Submitted:
19 March 2025
Posted:
19 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Inclusion and Exclusion Criteria
2.4. Data Processing and Analysis
2.5. Time Series Modeling
3. Results
3.1. Descriptive Statistics
3.2. Time Series Trend Analysis
3.3. Trend Model Comparisons
3.4. Forecasting Using the Cubic Model (2020-2021)
4. Discussion
5. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, J.I.; Cherry, J.D.; Chang, S.-J.; Partridge, S.; Lee, H.; Treanor, J.; Greenberg, D.P.; Keitel, W.; Barenkamp, S.; Bernstein, D.I.; et al. Efficacy of an Acellular Pertussis Vaccine among Adolescents and Adults. New Engl. J. Med. 2005, 353, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Muloiwa, R.; Kagina, B.M.; E Engel, M.; Hussey, G.D. The burden of pertussis in low- and middle-income countries since the inception of the Expanded Programme on Immunization (EPI) in 1974: a systematic review protocol. Syst. Rev. 2015, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Young, A.A.; Li, X. Applications and Comparisons of Four Time Series Models in Epidemiological Surveillance Data. PLOS ONE 2014, 9, e88075. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Nelson, T.A.; MacNab, Y.C.; Lawson, A.B. Review of methods for space–time disease surveillance. Spat. Spatio-temporal Epidemiology 2010, 1, 105–116. [Google Scholar] [CrossRef]
- Cazelles, B.; Chavez, M.; de Magny, G.C.; Guégan, J.-F.; Hales, S. Time-dependent spectral analysis of epidemiological time-series with wavelets. J. R. Soc. Interface 2007, 4, 625–636. [Google Scholar] [CrossRef]
- Cardinal M, Lambert J, Roy R. On the application of integer-valued time series models for the analysis of disease incidence. Stat Med. 1999;18:2025–39.
- Gentleman R, Hornik K, Parmigiani G. Epidemics Models and Data using R. 1st edition. University Park, PA, USA; 2018.
- Zeger, S.L.; Irizarry, R.; Peng, R.D. On Time Series Analysis of Public Health and Biomedical Data. Annu. Rev. Public Heal. 2006, 27, 57–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Bambrick, H.; Mengersen, K.; Tong, S.; Feng, L.; Liu, G.; Xu, A.; Zhang, L.; Hu, W. Association of weather variability with resurging pertussis infections among different age groups: A non-linear approach. Sci. Total. Environ. 2020, 719, 137510. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; Huang, G.; Xia, J.; Wang, X.; Zhang, Y.; Tang, W.; Zhou, H. Time series analysis of temporal trends in the pertussis incidence in Mainland China from 2005 to 2016. Sci. Rep. 2016, 6, srep32367–32367. [Google Scholar] [CrossRef]
- Huang, X.; Lambert, S.; Lau, C.; Magalhaes, R.J.S.; Marquess, J.; Rajmokan, M.; Milinovich, G.; Hu, W. Assessing the social and environmental determinants of pertussis epidemics in Queensland, Australia: a Bayesian spatio-temporal analysis. Epidemiology Infect. 2017, 145, 1221–1230. [Google Scholar] [CrossRef]
- Dafilis, M.P.; Frascoli, F.; McVernon, J.; Heffernan, J.M.; McCaw, J.M. The dynamical consequences of seasonal forcing, immune boosting and demographic change in a model of disease transmission. J. Theor. Biol. 2014, 361, 124–132. [Google Scholar] [CrossRef]
- Gomes, M.; Gomes, J.; Paulo, A. Diphtheria, pertussis, and measles in Portugal before and after mass vaccination: A time series analysis. Eur. J. Epidemiology 1999, 15, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Grenfell, B.T.; May, R.M. Oscillatory fluctuations in the incidence of infectious disease and the impact of vaccination: time series analysis. Epidemiology Infect. 1984, 93, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Skoff, T.H.; Hadler, S.; Hariri, S. The Epidemiology of Nationally Reported Pertussis in the United States, 2000–2016. Clin. Infect. Dis. 2018, 68, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Akhter, Y. Statistical Modeling for the Prediction of Infectious Disease Dissemination With Special Reference to COVID-19 Spread. Front. Public Heal. 2021, 9, 645405. [Google Scholar] [CrossRef]
- Kumar N, Susan S. COVID-19 Pandemic Prediction using Time Series Forecasting Models. 2020.
