1. Introduction
The introduction should briefly place the study in a broad context and highlight why it is important. It should define the purpose of the work and its significance. The current state of the research field should be carefully reviewed and key publications cited. Please highlight controversial and diverging hypotheses when necessary. Finally, briefly mention the main aim of the work and highlight the principal conclusions. As far as possible, please keep the introduction comprehensible to scientists outside your particular field of research. References should be numbered in order of appearance and indicated by a numeral or numerals in square brackets—e.g., [
1] or [
2,
3], or [
4,
5,
6]. See the end of the document for further details on references.
Cutting gaseous emissions into the atmosphere is the primary global climate objective today. To reach the goal to keep the rise of temperature below 1.5 °C drastic reductions in emissions must be accompanied by the removal of a large quantity of carbon dioxide from the atmosphere [
1]. According to the Intergovernmental Panel on Climate Change (IPCC) [
2] about 730 billion tons of CO
2 must be removed from atmosphere within this century. Most carbon is absorbed and fixed in terrestrial and aquatic ecosystems (
Table 1, [
3]).
Terrestrial ecosystems store about 2,100 GtC in living organisms, litter and soil organic matter, which is almost three times that currently exist in the atmosphere. The world’s forests store approximately 861 GtC, with 44% in soil (to one-meter depth), 42% in living biomass (above- and belowground), 8% in dead wood, and 5% in litter. In total, this is equivalent to nearly a century’s worth of current annual fossil fuel emissions at mondial level [
4]. Forests cover about 4 billion ha (30% of the world land surface) with 3,000 billion trees [
5] and store, in vegetation and soil, about half of the total terrestrial carbon.
Stopping deforestation, especially in the intertropical zone, improving existing forests and increasing their extension, is a fundamental strategy to fight climate change [
6] and is part of the European strategy for biodiversity, which provides for the planting of 3 billion trees by 2030. However, in the application of this strategy, limitations and trade-offs are encountered due to conflicts with socio-economic interests, ecological incompatibilities, limitation of resources and species-specific eco-physiological behaviors that induce unexpected responses or cause the opposite effect to what was expected.
The analysis of ecological trade-offs, the physiological mechanisms regulating the relationships and feedback between trees and climate, the ecological and socio-economic impacts of forest plantations on ecosystems should guide the selection of tree species in afforestation and forest management plans.
Planting trees for climate purposes implies a complex theoretical procedure to select the best suited species, sites and technical actions. In this paper we address these issues to draft a general framework for climate management of forests and tree plantations.
2. Trade-Off Between Tree Growth and Persistence
Trees absorb and store carbon based on two factors: their growth rate and size potential, and their longevity and persistence. However, enhancing one factor can negatively affect the other.
High growth rates in young trees are characteristic of early successional species [
7] and align with their strategy to quickly occupy available sites. These fast-growing trees thrive in environments rich in resources, require significant water, have low wood density [
8], and exhibit high susceptibility to pathogens and environmental challenges because the metabolic investment in growth reduces the allocation for defense substances [
9]. This inverse relationship, known as the growth-defense trade-off [
10], occurs because the resources and energy that could be allocated for defense are instead used for growth.
The persistence of trees is influenced by the ecological suitability of species for a given location and their lifespan. Species potentially persistent are typically late successional, characterized by slower growth rates and lower metabolic activity compared to early successional species [
7]. The longevity of trees can be explained by the "rate of living" theory [
11], which posits that lifespan is inversely related to metabolic activity, such as photosynthesis and respiration. Consequently, longer lifespans are associated with very slow growth, particularly in resource-poor environments. Factors contributing to tree longevity contrast with those promoting height and rapid growth, which are more prevalent in resource-rich areas [
12].
