1. Introduction
Glycemic measurement serves as an essential tool for diagnosing and monitoring various diseases in both humans and domestic animals [
1]. Most laboratories utilize automated analyzers, primarily based on hexokinase or glucose oxidase reactions, which are regarded as reference methods (RMs) for glycemic assessment [
2,
3].
In situations where RMs for glycemic measurement are not accessible, particularly in emergencies, PBGMs become invaluable. They provide immediate glycemic values, facilitating rapid clinical decisions, such as diagnosing diabetes mellitus or determining the necessity for glucose supplementation in a hypoglycemic animal [
4,
5].
Historically, PBGMs have been developed for measuring capillary glycemia in humans, with limited options available exclusively for veterinary use. As a result, veterinarians often resort to using PBGMs designed for humans on dogs and cats. One notable example is the AlphaTrak2®, a glucose meter recognized for its accuracy in measuring glycemic levels in canines and felines worldwide, although it is unavailable in Brazil. Meanwhile, a diverse array of human-use PBGMs is readily accessible, with new devices frequently entering the market and others being phased out, leading to significant turnover. However, it is crucial to evaluate the analytical accuracy and precision of these devices before incorporating them into routine veterinary clinical practice [
6,
7,
8].
Thus, the objective of this study was to evaluate the analytical accuracy and clinical precision of three human-use PBGMs for determining glycemia in dogs using whole blood samples.
2. Materials and Methods
2.1. Patients
This study was conducted with canine patients seen during routine service at the Veterinary Endocrinology and Metabolism Service (SEMV - PetEndocrine®) at the Veterinary Teaching Hospital of the Federal University of Rio Grande do Sul (HCV/UFRGS) in Brazil. The study included patients with a clinical indication for blood sampling, specifically for glycemic evaluation, resulting in a total of 69 dogs. Some of these patients participated at various points throughout the study. All dogs underwent a complete blood count (CBC) to assess their overall health status. Importantly, the devices used in this research work adequately in a hematocrit range between 10 - 68%. None of the patients exhibited hematocrit (HT) alterations that could potentially influence glycemic levels.
2.2. Blood Collection and Glycemia Determination
The samples were collected via venous puncture of the jugular vein using a 5 mL syringe equipped with a 30 x 0.7 mm hypodermic needle. Immediately after collection, the samples were aliquoted into three separate tubes (Vacutainer, BD®, New Jersey, USA), each containing the following: 1) EDTA (for complete blood count analysis), 2) a tube without anticoagulant (for specific biochemical determinations as needed), and 3) EDTA with sodium fluoride for glycemia measurement using the enzymatic colorimetric glucose oxidase method, performed with an automatic spectrophotometer (CM 200®, Wiener Lab Group, Argentina). To transfer the samples into the respective tubes, the syringe needle was detached, and after removing the caps, the blood was gently injected into each tube, allowing it to flow down the inner wall. The samples in the EDTA tubes were then homogenized. Additionally, a small final aliquot of whole blood was retained in the syringes for immediate glycemic measurement with the tested point-of-care blood glucose meters (PBGMs).
2.3. Technical Information of the Tested PBGMs
The devices utilized in this study were the Accu-Chek Guide® (ACG) and Accu-Chek Guide ME® (ACGM), both produced by Roche Diagnostics in Basel, as well as the EcoCheck® (EC) device from Eco Diagnóstica in Brazil. Each device requires a sample volume of 0.6 µL for glycemic readings and has an operational range of 10–600 mg/dL. Readings outside this range are indicated on the devices as "Low" or "High," respectively. According to the manufacturers, the ACG and ACGM devices are not influenced by hematocrit variations within the range of 10% to 65%, while the EC device is free from hematocrit interference within a range of 20% to 68%.
