Submitted:
19 March 2025
Posted:
20 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
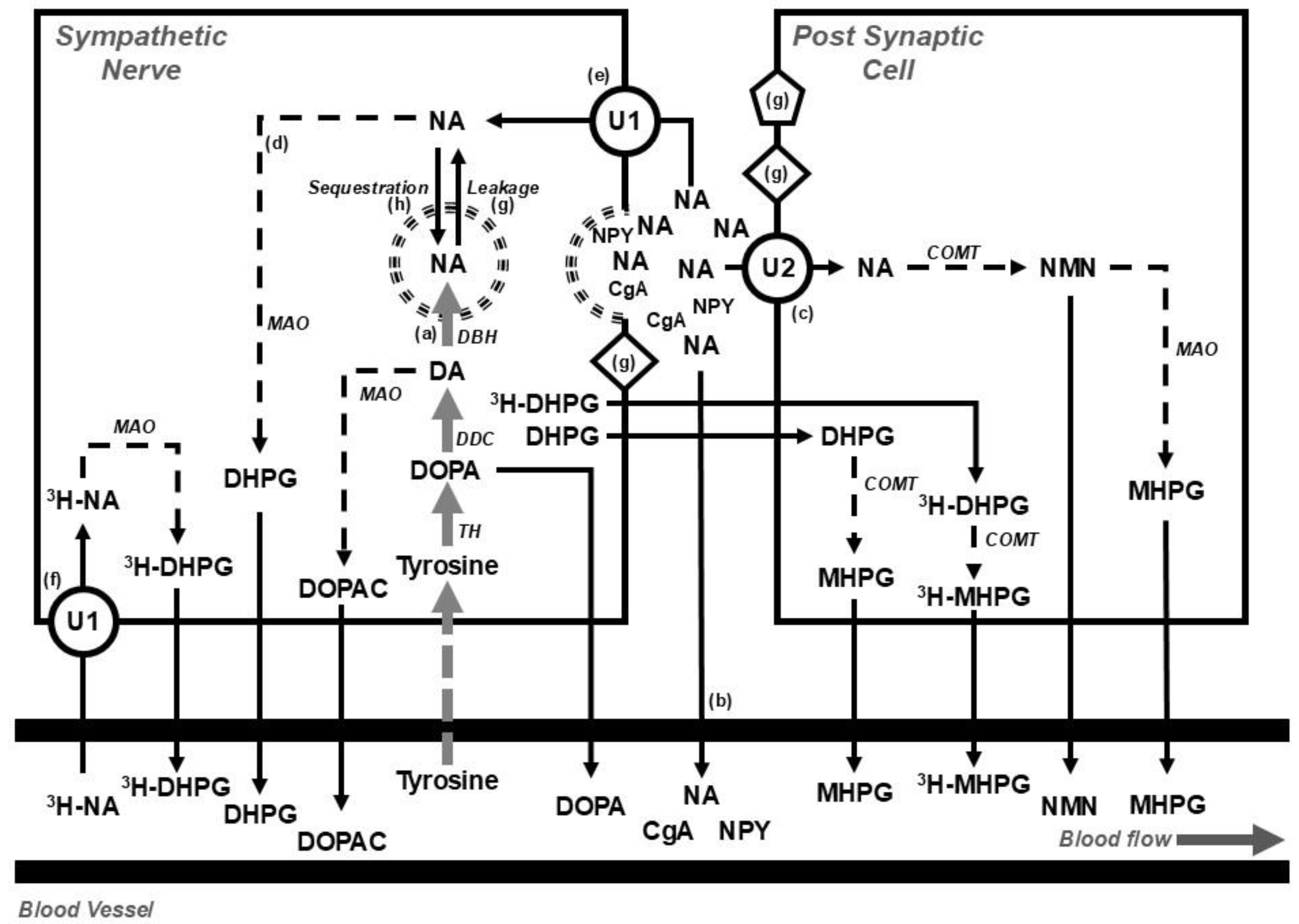
2. Assessing Sympathetic Nervous Activity
- Direct nerve recording using microneurography [14];
- Isotope dilution during a tracer dose infusion of radiolabeled noradrenaline for estimating the rate of release of neurotransmitter [15];
- Tissue microdialysis [16];
- I123-metaiodobenzylguanidine (MIBG) imaging of the heart [17];
- Visualizing catecholamine containing nerve fibers using glyoxylic acid histofluorescence [20];
3. Sympathetic Activity and Body Weight
3.1. Obesity and Weight Gain
3.2. Weight Loss
3.2.1. Lifestyle - Diet and Exercise
3.2.2. Bariatric Surgery
3.2.3. Anorexia Nervosa
3.2.4. Cachexia
3.2.5. Pharmacological Agents
Incretins
Glifozins
4. Brain Pathways Associated with Sympathetic Regulation
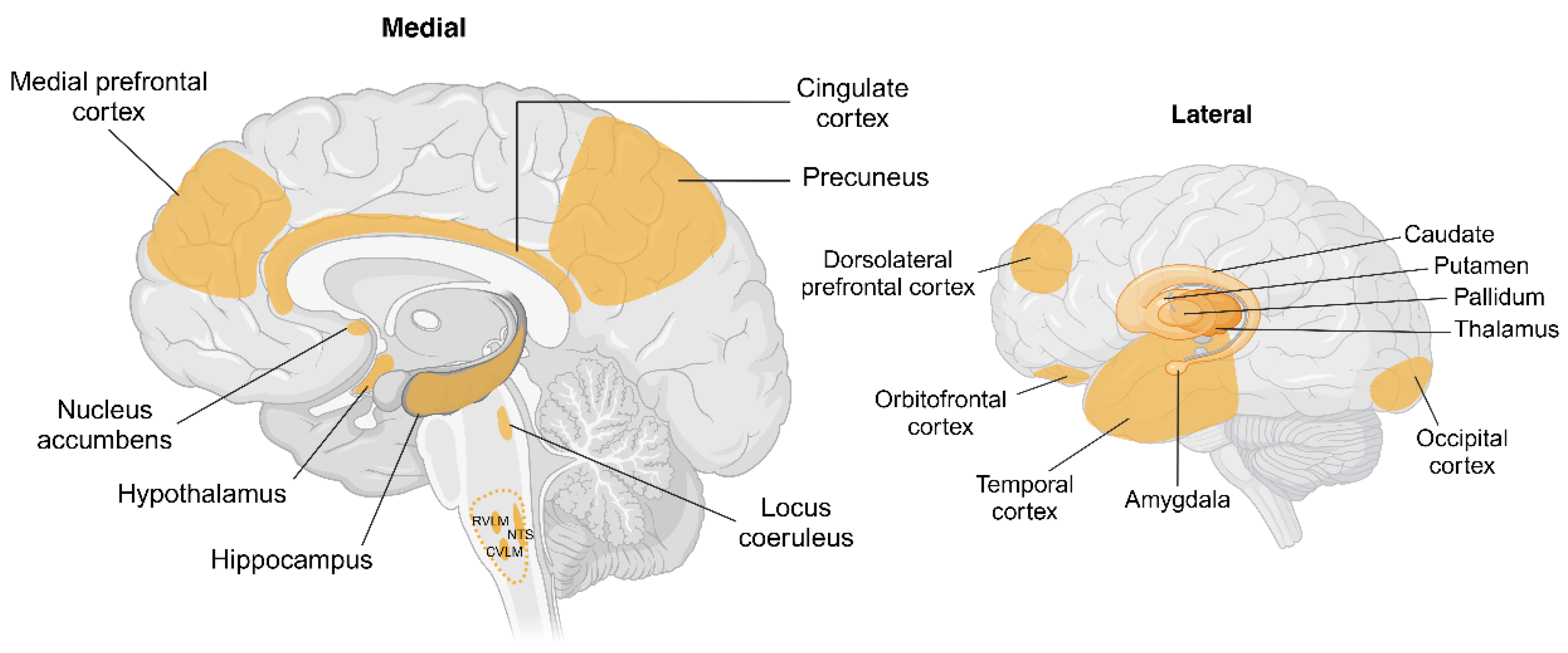
4.1. Brain Pathways Associated with Weight Gain
4.2. Brain Pathways Associated with Weight Loss
5. Regional Sympathetic Nervous Activity and Cardiometabolic Risk
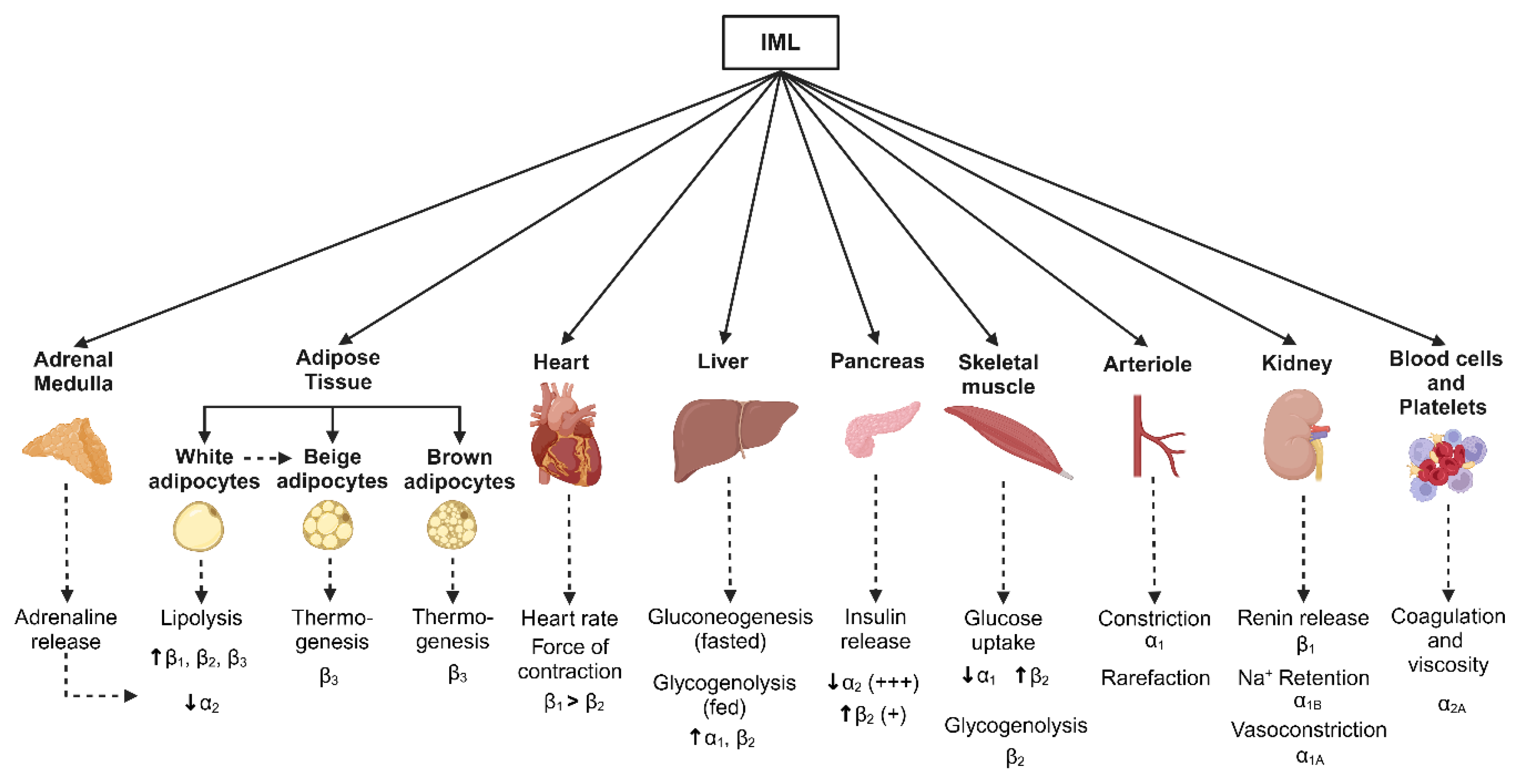
5.1. Adipose Tissue
5.2. Liver and Pancreas
5.3. Skeletal Muscle
5.4. Kidney
5.5. Heart and Vascular Function
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, C.D.; Higgins, M.; Donato, K.A.; Rohde, F.C.; Garrison, R.; Obarzanek, E.; Ernst, N.D.; Horan, M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res 2000, 8, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994, 17, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol 2025, 13, 221–262. [Google Scholar] [CrossRef]
- Folsom, A.R.; Kaye, S.A.; Sellers, T.A.; Hong, C.P.; Cerhan, J.R.; Potter, J.D.; Prineas, R.J. Body fat distribution and 5-year risk of death in older women. JAMA 1993, 269, 483–487. [Google Scholar] [CrossRef]
- Kwon, H.; Yun, J.M.; Park, J.H.; Cho, B.L.; Han, K.; Joh, H.K.; Son, K.Y.; Cho, S.H. Incidence of cardiovascular disease and mortality in underweight individuals. J Cachexia Sarcopenia Muscle 2021, 12, 331–338. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.H.; Han, S. Underweight: another risk factor for cardiovascular disease?: A cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine (Baltimore) 2017, 96, e8769. [Google Scholar] [CrossRef]
- von Euler, U.S. A specific sympathetic ergone in adrenergic nerve fibres (sympathin) and its relation to adrenaline and noradrenaline. Acta Physiol Scand 1946, 12, 73–97. [Google Scholar] [CrossRef]
- Brown, G.L.; Gillespie, J.S. The output of sympathetic transmitter from the spleen of the cat. J Physiol 1957, 138, 81–102. [Google Scholar] [CrossRef]
- Von Euler, U.S.; Hellner, S.; Purkhold, A. Excretion of noradrenaline in urine in hypertension. Scand J Clin Lab Invest 1954, 6, 54–59. [Google Scholar] [CrossRef]
- Kramer, R.S.; Mason, D.T.; Braunwald, E. Augmented sympathetic neurotransmitter activity in the peripheral vascular bed of patients with congestive heart failure and cardiac norepinephrine depletion. Circulation 1968, 38, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Engelman, K.; Portnoy, B.; Lovenberg, W. A sensitive and specific double-isotope derivative method for the determination of catecholamines in biological specimens. Am J Med Sci 1968, 255, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Zweifler, A.J.; Julius, S. Increased platelet catecholamine content in pheochromocytoma: a diagnostic test in patients with elevated plasma catecholamines. N Engl J Med 1982, 306, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Hagbarth, K.E.; Vallbo, A.B. Mechanoreceptor activity recorded percutaneously with semi-microelectrodes in human peripheral nerves. Acta Physiol Scand 1967, 69, 121–122. [Google Scholar] [CrossRef]
- Esler, M.; Jackman, G.; Bobik, A.; Kelleher, D.; Jennings, G.; Leonard, P.; Skews, H.; Korner, P. Determination of norepinephrine apparent release rate and clearance in humans. Life Sci 1979, 25, 1461–1470. [Google Scholar] [CrossRef]
- Gronlund, B.; Astrup, A.; Bie, P.; Christensen, N.J. Noradrenaline release in skeletal muscle and in adipose tissue studied by microdialysis. Clin Sci (Lond) 1991, 80, 595–598. [Google Scholar] [CrossRef]
- Wieland, D.M.; Brown, L.E.; Rogers, W.L.; Worthington, K.C.; Wu, J.L.; Clinthorne, N.H.; Otto, C.A.; Swanson, D.P.; Beierwaltes, W.H. Myocardial imaging with a radioiodinated norepinephrine storage analog. J Nucl Med 1981, 22, 22–31. [Google Scholar]
- DeQuattro, V.; Miura, Y.; Lurvey, A.; Cosgrove, M.; Mendez, R. Increased plasma catecholamine concentrations and vas deferens norepinephrine biosynthesis in men with elevated blood pressure. Circ Res 1975, 36, 118–126. [Google Scholar] [CrossRef]
- Guenter, J.; Lenartowski, R. Molecular characteristic and physiological role of DOPA-decarboxylase. Postepy Hig Med Dosw (Online) 2016, 70, 1424–1440. [Google Scholar] [CrossRef]
- Guidry, G. A method for counterstaining tissues in conjunction with the glyoxylic acid condensation reaction for detection of biogenic amines. J Histochem Cytochem 1999, 47, 261–264. [Google Scholar] [CrossRef]
- Morris, M.J.; Cox, H.S.; Lambert, G.W.; Kaye, D.M.; Jennings, G.L.; Meredith, I.T.; Esler, M.D. Region-specific neuropeptide Y overflows at rest and during sympathetic activation in humans. Hypertension 1997, 29, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Vaingankar, S.M.; Li, Y.; Biswas, N.; Gayen, J.; Choksi, S.; Rao, F.; Ziegler, M.G.; Mahata, S.K.; O'Connor, D.T. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J Hypertens 2010, 28, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Jennings, G.; Lambert, G.; Meredith, I.; Horne, M.; Eisenhofer, G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev 1990, 70, 963–985. [Google Scholar] [CrossRef] [PubMed]
- Meredith, I.T.; Eisenhofer, G.; Lambert, G.W.; Jennings, G.L.; Thompson, J.; Esler, M.D. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension 1992, 19, 628–633. [Google Scholar] [CrossRef]
- Pacholczyk, T.; Blakely, R.D.; Amara, S.G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 1991, 350, 350–354. [Google Scholar] [CrossRef]
- Esler, M.; Alvarenga, M.; Pier, C.; Richards, J.; El-Osta, A.; Barton, D.; Haikerwal, D.; Kaye, D.; Schlaich, M.; Guo, L.; et al. The neuronal noradrenaline transporter, anxiety and cardiovascular disease. J Psychopharmacol 2006, 20, 60–66. [Google Scholar] [CrossRef]
- Esler, M.D.; Wallin, G.; Dorward, P.K.; Eisenhofer, G.; Westerman, R.; Meredith, I.; Lambert, G.; Cox, H.S.; Jennings, G. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol 1991, 260, R817–R823. [Google Scholar] [CrossRef]
- Shannon, J.R.; Flattem, N.L.; Jordan, J.; Jacob, G.; Black, B.K.; Biaggioni, I.; Blakely, R.D.; Robertson, D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000, 342, 541–549. [Google Scholar] [CrossRef]
