Introduction
Bioethanol is a promising renewable energy source that can reduce global dependence on fossil fuels and mitigate greenhouse gas emissions. The global bioethanol market is projected to grow significantly, from USD 94.77 billion in 2025 to USD 158.02 billion by 2033, with a CAGR of 6.60% during this period (Bothare, 2025). This growth is driven by increasing concerns over carbon emissions and the transition towards renewable energy sources. Governments worldwide are implementing stringent environmental regulations and blending mandates, such as the Renewable Fuel Standard (RFS) in the U.S. and the European Union’s Renewable Energy Directive (RED), which are accelerating bioethanol adoption. Bioethanol is a cleaner and greener alternative to traditional fossil fuels. Its combustion generally produces lower greenhouse gas emissions than gasoline, contributing to a reduction in overall carbon footprint and mitigating the effects of climate change (Broda et al., 2022). Derived from biomass, bioethanol is particularly attractive because it can be produced from non-food sources, such as lignocellulosic materials, which are abundant and sustainable. Lignocellulosic biomass, including agricultural residues, forestry waste, and energy crops, represents a vast and underutilized resource for bioethanol production. Despite its potential, the conversion of lignocellulose to bioethanol faces significant challenges. The complex structure of lignocellulose, comprising cellulose, hemicellulose, and lignin, makes it highly recalcitrant to degradation (Broda et al., 2022). Additionally, the high costs of enzymatic hydrolysis and fermentation, as well as the formation of inhibitory compounds during pretreatment, hinder the economic viability of the process (Robak and Balcerek, 2020).
Biofilms, which are structured communities of microorganisms embedded in a self-produced extracellular polymeric substance (EPS) matrix, have gained attention for their potential in bioprocessing. Biofilms offer several advantages, including enhanced microbial stability, resistance to environmental stressors, and improved nutrient exchange (dos Santos et al., 2018). These properties make biofilms particularly suitable for industrial applications, including bioethanol production (Güneş et al. 2024; Patwardhan et al. 2024; Weiler et al. 2024). Fungal-bacterial biofilms is known to improve cellulolytic activity and ethanol yields compared to single cultures (Henagamage, 2022). Zymomonas mobilis biofilms formed on plastic composites exhibit higher ethanol production efficiency and tolerance to toxic inhibitors when using rice straw and rice bran hydrolysates as substrates (Todhanakasem et al., 2019). This review evaluates the role of biofilms in enhancing the conversion of lignocellulose to bioethanol. It explores the potential of biofilms in pretreatment, enzymatic hydrolysis, and fermentation, as well as their application in enzyme immobilization and bioreactor design.
Lignocellulose Structure and Its Recalcitrance
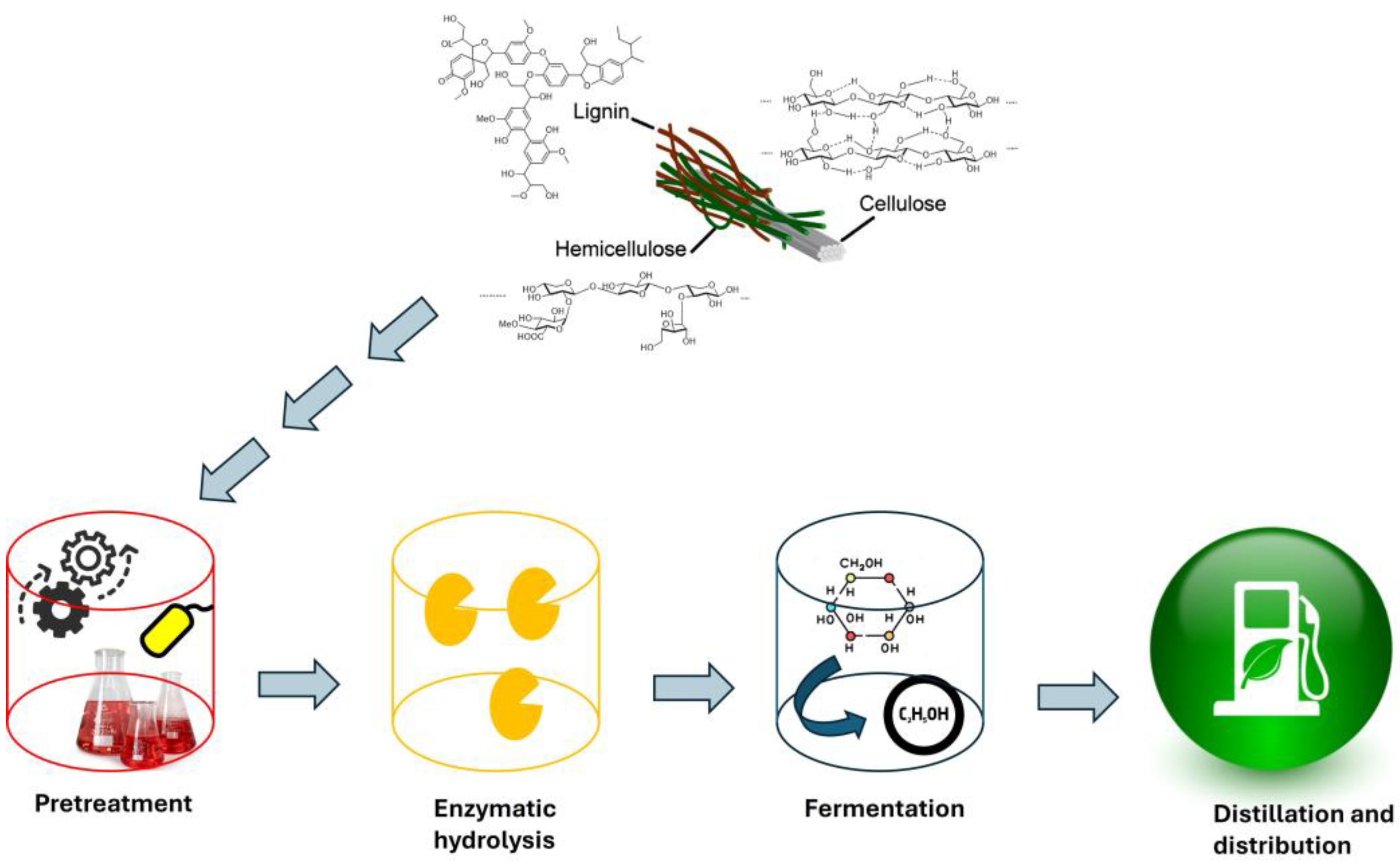
Lignocellulosic biomass is primarily composed of cellulose (40–50%), hemicellulose (20–30%), and lignin (15–25%) (Zoghlami and Paës 2019). Cellulose, a linear polymer of glucose, provides structural integrity, while hemicellulose, a heteropolymer of sugars like xylose and arabinose, acts as a matrix. Lignin, a complex aromatic polymer, provides rigidity and resistance to microbial degradation. The crystalline structure of cellulose and the protective barrier formed by lignin make lignocellulose highly recalcitrant to enzymatic and microbial degradation (Dong et al. 2023). Additionally, pretreatment processes often generate inhibitors, such as furfural and phenolic compounds, which can negatively impact downstream hydrolysis and fermentation.
