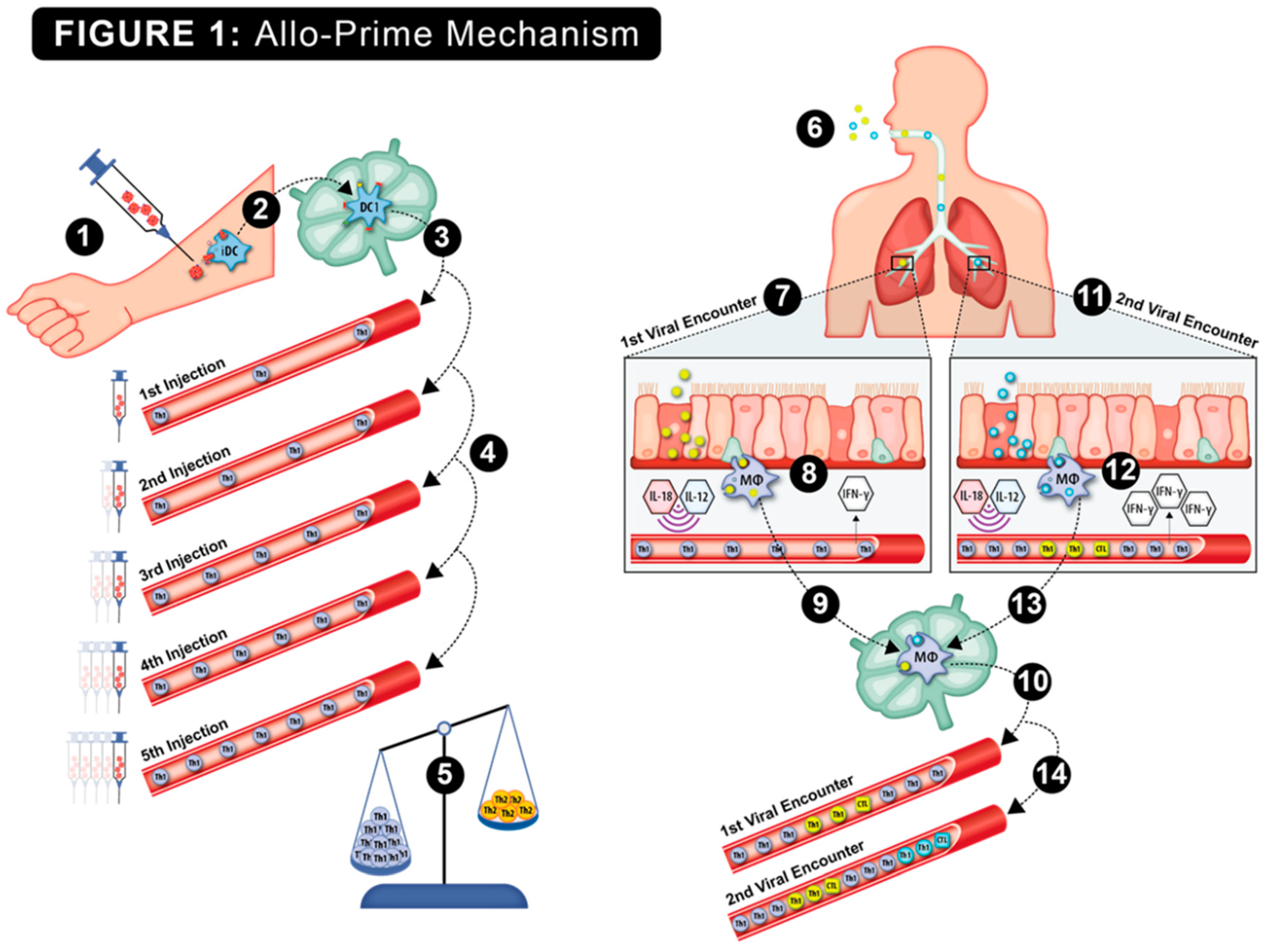
2.5. Mechanism of Action
2.6. Cellular Immune Function Assays
2.7. Flow Cytometry Analysis
3. Results
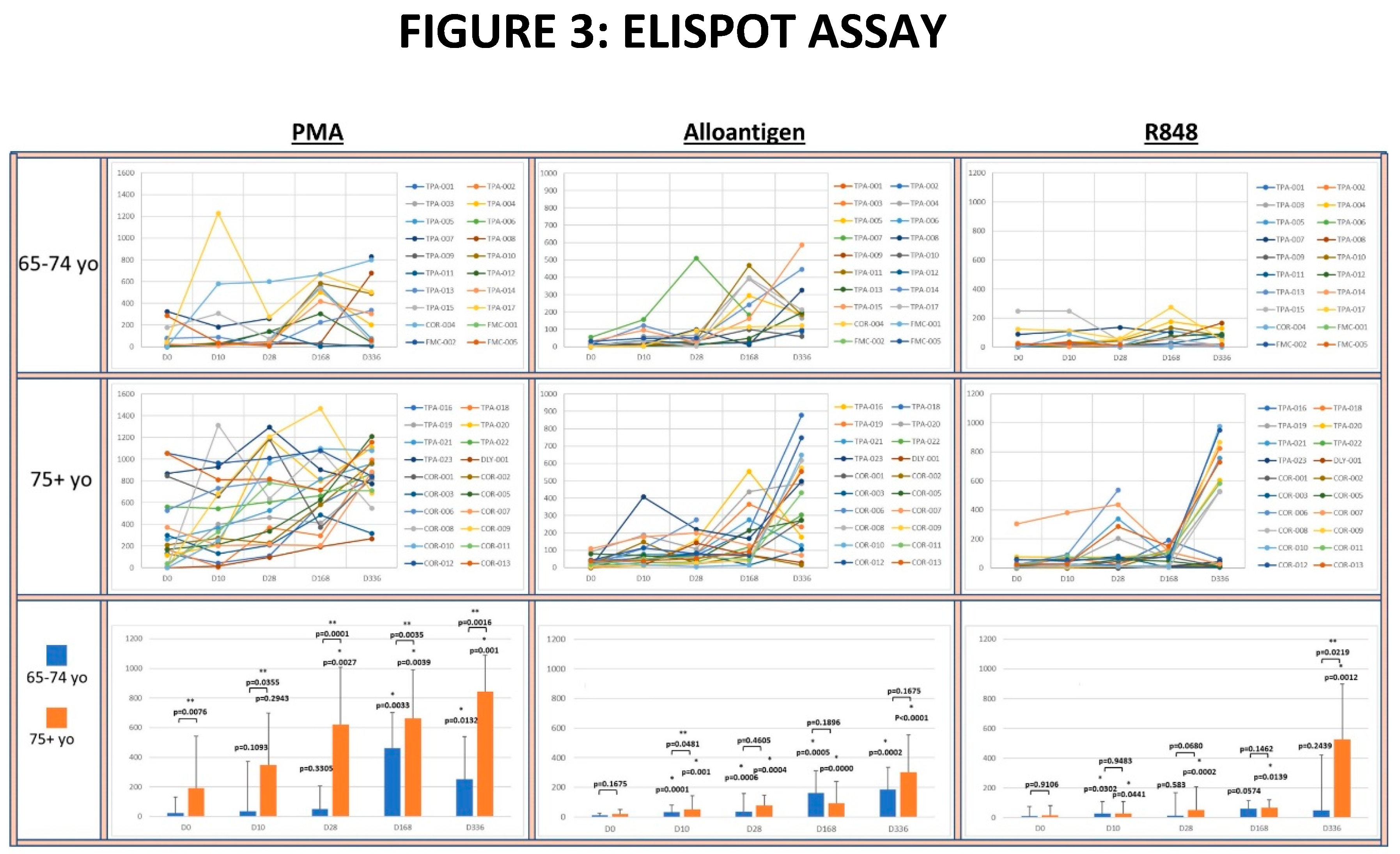
3.1. IFN-γ+ Th1 Cell Titers
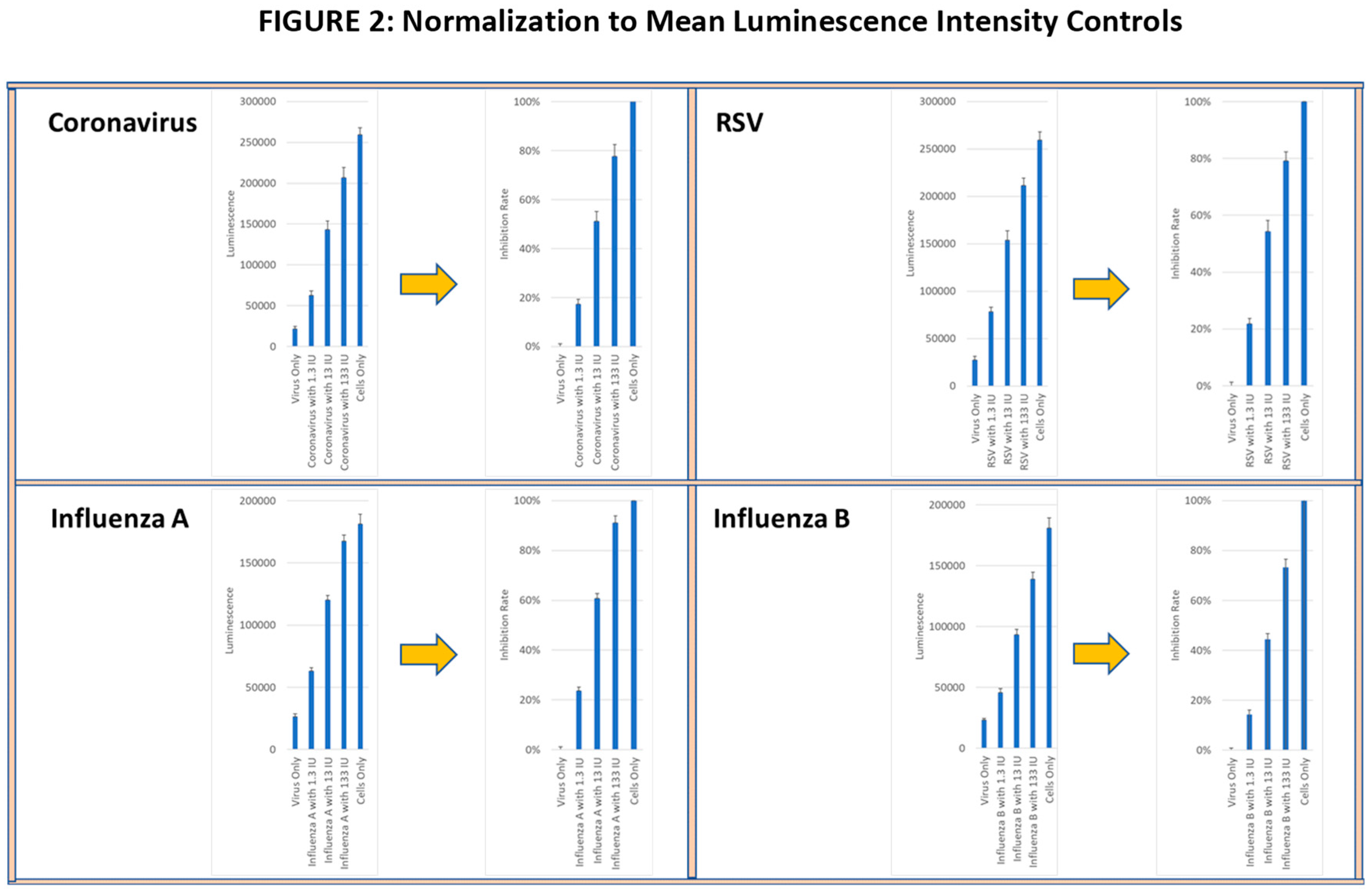
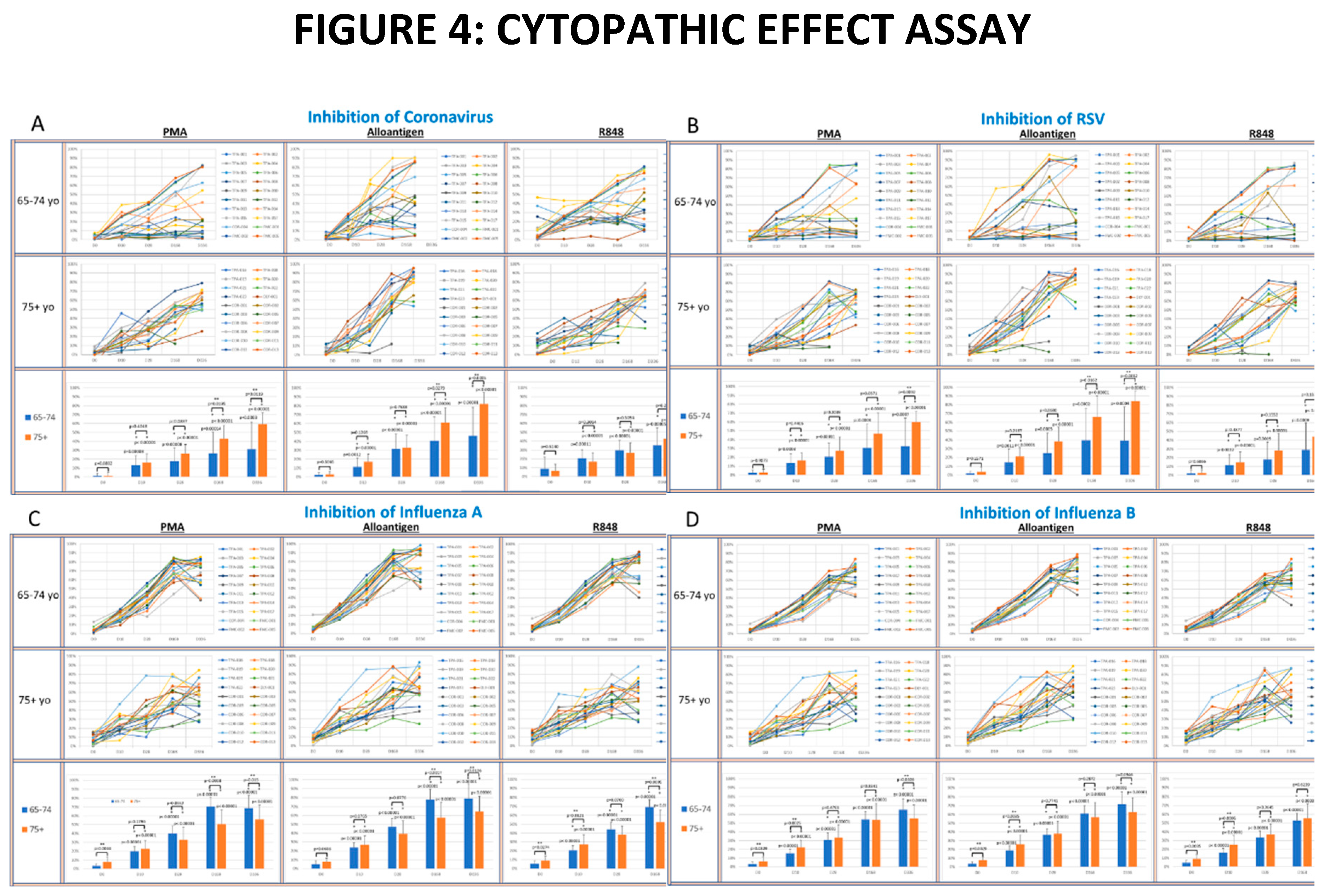
3.2. Viral Lytic Activity Suppression
3.3. Flow Cytometry
3.4. SARS-CoV-2 Vaccine IgG Titers
3.5. COVID-19 Incidence
3.6. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| Cat# | Catalog number |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTL | Cytotoxic T-lymphocyte |
| EDTA | Ethylenediaminetetraacetic acid |
| EIA | Enzyme Immunoassay |
| FACS | Fluorescence-activated cell sorting |
| Th1 | T helper 1 lymphocyte |
| Th2 | T helper 2 lymphocyte |
| TLR | Toll-Like Receptor |
| yo | Years Old |
References
- Luo, H.; Liu, S.; Wang, Y.; A Phillips-Howard, P.; Ju, S.; Yang, Y.; Wang, D. Age differences in clinical features and outcomes in patients with COVID-19, Jiangsu, China: a retrospective, multicentre cohort study. BMJ Open 2020, 10, e039887. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R. , Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med 2007, 28, 171–81. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Chemaitelly, H.; Seedat, S.; Mumtaz, G.R.; Makhoul, M.; Abu-Raddad, L.J. Age could be driving variable SARS-CoV-2 epidemic trajectories worldwide. PLOS ONE 2020, 15, e0237959. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- van der Heiden, M., et al., Multiple vaccine comparison in the same adults reveals vaccine-specific and age-related humoral response patterns: an open phase IV trial. Nat Commun 2024, 15, 6603. [CrossRef]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef]
- Cifuentes-Muñoz, N.; Dutch, R.E.; Cattaneo, R. Direct cell-to-cell transmission of respiratory viruses: The fast lanes. PLOS Pathog. 2018, 14, e1007015. [Google Scholar] [CrossRef]
- Woodland, D.L. Cell-mediated immunity to respiratory virus infections. Curr. Opin. Immunol. 2003, 15, 430–435. [Google Scholar] [CrossRef]
- Hilleman, M.R. Vaccines in historic evolution and perspective: a narrative of vaccine discoveries. Vaccine 2000, 18, 1436–1447. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Malinczak, C.-A. Harnessing Cellular Immunity for Vaccination against Respiratory Viruses. Vaccines 2020, 8, 783. [Google Scholar] [CrossRef]
- Dutta, A.; Huang, C.-T.; Lin, C.-Y.; Chen, T.-C.; Lin, Y.-C.; Chang, C.-S.; He, Y.-C. Sterilizing immunity to influenza virus infection requires local antigen-specific T cell response in the lungs. Sci. Rep. 2016, 6, 32973. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Smith, C.A.; Sakamoto, K.; Kaminski, N.; Koff, J.L.; Goldstein, D.R. Aging Impairs Alveolar Macrophage Phagocytosis and Increases Influenza-Induced Mortality in Mice. J. Immunol. 2017, 199, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.; Asghary, A.; Zolbanin, N.M.; Faraji, F.; Jafari, R. Immunosenescence and Inflammaging in COVID-19. Viral Immunol. 2023, 36, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: a review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar] [CrossRef]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef]
- Bandaranayake, T.; Shaw, A.C. Host Resistance and Immune Aging. Clin. Geriatr. Med. 2016, 32, 415–432. [Google Scholar] [CrossRef]
- Moss, P. , Cellular immune responses to influenza. Dev Biol (Basel) 2003, 115, 31–7. [Google Scholar]
- Lee, K.-A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef]
- Chovancová, Z. Immunosenescence - the sunset over the immune system. Vnitr Lek 2020, 66, 353–358. [Google Scholar] [CrossRef]
- Dhochak, N. , et al. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pediatr 2020, 87, 537–546. [Google Scholar] [CrossRef]
- Mihaescu, G.; Chifiriuc, M.C.; Filip, R.; Bleotu, C.; Ditu, L.M.; Constantin, M.; Cristian, R.-E.; Grigore, R.; Bertesteanu, S.V.; Bertesteanu, G.; et al. Role of interferons in the antiviral battle: from virus-host crosstalk to prophylactic and therapeutic potential in SARS-CoV-2 infection. Front. Immunol. 2023, 14, 1273604. [Google Scholar] [CrossRef] [PubMed]
- Murira, A.; Lamarre, A. Type-I Interferon Responses: From Friend to Foe in the Battle against Chronic Viral Infection. Front. Immunol. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Molony, R.D.; Nguyen, J.T.; Kong, Y.; Montgomery, R.R.; Shaw, A.C.; Iwasaki, A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.P.; Vrba, S.M.; Reynoso, G.V.; Wynne-Jones, E.; Kamenyeva, O.; Malo, C.S.; Cherry, C.R.; McManus, D.T.; Hickman, H.D. Group 1 innate lymphoid-cell-derived interferon-γ maintains anti-viral vigilance in the mucosal epithelium. Immunity 2021, 54, 276–290.e5. [Google Scholar] [CrossRef]
- Levy, D.E.; Garcia-Sastre, A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev 2001, 12, 143–56. [Google Scholar] [CrossRef]
- Yang, J.; Murphy, T.L.; Ouyang, W.; Murphy, K.M. Induction of interferon-γ production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur. J. Immunol. 1999, 29, 548–555. [Google Scholar] [CrossRef]
- Provinciali, M.; Moresi, R.; Donnini, A.; Lisa, R.M. Reference Values for CD4+ and CD8+ T Lymphocytes with Naïve or Memory Phenotype and Their Association with Mortality in the Elderly. Gerontology 2009, 55, 314–321. [Google Scholar] [CrossRef]