All devices were assessed for their analytical accuracy by the ISO 15197:2013 standard. To be deemed accurate, the devices must demonstrate a maximum variation of ± 15 mg/dL when glycemia is ≤ 100 mg/dL, or ± 15% when glycemia is ≥ 100 mg/dL, achieving this in at least 95% of measurements compared to the reference method (ISO, 2013). Furthermore, clinical precision was evaluated using the Parkes error grid, which is divided into five zones: A, B, C, D, and E [
9]. For a glucose meter to be recognized as accurate, ISO 2013 mandates that 99% of glycemic readings fall within zones A and B [
10]. The coefficient of variation (CV%) for each device was also calculated based on three repeated measurements of the same blood sample. Samples were categorized as hypoglycemic if the values were <60 mg/dL, normoglycemic if they fell between 60 and 116 mg/dL, and hyperglycemic if they exceeded 116 mg/dL [
11,
12].
2.4. Statistical Analyses
Statistical analysis was conducted using the GraphPad Prism software (version 6.05, San Diego, USA). The Pearson linear correlation coefficient was determined between the results obtained with the different glucose meters and the RM. Values of p < 0.05 were considered statistically significant. Additionally, the difference between the results determined by the PBGMs and the RM was represented through Bland-Altman plots [
13]. The results were distributed with each glucose meter in the Parkes error grid using the BD Error Grid spreadsheet for Excel on Windows [
9].
3. Results
The sample size tested with each glucose meter was not uniform, as the study initially concentrated on the analytical evaluation of the EC device (n = 204), followed by the inclusion of the ACG device (n = 146). However, the ACG was discontinued in Brazil during the study and was subsequently replaced by the ACGM device (n = 69). In total, 419 samples were assessed across the three glucose meters. Among these, 34 samples (8.1%) were classified as hypoglycemic (mean = 36.7 ± 14.15 mg/dL), 181 samples (43.1%) as normoglycemic (mean = 88 ± 14.12 mg/dL), and 204 samples (48.6%) as hyperglycemic (mean = 317.7 ± 126.11 mg/dL).
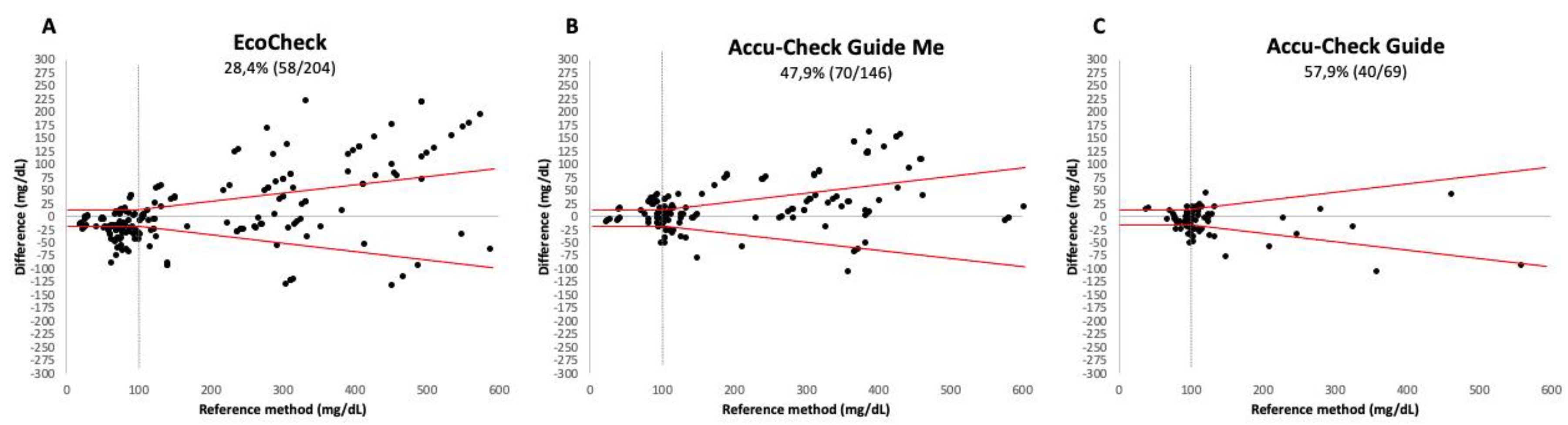
None of the three devices met the analytical accuracy criteria established by ISO 15197:2013 (see
Figure 1). For the EC device, only 28.4% (
Figure 1A) of the readings fell within the acceptable variation, while for the ACGM and ACG devices, this figure was 47.9% (
Figure 1B) and 57.9% (
Figure 1C), respectively.
