The patient, after 6 months of follow-up, is still in complete remission, under immunotherapy treatment, with no signs or symptoms of immunotherapy-related toxicity. Xeroderma pigmentosum (XP) is a rare autosomal recessive genetic disorder characterized by extreme sensitivity to UV light [
1]. The underlying defect involves germline biallelic loss-of-function mutations in genes of the nucleotide excision repair (NER) pathway, leading to an accumulation of unrepaired DNA damage [
2,
3,
4,
5]. Consequently, XP patients are highly predisposed to developing multiple cutaneous malignancies of different histological types [
6,
7]. Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor expressed on lymphocytes, and its ligands, PD-L1 and PD-L2, inhibit immune activation, promoting self-tolerance [
8,
9]. Tumor-infiltrating lymphocytes often exhibit high levels of PD-1, while cancer cells and elements of the tumor microenvironment frequently express PD-L1. This mechanism enables tumors to evade immune surveillance by suppressing T-cell activity. Given this, immune checkpoint inhibitors targeting PD-1/PD-L1 have emerged as an effective therapeutic strategy, restoring T-cell activation and triggering an anti-tumor immune response. Moreover, studies have demonstrated a correlation between response to checkpoint blockade therapy and tumor mutational burden (TMB) [
10,
11]. Since XP patients exhibit a severe DNA repair deficiency, it is hypothesized that their tumors harbor an elevated mutational load, potentially making them more responsive to immune checkpoint blockade. In this case, we administered nivolumab to a patient with metastatic localizations under the assumption that immune checkpoint inhibitors could enhance endogenous immune responses and that the high mutational burden associated with XP might be a predictive factor for response. Consistently, the patient demonstrated a remarkable therapeutic outcome, suggesting that the intrinsic DNA repair defect in XP may contribute to increased immunogenicity of the tumor, favoring sensitivity to PD-1 blockade. Notably, [
18F]FDG PET/CT imaging has been rarely utilized in XP patients, although its application in metastatic melanoma is increasing [
12,
13,
14,
15]. In our case, [
18F]FDG PET/CT played a crucial role in both staging and treatment monitoring. The decision to perform [
18F]FDG PET/CT, although not standard in XP management, was justified by indeterminate thoracic findings on CT. Pre-treatment PET/CT facilitated a more precise staging by identifying a previously unrecognized hypermetabolic lesion in the parotid gland, refining the assessment of disease burden. Additionally, PET/CT proved instrumental in evaluating the patient’s response to immunotherapy, demonstrating complete metabolic resolution of both the parotid and pulmonary lesions. This highlights the potential utility of [
18F]FDG PET/CT in XP patients with ambiguous radiological findings, particularly in guiding therapeutic decisions and monitoring treatment efficacy. In conclusion, this XP patient showed a remarkable response to nivolumab, reinforcing the potential impact of checkpoint inhibitors in this unique genetic context. We propose that the DNA repair deficiency in XP may lead to a heightened mutational load, enhancing tumor immunogenicity and responsiveness to PD-1 blockade. To validate this hypothesis, further studies should evaluate the immune landscape and mutational burden of XP-associated tumors. Additionally, identifying recurrent mutational patterns in different XP-related skin malignancies may provide insights into factors influencing sensitivity or resistance to nivolumab. Our experience underscores the need for further investigation into the optimal therapeutic strategies for XP-associated malignancies. While immune checkpoint inhibition appears promising, integrating advanced imaging modalities such as FDG PET/CT into routine clinical practice may enhance disease assessment, ultimately improving patient management and outcomes.