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A Short History of Skin Grafting in Burns: From the Gold Standard of Autologous Skin Grafting to the Possibilities of Allogeneic Skin Grafting with Immunomodulatory Approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef]
- Iborra, S.; Abánades, D.R.; Parody, N.; Carrión, J.; Risueño, R.M.; A Pineda, M.; Bonay, P.; Alonso, C.; Soto, M. The immunodominant T helper 2 (Th2) response elicited in BALB/c mice by theLeishmaniaLiP2a and LiP2b acidic ribosomal proteins cannot be reverted by strong Th1 inducers. Clin. Exp. Immunol. 2007, 150, 375–385. [Google Scholar] [CrossRef]
- Har-Noy, M.; Zeira, M.; Weiss, L.; Fingerut, E.; Or, R.; Slavin, S. Allogeneic CD3/CD28 cross-linked Th1 memory cells provide potent adjuvant effects for active immunotherapy of leukemia/lymphoma. Leuk. Res. 2009, 33, 525–538. [Google Scholar] [CrossRef]
- Har-Noy, M.; Or, R. Allo-priming as a universal anti-viral vaccine: protecting elderly from current COVID-19 and any future unknown viral outbreak. J. Transl. Med. 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baydemir, I.; Dulfer, E.A.; Netea, M.G.; Domínguez-Andrés, J. Trained immunity-inducing vaccines: Harnessing innate memory for vaccine design and delivery. Clin. Immunol. 