- Lambert, E.; Eikelis, N.; Esler, M.; Dawood, T.; Schlaich, M.; Bayles, R.; Socratous, F.; Agrotis, A.; Jennings, G.; Lambert, G.; et al. Altered sympathetic nervous reactivity and norepinephrine transporter expression in patients with postural tachycardia syndrome. Circ Arrhythm Electrophysiol 2008, 1, 103–109. [Google Scholar] [CrossRef]
- Mandela, P.; Ordway, G.A. The norepinephrine transporter and its regulation. J Neurochem 2006, 97, 310–333. [Google Scholar] [CrossRef]
- Marques, F.Z.; Eikelis, N.; Bayles, R.G.; Lambert, E.A.; Straznicky, N.E.; Hering, D.; Esler, M.D.; Head, G.A.; Barton, D.A.; Schlaich, M.P.; et al. A polymorphism in the norepinephrine transporter gene is associated with affective and cardiovascular disease through a microRNA mechanism. Mol Psychiatry 2017, 22, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, K.N.; Bayles, R.; Ciccotosto, G.D.; Maxwell, S.; Cappai, R.; Pelka, G.J.; Tam, P.P.; Christodoulou, J.; El-Osta, A. Alleviating transcriptional inhibition of the norepinephrine slc6a2 transporter gene in depolarized neurons. J Neurosci 2010, 30, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Esler, M.D.; Meredith, I.T.; Dart, A.; Cannon, R.O., 3rd; Quyyumi, A.A.; Lambert, G.; Chin, J.; Jennings, G.L.; Goldstein, D.S. Sympathetic nervous function in human heart as assessed by cardiac spillovers of dihydroxyphenylglycol and norepinephrine. Circulation 1992, 85, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Bell, C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes 2004, 53, 276–284. [Google Scholar] [CrossRef]
- Limberg, J.K.; Malterer, K.R.; Matzek, L.J.; Levine, J.A.; Charkoudian, N.; Miles, J.M.; Joyner, M.J.; Curry, T.B. Resting sympathetic activity is associated with the sympathetically mediated component of energy expenditure following a meal. Physiol Rep 2017, 5. [Google Scholar] [CrossRef]
- Weyer, C.; Pratley, R.E.; Snitker, S.; Spraul, M.; Ravussin, E.; Tataranni, P.A. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension 2000, 36, 531–537. [Google Scholar] [CrossRef]
- Lambert, E.; Straznicky, N.; Eikelis, N.; Esler, M.; Dawood, T.; Masuo, K.; Schlaich, M.; Lambert, G. Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens 2007, 25, 1411–1419. [Google Scholar] [CrossRef]
- Tank, J.; Heusser, K.; Diedrich, A.; Hering, D.; Luft, F.C.; Busjahn, A.; Narkiewicz, K.; Jordan, J. Influences of gender on the interaction between sympathetic nerve traffic and central adiposity. J Clin Endocrinol Metab 2008, 93, 4974–4978. [Google Scholar] [CrossRef]
- Esler, M.; Jennings, G.; Korner, P.; Willett, I.; Dudley, F.; Hasking, G.; Anderson, W.; Lambert, G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 1988, 11, 3–20. [Google Scholar] [CrossRef]
- D'Souza, A.W.; Moore, J.P.; Manabe, K.; Lawley, J.S.; Washio, T.; Hissen, S.L.; Sanchez, B.; Fu, Q. The interactive effects of posture and biological sex on the control of muscle sympathetic nerve activity during rhythmic handgrip exercise. Am J Physiol Regul Integr Comp Physiol 2024, 327, R133–R144. [Google Scholar] [CrossRef]
- Rundqvist, B.; Elam, M.; Bergmann-Sverrisdottir, Y.; Eisenhofer, G.; Friberg, P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation 1997, 95, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.W.; Kaye, D.M.; Lefkovits, J.; Jennings, G.L.; Turner, A.G.; Cox, H.S.; Esler, M.D. Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation 1995, 92, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Jennings, G.; Lambert, G. Measurement of overall and cardiac norepinephrine release into plasma during cognitive challenge. Psychoneuroendocrinology 1989, 14, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.; Ray, C.A. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 2009, 296, H847–H853. [Google Scholar] [CrossRef]
- Vollenweider, P.; Tappy, L.; Randin, D.; Schneiter, P.; Jequier, E.; Nicod, P.; Scherrer, U. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest 1993, 92, 147–154. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, E.A.; Grima, M.T.; Eikelis, N.; Richards, K.; Nestel, P.J.; Dawood, T.; Masuo, K.; Sari, C.I.; Dixon, J.B.; et al. The effects of dietary weight loss on indices of norepinephrine turnover: modulatory influence of hyperinsulinemia. Obesity 2014, 22, 652–662. [Google Scholar] [CrossRef]
- Prior, L.J.; Eikelis, N.; Armitage, J.A.; Davern, P.J.; Burke, S.L.; Montani, J.P.; Barzel, B.; Head, G.A. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 2010, 55, 862–868. [Google Scholar] [CrossRef]
- Gentile, C.L.; Orr, J.S.; Davy, B.M.; Davy, K.P. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol 2007, 292, R1834–R1838. [Google Scholar] [CrossRef]
- Hissen, S.L.; Takeda, R.; Badrov, M.B.; Arias-Franklin, S.; Patel, S.; Nelson, D.B.; Babb, T.G.; Fu, Q. Impact of maternal obesity on resting muscle sympathetic nerve activity during uncomplicated pregnancy: a longitudinal assessment. Am J Physiol Regul Integr Comp Physiol 2024, 326, R10–R18. [Google Scholar] [CrossRef]
- Grassi, G.; Biffi, A.; Seravalle, G.; Trevano, F.Q.; Dell'Oro, R.; Corrao, G.; Mancia, G. Sympathetic neural overdrive in the obese and overweight state. Hypertension 2019, 74, 349–358. [Google Scholar] [CrossRef]
- Quarti Trevano, F.; Dell'Oro, R.; Biffi, A.; Seravalle, G.; Corrao, G.; Mancia, G.; Grassi, G. Sympathetic overdrive in the metabolic syndrome: meta-analysis of published studies. J Hypertens 2020, 38, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Jennings, G.; Turner, A.; Cox, H.; Lambert, G.; Esler, M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 1997, 96, 3423–3429. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; Straznicky, N.; Schlaich, M.; Esler, M.; Dawood, T.; Hotchkin, E.; Lambert, G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 2007, 50, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.W.; Vaz, M.; Cox, H.S.; Turner, A.G.; Kaye, D.M.; Jennings, G.L.; Esler, M.D. Human obesity is associated with a chronic elevation in brain 5- hydroxytryptamine turnover. Clin Sci (Colch) 1999, 96, 191–197. [Google Scholar] [CrossRef]
- Ferrier, C.; Jennings, G.L.; Eisenhofer, G.; Lambert, G.; Cox, H.S.; Kalff, V.; Kelly, M.; Esler, M.D. Evidence for increased noradrenaline release from subcortical brain regions in essential hypertension. J Hypertens 1993, 11, 1217–1227. [Google Scholar] [CrossRef]
- Lambert, E.; Straznicky, N.; Sari, C.I.; Eikelis, N.; Hering, D.; Head, G.; Dixon, J.; Esler, M.; Schlaich, M.; Lambert, G. Dyslipidemia is associated with sympathetic nervous activation and impaired endothelial function in young females. Am J Hypertens 2013, 26, 250–256. [Google Scholar] [CrossRef]
- Eikelis, N.; Lambert, E.A.; Phillips, S.; Sari, C.I.; Mundra, P.A.; Weir, J.M.; Huynh, K.; Grima, M.T.; Straznicky, N.E.; Dixon, J.B.; et al. Muscle sympathetic nerve activity Is associated with elements of the plasma lipidomic profile in young Asian adults. J Clin Endocrinol Metab 2017, 102, 2059–2068. [Google Scholar] [CrossRef]