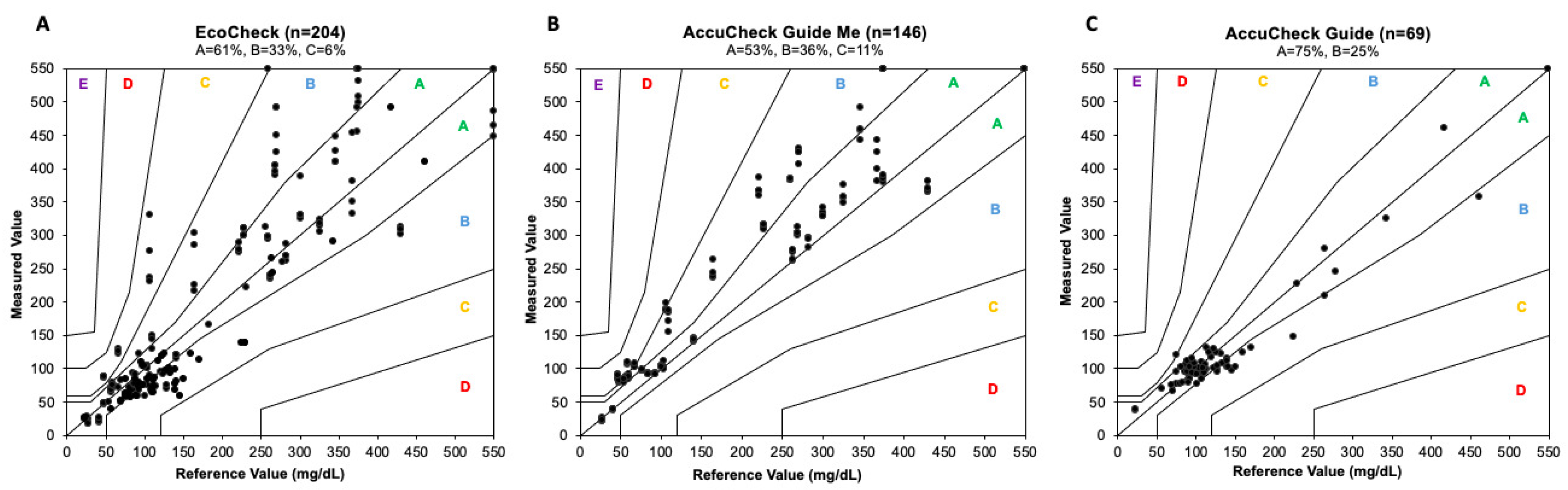
In terms of clinical accuracy, neither the EC nor the ACGM devices satisfied the ISO criteria, with only 94% of measurements falling within Zones A and B (61% in Zone A and 33% in Zone B) for the EC device and 89% (53% in Zone A and 36% in Zone B) for the ACGM. In contrast, the ACG device emerged as the most accurate PBGM in this study, achieving 100% of measurements within Zones A and B, with 75% in Zone A and 25% in Zone B (refer to
Figure 2A–C).