- Yu, G.; Feng, H.; Feng, S.; Zhao, J.; Xu, J. Forecasting hand-foot-and-mouth disease cases using wavelet-based SARIMA–NNAR hybrid model. PLOS ONE 2021, 16, e0246673. [Google Scholar] [CrossRef]
- Nobre, F.F.; Monteiro, A.B.S.; Telles, P.R.; Williamson, G.D. Dynamic linear model and SARIMA: a comparison of their forecasting performance in epidemiology. Stat. Med. 2001, 20, 3051–3069. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, D.; Zhu, Y.; Liu, L.; Li, C.; Wang, Z.; Li, X. SARFIMA model prediction for infectious diseases: application to hemorrhagic fever with renal syndrome and comparing with SARIMA. BMC Med Res. Methodol. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Allard, R. Update/Le point Use of time-series analysis in infectious disease surveillance. 1998.
- Wang, M.; Pan, J.; Li, X.; Li, M.; Liu, Z.; Zhao, Q.; Luo, L.; Chen, H.; Chen, S.; Jiang, F.; et al. ARIMA and ARIMA-ERNN models for prediction of pertussis incidence in mainland China from 2004 to 2021. BMC Public Heal. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Nann, D.; Walker, M.; Frauenfeld, L.; Ferenci, T.; Sulyok, M. Forecasting the future number of pertussis cases using data from Google Trends. Heliyon 2021, 7, e08386. [Google Scholar] [CrossRef]
- Raycheva, R.; Kevorkyan, A.; Stoilova, Y. Stochastic modelling of scalar time series of varicella incidence for a period of 92 years (1928-2019). Folia Medica 2022, 64, 624–632. [Google Scholar] [CrossRef]
- Campbell, P.T.; McCaw, J.M.; McVernon, J. Pertussis models to inform vaccine policy. Hum. Vaccines Immunother. 2015, 11, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Patel, V.; Duan, L.; Mikuliak, J.; Basran, J.; Osgood, N.D. Real-Time Epidemiology and Acute Care Need Monitoring and Forecasting for COVID-19 via Bayesian Sequential Monte Carlo-Leveraged Transmission Models. Int. J. Environ. Res. Public Heal. 2024, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Broutin, H.; Viboud, C.; Grenfell, B.T.; Miller, M.A.; Rohani, P. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proc. R. Soc. B: Biol. Sci. 2010, 277, 3239–3245. [Google Scholar] [CrossRef]
- Heininger U, Martini H, Eeuwijk J, Prokić I, Guignard AP, Turriani E, et al. Pertactin deficiency of Bordetella pertussis: Insights into epidemiology, and perspectives on surveillance and public health impact. Human Vaccines and Immunotherapeutics. 2024.