Efficient pretreatment and hydrolysis are essential to overcome the recalcitrance of lignocellulose. Pretreatment disrupts the lignocellulosic matrix, making cellulose and hemicellulose accessible for enzymatic hydrolysis (Sasmal and Mohanty 2018). Hydrolysis converts these polysaccharides into fermentable sugars, which are then fermented into bioethanol (Jørgensen et al. 2007). However, the high cost and low efficiency of these processes remain significant barriers to commercialization. For example, chemical pretreatments using acids, alkalis, or other solvents can add up to an additional 40% to the cost of bioethanol (Ceaser et al. 2024). Moreover, these methods often generate chemical waste and require substantial energy inputs, which raise environmental concerns and operational costs. The hydrolysis of pretreated biomass to release fermentable sugars is another critical step. Enzymatic hydrolysis, while effective, is expensive due to the high cost of enzymes. For instance, even at a relatively low cellulase dosage, the enzyme cost can account for 15.7% of the total bioethanol production cost bioethanol (Ceaser et al. 2024). Additionally, the commonly used yeast strain,
Saccharomyces cerevisiae, is only effective in fermenting glucose and not xylose, which limits the overall ethanol yield. The integration of pretreatment, hydrolysis, and fermentation processes is crucial for improving efficiency and reducing costs. However, achieving seamless integration on a commercial scale remains a challenge. For example, simultaneous saccharification and fermentation (SSF) or separate hydrolysis and fermentation (SHF) processes require careful optimization to maximize yields and minimize costs. Moreover, the variability in lignocellulosic biomass composition necessitates adaptable and scalable pretreatment technologies. The overall process of bioethanol production using lignocellulose is summarized in
Figure 1.
Pretreatment of Lignocellulose
Pretreatment is the first and most critical step in the conversion of lignocellulosic biomass into bioethanol. The primary objective of pretreatment is to break down the rigid structure of lignocellulose, disrupt the lignin barrier, and make cellulose and hemicellulose more accessible for enzymatic hydrolysis. Without effective pretreatment, the subsequent steps of hydrolysis and fermentation are inefficient. Pretreatment methods can be broadly categorized into physical, chemical, and biological approaches. Physical methods involve mechanical disruption of the biomass to reduce particle size and increase surface area. Common techniques include milling, grinding, and extrusion. These methods break hydrogen bonds and reduce the crystallinity of cellulose, making it more amenable to enzymatic attack. However, physical pretreatment is energy-intensive and often insufficient on its own to fully disrupt the lignocellulosic matrix. Chemical methods use acids, alkalis, or organic solvents to break down lignin and hemicellulose. Acid pretreatment, typically using dilute sulfuric acid, hydrolyzes hemicellulose into sugars and partially solubilizes lignin. Alkali pretreatment, using sodium hydroxide or ammonia, disrupts ester bonds between lignin and carbohydrates, solubilizing lignin and reducing cellulose crystallinity. Organosolv pretreatment employs organic solvents like ethanol to dissolve lignin and hemicellulose. While chemical methods are effective, they often generate inhibitory compounds (e.g., furfural, hydroxymethylfurfural) that can hinder downstream processes. Biological methods, such as fungal pretreatment, use microorganisms to degrade lignin and hemicellulose. Fungal pretreatment is an environmentally friendly and energy-efficient method for degrading lignin and hemicellulose in lignocellulosic biomass, making cellulose more accessible for biofuel production (Nadir et al., 2019). White rot fungi are particularly effective due to their unique ligninolytic enzyme systems that can selectively degrade lignin (Wan & Li, 2012). This biological pretreatment reduces biomass recalcitrance and can be performed under ambient conditions without chemical additives. However, the process is relatively slow compared to thermal or chemical methods. Factors influencing fungal pretreatment effectiveness include operating parameters and enzyme systems involved in biodegradation (Wan & Li, 2012). Combining fungal pretreatment with physical or chemical methods may further improve enzymatic hydrolysis and ethanol production.
Hydrolysis of Lignocellulose
Hydrolysis is the process of breaking down cellulose and hemicellulose into fermentable sugars, primarily glucose and xylose. This step is a critical step in converting cellulose and hemicellulose into fermentable sugars. Hydrolysis can be achieved through acid or enzymatic methods, each with distinct mechanisms and challenges. Acid hydrolysis uses concentrated or dilute acids to break the β-1,4-glycosidic bonds in cellulose and hemicellulose, releasing monosaccharides. Concentrated acids are highly effective but corrosive and difficult to handle, while dilute acids are less aggressive but generate inhibitory compounds like furfural and acetic acid. Acid hydrolysis is often used in combination with pretreatment to improve sugar yields. Enzymatic hydrolysis is the preferred method due to its specificity and mild reaction conditions. Cellulases, a group of enzymes including endoglucanases, exoglucanases, and β-glucosidases, work synergistically to hydrolyze cellulose into glucose. Similarly, hemicellulases, such as xylanases and mannanases, degrade hemicellulose into its constituent sugars. These enzymes are essential for unlocking the fermentable sugars trapped within the lignocellulosic matrix. The enzymes are often inhibited by the presence of lignin and other by-products generated during pretreatment, such as furfural and phenolic compounds. These inhibitors can bind to the active sites of enzymes, reducing their activity and stability. Furthermore, the crystalline nature of cellulose and the heterogeneous structure of hemicellulose make them difficult substrates for enzymatic attack, requiring high enzyme loadings and long reaction times. Cellulases and hemicellulases are the key enzymes involved, but their high cost and low stability limit their industrial application (Yang et al. 2011). Factors affecting enzymatic hydrolysis include substrate characteristics like crystallinity and accessible surface area, as well as the presence of lignin and oligomeric xylan. Efforts to improve enzyme efficiency should focus on optimizing enzyme mixtures, identifying novel enzymes, and enhancing enzyme stability and activity.