2024, 261, 109930. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y., C. Ma, and J. Wang, Cytopathic Effect Assay and Plaque Assay to Evaluate in vitro Activity of Antiviral Compounds Against Human Coronaviruses 229E, OC43, and NL63. Bio Protoc 2022, 12, e4314.
- Kelsall, B.L., et al., Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann N Y Acad Sci 1996, 795, 116–26. [CrossRef]
- Scott, P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol. 1991, 147, 3149–3155. [Google Scholar] [CrossRef]
- Cakman, I.; Rohwer, J.; Schütz, R.-M.; Kirchner, H.; Rink, L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech. Ageing Dev. 1996, 87, 197–209. [Google Scholar] [CrossRef]
- Lee, O.-J.; Cho, Y.-N.; Kee, S.-J.; Kim, M.-J.; Jin, H.-M.; Lee, S.-J.; Park, K.-J.; Kim, T.-J.; Lee, S.-S.; Kwon, Y.-S.; et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp. Gerontol. 2014, 49, 47–54. [Google Scholar] [CrossRef]
- Yasuda, T.; Ura, T.; Taniguchi, M.; Yoshida, H. Intradermal Delivery of Antigens Enhances Specific IgG and Diminishes IgE Production: Potential Use for Vaccination and Allergy Immunotherapy. PLOS ONE 2016, 11, e0167952. [Google Scholar] [CrossRef]
- Ginaldi, L., et al., The immune system in the elderly: III. Innate immunity. Immunol Res 1999, 20, 117–26.