- Sverrisdottir, Y.B.; Mogren, T.; Kataoka, J.; Janson, P.O.; Stener-Victorin, E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 2008, 294, E576–E581. [Google Scholar] [CrossRef]
- Lambert, E.A.; Teede, H.; Sari, C.I.; Jona, E.; Shorakae, S.; Woodington, K.; Hemmes, R.; Eikelis, N.; Straznicky, N.E.; De Courten, B.; et al. Sympathetic activation and endothelial dysfunction in polycystic ovary syndrome are not explained by either obesity or insulin resistance. Clinical endocrinology 2015, 83, 812–819. [Google Scholar] [CrossRef]
- Shorakae, S.; Jona, E.; de Courten, B.; Lambert, G.W.; Lambert, E.A.; Phillips, S.E.; Clarke, I.J.; Teede, H.J.; Henry, B.A. Brown adipose tissue thermogenesis in polycystic ovary syndrome. Clinical endocrinology 2019, 90, 425–432. [Google Scholar] [CrossRef]
- Shorakae, S.; Lambert, E.A.; Jona, E.; Ika Sari, C.; de Courten, B.; Dixon, J.B.; Lambert, G.W.; Teede, H.J. Effect of central sympathoinhibition with moxonidine on sympathetic nervous activity in polycystic ovary syndrome-A randomized controlled trial. Front Physiol 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.J.; Warnert, E.A.H.; Sverrisdottir, Y.; Wise, R.G.; Rees, D.A. Regional cerebral activation accompanies sympathoexcitation in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2019, 104, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Ayer, J.; Charakida, M.; Deanfield, J.E.; Celermajer, D.S. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J 2015, 36, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Woo, J.G.; Sinaiko, A.R.; Daniels, S.R.; Ikonen, J.; Juonala, M.; Kartiosuo, N.; Lehtimaki, T.; Magnussen, C.G.; Viikari, J.S.A.; et al. Childhood cardiovascular risk Factors and adult cardiovascular events. N Engl J Med 2022, 386, 1877–1888. [Google Scholar] [CrossRef]
- Goodman, E.; Dolan, L.M.; Morrison, J.A.; Daniels, S.R. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation 2005, 111, 1970–1977. [Google Scholar] [CrossRef]
- Esler, M.; Lambert, G.; Esler, D.; Ika Sari, C.; Guo, L.; Jennings, G. Evaluation of elevated heart rate as a sympathetic nervous system biomarker in essential hypertension. J Hypertens 2020, 38, 1488–1495. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Vanoli, J.; Facchetti, R.; Spaziani, D.; Mancia, G. Relationships between sympathetic markers and heart rate thresholds for cardiovascular risk in chronic heart failure. Clin Res Cardiol 2023, 112, 59–67. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; o'Hartaigh, B.; Janszky, I.; Romundstad, P.R.; Tonstad, S.; Vatten, L.J. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality - A systematic review and dose-response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis 2017, 27, 504–517. [Google Scholar] [CrossRef]
- Freitas Junior, I.F.; Monteiro, P.A.; Silveira, L.S.; Cayres, S.U.; Antunes, B.M.; Bastos, K.N.; Codogno, J.S.; Sabino, J.P.; Fernandes, R.A. Resting heart rate as a predictor of metabolic dysfunctions in obese children and adolescents. BMC Pediatr 2012, 12, 5. [Google Scholar] [CrossRef]
- Kwok, S.Y.; So, H.K.; Choi, K.C.; Lo, A.F.; Li, A.M.; Sung, R.Y.; Nelson, E.A. Resting heart rate in children and adolescents: association with blood pressure, exercise and obesity. Arch Dis Child 2013, 98, 287–291. [Google Scholar] [CrossRef]
- Rossi, R.C.; Vanderlei, L.C.; Goncalves, A.C.; Vanderlei, F.M.; Bernardo, A.F.; Yamada, K.M.; da Silva, N.T.; de Abreu, L.C. Impact of obesity on autonomic modulation, heart rate and blood pressure in obese young people. Auton Neurosci 2015, 193, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, F.; Sindhu, S.; Al Madhoun, A.; Ahmad, Z.; AlMekhled, D.; Azim, R.; Al-Kandari, S.; Wahid, M.A.; Al-Mulla, F.; Ahmad, R. Elevated resting heart rate as a predictor of inflammation and cardiovascular risk in healthy obese individuals. Sci Rep 2021, 11, 13883. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 2003, 177, 385–390. [Google Scholar] [CrossRef] [PubMed]
- O'Driscoll, D.M.; Horne, R.S.; Davey, M.J.; Hope, S.A.; Anderson, V.; Trinder, J.; Walker, A.M.; Nixon, G.M. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med 2011, 12, 483–488. [Google Scholar] [CrossRef]
- Cheng, E.T.W.; Chan, R.N.C.; Chan, K.C.C.; Au, C.T.; Li, A.M. Level of urinary catecholamine in children with sleep disordered breathing: A systematic review and meta-analysis. Sleep Med 2022, 100, 565–572. [Google Scholar] [CrossRef]
- Ji, J.; Wang, Y.; Chen, B.; Xu, X.; Lv, M. Is there an association between cognitive impairment and urinary adrenaline, norepinephrine, gamma-aminobutyric acid, and taurine levels in children with obstructive sleep apnea?: A case control study. BMC Pediatr 2025, 25, 156. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of adult disease. J Am Coll Nutr 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- Huxley, R.R.; Shiell, A.W.; Law, C.M. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens 2000, 18, 815–831. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Sachdev, H.S.; Fall, C.H.; Osmond, C.; Lakshmy, R.; Barker, D.J.; Biswas, S.K.; Ramji, S.; Prabhakaran, D.; Reddy, K.S. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004, 350, 865–875. [Google Scholar] [CrossRef]
- Mzayek, F.; Cruickshank, J.K.; Amoah, D.; Srinivasan, S.; Chen, W.; Berenson, G.S. Birth weight was longitudinally associated with cardiometabolic risk markers in mid-adulthood. Ann Epidemiol 2016, 26, 643–647. [Google Scholar] [CrossRef]
- Ijzerman, R.G.; Stehouwer, C.D.; de Geus, E.J.; van Weissenbruch, M.M.; Delemarre-van de Waal, H.A.; Boomsma, D.I. Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation 2003, 108, 566–571. [Google Scholar] [CrossRef]
- Weitz, G.; Deckert, P.; Heindl, S.; Struck, J.; Perras, B.; Dodt, C. Evidence for lower sympathetic nerve activity in young adults with low birth weight. J Hypertens 2003, 21, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Lambert, G.W. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3-4 months of age. J Hypertens 1999, 17, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Denton, K.M.; Flower, R.L.; Stevenson, K.M.; Anderson, W.P. Adult rabbit offspring of mothers with secondary hypertension have increased blood pressure. Hypertension 2003, 41, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, J.M.; Huskey, K.W.; Davis, R.B.; Wee, C.C. Successful weight loss among obese U.S. adults. Am J Prev Med 2012, 42, 481–485. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Colombo, M.; Bolla, G.; Cattaneo, B.M.; Cavagnini, F.; Mancia, G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 1998, 97, 2037–2042. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, E.A.; Lambert, G.W.; Masuo, K.; Esler, M.D.; Nestel, P.J. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab 2005, 90, 5998–6005. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, E.A.; Nestel, P.J.; McGrane, M.T.; Dawood, T.; Schlaich, M.P.; Masuo, K.; Eikelis, N.; de Courten, B.; Mariani, J.A.; et al. Sympathetic neural adaptation to hypocaloric diet with or without exercise training in obese metabolic syndrome subjects. Diabetes 2010, 59, 71–79. [Google Scholar] [CrossRef]