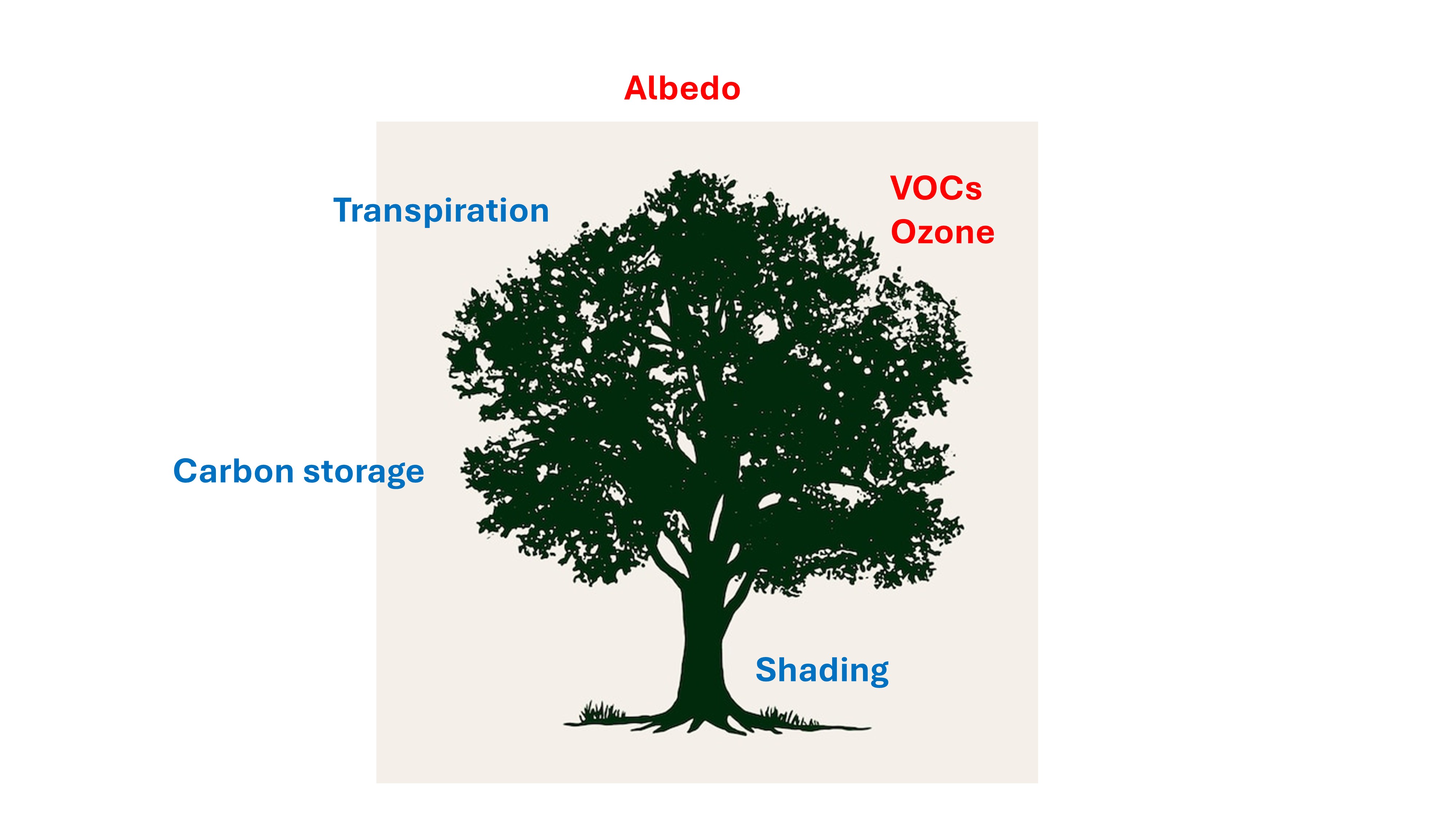
3. Positive and Negative Influences of Trees on Climate
Trees influence the climate through different mechanisms [
13], with positive (mitigation of temperatures) or negative (increase of temperatures) effects, depending on tree species and site-species interactions (Table 2).
Atmospheric CO2 sequestration. The pre-industrial concentrations of CO
2, at world level, were about 280 ppm, whereas at the present (January 2025) they reached 426,65 ppm at the Mauna Loa observatory (Hawaii, USA) [
14]. CO
2 is a greenhouse gas because it absorbs and re-emits IR (thermal) radiation. Vegetation absorbs CO
2 through the photosynthetic function. Trees are especially effective because they can store carbon products (cellulose, lignin) in their woody tissues for a long time [
15]. In addition, forests and natural ecosystems store permanently carbon in the soil [
16].
Stomatal transpiration of water by stomata is a fundamental cooling mechanism. This process is energy consuming as latent heat, and can have a relevant local impact on temperatures, with local reductions until 8°C [
17]. Alongside transpiration, an important local action on climate is made by shading. Trees reduce temperatures under their canopies by an average of 4.1°C [
18]. During extremely hot summer days, the reduction can be as much as 10°C. This effect is relevant for the understory forest layer, favoring regeneration and biodiversity [
19].
Albedo effect refers to the phenomenon where forests absorb more solar radiation, leading to a local warming influence. This effect can diminish or counteract the climatic benefits of carbon sequestration by forests. Afforestation in boreal and high-latitude temperate regions can result in near-term warming due to reduced surface albedo, which may exceed the cooling benefits from carbon sequestration [
20]. The albedo effect can be relevant in all the conditions in which the bare soil is more reflective than the crowns of trees. In most cases there is at least a 20% albedo offset in temperature regulation [
21].