- Deng, Y.; Jing, Y.; Campbell, A.E.; Gravenstein, S. Age-Related Impaired Type 1 T Cell Responses to Influenza: Reduced Activation Ex Vivo, Decreased Expansion in CTL Culture In Vitro, and Blunted Response to Influenza Vaccination In Vivo in the Elderly. J. Immunol. 2004, 172, 3437–3446. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J. , et al. , Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun 2014, 6, 152–8. [Google Scholar] [PubMed]
- Freyne, B., A. Marchant, and N. Curtis, BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans R Soc Trop Med Hyg 2015, 109, 287. [Google Scholar] [CrossRef] [PubMed]
- Bangs, S.C.; Baban, D.; Cattan, H.J.; Li, C.K.-F.; McMichael, A.J.; Xu, X.-N. Human CD4+ Memory T Cells Are Preferential Targets for Bystander Activation and Apoptosis. J. Immunol. 2009, 182, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Brugnolo, F.; Sampognaro, S.; Liotta, F.; Cosmi, L.; Annunziato, F.; Manuelli, C.; Campi, P.; Maggi, E.; Romagnani, S.; Parronchi, P. The novel synthetic immune response modifier R-848 (Resiquimod) shifts human allergen-specific CD4+ TH2 lymphocytes into IFN-γ–producing cells. J. Allergy Clin. Immunol. 2003, 111, 380–388. [Google Scholar] [CrossRef]
- Mbawuike, I.N.; Acuna, C.L.; Walz, K.C.; Atmar, R.L.; Greenberg, S.B.; Couch, R.B. Cytokines and impaired CD8+ CTL activity among elderly persons and the enhancing effect of IL-12. Mech. Ageing Dev. 1997, 94, 25–39. [Google Scholar] [CrossRef]
- Eberl, M.; Beck, E.; Coulson, P.S.; Okamura, H.; Wilson, R.; Mountford, A.P. IL-18 potentiates the adjuvant properties of IL-12 in the induction of a strong Th1 type immune response against a recombinant antigen. Vaccine 2000, 18, 2002–2008. [Google Scholar] [CrossRef]
- Thibaut, R.; Bost, P.; Milo, I.; Cazaux, M.; Lemaître, F.; Garcia, Z.; Amit, I.; Breart, B.; Cornuot, C.; Schwikowski, B.; et al. Bystander IFN-γ activity promotes widespread and sustained cytokine signaling altering the tumor microenvironment. Nat. Cancer 2020, 1, 302–314. [Google Scholar] [CrossRef]
- Yin, L.-F.; Fan, Y.-Y.; Li, L.; Wu, C.-Y. [Effect of TLR ligand (R-848) and IL-12 on the production of IFN-gamma by human NK cell subsets]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2007, 23, 623–6. [Google Scholar]
- Chidrawar, S.M.; Khan, N.; Chan, Y.L.T.; Nayak, L.; Moss, P.A. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun. Ageing 2006, 3, 10. [Google Scholar] [CrossRef]
- Bahl, A.; Pandey, S.; Rakshit, R.; Kant, S.; Tripathi, D. Infection-induced trained immunity: a twist in paradigm of innate host defense and generation of immunological memory. Infect. Immun. 2025, 93, e0047224. [Google Scholar] [CrossRef]
- Garn, H.; Potaczek, D.P.; Pfefferle, P.I. The Hygiene Hypothesis and New Perspectives—Current Challenges Meeting an Old Postulate. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Haspeslagh, E.; Heyndrickx, I.; Hammad, H.; Lambrecht, B.N. The hygiene hypothesis: immunological mechanisms of airway tolerance. Curr. Opin. Immunol. 2018, 54, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of trained immunity by influenza vaccination - impact on COVID-19. PLOS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Arts, R.J.; Bekkering, S.; Bahrar, H.; Blok, B.A.; de Bree, L.C.J.; Bruno, M.; Bulut, Ö.; Debisarun, P.A.; Dijkstra, H.; et al. In vitro induction of trained immunity in adherent human monocytes. STAR Protoc. 2021, 2, 100365. [Google Scholar] [CrossRef]