- Meredith, I.T.; Friberg, P.; Jennings, G.L.; Dewar, E.M.; Fazio, V.A.; Lambert, G.W.; Esler, M.D. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension 1991, 18, 575–582. [Google Scholar] [CrossRef]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraca, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: An overview of 12 systematic reviews and 149 studies. Obes Rev 2021, 22 Suppl 4, e13256. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Emadi, A.; Zargar, M.S.; Najafi, A. Aerobic exercise and weight loss in adults: A systematic review and dose-response meta-analysis. JAMA Netw Open 2024, 7, e2452185. [Google Scholar] [CrossRef] [PubMed]
- Straznicky, N.E.; Eikelis, N.; Nestel, P.J.; Dixon, J.B.; Dawood, T.; Grima, M.T.; Sari, C.I.; Schlaich, M.P.; Esler, M.D.; Tilbrook, A.J.; et al. Baseline sympathetic nervous system activity predicts dietary weight loss in obese metabolic syndrome subjects. J Clin Endocrinol Metab 2012, 97, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Straznicky, N.E.; Lambert, E.A.; Schlaich, M.P.; Lambert, G.W. Surgical approaches to the treatment of obesity. Nat Rev Gastroenterol Hepatol 2011, 8, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L.; Narbro, K.; Sjostrom, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007, 357, 741–752. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity 2013, 21 Suppl 1, S1–27. [Google Scholar] [CrossRef]
- Lambert, E.A.; Rice, T.; Eikelis, N.; Straznicky, N.E.; Lambert, G.W.; Head, G.A.; Hensman, C.; Schlaich, M.P.; Dixon, J.B. Sympathetic activity and markers of cardiovascular risk in nondiabetic severely obese patients: the effect of the initial 10% weight loss. Am J Hypertens 2014, 27, 1308–1315. [Google Scholar] [CrossRef]
- Seravalle, G.; Colombo, M.; Perego, P.; Giardini, V.; Volpe, M.; Dell'Oro, R.; Mancia, G.; Grassi, G. Long-term sympathoinhibitory effects of surgically induced weight loss in severe obese patients. Hypertension 2014, 64, 431–437. [Google Scholar] [CrossRef]
- Jenkins, Z.M.; Castle, D.J.; Eikelis, N.; Phillipou, A.; Lambert, G.W.; Lambert, E.A. Autonomic nervous system function in women with anorexia nervosa. Clin Auton Res 2022, 32, 29–42. [Google Scholar] [CrossRef]
- Gross, H.A.; Lake, C.R.; Ebert, M.H.; Ziegler, M.G.; Kopin, I.J. Catecholamine metabolism in primary anorexia nervosa. J Clin Endocrinol Metab 1979, 49, 805–809. [Google Scholar] [CrossRef]
- Sachs, K.V.; Harnke, B.; Mehler, P.S.; Krantz, M.J. Cardiovascular complications of anorexia nervosa: A systematic review. Int J Eat Disord 2016, 49, 238–248. [Google Scholar] [CrossRef]
- Zamboni, M.; Armellini, F.; Turcato, E.; Todisco, P.; Gallagher, D.; Dalle Grave, R.; Heymsfield, S.; Bosello, O. Body fat distribution before and after weight gain in anorexia nervosa. Int J Obes Relat Metab Disord 1997, 21, 33–36. [Google Scholar] [CrossRef] [PubMed]
- El Ghoch, M.; Calugi, S.; Lamburghini, S.; Dalle Grave, R. Anorexia nervosa and body fat distribution: a systematic review. Nutrients 2014, 6, 3895–3912. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; Walsh, B.T.; Pierson, R.N., Jr.; Heymsfield, S.B.; Gallagher, D.; Wang, J.; Parides, M.K.; Leibel, R.L.; Warren, M.P.; Killory, E.; et al. Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr 2005, 81, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Muller, T.D.; Holtkamp, K.; Herpertz-Dahlmann, B. The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 2007, 12, 23–35. [Google Scholar] [CrossRef]
- Prioletta, A.; Muscogiuri, G.; Sorice, G.P.; Lassandro, A.P.; Mezza, T.; Policola, C.; Salomone, E.; Cipolla, C.; Della Casa, S.; Pontecorvi, A.; et al. In anorexia nervosa, even a small increase in abdominal fat is responsible for the appearance of insulin resistance. Clinical endocrinology 2011, 75, 202–206. [Google Scholar] [CrossRef]
- Diba, P.; Sattler, A.L.; Korzun, T.; Habecker, B.A.; Marks, D.L. Unraveling the lost balance: Adrenergic dysfunction in cancer cachexia. Auton Neurosci 2024, 251, 103136. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 2014, 20, 433–447. [Google Scholar] [CrossRef]
- Xie, H.; Heier, C.; Meng, X.; Bakiri, L.; Pototschnig, I.; Tang, Z.; Schauer, S.; Baumgartner, V.J.; Grabner, G.F.; Schabbauer, G.; et al. An immune-sympathetic neuron communication axis guides adipose tissue browning in cancer-associated cachexia. Proc Natl Acad Sci U S A 2022, 119. [Google Scholar] [CrossRef]
- Laird, B.J.A.; Skipworth, R.; Bonomi, P.D.; Fallon, M.; Kaasa, S.; Giorgino, R.; McMillan, D.C.; Currow, D.C. Anamorelin efficacy in non-small-cell lung cancer patients with cachexia: InsightsfFrom ROMANA 1 and ROMANA 2. J Cachexia Sarcopenia Muscle 2025, 16, e13732. [Google Scholar] [CrossRef]
- Kaye, D.M.; Lefkovits, J.; Jennings, G.L.; Bergin, P.; Broughton, A.; Esler, M.D. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 1995, 26, 1257–1263. [Google Scholar] [CrossRef]
- Petersson, M.; Friberg, P.; Eisenhofer, G.; Lambert, G.; Rundqvist, B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J 2005, 26, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Joho, S.; Ushijima, R.; Nakagaito, M.; Kinugawa, K. Sympathetic overactivation predicts body weight loss in patients with heart failure. Auton Neurosci 2020, 223, 102625. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.A.; Esler, M.D.; Schlaich, M.P.; Dixon, J.; Eikelis, N.; Lambert, G.W. Obesity-associated organ damage and sympathetic nervous activity. Hypertension 2019, 73, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chen, J.; Wang, W.; Li, Q.; Yin, X.; Zhuang, G.; Zhou, H.; Zeng, W. Sympathetic nerve-enteroendocrine L cell communication modulates GLP-1 release, brain glucose utilization, and cognitive function. Neuron 2024, 112, 972–990. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Pauza, A.G.; Thakkar, P.; Tasic, T.; Felippe, I.; Bishop, P.; Greenwood, M.P.; Rysevaite-Kyguoliene, K.; Ast, J.; Broichhagen, J.; Hodson, D.J.; et al. GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ Res 2022, 130, 694–707. [Google Scholar] [CrossRef]