Biogenic emissions. Some tree species emit biogenic substances (BVOCs, biogenic volatile organic substances) that, through atmospheric chemical reactions, produce greenhouse gases and toxic substances like ozone [
22;
23]. The emission of BVOCs, for example isoprene, is in turn influenced by factors related to climate change, such as CO
2 and high temperatures, that enhance enzymatic activity. In the last 30 years the production of BVOCs increased of 10% and a further increase of temperatures (2-3 °C) may lead to +30-45% of BVOC emissions [
22]. BVOC emissions represent a small but significant component of the carbon cycle. They can account for up to 5-10% of total net carbon exchange, particularly under stress conditions.
4. Stimulation and Limitation of Tree Growth Induced by Climate Change Factors
Trees influence the climate at global and local levels, and, in turn, they are influenced by climate change. The responses of trees to climate change factors (increased CO2 concentrations, increased temperature, increased N deposition) can determine negative feedback, i.e., stimulate the growth rates and carbon uptake. This beneficial effect, however, can be threatened by concurrent trade-offs and limitations.
Higher atmospheric CO
2 concentration increases the rate of photosynthesis by improving carbon assimilation as well as water use efficiency (iWUE) and resistance to drought [
24]. Higher CO
2 concentration increased global annual terrestrial photosynthesis by 13.5 ± 3.5% between 1981 and 2020, contributing significantly to the overall terrestrial carbon sink [
25]. On the other hand, growth stimulation by CO
2 reduces tree longevity [
26].
Rising temperatures, at least until reaching the optimum for photosynthesis [
27], stimulate the enzymatic activity of the Calvin cycle of photosynthesis. Higher temperatures also advance bud break and delay leaf abscission, thus extending the growing season [
28]. However, globally the average temperature of the warmest quarter is very close to exceeding the optimum [
29] and with further temperature increases, the capacity of terrestrial vegetation to absorb carbon will significantly decrease and forests could become a carbon source by 2040, if emissions continue at current rates.
Increased winter temperatures can cause delayed sprouting because it takes longer to meet the demand for vernalization [
30]. Furthermore, high temperatures and summer drought can cause premature senescence in deciduous species, effectively shortening the growing season [
31].
Increased temperatures, moreover, cause increased production of BVOCs and biogenic ozone. Background concentrations of ozone constitute a signal inducing plant resistance thanks to the production of defense compounds, such as secondary metabolites [
32]. However, increasing ozone concentrations have phytotoxic effects on tree growth and health [
33].
Elevated nitrogen (N) deposition, resulting from anthropogenic activities, can lead to nitrogen saturation in forest ecosystems. While initial nitrogen addition may stimulate growth, the excess N supply can cause a net decrease in tree vitality via complex and interlinked mechanisms, including increased susceptibility to insect attacks, pathogens, frost and storm damages [
34]. Moreover, the increased nitrogen availability can disrupt the balance of other essential nutrients, particularly phosphorus, leading to reduced phosphorus concentrations in foliage and subsequent growth limitations [
35;
36].
Tree growth stimulation by carbon fertilization, nitrogen deposition and higher temperatures is declining globally [
37;
38;
39], mainly due to two limiting factors: (i) soil limitations and (ii) water limitations. Increased carbon availability cannot be converted into organic compounds due to the poor fertility of forest soils; moreover reduced precipitation and increased air dryness that causes stomatal closure and carbon starvation in trees.
Table 2.
Main trade-offs and limitations concerning the effectiveness of tree planting to counteract climate change.
Table 2.
Main trade-offs and limitations concerning the effectiveness of tree planting to counteract climate change.
| ▪Albedo and VOCs production offset the mitigation of climate operated by carbon storage, transpiration and shading. |
| ▪ CO2 and nitrogen fertilization, and higher temperatures, stimulate growth and carbon storage, but reduce resistance and persistence. |
| ▪ Water and soil limitations, as well as above optimal temperatures, offset the carbon gains due to carbon and nitrogen fertilization. |
| ▪ Tree plantation in inappropriate sites and with inappropriate species may deplete soil resources (water, nutrients), threat biodiversity and provoke carbon loss from soil. |
| ▪ Species resistant and adapted to future climatic conditions have lower growth potential and store carbon in soil more than in trees. |
| ▪ Climate change driven forest disturbance enhances carbon loss from plantations and natural forests. |
5. Tree Plantations
Global potential tree coverage shows a potential of 4.400 MHa of canopy cover at global level [
40]. Excluding already existing forests, as well as agricultural and urban areas, there is room for an extra 900 MHa of canopy cover, which could store an additional 205 Gtons of carbon over a 100-year period [
40]. This value represents about a third of the carbon emissions that have occurred to date. Most of the areas susceptible to reforestation are in the intertropical zone, where deforestation was more intense in the past. In the temperate mediterranean regions the existing forests appear to be expanding spontaneously [
41], and forestation interventions can be made especially in urban and peri-urban areas, where the potential beneficial effects on the climate and local ecosystem services are greater [
42]. About 141 to 322 Mha are globally located in urban or peri-urban areas [
42].