Fermentation of Sugars to Bioethanol
Fermentation is a critical step in the conversion of lignocellulosic sugars into bioethanol. This process involves the microbial conversion of fermentable sugars, such as glucose and xylose, into ethanol and carbon dioxide (Dionisi et al. 2015). Fermentation can be performed using different strategies, including separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), and consolidated bioprocessing (CBP). In SHF, hydrolysis and fermentation are performed in separate steps. Enzymatic hydrolysis produces sugars, which are then fermented by yeast (e.g., Saccharomyces cerevisiae) or bacteria (e.g., Zymomonas mobilis). SHF allows for optimized conditions for each step but is limited by end-product inhibition of enzymes and longer processing times. In SSF, hydrolysis and fermentation occur in the same reactor. Enzymes and fermenting microorganisms work simultaneously, reducing end-product inhibition and processing time. SSF is more efficient but requires careful optimization of conditions to balance enzyme and microorganism activity. CBP uses a single microorganism or microbial consortium to produce enzymes, hydrolyze biomass, and ferment sugars. Engineered strains like Clostridium thermocellum are capable of performing all steps in one reactor. CBP has the potential to significantly reduce costs and processing time but requires the development of robust and efficient microbial strains. While S. cerevisiae remains the primary microorganism for bioethanol production, researchers are exploring alternative yeasts and bacteria to address challenges in second-generation bioethanol fermentation (Radecka et al. 2015). Non-conventional yeasts like Zygosaccharomyces rouxii and Kluyveromyces marxianus show promise for their tolerance to osmotic stress and high temperatures, respectively (Radecka et al. 2015). Various substrates, including lignocellulosic biomass and agricultural wastes, are being investigated for ethanol production (Nigam, 2017). The selection of suitable microorganisms, whether Saccharomyces or non-Saccharomyces, is crucial for efficient ethanol fermentation from various substrates (Nigam, 2017).
Biofilm Structure and Its Role in Bioethanol Production
Biofilms are complex, structured communities of microorganisms that adhere to surfaces and are embedded in a self-produced extracellular matrix. This matrix is composed of extracellular polymeric substances (EPS), which play a critical role in the biofilm's stability, functionality, and resistance to environmental stresses. The EPS is a complex mixture of biopolymers, including polysaccharides, proteins, nucleic acids, and lipids (Yahya et al. 2017; Yahya et al. 2018; Yaacob et al. 2021; Kamaruzzaman et al. 2022; Johari et al. 2023; Hamdan et al. 2024). Its composition varies depending on the microbial species and environmental conditions. Biofilms are metabolically active and often exhibit phenotypic changes compared to their planktonic (free-floating) counterparts. Biofilms are not solid masses but rather porous structures with a network of water channels (Quan et al. 2021). These channels facilitate the transport of nutrients, oxygen, and waste products, ensuring the survival of cells within the biofilm. Biofilm structure is commonly investigated using microplate include light microscopy, confocal laser scanning microscopy (CLSM), scanning electron microscopy (SEM), atomic force microscopy (AFM), and FTIR (Fourier-transform infrared) spectroscopy (Amran et al. 2024). Biofilms exhibit chemical and physiological gradients, such as oxygen, pH, and nutrient gradients, due to the diffusion limitations within the matrix. These gradients create microenvironments that influence microbial activity and diversity. Biofilm-forming microorganisms, such as certain fungi and bacteria, have shown promise in biological pretreatment of lignocellulose (Narayanaswamy et al. 2013). These microorganisms produce ligninolytic enzymes that degrade lignin, enhancing cellulose accessibility. Biofilms also provide a protective environment for microorganisms, enabling them to withstand harsh conditions and inhibitors generated during pretreatment. In addition, biofilms facilitate nutrient exchange and microbial cooperation, making them highly efficient for industrial applications (dos Santos et al. 2018).
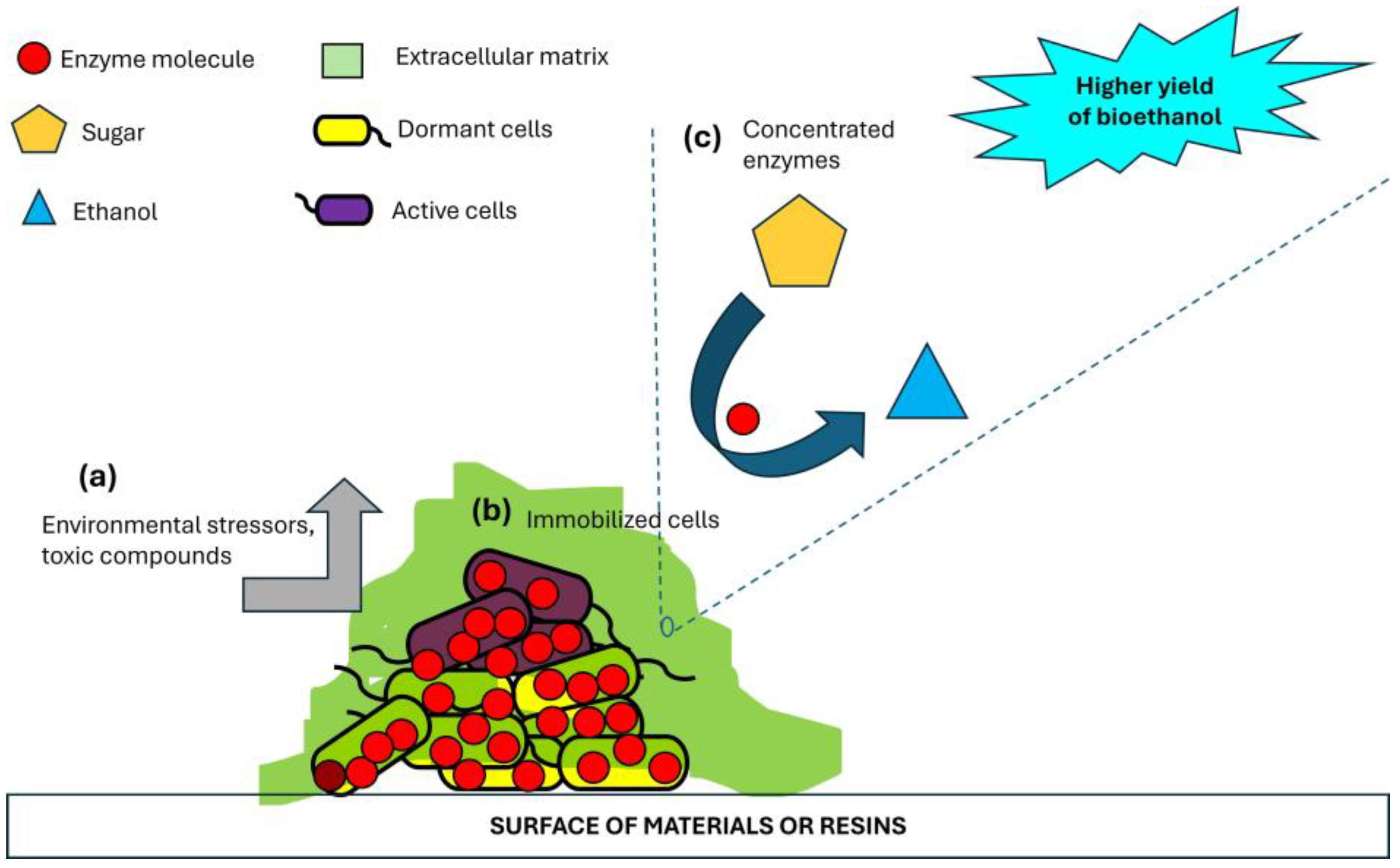
Cellulolytic biofilms enhance enzyme concentration at the substrate-liquid interface, facilitating more efficient hydrolysis and capture of products (Brethauer et al. 2020). According to Shukla et al. (2023), biofilms enhance the concentration of enzymes at the substrate-liquid interface. This high concentration of cellulolytic enzymes facilitates more efficient hydrolysis and capture of products, leading to higher yields of fermentable sugars. Biofilms play a crucial role in the hydrolysis of lignocellulose by enabling efficient degradation and conversion of cellulose. The biofilm matrix can absorb exogenous cellulases and retain self-produced enzymes, enhancing cellulose degradation (Deng & Wang, 2022). Biofilm formation is synchronized with cellulose degradation, characterized by crater-like depressions on the cellulose surface (Wang et al., 2011). Clostiridium thermocellum, one of the most extensively researched cellulolytic anaerobic bacteria, is a highly promising candidate for the direct conversion of lignocellulose into fuels and chemicals (Dumitrache et al. 2023). This is largely due to its rapid growth rate (0.1–0.16 h⁻¹) on crystalline cellulose. In flow cell systems, where the cellulosic substrate is retained but the dilution rate significantly exceeds the growth rate of planktonic cells, the behaviour of substrate-bound cells can be studied without interference from planktonic cells, as the latter are washed out of the system. Research has demonstrated that C. thermocellum biofilms alone are capable of achieving nearly complete substrate hydrolysis under such flow cell conditions.