- Drucker, D.J. Efficacy and safety of GLP-1 medicines for type 2 diabetes and obesity. Diabetes Care 2024, 47, 1873–1888. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kaplan, L.M.; Frias, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L.; et al. Triple-hormone-receptor agonist retatrutide for obesity - A phase 2 trial. N Engl J Med 2023, 389, 514–526. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Kaplan, L.M.; Frias, J.P.; Brouwers, B.; Wu, Q.; Thomas, M.K.; Harris, C.; Schloot, N.C.; Du, Y.; Mather, K.J.; et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat Med 2024, 30, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Lawson, F.; Owens, D.; Raccah, D.; Roy-Duval, C.; Lehmann, A.; Perfetti, R.; Blonde, L. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Lubberding, A.F.; Veedfald, S.; Achter, J.S.; Nissen, S.D.; Soattin, L.; Sorrentino, A.; Vega, E.T.; Linz, B.; Eggertsen, C.H.E.; Mulvey, J.; et al. Glucagon-like peptide-1 increases heart rate by a direct action on the sinus node. Cardiovasc Res 2024, 120, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Charkoudian, N.; Andrews, C.N.; Camilleri, M.; Sletten, D.; Zinsmeister, A.R.; Low, P.A. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol 2008, 295, R874–R880. [Google Scholar] [CrossRef]
- Chao, E.C. SGLT-2 Inhibitors: A new mechanism for glycemic control. Clin Diabetes 2014, 32, 4–11. [Google Scholar] [CrossRef]
- Patel, S.M.; Kang, Y.M.; Im, K.; Neuen, B.L.; Anker, S.D.; Bhatt, D.L.; Butler, J.; Cherney, D.Z.I.; Claggett, B.L.; Fletcher, R.A.; et al. Sodium-glucose cotransporter-2 inhibitors and major adverse cardiovascular outcomes: A SMART-C collaborative meta-analysis. Circulation 2024, 149, 1789–1801. [Google Scholar] [CrossRef]
- Cheong, A.J.Y.; Teo, Y.N.; Teo, Y.H.; Syn, N.L.; Ong, H.T.; Ting, A.Z.H.; Chia, A.Z.Q.; Chong, E.Y.; Chan, M.Y.; Lee, C.H.; et al. SGLT inhibitors on weight and body mass: A meta-analysis of 116 randomized-controlled trials. Obesity 2022, 30, 117–128. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-induced sympathoinhibition: A novel mechanism for cardiorenal protection. JACC Basic Transl Sci 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Oshima, N.; Onimaru, H.; Yamashiro, A.; Goto, H.; Tanoue, K.; Fukunaga, T.; Sato, H.; Uto, A.; Matsubara, H.; Imakiire, T.; et al. SGLT2 and SGLT1 inhibitors suppress the activities of the RVLM neurons in newborn Wistar rats. Hypertens Res 2024, 47, 46–54. [Google Scholar] [CrossRef]
- Nguyen, T.; Wen, S.; Gong, M.; Yuan, X.; Xu, D.; Wang, C.; Jin, J.; Zhou, L. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting SGLT-2 in mice. Diabetes Metab Syndr Obes 2020, 13, 2781–2799. [Google Scholar] [CrossRef]
- Jordan, J.; Tank, J.; Heusser, K.; Heise, T.; Wanner, C.; Heer, M.; Macha, S.; Mattheus, M.; Lund, S.S.; Woerle, H.J.; et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J Am Soc Hypertens 2017, 11, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Heusser, K.; Tank, J.; Diedrich, A.; Fischer, A.; Heise, T.; Jordan, J. Randomized trial comparing SGLT2 inhibition and hydrochlorothiazide on sympathetic traffic in type 2 diabetes. Kidney Int Rep 2023, 8, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Strack, A.M.; Sawyer, W.B.; Platt, K.B.; Loewy, A.D. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res 1989, 491, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Dampney, R.A.; Polson, J.W.; Potts, P.D.; Hirooka, Y.; Horiuchi, J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol 2003, 23, 597–616. [Google Scholar] [CrossRef]
- Lindberg, D.; Chen, P.; Li, C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J Comp Neurol 2013, 521, 3167–3190. [Google Scholar] [CrossRef]
- Van Huysse, J.W.; Bealer, S.L. Central nervous system norepinephrine release during hypotension and hyperosmolality in conscious rats. Am J Physiol 1991, 260, R1071–R1076. [Google Scholar] [CrossRef]
- Huangfu, D.H.; Koshiya, N.; Guyenet, P.G. A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am J Physiol 1991, 261, R393–R402. [Google Scholar] [CrossRef]
- Singewald, N.; Philippu, A. Catecholamine release in the locus coeruleus is modified by experimentally induced changes in haemodynamics. Naunyn Schmiedebergs Arch Pharmacol 1993, 347, 21–27. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Lambert, G.; Secher, N.H.; Raven, P.B.; van Lieshout, J.; Esler, M.D. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J Physiol 2009, 587, 2589–2597. [Google Scholar] [CrossRef]
- Foote, S.L.; Bloom, F.E.; Aston-Jones, G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 1983, 63, 844–914. [Google Scholar] [CrossRef]
- Hauglund, N.L.; Andersen, M.; Tokarska, K.; Radovanovic, T.; Kjaerby, C.; Sorensen, F.L.; Bojarowska, Z.; Untiet, V.; Ballestero, S.B.; Kolmos, M.G.; et al. Norepinephrine-mediated slow vasomotion drives glymphatic clearance during sleep. Cell 2025, 188, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Macefield, V.G.; Henderson, L.A. Real-time imaging of the medullary circuitry involved in the generation of spontaneous muscle sympathetic nerve activity in awake subjects. Hum Brain Mapp 2010, 31, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Macefield, V.G.; Henderson, L.A. Identification of the human sympathetic connectome involved in blood pressure regulation. Neuroimage 2019, 202, 116119. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Macefield, V.G.; Henderson, L.A. Real-time imaging of cortical and subcortical control of muscle sympathetic nerve activity in awake human subjects. Neuroimage 2013, 70, 59–65. [Google Scholar] [CrossRef]
- Wang, G.-J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain dopamine and obesity. The Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Carnell, S.; Gibson, C.; Benson, L.; Ochner, C.N.; Geliebter, A. Neuroimaging and obesity: current knowledge and future directions. 2012, 13, 43–56. [CrossRef]
- Zhang, P.; Wu, G.W.; Yu, F.X.; Liu, Y.; Li, M.Y.; Wang, Z.; Ding, H.Y.; Li, X.S.; Wang, H.; Jin, M.; et al. Abnormal regional neural activity and reorganized neural network in obesity: Evidence from resting-state fMRI. Obesity 2020, 28, 1283–1291. [Google Scholar] [CrossRef]
- Gómez-Apo, E.; Mondragón-Maya, A.; Ferrari-Díaz, M.; Silva-Pereyra, J. Structural brain changes associated with overweight and obesity. J Obes 2021, Jul 16, 6613385. [Google Scholar] [CrossRef]
- Li, G.; Hu, Y.; Zhang, W.; Wang, J.; Ji, W.; Manza, P.; Volkow, N.D.; Zhang, Y.; Wang, G.J. Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions. Mol Psychiatry 2023, 28, 1466–1479. [Google Scholar] [CrossRef]
- Patel, M.; Braun, J.; Lambert, G.W.; Kameneva, T.; Keatch, C.; Lambert, E. Central mechanisms in sympathetic nervous dysregulation in obesity. J Neurophysiol 2023. [Google Scholar] [CrossRef]
- Tadross, J.A.; Steuernagel, L.; Dowsett, G.K.C.; Kentistou, K.A.; Lundh, S.; Porniece, M.; Klemm, P.; Rainbow, K.; Hvid, H.; Kania, K.; et al. A comprehensive spatio-cellular map of the human hypothalamus. Nature 2025. [Google Scholar] [CrossRef]
- Buonfiglio, D.; Tchio, C.; Furigo, I.; Donato, J., Jr.; Baba, K.; Cipolla-Neto, J.; Tosini, G. Removing melatonin receptor type 1 signaling leads to selective leptin resistance in the arcuate nucleus. J Pineal Res 2019, 67, e12580. [Google Scholar] [CrossRef] [PubMed]