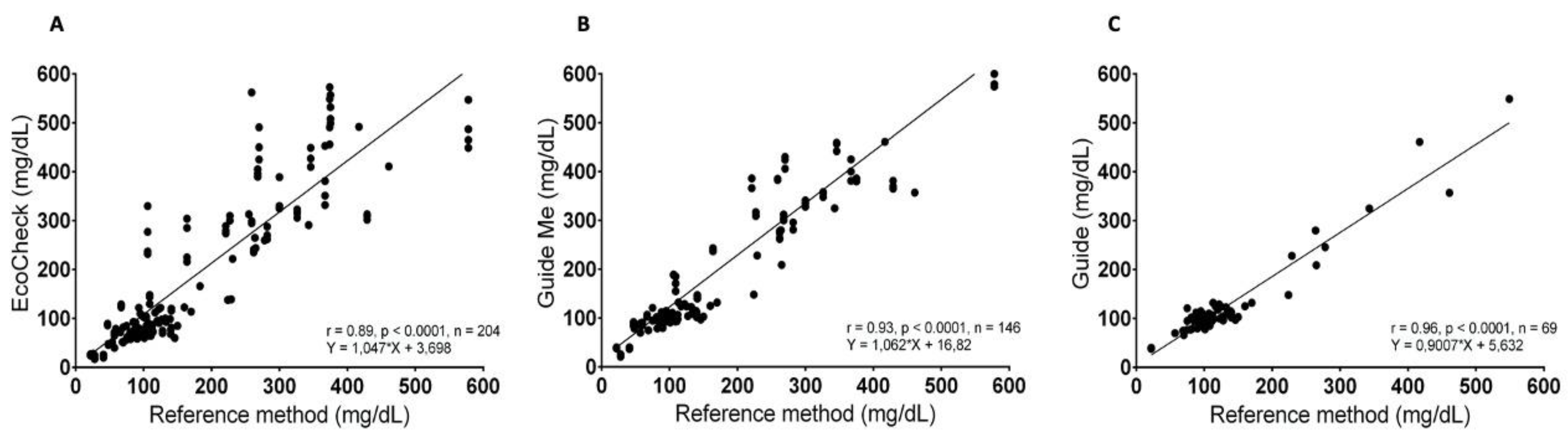
The three PBGMs exhibited a notable positive correlation, as indicated by Pearson's correlation coefficient (
Figure 3). Specifically, for the ACG PBGM, the correlation coefficient was r = 0.96 (95% CI = 0.93 to 0.97; p < 0.0001). The ACGM PBGM demonstrated a coefficient of r = 0.93 (95% CI = 0.90 to 0.95; p < 0.0001), while the EC PBGM showed a coefficient of r = 0.89 (95% CI = 0.87 to 0.92; p < 0.0001).
Regarding the coefficient of variation (CV%), no significant difference was observed among the CVs of the different PBGMs evaluated (p = 0.077), despite the wide variability documented in some readings of the EC device, as shown in
Table 1.
4. Discussion
PBGMs play a crucial role in small animal clinical practice. However, in certain countries, the absence of devices specifically designed for canine and feline species makes it necessary to use human devices as an alternative. This is acceptable as long as these devices are validated for use in animals or at least do not result in erroneous decision-making regarding patient management [
1,
15,
16,
17,
18]. The validity of various human-use PBGMs for application in dogs is a topic of frequent study [
19,
20].
The validation of various human-use point-of-care PBGMs for application in dogs is a subject of frequent research [
1,
4,
14,
15,
16,
17,
18,
19,
20]. One study conducted a comparison between the Accu-Chek Guide® (ACG) glucose meter and the One Call Plus II® (OCP). Despite some differences in statistical analysis and variations in glycemic ranges between that study and ours, the ACG was found to be more accurate and precise than the OCP, which aligns with the results of our research [
21]. As of now, no other studies have assessed the analytical accuracy of the EcoCheck® (EC) and Accu-Chek Guide ME® (ACGM) glucose meters.
According to Gerber & Freeman [
7], several factors can interfere with glycemic measurement, including lipemia, the presence of plasma pigments such as bilirubin, blood pH, partial oxygen pressure (PO2), and hematocrit levels. A limitation of our study was that, among these variables, only the hematocrit of the patients was systematically assessed across all samples. Importantly, 100% of the samples exhibited hematocrit readings within the range deemed interference-free by the manufacturers (data not shown).
Documenting hematocrit levels and understanding the interference-free range of the PBGM is essential, as anemic patients may display overestimated glycemic readings, while those with elevated hematocrit levels could show underestimated readings. These discrepancies arise from the differential plasma contact with the reagent strip, leading to either increased or decreased measurement accuracy [
14,
18,
22,
23].
The findings of this study reveal that, although there is a strong correlation between the results obtained from the human-use PBGMs ACG, ACGM, and EC compared to the laboratory method, none of these devices entirely satisfied the analytical accuracy recommendations outlined in ISO 15197:2013. This ISO standard has served as a foundational reference for research assessing the clinical and analytical accuracy of human glucose meters, and it is commonly utilized in veterinary medicine studies to evaluate these parameters [
10].