The EPS matrix provides a protective environment for microbial cells, enhancing their resistance to environmental stressors, such as inhibitors, ethanol toxicity, and pH fluctuations (Yin et al. 2019). The EPS matrix also acts as a barrier, preventing toxic compounds from reaching the microbial cells (Koechler et al. 2015). Additionally, biofilm communities often exhibit metabolic diversity, enabling them to detoxify or tolerate inhibitors more effectively than planktonic cells. The explicit roles of biofilm in lignocellulose-to-bioethanol conversion are summarized in
Table 1 while the advantage of biofilm structure in bioethanol production is shown in
Figure 2.
Biofilm Reactor
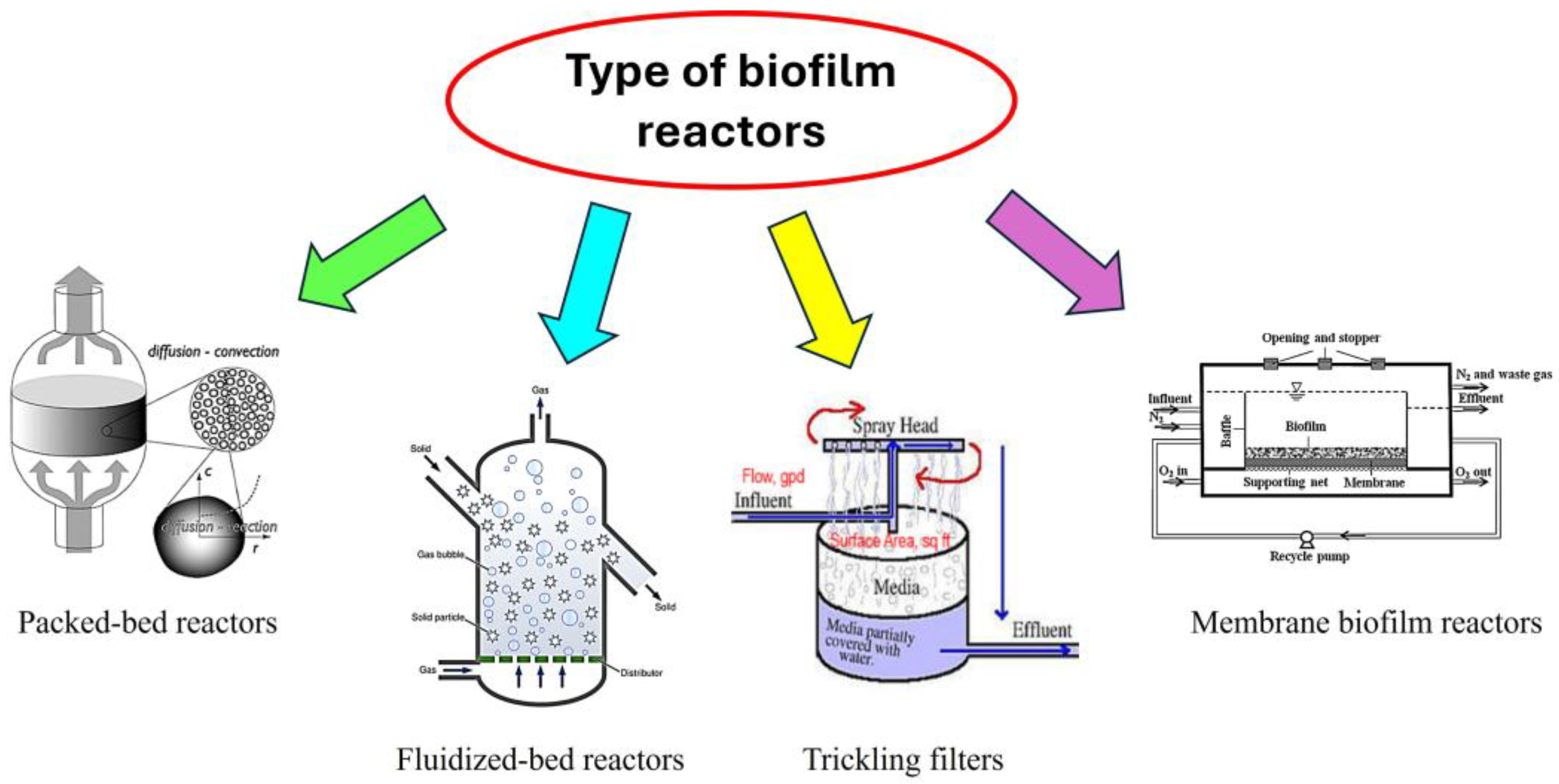
A biofilm reactor is a specialized system designed to cultivate and utilize biofilms—structured communities of microorganisms attached to surfaces and embedded in a self-produced extracellular matrix. These reactors are engineered to optimize the growth, stability, and functionality of biofilms for various applications, including bioethanol production (Vega et al. 1988). Biofilm reactors leverage the natural ability of microorganisms to form biofilms, which are highly resistant to environmental stresses and can perform complex biochemical processes efficiently. The design of a biofilm reactor for lignocellulose degradation depends on the specific application, scale, and type of biomass. Common reactor configurations include packed-bed reactors, fluidized-bed reactors, trickling filters, and membrane biofilm reactors (
Figure 3). In the packed-bed reactors, the reactor is filled with a solid support material (e.g., plastic beads, wood chips, or ceramic particles) that provides a surface for biofilm attachment (Li et al. 2021). Lignocellulosic biomass is passed through the bed, allowing microbial biofilms to degrade the substrate. Packed-bed reactors are simple and cost-effective but can suffer from clogging and uneven flow distribution. The fluidized-bed reactors use a suspended support material (e.g., sand or granular activated carbon) that is fluidized by the upward flow of liquid (Coelhoso et al. 1992). The fluidized bed provides a large surface area for biofilm formation and ensures good mixing and mass transfer. However, fluidized-bed reactors require careful control of flow rates to maintain fluidization. In trickling filters, lignocellulosic biomass is trickled over a stationary bed of support material, while air is supplied from below (Liu et al. 2016). Biofilms grow on the support material and degrade the biomass as it passes through. Trickling filters are energy-efficient but may require large footprints. Membrane biofilm reactors use membranes to support biofilm growth while allowing the passage of nutrients and products (Abera et al. 2024). Membranes can be designed to selectively retain enzymes and microbial cells, enhancing degradation efficiency. However, membrane reactors are expensive and prone to fouling.