- Enriori, P.J.; Sinnayah, P.; Simonds, S.E.; Garcia Rudaz, C.; Cowley, M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci 2011, 31, 12189–12197. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; Fontes, M.A.; Killinger, S.; Pawlak, D.B.; Polson, J.W.; Dampney, R.A. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension 2003, 42, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.R.; Miller, J.W.; Keogh, J.M.; Henning, E.; Satterwhite, J.H.; Cameron, G.S.; Astruc, B.; Mayer, J.P.; Brage, S.; See, T.C.; et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 2009, 360, 44–52. [Google Scholar] [CrossRef]
- Lim, K.; Barzel, B.; Burke, S.L.; Armitage, J.A.; Head, G.A. Origin of aberrant blood pressure and sympathetic regulation in diet-induced obesity. Hypertension 2016, 68, 491–500. [Google Scholar] [CrossRef]
- Eikelis, N.; Lambert, G.; Wiesner, G.; Kaye, D.; Schlaich, M.; Morris, M.; Hastings, J.; Socratous, F.; Esler, M. Extra-adipocyte leptin release in human obesity and its relation to sympathoadrenal function. Am J Physiol Endocrinol Metab 2004, 286, E744–E752. [Google Scholar] [CrossRef]
- Myers, M.G., Jr.; Affinati, A.H.; Richardson, N.; Schwartz, M.W. Central nervous system regulation of organismal energy and glucose homeostasis. Nat Metab 2021, 3, 737–750. [Google Scholar] [CrossRef]
- Sewaybricker, L.E.; Huang, A.; Chandrasekaran, S.; Melhorn, S.J.; Schur, E.A. The significance of hypothalamic inflammation and gliosis for the pathogenesis of obesity in humans. Endocr Rev 2023, 44, 281–296. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, G.W.; Masuo, K.; Dawood, T.; Eikelis, N.; Nestel, P.J.; McGrane, M.T.; Mariani, J.A.; Socratous, F.; Chopra, R.; et al. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am J Clin Nutr 2009, 89, 27–36. [Google Scholar] [CrossRef]
- Guimaraes, P.S.; Huber, D.A.; Campagnole-Santos, M.J.; Schreihofer, A.M. Development of attenuated baroreflexes in obese Zucker rats coincides with impaired activation of nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 2014, 306, R681–R692. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Asirvatham-Jeyaraj, N.; Monteiro, R.; Sivasubramanian, M.K.; Hall, D.; Subramanian, M. Obesity-induced sympathoexcitation is associated with Nrf2 dysfunction in the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 2020, 318, R435–R444. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Ogawa, K.; Konno, S.; Sunagawa, K. Calorie restriction inhibits sympathetic nerve activity via anti-oxidant effect in the rostral ventrolateral medulla of obesity-induced hypertensive rats. Clin Exp Hypertens 2011, 33, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.A.; Sari, C.I.; Eikelis, N.; Phillips, S.E.; Grima, M.; Straznicky, N.E.; Dixon, J.B.; Esler, M.; Schlaich, M.P.; Head, G.A.; et al. Effects of moxonidine and low-calorie diet: Cardiometabolic benefits from combination of both therapies. Obesity 2017, 25, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, Y.; Zhang, W.; Ding, Y.; Wang, Y.; Wang, J.; He, Y.; Lv, G.; von Deneen, K.M.; Zhao, Y.; et al. Resting activity of the hippocampus and amygdala in obese individuals predicts their response to food cues. Addict Biol 2021, 26, e12974. [Google Scholar] [CrossRef]
- Li, G.; Ji, G.; Hu, Y.; Liu, L.; Jin, Q.; Zhang, W.; Liu, L.; Wang, Y.; Zhao, J.; von Deneen, K.M.; et al. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrinology 2019, 100, 229–236. [Google Scholar] [CrossRef]
- Lambert, E.; Lambert, G.; Ika-Sari, C.; Dawood, T.; Lee, K.; Chopra, R.; Straznicky, N.; Eikelis, N.; Drew, S.; Tilbrook, A.; et al. Ghrelin modulates sympathetic nervous system activity and stress response in lean and overweight men. Hypertension 2011, 58, 43–50. [Google Scholar]
- Wang, Y.; Ji, G.; Hu, Y.; Li, G.; Ding, Y.; Hu, C.; Liu, L.; Zhang, W.; von Deneen, K.M.; Han, Y.; et al. Laparoscopic sleeve gastrectomy induces sustained changes in gray and white matter brain volumes and resting functional connectivity in obese patients. Surg Obes Relat Dis 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Custers, E.; Vreeken, D.; Kleemann, R.; Kessels, R.P.C.; Duering, M.; Brouwer, J.; Aufenacker, T.J.; Witteman, B.P.L.; Snabel, J.; Gart, E.; et al. Long-term brain structure and cognition following bariatric surgery. JAMA Netw Open 2024, 7, e2355380. [Google Scholar] [CrossRef]
- Simon, J.J.; Becker, A.; Sinno, M.H.; Skunde, M.; Bendszus, M.; Preissl, H.; Enck, P.; Herzog, W.; Friederich, H.C. Neural food reward processing in successful and unsuccessful weight maintenance. Obesity 2018, 26, 895–902. [Google Scholar] [CrossRef]
- Alhadeff, A.L.; Rupprecht, L.E.; Hayes, M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012, 153, 647–658. [Google Scholar] [CrossRef]
- Rinaman, L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 2010, 1350, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 receptor agonist-induced weight loss: A review of central and peripheral pathways in appetite and energy regulation. Am J Med 2025. [Google Scholar] [CrossRef]
- Suda, Y.; Nakamura, K.; Matsuyama, F.; Hamada, Y.; Makabe, H.; Narita, M.; Nagumo, Y.; Mori, T.; Kuzumaki, N.; Narita, M. Peripheral-central network analysis of cancer cachexia status accompanied by the polarization of hypothalamic microglia with low expression of inhibitory immune checkpoint receptors. Mol Brain 2024, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.; Bernardoni, F.; Batury, V.L.; Bahnsen, K.; Lariviere, S.; Abbate-Daga, G.; Andres-Perpina, S.; Bang, L.; Bischoff-Grethe, A.; Brooks, S.J.; et al. Brain structure in acutely underweight and partially weight-restored individuals with anorexia nervosa: A coordinated analysis by the ENIGMA Eating Disorders Working Group. Biol Psychiatry 2022, 92, 730–738. [Google Scholar] [CrossRef]
- Papassotiriou, I.; Spiliopoulou, S.; Dragonas, D.; Tsoutsoura, N.; Korompoki, E.; Manios, E. The relation between body mass index and target organ damage and the mediating role of blood pressure. Eur J Clin Nutr 2025. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-Garcia, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol 2023, 20, 475–494. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Alvarez, G.E.; Beske, S.D.; Ballard, T.P.; Davy, K.P. Sympathetic neural activation in visceral obesity. Circulation 2002, 106, 2533–2536. [Google Scholar] [CrossRef]
- Hillebrand, S.; de Mutsert, R.; Christen, T.; Maan, A.C.; Jukema, J.W.; Lamb, H.J.; de Roos, A.; Rosendaal, F.R.; den Heijer, M.; Swenne, C.A.; et al. Body fat, especially visceral fat, is associated with electrocardiographic measures of sympathetic activation. Obesity 2014, 22, 1553–1559. [Google Scholar] [CrossRef]