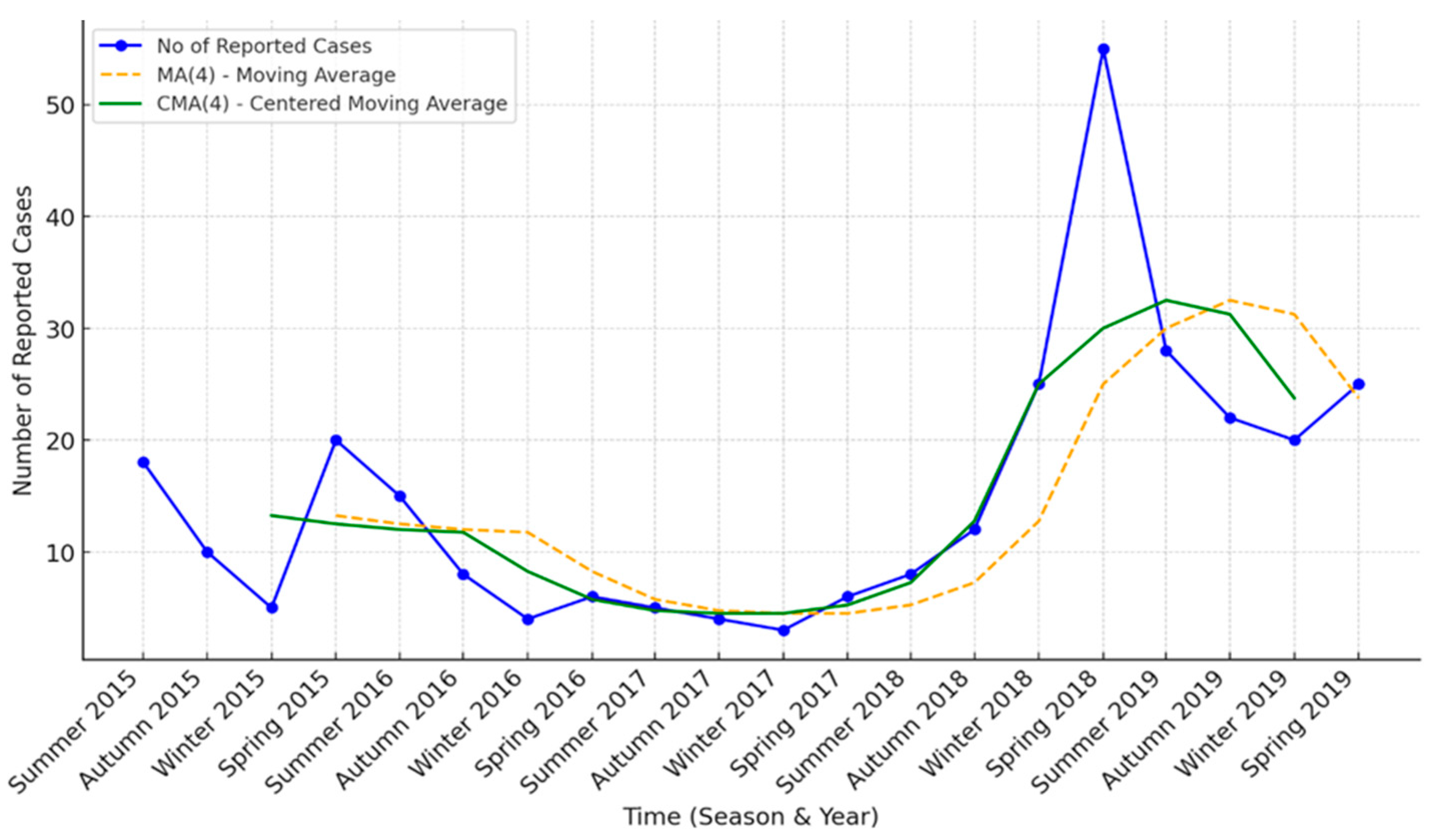
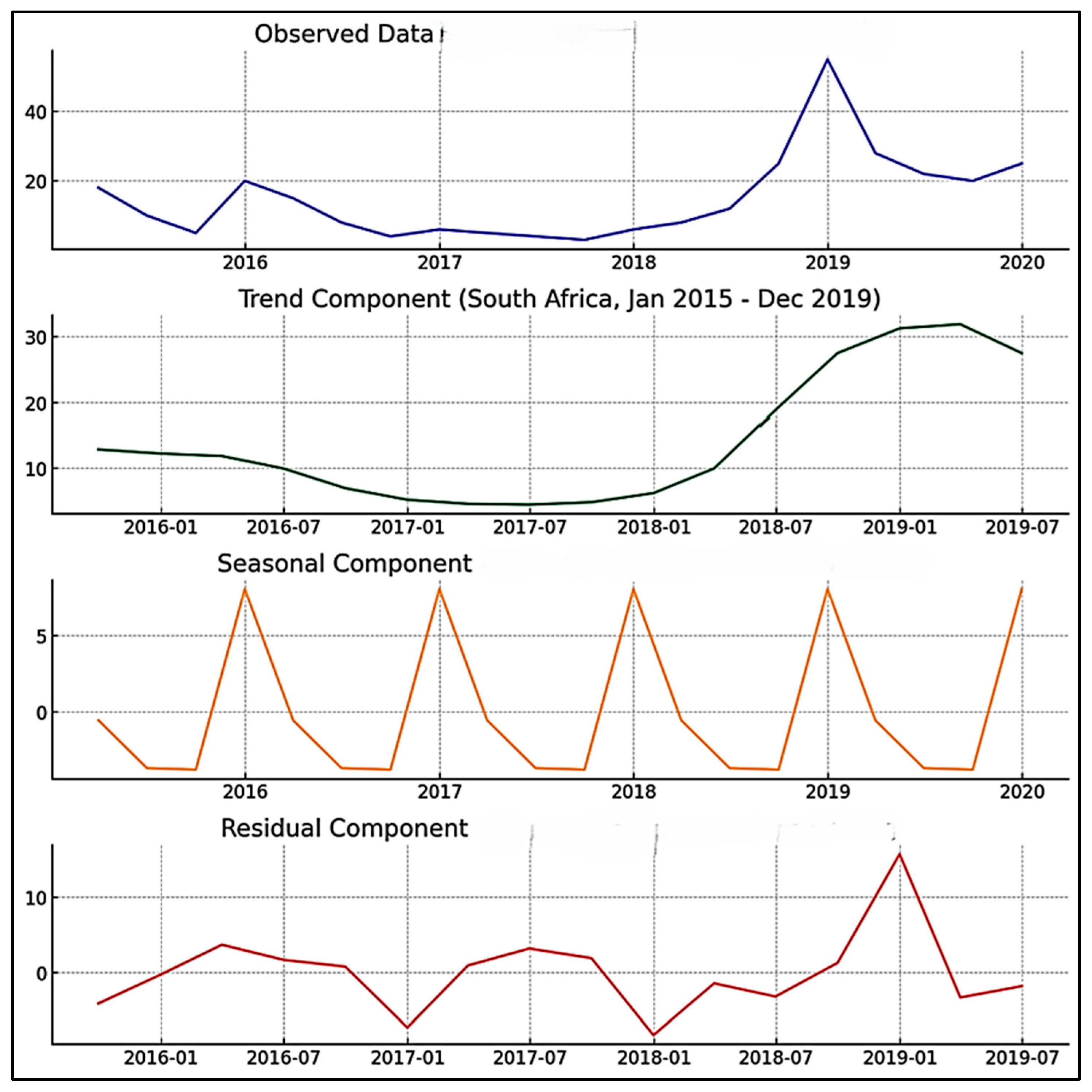
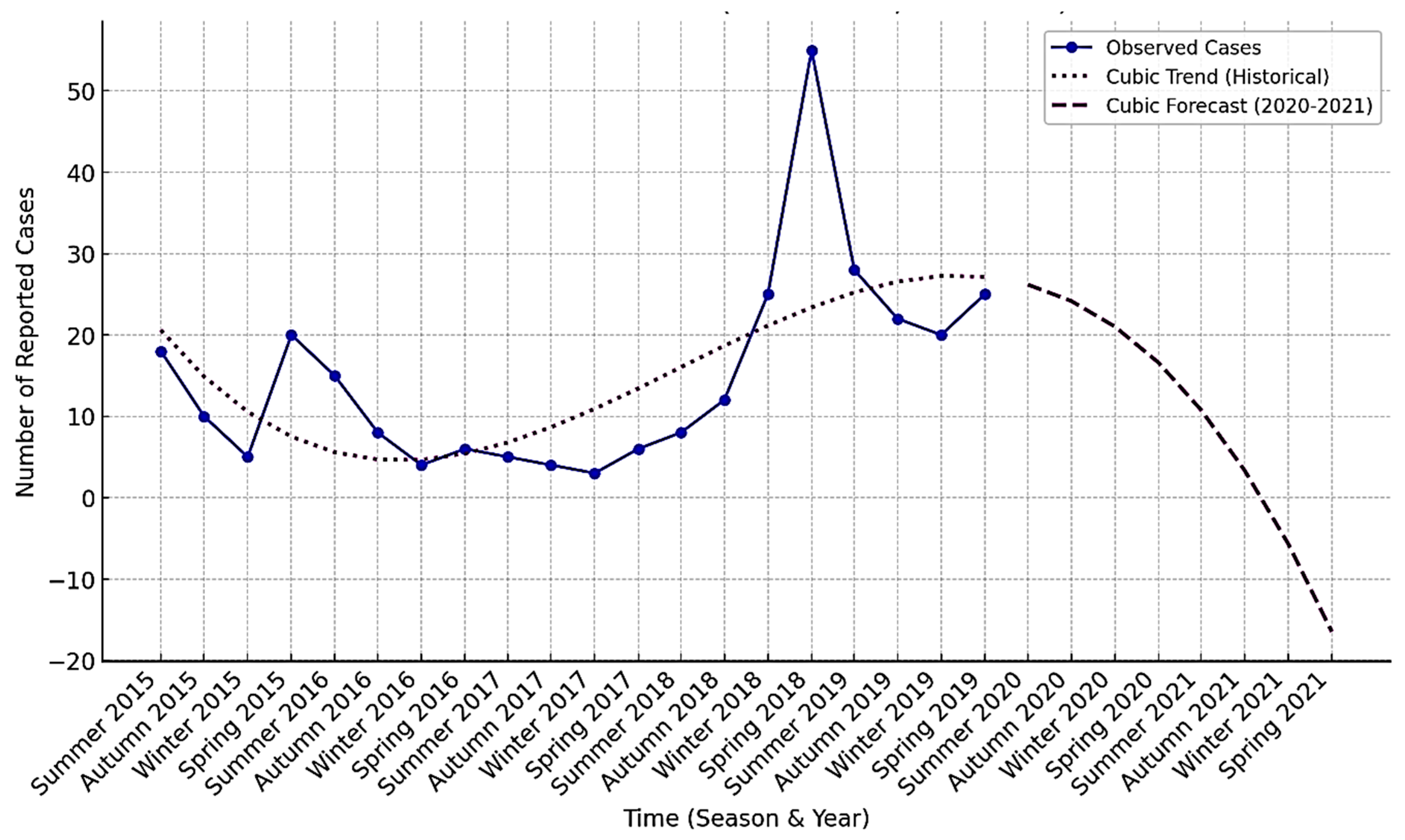
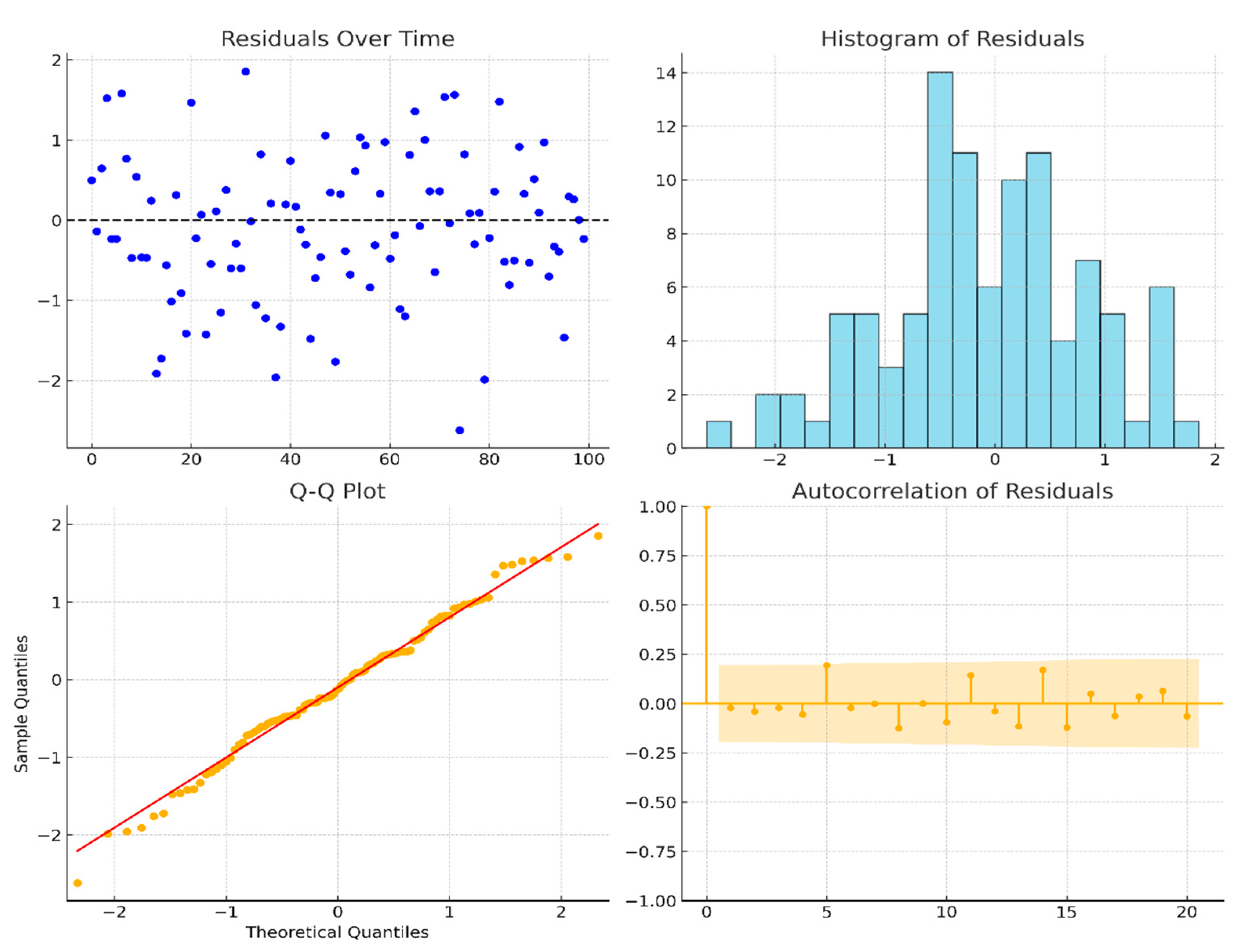
| Season | Mean Cases | Std. Dev. | Min Cases | Max Cases |