Biofilm reactor has emerged as a promising approach for enhancing bioethanol production. Biofilms provide a natural and renewable architecture for enzyme immobilization, offering reduced diffusional resistance and high productivities (Vega et al. 1988). Previous studies have demonstrated the feasibility of using bacterial biofilms to immobilize multienzyme complexes, resulting in increased reaction rates and improved stability compared to free enzyme mixtures (Liu et al. 2021). For yeast-based ethanol production, biofilms on modified carriers can enhance fermentation efficiency and tolerance to inhibitors in lignocellulosic hydrolysates (Saeed et al. 2021). Many anaerobic, cellulolytic bacteria form biofilms on cellulosic substrates, such as
Clostridium phytofermentans,
Fibrobacter succinogenes and
Ruminococcus albus (Brethauer et al. 2020). The biofilm allows for a high concentration of cellulases at the boundary layer and a more complete capture of hydrolysis products directly at the hydrolysis site, which is energetically favourable. Enzyme immobilization techniques have been applied to various stages of bioethanol production, including pretreatment and hydrolysis, with laccases and cellulases being the most explored enzymes (de Araújo et al., 2023). These advancements in biofilm immobilization technology offer potential for improving the efficiency and reducing the costs of bioethanol production processes. Other works using biofilms to improve bioethanol yield are summarized in
Table 2.
Industrial Application of Biofilm-Based System
Biofilms have several industrial applications in bioethanol production, enhancing efficiency and sustainability. Biofilm-immobilized continuous fermentation has shown promise in enhancing cellular environmental tolerance, maintaining cell activity, and improving production efficiency. For example, a study by Wang et al. (2023) engineered Saccharomyces cerevisiae strains to overexpress biofilm-forming genes such as FLO5, FLO8, and FLO10. These engineered strains significantly improved biofilm formation, reduced the density of cells dispersed in the fermentation broth, and increased ethanol production during biofilm-immobilized continuous fermentation. This method also reduces the contamination of separation membranes when coupled with membrane separation, enhancing the effectiveness and stability of the process.
Mixed-species biofilms have also been explored for high-cell-density applications in bioethanol production. Mixed-species biofilms have shown promise for high-cell-density applications in various biotechnological processes. Hoschek et al. (2019) demonstrated the use of a mixed-species biofilm of Synechocystis and Pseudomonas for continuous cyclohexane oxidation, achieving high cell densities and maintained productivity. In microalgal biorefineries, mixed-species biofilms can address challenges related to resource utilization, biomass production, and harvesting (Wicker et al. 2022). These biofilms exhibit distinct structures and enhanced stress resistance compared to single-species biofilms. For instance, mixed-species biofilms showed increased resistance to antimicrobials through community-level interactions. In the brewing industry, mixed-species biofilms formed by direct cell-cell contact between yeasts and lactic acid bacteria have been observed (Furukawa et al. 2010). These studies highlight the potential of mixed-species biofilms for improving productivity and resilience in various biotechnological applications, including bioethanol production.
In another application, biofilms are used in combination with pervaporation membrane separation technology to enhance bioethanol productivity. Yeast-immobilized catalytically active membranes have shown promising results in fermentation-pervaporation coupling processes for bioethanol production. These systems enhance ethanol productivity by efficiently removing extracellular ethanol, reducing inhibition and improving yeast viability (Cao et al. 2020). Similar benefits have been observed in lipase-immobilized membranes for ester synthesis, demonstrating improved thermal stability and reusability compared to free enzymes (Zhang et al. 2014). Integrated systems combining immobilized yeast reactors with pervaporation modules allow for separate optimization of fermentation and separation parameters. Recent advancements include the use of composite membranes, such as silicalite-1/polydimethylsiloxane/polyvinylidene fluoride, which have demonstrated high ethanol productivity, separation factors, and permeate ethanol concentrations in both fed-batch and continuous fermentation-pervaporation processes (Cai et al. 2016). These developments highlight the potential of immobilized enzyme systems coupled with pervaporation for enhancing bioethanol production and other biocatalytic processes.
Challenges and Future Directions
The conversion of lignocellulose to ethanol using microbial biofilms is a promising approach for sustainable biofuel production. However, this process faces several challenges related to pretreatment, hydrolysis, and fermentation. Addressing these challenges is critical for improving the efficiency, scalability, and economic viability of bioethanol production.
Pretreatment is essential for breaking down the rigid structure of lignocellulose and making cellulose and hemicellulose accessible for enzymatic hydrolysis. However, the use of microorganisms, particularly biofilm-forming species, for pretreatment faces several challenges. Microbial pretreatment, especially by lignin-degrading fungi and bacteria, is often slower than chemical or physical methods. This limits its application in industrial-scale processes where high throughput is required. Microorganisms may not fully degrade lignin, leaving behind recalcitrant components that hinder subsequent hydrolysis. The growth and activity of biofilm-forming microorganisms depend on specific environmental conditions (such as pH, temperature, oxygen levels), which must be carefully controlled. To overcome these problems, researchers can develop genetically modified microorganisms with enhanced lignin-degrading capabilities and tolerance to inhibitors (Almeida and Hahn-Hägerdal 2009).