- Flores-Opazo, M.; Kopinke, D.; Helmbacher, F.; Fernandez-Verdejo, R.; Tunon-Suarez, M.; Lynch, G.S.; Contreras, O. Fibro-adipogenic progenitors in physiological adipogenesis and intermuscular adipose tissue remodeling. Mol Aspects Med 2024, 97, 101277. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Grima, M.T.; Eikelis, N.; Nestel, P.J.; Dawood, T.; Schlaich, M.P.; Chopra, R.; Masuo, K.; Esler, M.D.; Sari, C.I.; et al. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J Clin Endocrinol Metab 2011, 96, E503–508. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Maretich, P.; Kajimura, S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Yahagi, N. Hepatic control of energy metabolism via the autonomic nervous system. J Atheroscler Thromb 2017, 24, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Liu, Y.; Shrestha, Y.B.; Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 2014, 35, 473–493. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Vaughan, C.H.; Zarebidaki, E.; Ehlen, J.C.; Bartness, T.J. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol 2014, 537, 199–225. [Google Scholar] [CrossRef]
- Admiraal, W.M.; Holleman, F.; Bahler, L.; Soeters, M.R.; Hoekstra, J.B.; Verberne, H.J. Combining 123I-metaiodobenzylguanidine SPECT/CT and 18F-FDG PET/CT for the assessment of brown adipose tissue activity in humans during cold exposure. J Nucl Med 2013, 54, 208–212. [Google Scholar] [CrossRef]
- Green, A.L.; Bagci, U.; Hussein, S.; Kelly, P.V.; Muzaffar, R.; Neuschwander-Tetri, B.A.; Osman, M.M. Brown adipose tissue detected by PET/CT imaging is associated with less central obesity. Nucl Med Commun 2017, 38, 629–635. [Google Scholar] [CrossRef]
- Carey, A.L.; Formosa, M.F.; Van Every, B.; Bertovic, D.; Eikelis, N.; Lambert, G.W.; Kalff, V.; Duffy, S.J.; Cherk, M.H.; Kingwell, B.A. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 2013, 56, 147–155. [Google Scholar] [CrossRef]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Bahler, L.; Verberne, H.J.; Admiraal, W.M.; Stok, W.J.; Soeters, M.R.; Hoekstra, J.B.; Holleman, F. Differences in sympathetic nervous stimulation of brown adipose tissue between the young and old, and the lean and obese. J Nucl Med 2016, 57, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The beta3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest 2020, 130, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Minocci, A.; Savia, G.; Lucantoni, R.; Berselli, M.E.; Tagliaferri, M.; Calo, G.; Petroni, M.L.; de Medici, C.; Viberti, G.C.; Liuzzi, A. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord 2000, 24, 1139–1144. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Peskind, E.; Raskind, M.; Boyko, E.J.; Porte, D., Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 1996, 2, 589–593. [Google Scholar] [CrossRef]
- Rahmouni, K. Leptin-induced sympathetic nerve activation: Signaling mechanisms and cardiovascular consequences in obesity. Curr Hypertens Rev 2010, 6, 104–209. [Google Scholar] [CrossRef]
- Rahmouni, K.; Morgan, D.A. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 2007, 49, 647–652. [Google Scholar] [CrossRef]
- Rumantir, M.S.; Vaz, M.; Jennings, G.L.; Collier, G.; Kaye, D.M.; Seals, D.R.; Wiesner, G.H.; Brunner-La Rocca, H.P.; Esler, M.D. Neural mechanisms in human obesity-related hypertension. J Hypertens 1999, 17, 1125–1133. [Google Scholar] [CrossRef]
- Grassi, G.; Colombo, M.; Seravalle, G.; Spaziani, D.; Mancia, G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension 1998, 31, 64–67. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suarez, V.J.; Redondo-Florez, L.; Beltran-Velasco, A.I.; Martin-Rodriguez, A.; Martinez-Guardado, I.; Navarro-Jimenez, E.; Laborde-Cardenas, C.C.; Tornero-Aguilera, J.F. The role of adipokines in health and disease. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Sommer, M.; Strobel, S.; Thrum, S.; Bluher, M.; Wagner, U.; Rossol, M. Perturbation of the monocyte compartment in human obesity. Front Immunol 2019, 10, 1874. [Google Scholar] [CrossRef] [PubMed]
- Larabee, C.M.; Neely, O.C.; Domingos, A.I. Obesity: A neuroimmunometabolic perspective. Nat Rev Endocrinol 2020, 16, 30–43. [Google Scholar] [CrossRef]
- Huang, F.; Del-Rio-Navarro, B.E.; Leija-Martinez, J.; Torres-Alcantara, S.; Ruiz-Bedolla, E.; Hernandez-Cadena, L.; Barraza-Villarreal, A.; Romero-Nava, R.; Sanchez-Munoz, F.; Villafana, S.; et al. Effect of omega-3 fatty acids supplementation combined with lifestyle intervention on adipokines and biomarkers of endothelial dysfunction in obese adolescents with hypertriglyceridemia. J Nutr Biochem 2019, 64, 162–169. [Google Scholar] [CrossRef]
- Akiyoshi, H.; Gonda, T.; Terada, T. A comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig, dog and human livers. Liver 1998, 18, 352–359. [Google Scholar] [CrossRef]
- Jarhult, J.; Falck, B.; Ingemansson, S.; Nobin, A. The functional importance of sympathetic nerves to the liver and endocrine pancreas. Ann Surg 1979, 189, 96–100. [Google Scholar] [CrossRef]
- Mizuno, K.; Ueno, Y. Autonomic nervous system and the liver. Hepatol Res 2017, 47, 160–165. [Google Scholar] [CrossRef]
- Basu, R.; Chandramouli, V.; Dicke, B.; Landau, B.; Rizza, R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 2005, 54, 1942–1948. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Grima, M.T.; Sari, C.I.; Eikelis, N.; Lambert, E.A.; Nestel, P.J.; Esler, M.D.; Dixon, J.B.; Chopra, R.; Tilbrook, A.J.; et al. Neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes 2012, 61, 2506–2516. [Google Scholar] [CrossRef]
- Huggett, R.J.; Scott, E.M.; Gilbey, S.G.; Stoker, J.B.; Mackintosh, A.F.; Mary, D.A. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation 2003, 108, 3097–3101. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Takamura, T.; Takeshita, Y.; Ryu, Y.; Misu, H.; Ota, T.; Tokuyama, K.; Nagasaka, S.; Matsuhisa, M.; Matsui, O.; et al. Ectopic fat accumulation and distant organ-specific insulin resistance in Japanese people with nonalcoholic fatty liver disease. PLoS One 2014, 9, e92170. [Google Scholar] [CrossRef] [PubMed]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J Physiol 2019, 597, 4565–4580. [Google Scholar] [CrossRef] [PubMed]
- Hampton, R.F.; Jimenez-Gonzalez, M.; Stanley, S.A. Unravelling innervation of pancreatic islets. Diabetologia 2022, 65, 1069–1084. [Google Scholar] [CrossRef]
- Almaca, J.; Weitz, J.; Rodriguez-Diaz, R.; Pereira, E.; Caicedo, A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 2018, 27, 630–644. [Google Scholar] [CrossRef]
- Mateus Goncalves, L.; Almaca, J. Functional characterization of the human islet microvasculature using living pancreas slices. Front Endocrinol (Lausanne) 2020, 11, 602519. [Google Scholar] [CrossRef]
- Sakamoto, K.; Butera, M.A.; Zhou, C.; Maurizi, G.; Chen, B.; Ling, L.; Shawkat, A.; Patlolla, L.; Thakker, K.; Calle, V.; et al. Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity. Cell Metab 2025, 37, 121–137. [Google Scholar] [CrossRef]
- Scherrer, U.; Sartori, C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation 1997, 96, 4104–4113. [Google Scholar] [CrossRef]
- Roatta, S.; Farina, D. Sympathetic actions on the skeletal muscle. Exerc Sport Sci Rev 2010, 38, 31–35. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 Suppl 2, S157–163. [Google Scholar] [CrossRef]