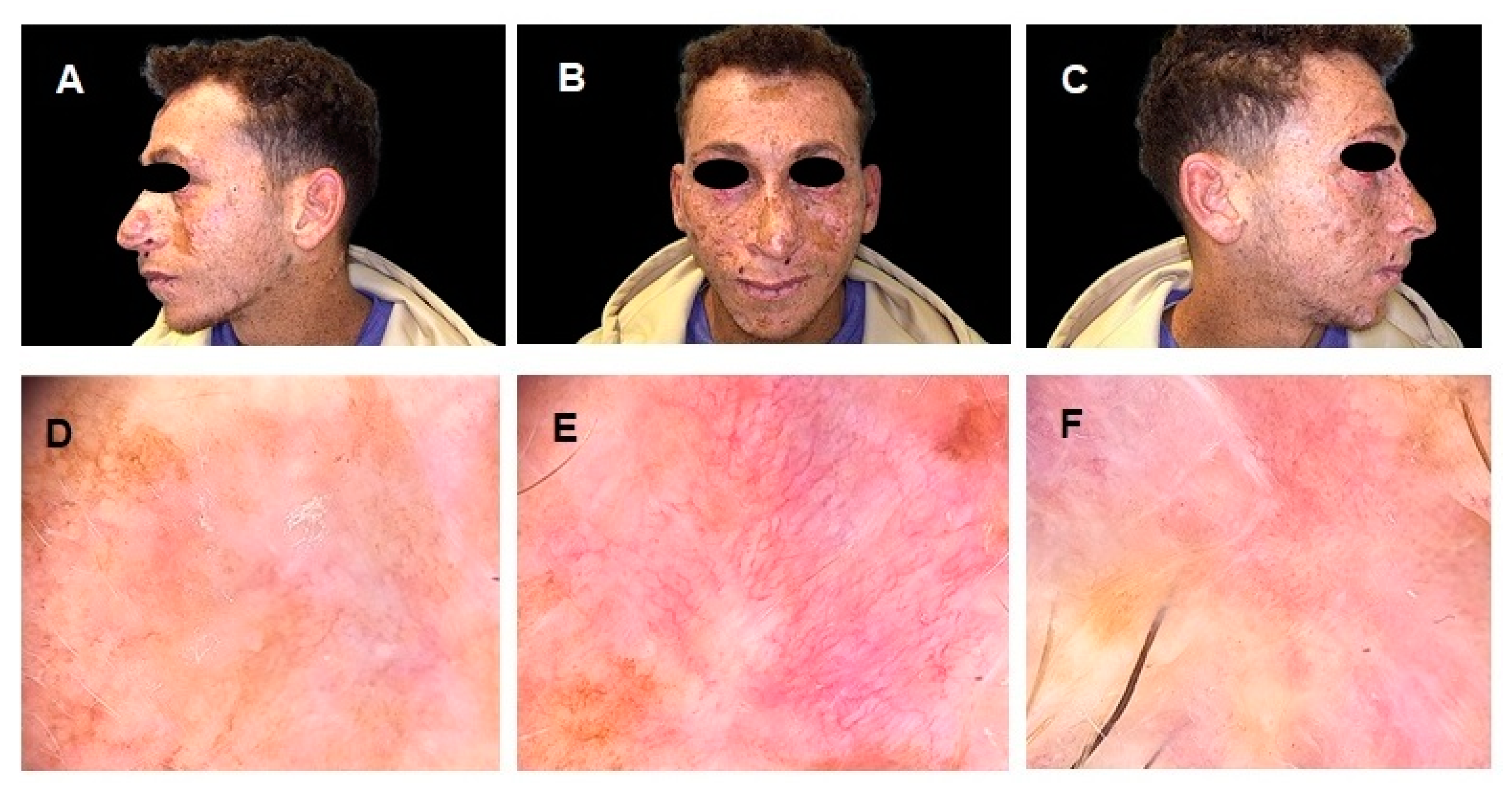
Figure 1.
(A–C) Clinical images of a 26-year-old Egyptian male with a history of Xeroderma Pigmentosum (XP), a rare autosomal recessive disorder characterized by extreme sensitivity to ultraviolet (UV) radiation, leading to early-onset cutaneous malignancies. The patient exhibits multiple pigmented lesions, extensive freckling, and actinic damage, particularly in sun-exposed areas. (D–F) Dermoscopic images of different skin malignancies. Image (D) shows a Superficial Spreading Melanoma (SSM), displaying an irregular pigment network, asymmetry, multiple colors, and regression structures. Image (E) depicts a Basal Cell Carcinoma (BCC), characterized by arborizing vessels, a pink background, and superficial scaling. Image (F) illustrates a Squamous Cell Carcinoma (SCC), presenting a central keratinous core, ulceration, and peripheral erythema, indicative of an advanced lesion. The SSM was surgically excised and histologically confirmed, while the BSC and SCC were deemed unresectable. Given the complexity and rarity of the patient’s clinical condition, further diagnostic examinations were conducted, including ultrasound of the head and neck lymph nodes, both of which were negative for metastatic recurrence. However, a chest CT scan revealed a nodular formation with a maximum diameter of 16 mm in the lower lobe of the left lung, suspected to be a metastatic lesion. To achieve a metabolic characterization of the lung nodule, a PET/CT scan with 18F-fluorodeoxyglucose ([18F]FDG) was requested.