|---|---|---|---|---|
| Autumn | 11.6 | 6.5 | 4 | 20 |
| Winter | 16.6 | 12.0 | 2 | 30 |
| Spring | 19.8 | 21.5 | 1 | 54 |
| Summer | 6.0 | 7.4 | 1 | 19 |
| Trend Model | Mean Squared Error (MSE) |
|---|---|
| Linear | 114.95 |
| Cubic | 83.49 |
| Exponential | 122.16 |
| Date | Forecasted Cases (Cubic) |
|---|---|
| March 2020 | 26.18 |
| June 2020 | 24.17 |
| September 2020 | 21.02 |
| December 2020 | 16.58 |
| March 2021 | 10.75 |
| Variable | Coefficient | Std. Error | t-Statistic | p-Value |
|---|---|---|---|---|
| AR(1) | 0.797*** | 0.167 | 4.77 | 0.000 |
| MA(1) | -0.698*** | 0.189 | -3.69 | 0.001 |
| Constant | 21.77*** | 1.417 | 15.37 | 0.000 |
| Statistic | Value |
|---|---|
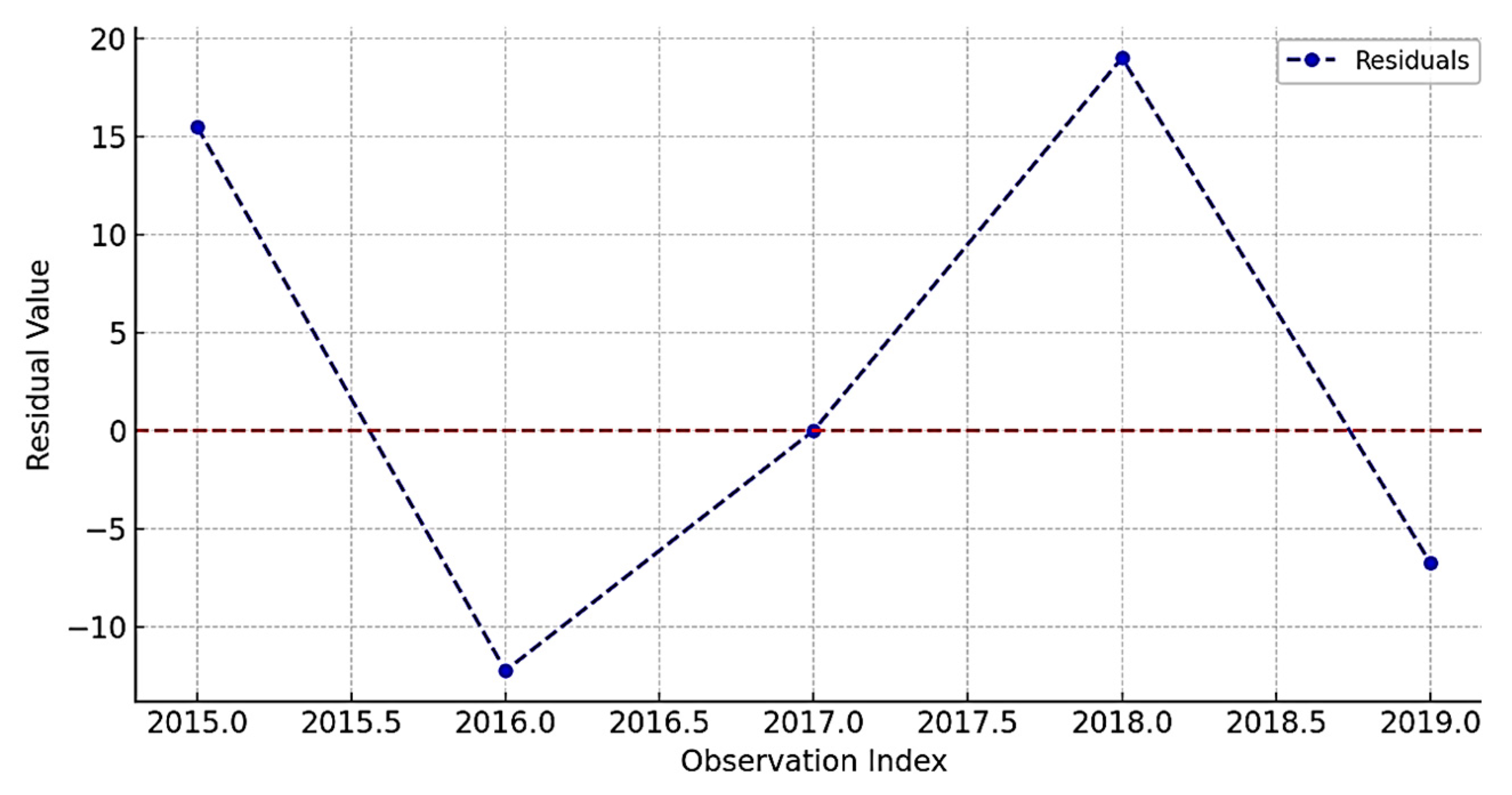
| Mean Residual | 0.002 |
| Std. Deviation | 3.78 |
| Ljung-Box Test (p-value) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