Hydrolysis involves the enzymatic breakdown of cellulose and hemicellulose into fermentable sugars. While biofilms can enhance hydrolysis by retaining enzymes and providing a stable environment, the production and use of cellulases and hemicellulases are expensive, contributing significantly to the overall cost of bioethanol production. In biofilm systems, poor mass transfer of substrates and enzymes within the biofilm can also limit hydrolysis rates. To address these challenges, researchers can develop advanced immobilization methods to retain enzymes within the biofilm and enhance their stability. For example, Dong et al. (2021) developed E. coli BL21::ΔCsgA-CsgB-CsgALBP2 (LBP2-functionalized) biofilms as surface display platforms to maximize the catalytic performance of lipase (Lip181). After immobilization onto LBP2-functionalized biofilm materials, Lip181 showed increased thermostability, pH, and storage stability.
Fermentation converts the sugars released during hydrolysis into ethanol. While biofilms can improve fermentation efficiency by immobilizing microorganisms and protecting them from inhibitors, many fermentative microorganisms, particularly yeast, cannot efficiently ferment pentose sugars (e.g., xylose), leading to incomplete sugar utilization and lower ethanol yields. Maintaining stable and active biofilms over long fermentation periods can be challenging due to biofilm detachment or overgrowth. To address these challenges, researchers can employ microbial consortia or co-cultures, which allow for the simultaneous fermentation of all available sugars. Additionally, the use of advanced biofilm reactors, such as rotating biological contactors or membrane biofilm reactors, can enhance scalability and efficiency (Abera et al. 2024).
Conclusions
Biofilms play a critical role in enhancing the conversion of lignocellulose to bioethanol. They improve pretreatment efficiency, stabilize enzymes, and enhance microbial fermentation, offering a promising solution to the challenges of lignocellulosic bioethanol production. Biofilm-based systems have the potential to revolutionize bioethanol production by improving process efficiency, reducing costs, and enhancing sustainability. Interdisciplinary collaboration between microbiologists, engineers, and biotechnologists is essential to fully realize the potential of biofilm technology in bioethanol production.
Author Contributions
Anati Abd Rashid Syaida carried out the research, wrote and revised the article. Mohd Taufiq Mat Jalil and Mohd Fakharul Zaman Raja Yahya conceptualised the central research idea, provided the theoretical framework and approved the article submission.
Funding
The authors would like to acknowledge the support of Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam, Selangor, Malaysia for providing the research facilities.
Acknowledgements
The authors would like to acknowledge the support of Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam, Selangor, Malaysia for providing the research facilities.
Conflict of Interest
The authors agree that this research was conducted in the absence of any self-benefits, commercial or financial conflicts and declare the absence of conflicting interests with the funders.
References
- Abera, G. B., Trømborg, E., Solli, L., Walter, J. M., Wahid, R., Govasmark, E.,... & Feng, L. (2024). Biofilm application for anaerobic digestion: a systematic review and an industrial scale case. Biotechnology for Biofuels and Bioproducts, 17(1), 145. [CrossRef]
- Ahmad, M., Taylor, C. R., Pink, D., Burton, K., Eastwood, D., Bending, G. D., & Bugg, T. D. (2010). Development of novel assays for lignin degradation: comparative analysis of bacterial and fungal lignin degraders. Molecular biosystems, 6(5), 815-821. [CrossRef]
- Almeida, J. R., & Hahn-Hägerdal, B. (2009). Developing Saccharomyces cerevisiae strains for second generation bioethanol: improving xylose fermentation and inhibitor tolerance.
- Amran, S. S. D., Syaida, A. A. R., Jalil, M. T. M., Nor, N. H. M., & Yahya, M. F. Z. R. (2024). Preparation of Biofilm Assay Using 96-Well and 6-Well Microplates for Quantitative Measurement and Structural Characterization: A Review. Science Letters, 18(2), 121-134.
- Bader, N. B., Germec, M., & Turhan, I. (2021). Scheffersomyces stipitis biofilm reactor for ethanol production from acid-pretreated/detoxified and glucose-or xylose-enriched rice husk hydrolysate under a continuous process. Biomass Conversion and Biorefinery, 11, 2909-2921. [CrossRef]
- Blume, L. R., Noronha, E. F., Leite, J., Queiroz, R. M., Ricart, C. A. O., de Sousa, M. V., & Felix, C. R. (2013). Characterization of Clostridium thermocellum isolates grown on cellulose and sugarcane bagasse. BioEnergy Research, 6, 763-775. [CrossRef]
- Bothare, V. (2025). Bioethanol market size, share & trends analysis report by feedstock (starch-based, sugar based, others), by blend (E10, E20 & E25, E70 & E75, E85, others, by application (transportation fuel, power generation, cosmetics, pharmaceutical, others) and by region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033 (SRCH56939DR). Straits Research. https://straitsresearch.com/report/bioethanol-market.
- Brethauer, S., Shahab, R. L., & Studer, M. H. (2020). Impacts of biofilms on the conversion of cellulose. Applied Microbiology and Biotechnology, 104, 5201-5212. [CrossRef]
- Brethauer, S., Shahab, R. L., & Studer, M. H. (2020). Impacts of biofilms on the conversion of cellulose. Applied Microbiology and Biotechnology, 104, 5201-5212. [CrossRef]
- Broda, M., Yelle, D. J., & Serwańska, K. (2022). Bioethanol production from lignocellulosic biomass—challenges and solutions. Molecules, 27(24), 8717. [CrossRef]
- Cai, D., Hu, S., Chen, C., Wang, Y., Zhang, C., Miao, Q., Qin, P., & Tan, T. (2016). Immobilized ethanol fermentation coupled to pervaporation with silicalite-1/polydimethylsiloxane/polyvinylidene fluoride composite membrane. Bioresource Technology, 220, 124-131. [CrossRef]
- Cao, Z., Xia, C., Jia, W., Qing, W., & Zhang, W. (2020). Enhancing bioethanol productivity by a yeast-immobilized catalytically active membrane in a fermentation-pervaporation coupling process. Journal of Membrane Science, 595, 117485. [CrossRef]
- Ceaser, R., Montané, D., Constantí, M., & Medina, F. (2024). Current progress on lignocellulosic bioethanol including a technological and economical perspective. Environment, Development and Sustainability. [CrossRef]
- Chacón-Navarrete, H., Martín, C., & Moreno-García, J. (2021). Yeast immobilization systems for second-generation ethanol production: Actual trends and future perspectives. Biofuels, Bioproducts and Biorefining, 15(5), 1549-1565. [CrossRef]
- Coelhoso, I., Boaventura, R., & Rodrigues, A. (1992). Biofilm reactors: an experimental and modeling study of wastewater denitrification in fluidized-bed reactors of activated carbon particles. Biotechnology and bioengineering, 40(5), 625-633. [CrossRef]
- de Araújo, B. M., Costa, I. O., de Brito, H. G., Rios, N. S., & dos Santos, E. S. (2023). Enzyme technology in bioethanol production from lignocellulosic biomass: Recent trends with a focus on immobilized enzymes. Bioresources, 18(4), 8653-8687.