Figure 1.
(A–C) Clinical images of a 26-year-old Egyptian male with a history of Xeroderma Pigmentosum (XP), a rare autosomal recessive disorder characterized by extreme sensitivity to ultraviolet (UV) radiation, leading to early-onset cutaneous malignancies. The patient exhibits multiple pigmented lesions, extensive freckling, and actinic damage, particularly in sun-exposed areas. (D–F) Dermoscopic images of different skin malignancies. Image (D) shows a Superficial Spreading Melanoma (SSM), displaying an irregular pigment network, asymmetry, multiple colors, and regression structures. Image (E) depicts a Basal Cell Carcinoma (BCC), characterized by arborizing vessels, a pink background, and superficial scaling. Image (F) illustrates a Squamous Cell Carcinoma (SCC), presenting a central keratinous core, ulceration, and peripheral erythema, indicative of an advanced lesion. The SSM was surgically excised and histologically confirmed, while the BSC and SCC were deemed unresectable. Given the complexity and rarity of the patient’s clinical condition, further diagnostic examinations were conducted, including ultrasound of the head and neck lymph nodes, both of which were negative for metastatic recurrence. However, a chest CT scan revealed a nodular formation with a maximum diameter of 16 mm in the lower lobe of the left lung, suspected to be a metastatic lesion. To achieve a metabolic characterization of the lung nodule, a PET/CT scan with 18F-fluorodeoxyglucose ([18F]FDG) was requested.

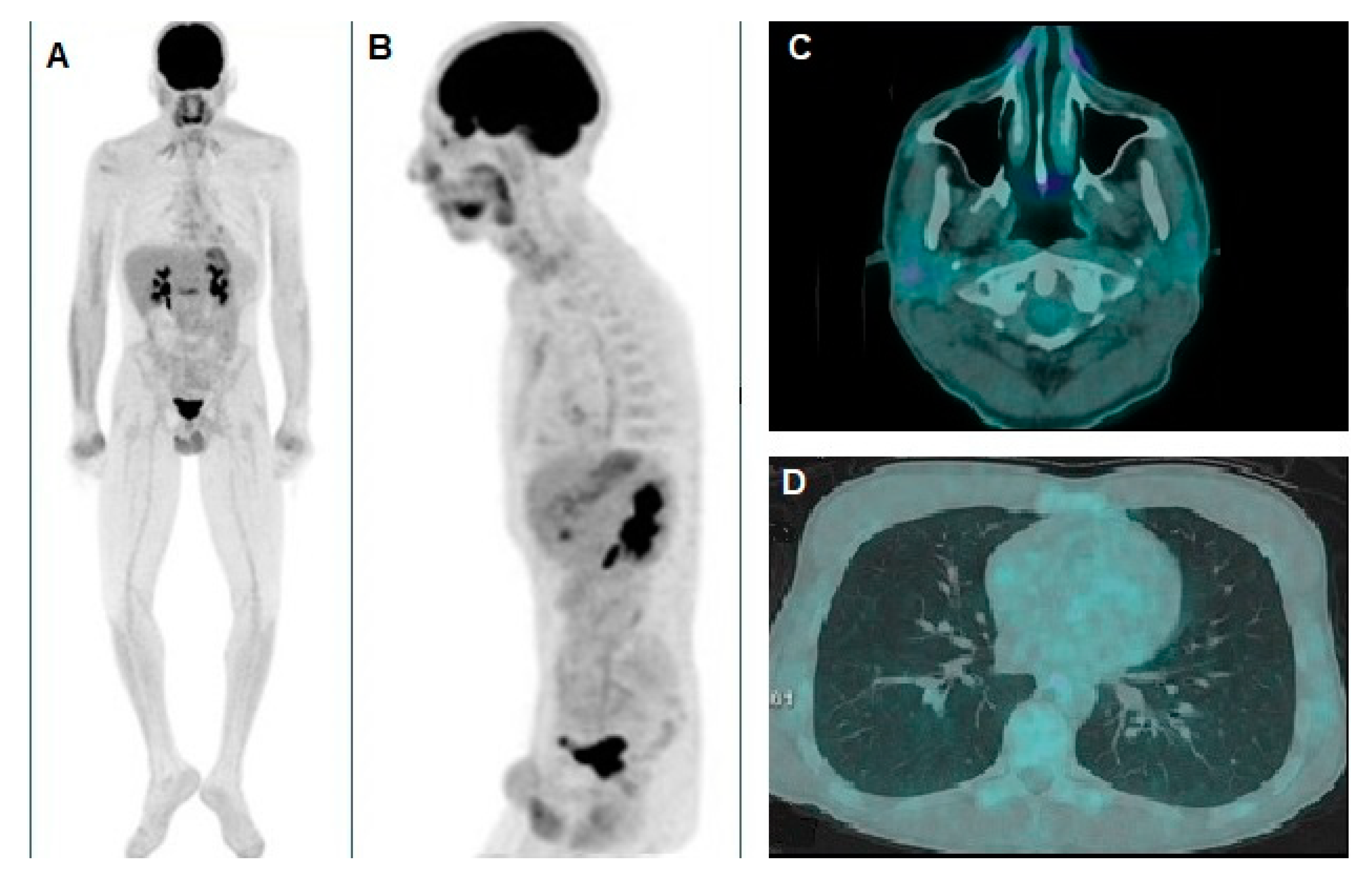
Figure 2.