- Rakebrandt, N.; Yassini, N.; Kolz, A.; Schorer, M.; Lambert, K.; Goljat, E.; Brull, A.E.; Rauld, C.; Balazs, Z.; Krauthammer, M.; et al. Innate acting memory Th1 cells modulate heterologous diseases. Proc. Natl. Acad. Sci. USA 2024, 121. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A. Trained Immunity and Local Innate Immune Memory in the Lung. Cell 2018, 175, 1463–1465. [Google Scholar] [CrossRef]
- Pusch, E., H. Renz, and C. Skevaki, Respiratory virus-induced heterologous immunity: Part of the problem or part of the solution? Allergo J 2018, 27, 28–45. [Google Scholar] [CrossRef]
- Gyssens, I.; Netea, M. Heterologous effects of vaccination and trained immunity. Clin. Microbiol. Infect. 2019, 25, 1457–1458. [Google Scholar] [CrossRef]
- Blossey, A.M.; Brückner, S.; May, M.; Parzmair, G.P.; Sharma, H.; Shaligram, U.; Grode, L.; E Kaufmann, S.H.; Netea, M.G.; Schindler, C. VPM1002 as Prophylaxis Against Severe Respiratory Tract Infections Including Coronavirus Disease 2019 in the Elderly: A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Study. Clin. Infect. Dis. 2022, 76, 1304–1310. [Google Scholar] [CrossRef]
- Berendsen, M.L.T.; Bles, P.; de Bree, L.C.J.; Jensen, K.J.; Jensen, C.C.; Wejse, C.; Mendes, D.V.; Netea, M.G.; Benn, C.S. Bacillus Calmette-Guérin vaccination induces a trained innate immunity phenotype in adults over 50 years of age: A randomized trial in Guinea-Bissau. Vaccine 2024, 42, 126439. [Google Scholar] [CrossRef]
- Kumar, N.P.; Padmapriyadarsini, C.; Rajamanickam, A.; Bhavani, P.K.; Nancy, A.; Jeyadeepa, B.; Renji, R.M.; Babu, S. BCG vaccination induces enhanced humoral responses in elderly individuals. Tuberculosis 2023, 139, 102320. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Saavedra-Avila, N.A.; Tiwari, S.; Porcelli, S.A. A century of BCG vaccination: Immune mechanisms, animal models, non-traditional routes and implications for COVID-19. Front. Immunol. 2022, 13, 959656. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-N.; Liu, P.-P.; Li, X.-G.; Zhou, S.-J.; Li, H.; Wang, Z.-Y.; Shen, F.; Lu, B.-C.; Long, Y.; Xiao, X.; et al. Neutralizing Antibodies and Cellular Immune Responses Against SARS-CoV-2 Sustained One and a Half Years After Natural Infection. Front. Microbiol. 2021, 12, 803031. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A., et al., COVID-19-Associated Hospitalizations Among U.S. Adults Aged >/=65 Years - COVID-NET, 13 States, January-August 2023. MMWR Morb Mortal Wkly Rep 2023, 72, 1089–1094. [CrossRef]
- Kelleni, M.T. Could the next disease X be pandemic of virus-induced encephalitis? what should be our first medical response? Expert Rev Anti Infect Ther 2025, 23, 1–3. [Google Scholar] [CrossRef]
| D0 | D10 | D28 | D168 | D336 | ||
|---|---|---|---|---|---|---|
|
65-74yo (n=20) |
%CD4+ CD45RO+ | 65.3±15.5 | 63.0±13.2 | 69.3±12.0 | 56.7±8.3 | 57.8±13.5 |
| %CD8+ CD45RO+ | 39.2±20.8 | 34.5±14.8 | 40.1±19.1 | 33.3±12.6 | 36.7±18.0 | |
|
75yo+ (n=20) |
%CD4+ CD45RO+ | 62.9±14.9 | 66.9±15.7 | 69.0±13.6 | 69.3±16.6 | 63.5±17.3 |
| %CD8+ CD45RO+ | 41.3±16.9 | 43.8±16.1 | 43.1±20.5 | 43.8±18.9 | 34.5±2.6 | |
| Event | Grade |
#subjects (percent) |
| Rash | 1 | 4 (10%) |
| Non-COVID Flu symptoms | 1 | 3 (7.5%) |
| Back Pain | 1 | 2 (5%) |
| Anemia | 1 | 2 (5%) |
| Urinary Tract Infection | 1 | 1 (2.5%) |
| Vasovagal Syndrome | 1 | 1 (2.5%) |
| Epistaxis | 1 | 1 (2.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