The research conducted by Brito-Casillas et al. [
14] assessed the accuracy of nine human-use glucose meters in dogs, adhering to this standard. This study was pioneering in its adoption and promotion of the standard within veterinary medicine, thus setting a benchmark for future research aimed at examining the accuracy and precision of human glucose meters in animals [
10,
14].
In terms of clinical accuracy, only the ACG device met the necessary criteria to be classified as clinically precise according to the Parkes error grid, with 100% of its measurements falling within zones A and B. In contrast, neither of the other two evaluated devices fulfilled the minimum requirements established by ISO 15197:2013. This indicates that making therapeutic decisions based on glycemic results from the ACGM and EC devices could potentially lead to inappropriate actions for patients, although errors categorized in zone C of the error grid are considered low-risk [
14].
The evaluation of three glucose meters revealed a significant positive correlation, as indicated by Pearson's correlation coefficient. The correlation was classified as very strong (> 0.9) for the PBGMs ACG (r = 0.96) and ACGM (r = 0.93). In contrast, the correlation for the EC glucose meter was categorized as merely "strong" (r = 0.89). Additionally, an analysis of the coefficient of variation (CV%) showed that, on average, although there were no significant differences among the three devices tested, the readings from the EC PBGM demonstrated the highest dispersion index, with differences reaching up to 36% among readings of the same sample. Understanding the CV% of PBGMs is crucial in clinical practice, as glycemic readings are frequently taken as single-point measurements, and results considered normal are seldom confirmed subsequently [
24].
5. Conclusions
The significance and relevance of portable glucose meters in clinical practice are clear. However, selecting these devices should not be done indiscriminately or haphazardly. The frequent introduction of new models and the phasing out of older ones contribute to a rapidly evolving market. Consequently, this study underscores the necessity of rigorously assessing both the analytical and clinical accuracy of the available portable blood glucose meters (PBGMs), particularly those intended for human use, given the limited availability of veterinary-specific options in Brazil.
In our research, none of the glucose meters met the ISO 15197:2013 standards, resulting in their analytical inaccuracy. However, the Accu-Chek Guide® successfully fulfilled the required specifications for clinical accuracy, making it the superior choice among the devices tested for routine glycemic monitoring in dogs, delivering prompt and reliable results. Nonetheless, its discontinuation in the Brazilian market indicates that its successor, the Accu-Chek Guide ME, emerges as the most suitable option among the PBGMs evaluated.
Author Contributions
J.L.X.L. and A.G.P. were responsible for data analysis, statistical analysis, writing, sample collection, and manuscript reviewing. T.B.N, L.R, V.S.W, D.I.d.S, and B.d.S.M were responsible for sample collection. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
For this study, a specific protocol number was not generated because the animals used were routine patients at SEMV, whose glucose measurements were essential for their management and clinical treatment. These animals were not subjected to any additional experiments beyond the blood collection for glucose monitoring, a standard and crucial procedure for the management of their clinical cases. Blood collection was conducted according to established veterinary norms and practices, ensuring the well-being of the animals and the integrity of the results obtained.
Informed Consent Statement
Not applicable. The animals were from the university lab. Data Availability Statement: Materials and data sheets are available upon request to interested researchers.
Acknowledgments
The authors would like to thank the “Programa de Ações em Endocrinologia Veterinária (PetEndocrine)” from the Universidade Federal do Rio Grande do Sul for supporting this manuscript and the Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PBGM |
Portable blood glucose meters |
| ACGM |
Accu-Chek Guide Me® |
| EC |
EcoCheck® |
ACG
GOM
ISO
RM
CBC
HT
CV%
OCP
PO2 |
Accu-Chek Guide®
Glucose oxidase method
International Organization for Standardization
Reference method
Complete blood count
Hematocrit
Coefficient of variation
One Call Plus II®
Partial oxygen pressure |
References
- Cohn, L. A.; McCaw, D.L.; Tate, D.J.; Johnson, J.C. Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs. J Am Vet Med Assoc, Columbia, v. 216, n. 2, p. 198-202, Jan. 2000. Available at: https://pubmed.ncbi.nlm.nih.gov/10649753/. Accessed on: 10 jan. 2024. [CrossRef]
- Sonagra, A.D.; Zubair, M.; Motiani, A. Hexoquinase Method. Stat Pearls, Treasure Island; 2025. Available at: https://www.ncbi.nlm.nih.gov/books/NBK587446/. Acesso em: 24 jun 2024.