(A) Whole-body [18F]FDG PET/CT revealed significant hypermetabolism in the right parotid gland, as indicated by the black arrow, suggesting a metabolically active lesion. (B) Oblique sagittal reconstruction, confirmed pathological [18F]FDG uptake both in the parotid gland and in an additional focal area in the left hemithorax, compatible with a pulmonary nodule. The axial PET/CT fusion images of the skull (C) provided further characterization of the lesions, showing hypermetabolism in a small nodular formation within the right parotid gland, with a maximum standardized uptake value (SUVmax) of 6.9 and a metabolic tumor volume (MTV) of approximately 3 cubic centimeters (cc). Additionally, (D) the known pulmonary nodule in the apical segment of the left lower lobe, previously identified on chest CT, exhibited an SUVmax of 4.8 and an MTV of about 0.5 cc. Considering the patient's clinical history, both lesions were interpreted as metastatic disease from the XP-associated melanoma. Consequently, the patient started anti-programmed cell death protein 1 (anti-PD-1) therapy with nivolumab at a dose of 480 mg every four weeks, which was well tolerated without significant adverse effects.
Figure 2.
(A) Whole-body [18F]FDG PET/CT revealed significant hypermetabolism in the right parotid gland, as indicated by the black arrow, suggesting a metabolically active lesion. (B) Oblique sagittal reconstruction, confirmed pathological [18F]FDG uptake both in the parotid gland and in an additional focal area in the left hemithorax, compatible with a pulmonary nodule. The axial PET/CT fusion images of the skull (C) provided further characterization of the lesions, showing hypermetabolism in a small nodular formation within the right parotid gland, with a maximum standardized uptake value (SUVmax) of 6.9 and a metabolic tumor volume (MTV) of approximately 3 cubic centimeters (cc). Additionally, (D) the known pulmonary nodule in the apical segment of the left lower lobe, previously identified on chest CT, exhibited an SUVmax of 4.8 and an MTV of about 0.5 cc. Considering the patient's clinical history, both lesions were interpreted as metastatic disease from the XP-associated melanoma. Consequently, the patient started anti-programmed cell death protein 1 (anti-PD-1) therapy with nivolumab at a dose of 480 mg every four weeks, which was well tolerated without significant adverse effects.