- Danso, B., Ali, S. S., Xie, R., & Sun, J. (2022). Valorisation of wheat straw and bioethanol production by a novel xylanase-and cellulase-producing Streptomyces strain isolated from the wood-feeding termite, Microcerotermes species. Fuel, 310, 122333. [CrossRef]
- Deng, Y., & Wang, S. Y. (2022). Sorption of cellulases in biofilm enhances cellulose degradation by Bacillus subtilis. Microorganisms, 10(8), 1505. [CrossRef]
- Dionisi, D., Anderson, J. A., Aulenta, F., McCue, A., & Paton, G. (2015). The potential of microbial processes for lignocellulosic biomass conversion to ethanol: A review. Journal of Chemical Technology & Biotechnology, 90(3), 366-383. [CrossRef]
- Dong, J., Kim, D., & Yoo, C. G. (2023). Biochemical/biomaterial production from lignocellulosic biomass. Frontiers in Chemical Engineering, 5, 1266904. [CrossRef]
- Dong, H., Zhang, W., Xuan, Q., Zhou, Y., Zhou, S., Huang, J., & Wang, P. (2021). Binding peptide-guided immobilization of lipases with significantly improved catalytic performance using Escherichia coli BL21 (DE3) biofilms as a platform. ACS Applied Materials & Interfaces, 13(5), 6168-6179. [CrossRef]
- dos Santos, A. L. S., Galdino, A. C. M., de Mello, T. P., de Souza Ramos, L., Branquinha, M. H., Bolognese, A. M., neto, J. C., & Roudbary, M. (2018). What are the advantages of living in a community? A microbial biofilm perspective!. Memórias do Instituto Oswaldo Cruz, 113(9), e180212. [CrossRef]
- Dumitrache, A., Wolfaardt, G., Allen, G., Liss, S. N., & Lynd, L. R. (2013). Form and function of Clostridium thermocellum biofilms. Applied and Environmental Microbiology, 79(1), 231-239. [CrossRef]
- Furukawa, S., Yoshida, K., Ogihara, H., Yamasaki, M., & Morinaga, Y. (2010). Mixed-species biofilm formation by direct cell-cell contact between brewing yeasts and lactic acid bacteria. Bioscience, Biotechnology, and Biochemistry, 74(11), 2316-2319. [CrossRef]
- Güneş, K., Kaplangı, B. B., Özkan, A., & Kucuker, M. A. (2024). Algal Biofilm and Phycoremediation. In Phycoremediation of Wastewater (pp. 250-276). CRC Press.
- Hamann, P. R. V., Serpa, D. L., da Cunha, A. S. B., de Camargo, B. R., Osiro, K. O., de Sousa, M. V.,... & Noronha, E. F. (2015). Evaluation of plant cell wall degrading enzyme production by Clostridium thermocellum B8 in the presence of raw agricultural wastes. International Biodeterioration & Biodegradation, 105, 97-105.
- Hamdan, H. F., Ross, E. E. R., Jalil, M. T. M., Hashim, M. A., & Yahya, M. F. Z. R. (2024). Antibiofilm efficacy and mode of action of Etlingera elatior extracts against Staphylococcus aureus. Malaysian Applied Biology, 53(1), 27-34. [CrossRef]
- Henagamage, A. P. (2022). Evaluation of cellulolytic fungal-bacterial biofilms for the enhancement of bioethanol production. Journal of Science, 13(2), 13-28. [CrossRef]
- Hoschek, A., Heuschkel, I., Schmid, A., Bühler, B., Karande, R., & Bühler, K. (2019). Mixed-species biofilms for high-cell-density application of Synechocystis sp. PCC 6803 in capillary reactors for continuous cyclohexane oxidation to cyclohexanol. Bioresource Technology, 282, 171-178. [CrossRef]
- Ivanova, V., Petrova, P., & Hristov, J. (2011). Application in the ethanol fermentation of immobilized yeast cells in matrix of alginate/magnetic nanoparticles, on chitosan-magnetite microparticles and cellulose-coated magnetic nanoparticles. International Review of Chemical Engineering, 3(2), 289-299.
- Jørgensen, H., Kristensen, J. B., & Felby, C. (2007). Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels, Bioproducts and Biorefining, 1(2), 119-134. [CrossRef]
- Johari, N. A., Aazmi, M. S., & Yahya, M. F. Z. R. (2023). FTIR spectroscopic study of inhibition of chloroxylenol-based disinfectant against Salmonella enterica serovar Thyphimurium biofilm. Malaysian Applied Biology, 52(2), 97-107. [CrossRef]
- Kamaruzzaman, A.N.A., Mulok, T.E.T.Z., Nor, N.H.M., & Yahya, M.F.Z.R. (2022). FTIR spectral changes in Candida albicans biofilm following exposure to antifungals. Malaysian Applied Biology, 51(4), 57-66. [CrossRef]
- Koechler, S., Farasin, J., Cleiss-Arnold, J., & Arsène-Ploetze, F. (2015). Toxic metal resistance in biofilms: diversity of microbial responses and their evolution. Research in Microbiology, 166(10), 764-773. [CrossRef]
- Li, Y. Y., Huang, X. W., & Li, X. Y. (2021). Use of a packed-bed biofilm reactor to achieve rapid formation of anammox biofilms for high-rate nitrogen removal. Journal of Cleaner Production, 321, 128999. [CrossRef]
- Liu, B., Jarvis, I., Ren, H., Quang, C. N. D., Terashima, M., & Yasui, H. (2016). Biofilm Modelling and Kinetics in a Trickling Filter Process. Journal of Water and Environment Technology, 14(3), 200-210. [CrossRef]
- Liu, M., Han, P., Zhang, L., Zhong, C., & You, C. (2021). Biofilm-mediated immobilization of a multienzyme complex for accelerating inositol production from starch. Bioconjugate Chemistry, 32(9), 2032-2042. [CrossRef]
- Narayanaswamy, N., Dheeran, P., Verma, S., & Kumar, S. (2013). Biological pretreatment of lignocellulosic biomass for enzymatic saccharification. Pretreatment techniques for biofuels and biorefineries, 3-34.
- Nadir, N., Ismail, N. L., & Hussain, A. S. (2019). Fungal pretreatment of lignocellulosic materials. In: Abomohra, A. E. (Eds), Biomass for bioenergy-recent trends and future challenges. IntechOpen.