- Eichmann, A.; Brunet, I. Arterial innervation in development and disease. Sci Transl Med 2014, 6, 252ps259. [Google Scholar] [CrossRef] [PubMed]
- Straka, T.; Vita, V.; Prokshi, K.; Horner, S.J.; Khan, M.M.; Pirazzini, M.; Williams, M.P.I.; Hafner, M.; Zaglia, T.; Rudolf, R. Postnatal development and distribution of sympathetic innervation in mouse skeletal muscle. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Delbono, O.; Rodrigues, A.C.Z.; Bonilla, H.J.; Messi, M.L. The emerging role of the sympathetic nervous system in skeletal muscle motor innervation and sarcopenia. Ageing Res Rev 2021, 67, 101305. [Google Scholar] [CrossRef] [PubMed]
- Bacurau, A.V.; Jardim, M.A.; Ferreira, J.C.; Bechara, L.R.; Bueno, C.R., Jr.; Alba-Loureiro, T.C.; Negrao, C.E.; Casarini, D.E.; Curi, R.; Ramires, P.R.; et al. Sympathetic hyperactivity differentially affects skeletal muscle mass in developing heart failure: role of exercise training. J Appl Physiol (1985) 2009, 106, 1631–1640. [Google Scholar] [CrossRef]
- Fonseca, G.; Santos, M.R.D.; Souza, F.R.; Costa, M.; Haehling, S.V.; Takayama, L.; Pereira, R.M.R.; Negrao, C.E.; Anker, S.D.; Alves, M. Sympatho-vagal imbalance is associated with sarcopenia in male patients with heart failure. Arq Bras Cardiol 2019, 112, 739–746. [Google Scholar] [CrossRef]
- Rodrigues, A.C.Z.; Messi, M.L.; Wang, Z.M.; Abba, M.C.; Pereyra, A.; Birbrair, A.; Zhang, T.; O'Meara, M.; Kwan, P.; Lopez, E.I.S.; et al. The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta Physiol (Oxf) 2019, 225, e13195. [Google Scholar] [CrossRef]
- Seals, D.R.; Esler, M.D. Human ageing and the sympathoadrenal system. J Physiol 2000, 528, 407–417. [Google Scholar] [CrossRef]
- Esler, M.D.; Turner, A.G.; Kaye, D.M.; Thompson, J.M.; Kingwell, B.A.; Morris, M.; Lambert, G.W.; Jennings, G.L.; Cox, H.S.; Seals, D.R. Aging effects on human sympathetic neuronal function. Am J Physiol 1995, 268, R278–R285. [Google Scholar] [CrossRef]
- Esler, M.D.; Thompson, J.M.; Kaye, D.M.; Turner, A.G.; Jennings, G.L.; Cox, H.S.; Lambert, G.W.; Seals, D.R. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation 1995, 91, 351–358. [Google Scholar] [CrossRef]
- Kaye, D.M.; Vaddadi, G.; Gruskin, S.L.; Du, X.J.; Esler, M.D. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ Res 2000, 86, E80–E84. [Google Scholar] [CrossRef]
- Iseki, K.; Ikemiya, Y.; Kinjo, K.; Inoue, T.; Iseki, C.; Takishita, S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 2004, 65, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; McCulloch, C.E.; Iribarren, C.; Darbinian, J.; Go, A.S. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006, 144, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chronic Kidney Disease Prognosis, C.; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Tu, J.; Chen, H.; Zeng, Q.; Chen, L.; Guo, Y.; Chen, K. Trends in obesity prevalence among adults with hypertension in the United States, 2001 to 2023. Hypertension 2025, 82, 498–508. [Google Scholar] [CrossRef]
- Lambert, E.; Sari, C.I.; Dawood, T.; Nguyen, J.; McGrane, M.; Eikelis, N.; Chopra, R.; Wong, C.; Chatzivlastou, K.; Head, G.; et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension 2010, 56, 351–358. [Google Scholar]
- Chagnac, A.; Zingerman, B.; Rozen-Zvi, B.; Herman-Edelstein, M. Consequences of glomerular hyperfiltration: The role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 2019, 143, 38–42. [Google Scholar] [CrossRef]
- Agarwal, R.; Fouque, D. The foundation and the four pillars of treatment for cardiorenal protection in people with chronic kidney disease and type 2 diabetes. Nephrol Dial Transplant 2023, 38, 253–257. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N Engl J Med 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Wong, C.Y.; O'Moore-Sullivan, T.; Leano, R.; Byrne, N.; Beller, E.; Marwick, T.H. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004, 110, 3081–3087. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Kaye, D.M.; Lambert, E.; Hastings, J.; Campbell, D.J.; Lambert, G.; Esler, M.D. Angiotensin II and norepinephrine release: interaction and effects on the heart. J Hypertens 2005, 23, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Schmermund, A.; Lerman, L.O.; Ritman, E.L.; Rumberger, J.A. Cardiac production of angiotensin II and its pharmacologic inhibition: effects on the coronary circulation. Mayo Clin Proc 1999, 74, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Ball, S.G.; Worthy, G.; Struthers, A.D.; Mary, D.A.; Greenwood, J.P. Hypertensive left ventricular hypertrophy: a mechanistic approach to optimizing regression assessed by cardiovascular magnetic resonance. J Hypertens 2012, 30, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.Y.; Climie, R.E.; Shu, M.; Grieve, S.M.; Kozor, R.; Figtree, G.A. Vascular aging and cardiovascular disease: pathophysiology and measurement in the coronary arteries. Front Cardiovasc Med 2023, 10, 1206156. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Oren, A.; Vos, L.E.; Uiterwaal, C.S.; Grobbee, D.E.; Bots, M.L. Aortic stiffness and carotid intima-media thickness: two independent markers of subclinical vascular damage in young adults? Eur J Clin Invest 2003, 33, 949–954. [Google Scholar] [CrossRef]
- Sverrisdottir, Y.B.; Jansson, L.M.; Hagg, U.; Gan, L.M. Muscle sympathetic nerve activity is related to a surrogate marker of endothelial function in healthy individuals. PLoS ONE 2010, 5, e9257. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Sert-Kuniyoshi, F.H.; Sierra-Johnson, J.; Orban, M.; Gami, A.; Davison, D.; Singh, P.; Pusalavidyasagar, S.; Huyber, C.; Votruba, S.; et al. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J Am Coll Cardiol 2010, 56, 662–666. [Google Scholar] [CrossRef]
- Huang, W.; Gan, Z.; Gao, Z.; Lin, Q.; Li, X.; Xie, W.; Gao, Z.; Zhou, Z.; Qiu, Z.; Qiu, W.; et al. Discrepancies between general and central obesity in arterial stiffness: observational studies and Mendelian randomization study. BMC Med 2024, 22, 325. [Google Scholar] [CrossRef]
- Lambert, E.A.; Phillips, S.; Belski, R.; Tursunalieva, A.; Eikelis, N.; Sari, C.I.; Dixon, J.B.; Straznicky, N.; Grima, M.; Head, G.A.; et al. Endothelial function in healthy young individuals Is associated with dietary consumption of saturated fat. Front Physiol 2017, 8, 876. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol Rev 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.M.; Charlton, P.H.; Grillo, A.; et al. Vascular ageing: moving from bench towards bedside. Eur J Prev Cardiol 2023, 30, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, S.W.; Luehrs, R.E.; DuBose, L.; Collins, M.T.; Wooldridge, N.A.; Stroud, A.K.; Fadel, P.J.; Abboud, F.M.; Pierce, G.L. Elevated muscle sympathetic nerve activity contributes to central artery stiffness in young and middle-age/older adults. Hypertension 2019, 73, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