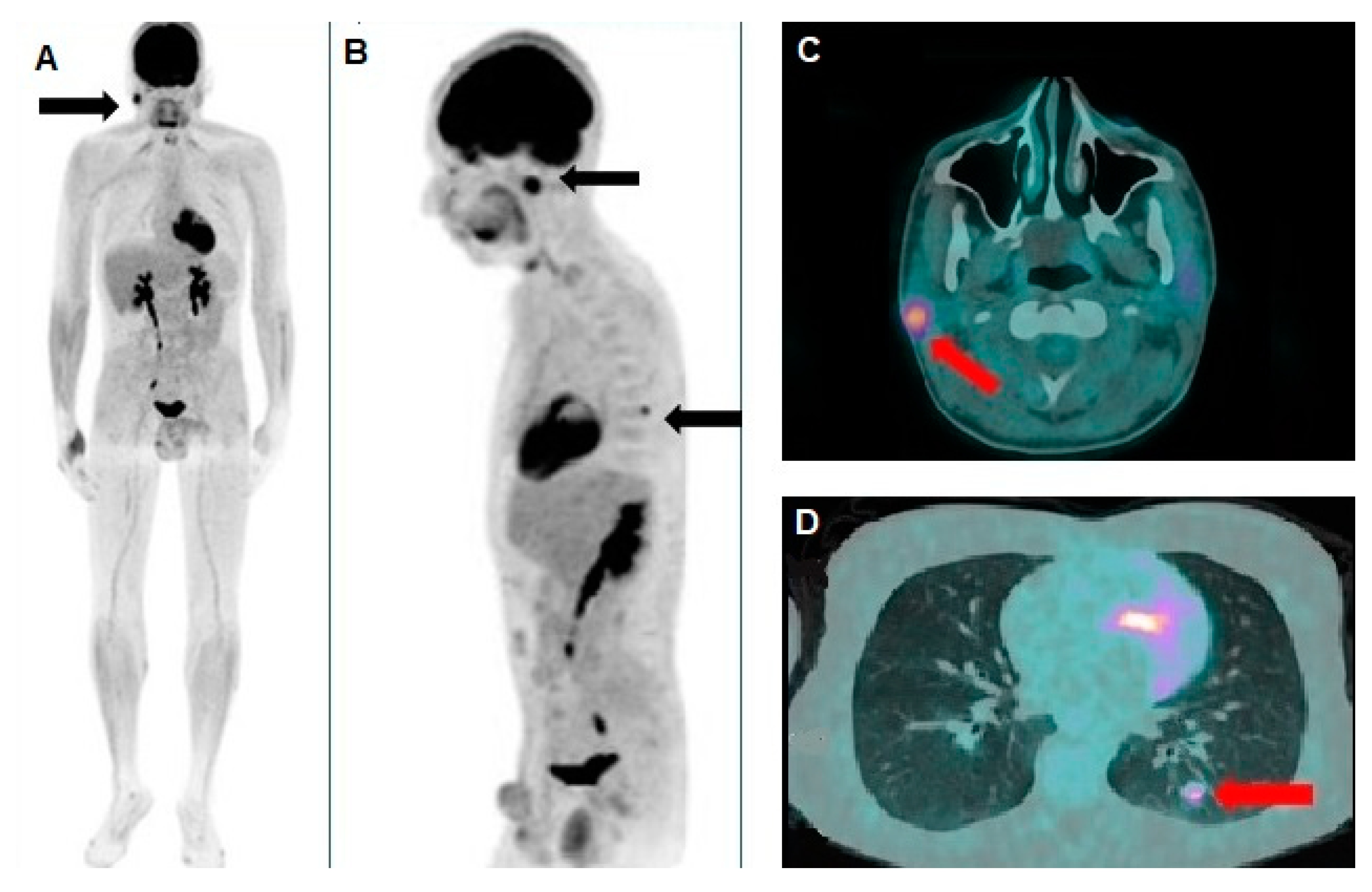
Figure 3.
Three months after the start of therapy, the patient was clinically re-evaluated. (A-C) The physical examination showed a significant improvement in previously observed skin lesions, with no signs of relapse of the previously excised SSM (D) and a marked regression, under dermoscopic examination, of both the BSC (E) and the SCC lesions (F).To assess the response to systemic therapy, the patient also underwent a follow-up PET/CT scan.
Figure 3.
Three months after the start of therapy, the patient was clinically re-evaluated. (A-C) The physical examination showed a significant improvement in previously observed skin lesions, with no signs of relapse of the previously excised SSM (D) and a marked regression, under dermoscopic examination, of both the BSC (E) and the SCC lesions (F).To assess the response to systemic therapy, the patient also underwent a follow-up PET/CT scan.
Figure 4.
(A) Whole-body and (B) oblique sagittal [18F]FDG PET/CT demonstrated complete resolution of the pathological foci of tracer uptake in the thorax and the right parotid gland, as documented by the pre-treatment scan. The axial PET/CT fusion images also clearly depict the corresponding complete disappearance of the nodules in the right parotid gland (C) and the left lung (D).
Figure 4.
(A) Whole-body and (B) oblique sagittal [18F]FDG PET/CT demonstrated complete resolution of the pathological foci of tracer uptake in the thorax and the right parotid gland, as documented by the pre-treatment scan. The axial PET/CT fusion images also clearly depict the corresponding complete disappearance of the nodules in the right parotid gland (C) and the left lung (D).