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem, v. 6, p. 24–27, 1969. Available at: https://journals.sagepub.com/doi/abs/10.1177/000456326900600108. Accessed on: 20 dez. 2023. doi: 10.1177/000456326900600108. [CrossRef]
- Jamaluddin, F.A.; Gunavathy, M.; Yean, C.Y.; Thevarajah, M. Variability of point-of-care testing blood glucometers versus the laboratory reference method in a tertiary teaching hospital. Asian Biomedicine, 6(1), 67–74. 10.5372/1905-7415.0506.128, 2017. Available at: https://sciendo.com/article/10.5372/1905-7415.0506.128. Accessed on: 19 dez. 2023. [CrossRef]
- Casella, M.; Hässig, M.; Reusch, C.E. Home-monitoring of blood glucose in cats with diabetes mellitus: Evaluation over a 4-month period. J Fel Med Sur, 7(3), 2005. Available at: https://journals.sagepub.com/doi/10.1016/j.jfms.2004.08.006. Accessed on: 12 dez. 2023. [CrossRef]
- Behrend, E.; Holford, A.; Lathan, P.; Rucinsky, R.; Schulman, R. AAHA Diabetes Management Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2018 Jan/Feb;54(1):1-21. doi: 10.5326/JAAHA-MS-6822. Available at: https://pubmed.ncbi.nlm.nih.gov/29314873/. Accessed on: 12 Jan. 2024. [CrossRef] [PubMed]
- Gerber, K.L.; Freeman, K.P. ASVCP guidelines: Quality assurance for portable blood glucose meter (glucometer) use in veterinary medicine. Vet. Clin. Pat., 1(45), 10–27. 10.1111/cp.12310, 2016. Available at: https://pubmed.ncbi.nlm.nih.gov/26748942/. Accessed on: 09 dez. 2023. [CrossRef]
- Shapiro, B.; Savage, P.J.; Lomatach, D.; Gniadek, T.; Forbes, R.; Mitchell, R.; Hein, K.; Starr, R.; Nutter, M.; Scherdt, B. A comparison of accuracy and estimated cost of methods for home blood glucose monitoring. Diabetes Care, 4(3), 396–403. 10.2337/diacare.4.3.396-403, 1981. Available at: https://pubmed.ncbi.nlm.nih.gov/7344886/. Accessed on: 09 dez. 2023. [CrossRef]
- Parkes, J.L.; Slatin, S.L.; Pardo, S.; Ginsberg, B.H. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000 Aug;23(8):1143-8. doi: 10.2337/diacare.23.8.1143. Available at: https://pubmed.ncbi.nlm.nih.gov/10937512/. Accessed on: 09 dez. 2023. [CrossRef]
- International organization for standardization (ISO). In VitroDiagnostic Test Systems—Requirements for Blood-glucose Monitoring Systems for Self-testing in Managing Diabetes Mellitus.European Committee for Standardization (CEN): Brussels; 2013. Available at: https://www.iso.org/standard/54976.html Accessed on: 29 Nov. 2023.
- Idowu, O.; Heading, K. Hypoglycemia in dogs: Causes, management, and diagnosis. Can Vet J. 2018 Jun;59(6):642-649. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5949948/. Accessed on: 26 Jun. 2024.