- Nigam, P. S. (2017). An overview of microorganisms' contribution and performance in alcohol fermentation processing a variety of substrates. Current Biotechnology, 6(1), 9-16. [CrossRef]
- Patwardhan, S. B., Pandit, S., Ghosh, D., Dhar, D. W., Banerjee, S., Joshi, S.,... & Kumar Kesari, K. (2024). A concise review on the cultivation of microalgal biofilms for biofuel feedstock production. Biomass Conversion and Biorefinery, 14(6), 7219-7236. [CrossRef]
- Quan, K., Hou, J., Zhang, Z., Ren, Y., Peterson, B. W., Flemming, H. C., … van der Mei, H. C. (2021). Water in bacterial biofilms: pores and channels, storage and transport functions. Critical Reviews in Microbiology, 48(3), 283–302. [CrossRef]
- Radecka, D., Mukherjee, V., Mateo, R. Q., Stojiljkovic, M., Foulquié-Moreno, M. R., & Thevelein, J. M. (2015). Looking beyond Saccharomyces: The potential of non-conventional yeast species for desirable traits in bioethanol fermentation. FEMS Yeast Research, 15(6), fov053. [CrossRef]
- Robak, K., & Balcerek, M. (2020). Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiological Research, 240, 126534. [CrossRef]
- Saeed, Z., Palamae, S., Intharapat, P., Khundamri, N., Tanrattanakul, V., Tirawanichakul, Y., & Suttinun, O. (2021). Enhanced ethanol production from lignocellulosic hydrolysate using Meyerozyma caribbica biofilm immobilized on modified epoxy foam. Biomass and Bioenergy, 154, 106267. [CrossRef]
- Sasmal, S., & Mohanty, K. (2018). Pretreatment of lignocellulosic biomass toward biofuel production. In: Kumar, S., & Sani, R. (Eds), Biorefining of biomass to biofuels: Opportunities and perception (pp. 203-221). Springer, Cham.
- Shao, Q., Li, X., Chen, Y., Zhang, Z., Cui, Y., Fan, H., & Wei, D. (2022). Investigations on the Fusants From Wide Cross Between White-Rot Fungi and Saccharomyces cerevisiae Reveal Unknown Lignin Degradation Mechanism. Frontiers in Microbiology, 13, 935462. [CrossRef]
- Shukla, A., Kumar, D., Girdhar, M., Kumar, A., Goyal, A., Malik, T., & Mohan, A. (2023). Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnology for Biofuels and Bioproducts, 16(1), 44. [CrossRef]
- Shukla, S. K., Manobala, T., & Rao, T. S. (2021). Biofilms: Naturally immobilized microbial cell factories. In: Tripathi, A., Melo, J. S. (Eds), Immobilization Strategies: Biomedical, Bioengineering and Environmental Applications (pp. 535-555). Springer, Singapore.
- Tian, J., Lin, Y., Su, X., Tan, H., Gan, C., & Ragauskas, A. J. (2023). Effects of Saccharomyces cerevisiae quorum sensing signal molecules on ethanol production in bioethanol fermentation process. Microbiological Research, 271, 127367. [CrossRef]
- Todhanakasem, T., Narkmit, T., Areerat, K., & Thanonkeo, P. (2015). Fermentation of rice bran hydrolysate to ethanol using Zymomonas mobilis biofilm immobilization on DEAE-cellulose. Electronic Journal of Biotechnology, 18(3), 196-201. [CrossRef]
- Todhanakasem, T., Salangsing, O. L., Koomphongse, P., Kaewket, S., Kanokratana, P., & Champreda, V. (2019). Zymomonas mobilis biofilm reactor for ethanol production using rice straw hydrolysate under continuous and repeated batch processes. Frontiers in Microbiology, 10, 1777. [CrossRef]
- Van Kuijk, S. J. A., Sonnenberg, A. S. M., Baars, J. J. P., Hendriks, W. H., & Cone, J. W. (2015). Fungal treated lignocellulosic biomass as ruminant feed ingredient: a review. Biotechnology advances, 33(1), 191-202. [CrossRef]
- Vega, J. L., Clausen, E. C., & Gaddy, J. L. (1988). Biofilm reactors for ethanol production. Enzyme and Microbial Technology, 10(7), 390-402. [CrossRef]
- Wan, C., & Li, Y. (2012). Fungal pretreatment of lignocellulosic biomass. Biotechnology Advances, 30(6), 1447-1457. [CrossRef]
- Wang, Z., Lee, S., Elkins, J., & Morrell-Falvey, J. L. (2011). Spatial and temporal dynamics of cellulose degradation and biofilm formation by Caldicellulosiruptor obsidiansis and Clostridium thermocellum. AMB Express, 1, 30. [CrossRef]
- Wang, Z., Xu, W., Gao, Y., Zha, M., Zhang, D., Peng, X., Zhang, H., Wang, C., Xu, C., Zhou, T., Liu, D., Niu, H., Liu, Q., Chen, Y., Zhu, C., Guo, T., & Ying, H. (2023). Engineering Saccharomyces cerevisiae for improved biofilm formation and ethanol production in continuous fermentation. Biotechnology for Biofuels and Bioproducts, 16(1), 119. [CrossRef]
- Weiler, J., Edel, M., & Gescher, J. (2024). Biofilms for production of chemicals and energy. Annual review of chemical and biomolecular engineering, 15.
- Wicker, R. J., Kwon, E., Khan, E., Kumar, V., & Bhatnagar, A. (2023). The potential of mixed-species biofilms to address remaining challenges for economically-feasible microalgal biorefineries: A review. Chemical Engineering Journal, 451(Part 1), 138481. [CrossRef]
- Yaacob, M. F., Murata, A., Nor, N. H. M., Jesse, F. F. A., & Yahya, M. F. Z. R. (2021). Biochemical composition, morphology and antimicrobial susceptibility pattern of Corynebacterium pseudotuberculosis biofilm. Journal of King Saud University-Science, 33(1), 101225. [CrossRef]
- Yahya, M. F. Z. R., Alias, Z., & Karsani, S. A. (2017). Subtractive protein profiling of Salmonella typhimurium biofilm treated with DMSO. The Protein Journal, 36, 286-298. [CrossRef]
- Yahya, M. F. Z. R., Alias, Z., & Karsani, S. A. (2018). Antibiofilm activity and mode of action of DMSO alone and its combination with afatinib against Gram-negative pathogens. Folia microbiologica, 63, 23-30. [CrossRef]
- Yang, B., Dai, Z., Ding, S. Y., & Wyman, C. E. (2011). Enzymatic hydrolysis of cellulosic biomass. Biofuels, 2(4), 421-450. [CrossRef]
- Yin, W., Wang, Y., Liu, L., & He, J. (2019). Biofilms: the microbial “protective clothing” in extreme environments. International journal of molecular sciences, 20(14), 3423. [CrossRef]
- Zhang, W., Qing, W., Ren, Z., Li, W., & Chen, J. (2014). Lipase immobilized catalytically active membrane for synthesis of lauryl stearate in a pervaporation membrane reactor. Bioresource Technology, 172, 16-21. [CrossRef]
- Zoghlami, A., & Paës, G. (2019). Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Frontiers in Chemistry, 7, 874. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).