Author Contributions
Conceptualization, I.P. and L.F.; methodology, I.P., L.F., E.C. and R.P.; writing—original draft preparation, E.C., G.A., V.C. and M.E.G.; writing—review and editing, L.F., R.P. and C.P.; supervision, I.P. and L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because every single clinical case was discussed by a panel of experts during the periodical meeting held in Terracina Hospital.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Black, J.O. Xeroderma Pigmentosum. Head and Neck Pathol 2016, 10, 139–144. [CrossRef]
- Kleijer, W.J.; Laugel, V.; Berneburg, M.; Nardo, T.; Fawcett, H.; Gratchev, A.; Jaspers, N.G.J.; Sarasin, A.; Stefanini, M.; Lehmann, A.R. Incidence of DNA Repair Deficiency Disorders in Western Europe: Xeroderma Pigmentosum, Cockayne Syndrome and Trichothiodystrophy. DNA Repair 2008, 7, 744–750. [CrossRef]
- Cleaver, J.E. Defective Repair Replication of DNA in Xeroderma Pigmentosum. Nature 1968, 218, 652–656. [CrossRef]
- Setlow, R.B.; Regan, J.D.; German, J.; Carrier, W.L. EVIDENCE THAT XERODERMA PIGMENTOSUM CELLS DO NOT PERFORM THE FIRST STEP IN THE REPAIR OF ULTRAVIOLET DAMAGE TO THEIR DNA. Proc. Natl. Acad. Sci. U.S.A. 1969, 64, 1035–1041. [CrossRef]
- Epstein, J.H.; Fukuyama, K.; Reed, W.B.; Epstein, W.L. Defect in DNA Synthesis in Skin of Patients with Xeroderma Pigmentosum Demonstrated in Vivo. Science 1970, 168, 1477–1478. [CrossRef]
- Paszkowska-Szczur, K.; Scott, R.J.; Serrano-Fernandez, P.; Mirecka, A.; Gapska, P.; Górski, B.; Cybulski, C.; Maleszka, R.; Sulikowski, M.; Nagay, L.; et al. Xeroderma Pigmentosum Genes and Melanoma Risk. Intl Journal of Cancer 2013, 133, 1094–1100. [CrossRef]
- Wade, M.H.; Plotnick, H. Xeroderma Pigmentosum and Squamous Cell Carcinoma of the Tongue. Journal of the American Academy of Dermatology 1985, 12, 515–521. [CrossRef]
- Chambon, F.; Osdoit, S.; Bagny, K.; Moro, A.; Nguyen, J.; Réguerre, Y. Dramatic Response to Nivolumab in Xeroderma Pigmentosum Skin Tumor. Pediatric Blood & Cancer 2018, 65, e26837. [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer 2012, 12, 252–264. [CrossRef]
- for the Organizing Committee of the 2013 SITC Workshop on Personalized Immunotherapy; Overwijk, W.W.; Wang, E.; Marincola, F.M.; Rammensee, H.-G.; Restifo, N.P. Mining the Mutanome: Developing Highly Personalized Immunotherapies Based on Mutational Analysis of Tumors. j. immunotherapy cancer 2013, 1, 11. [CrossRef]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Geukes Foppen, M.H.; Goldinger, S.M.; et al. Genomic Correlates of Response to CTLA-4 Blockade in Metastatic Melanoma. Science 2015, 350, 207–211. [CrossRef]
- Zalaquett, N.G.; Kreidieh, L.; Youssef, B.; Mourad, M.; Kreidieh, F. Case Report: Neoadjuvant-Intent Pembrolizumab Resulted in Complete Response in a Xeroderma Pigmentosum Patient with Locally Advanced Resectable Cutaneous Squamous Cell Carcinoma of the Nose. Front. Med. 2024, 11, 1488400. [CrossRef]
- Kaste, S.C. Imaging of Pediatric Cutaneous Melanoma. Pediatr Radiol 2019, 49, 1476–1487. [CrossRef]
- Filippi, L.; Bianconi, F.; Schillaci, O.; Spanu, A.; Palumbo, B. The Role and Potential of 18F-FDG PET/CT in Malignant Melanoma: Prognostication, Monitoring Response to Targeted and Immunotherapy, and Radiomics. Diagnostics 2022, 12, 929. [CrossRef]
- Sachpekidis, C.; Weru, V.; Kopp-Schneider, A.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. The Prognostic Value of [18F]FDG PET/CT Based Response Monitoring in Metastatic Melanoma Patients Undergoing Immunotherapy: Comparison of Different Metabolic Criteria. Eur J Nucl Med Mol Imaging 2023, 50, 2699–2714. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).