- Suchowersky, N. D.; Carlson, E.A.; Lee, H.P.; Behrend, E.N. Comparison of glucose concentrations in canine whole blood, plasma, and serum measured with a veterinary point-of-care glucometer. J Vet Diagn Invest. 2021 Jul;33(4):695-702. Epub 2021 Jun 2. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8229823/. Accessed on: 26 Jun. 2024. [CrossRef]
- Bland, J.M.; Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet, v.327, n.8476, p.307-310, 1986. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(86)90837-8/fulltext. Accessed: Jan 24, 2024. [CrossRef]
- Brito-Casillas, Y.; Figueirinhas, P.; Wiebe, J.C.; López-Rios, L.; Pérez-Barreto, D.; Mélian, C.; Wägner, A.M. ISO-based assessment of accuracy and precision of glucose meters in dogs. J Vet Intern Med. 2014. Available from: https://pubmed.ncbi.nlm.nih.gov/24990398/. Accessed: 02 dez. 2023. [CrossRef]
- Johnson, B.B.; Fry, M.M.; Flatland, B.; Kirk, C.A. Comparison of a human portable blood glucose meter, veterinary portable blood glucose meter, and automated chemistry analyzer for measurement of blood glucose concentrations in dogs. J. Am. Vet. Med. Assoc. Knoxville, v. 235, n. 11, December 1, 2009. Available from: https://pubmed.ncbi.nlm.nih.gov/19951099/. Accessed: 11 dez. 2023. [CrossRef]
- Dobromylskyj, M.J.; Sparkes, A.H. Assessing portable blood glucose meters for clinical use in cats in the United Kingdom. Vet Rec, 18(167), 438–442, 2010. Available from: https://pubmed.ncbi.nlm.nih.gov/20852246/. Accessed: 09 Jan. 2024. [CrossRef]
- Kang, M.H.; Kim, D.H.; Jeong, I.S.; Choi, G.C.; Park, H.M. Evaluation of four portable blood glucose meters in diabetic and non-diabetic dogs and cats. Vet Q, 36(1), 2–9. 2016. Available from: https://pubmed.ncbi.nlm.nih.gov/26442786/. Accessed: 13 Jan. 2024. [CrossRef]
- Wess, G.; Reusch, C. Assessment of five portable blood glucose meters for use in cats. J. Am. Vet. Med. Assoc, v.61, n.12, p.1587-1592, Dec.2000. Available from:: https://avmajournals.avma.org/doi/ pdf/10.2460/ajvr.2000.61.1587. Accessed:dez. 01, 2023. [CrossRef]
- Ismail-Hamdi, S.; Romdane, M.N.; Romdhane, S.B. Comparison of a human portable blood glucose meter and automated chemistry analyser for measurement of blood glucose concentrations in healthy dogs. Vet Med Sci, 2021.nov. Available from: https://pubmed.ncbi.nlm.nih.gov/34352158/. Accessed: 15 jan. 2024. [CrossRef]
- Dos Santos, M.A.B.; Vargas, A.M.; Rosato, P.N.; Andrade, C.G.; Martins, C.M.; Petri, G. Evaluation of three human-use glucometers for blood glucose measurement in dogs. Vet. Med. Int. 2022. Available from: https://pubmed.ncbi.nlm.nih.gov/36465855/. Accessed: 19 Jan. 2024. [CrossRef]
- Souza, K. T. R. de.; Monteiro, L. F.; Knackfus, F. B.; Monteiro, L. M. V. W. Study of data obtained in the evaluation of two portable glucometers for human use in dogs. Multidisciplinary Science Journal, 3(4), 2021. Available from: https://malque.pub/ojs/index.php/msj/article/view/190. Accessed: 15 jan. 2024.
- Mori, A.; Lee, P.; Yokoyama, T.; Oda, H.; Saeki, K.; Miki, Y.; Nozawa, S.; Azakami, D.; Momota, Y.; Makino, Y.; Matsubara, T.; Osaka, M.; Ishioka, K.; Arai, T.; Sako, T. Evaluation of artificial pancreas technology for continuous blood glucose monitoring in dogs. J. Artif. Organs 14: 133–139. Available from: https://pubmed.ncbi.nlm.nih.gov/21491113/. Accessed: 19 dez. 2023. [CrossRef]
- Nichols, J.H.; Howard, C.; Loman, K.; Miller, C.; Nyberg, D.; Chan, D.W. Laboratory and bedside evaluation of portable glucose meters. Am. J. Clin. Pathol. 103: 244–251, 1995. Available from: https://pubmed.ncbi.nlm.nih.gov/7856571/. [CrossRef]
- Vieira, S. Introdução à bioestatística, 5ª ed. Elsevier: Rio de Janeiro, Brasil, 2016. pp. 120-130.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).