According to Veldman [
43], the estimates reported by [
40] are too optimistic and propose a more conservative assessment, predicting a removal of 40 to 100 Gt of carbon until maturity of the trees on 900 million hectares. This amount, although significant, would only represent the value of 10 years of emissions at the current rate. Furthermore, the estimates presented do not always consider the socio-economic, physical and ecological limits that prevent or discourage the planting of new forests, and slow down the growth of trees, so the potential carbon storage is often overestimated [
44].
The area of planted forests increased from 167.5 to 277.9 M hectares, representing 4.06% to 6.95% of total forest area [
45]. Annual rates of increase in forested area were highest in the 1990–2000 period (2.0%) and the 2000–2005 period (2.7%) but dropped in 2005–2010 (1.9%) and further in 2010–2015 to 1.2% [
45]. The socio-economic factors limiting tree plantations [
46] concern the rupture with the traditional landscape and uses of the landscape, as well as the limitation to usual agricultural activities. The ecological disadvantages [
45] may be related to the high demand of water and nutrient of plantations so reducing the fertility of soils, the degradation of soil in consequence of the works for establishment and maintenance, the loss of biodiversity and the impact on previously existing ecosystems. These socio-economic and ecological disadvantages highlight the need for careful planning and management of tree plantations to mitigate their negative environmental impacts. Tree plantation, on the other hand, allows to reduce the pressure on natural forests for woody products.
Fast-growing tree species are commonly used for commercial plantings. Monocultures of fast-growing species can sequester carbon more rapidly than naturally regenerating forests, especially during the early phases of establishment, and under optimal cultivation conditions they can constitute an effective sink [
47;
48;
49]. According to [
47], a national planting strategy of commercial forests in the UK could achieve cumulative GHG mitigation of up to 1.64 Pg CO
2e by 2120. This is compared to 0.54 Pg CO
2e for semi-natural broadleaf conservation forests and 1.09 Pg CO
2e for a mixed planting strategy of commercial and conservation forests. The long-term terrestrial carbon storage is reduced by 61% due to harvesting, but the overall mitigation is enhanced by harvested carbon storage in wood products and the substitution of fossil fuels. Moreover, frequent harvesting and replanting operations favor the release of carbon from soil and reduce the belowground organic biomass.
Which species for the future
The selection of species for new plantations considers the climatic adaptability of species to both current and future conditions expected for the twenty-first century [
50]. To select tree species suitable for climate change, it is necessary to use an approach based on climate and ecological models that project the redistribution of bioclimatic units and evaluate the feasibility of species based on future climate scenarios. This involves validating the selected species and provenances through common garden experiments [
51]. These experiments allow the study of tree species performance in response to various environmental factors, such as climate, and are used to identify species and provenances that can best adapt to future climate conditions. Applying these criteria within an assisted migration framework [
52;
53;
54] enables proactive anticipation of vegetation changes and informs forestation decisions. Promoting invasion by non-native trees into areas with treeless vegetation could contribute to climate-change mitigation by increasing carbon (C) sequestration; however, the adverse effects of tree invasions on biodiversity, economic opportunities, and water yield may counterbalance any positive impacts on C sequestration [
55].
6. Managed and Unmanaged Forests
Managed forests
Globally, managed forests are about 50 years younger, have 25% more coniferous stands, and possess 50% less carbon stocks than unmanaged forests [
56]. While gross primary productivity (GPP) and net primary productivity (NPP) are similar between managed and unmanaged forests, managed forests allocate more assimilated carbon to aboveground pools and less to fine roots and rhizosymbionts. Managed forests also exhibit higher heterotrophic respiration (Rh), indicating greater soil carbon decomposition, potentially resulting in a carbon deficit compared to natural forests. Although fertilization and nutrient availability boost soil productivity in managed forests, maximizing merchantable productivity may significantly impact soil carbon levels.
Close to Nature Silviculture (CNS) is considered a useful tool for adapting European temperate forests to climate change [
57]. CNS involves interventions that preserve or increase taxonomic and structural diversity and improve the ability of trees to respond to environmental stresses. Specific objectives are: (i) to preserve or increase carbon stock by promoting multi-layered and highly diverse formations that allow the occupation of different ecological niches; (ii) to maintain canopy cover to limit soil respiration and protect the understory microclimate to promote natural regeneration and biodiversity; (iii) to control (at least in the most critical environments) competition for resources through thinning [
58]. Thinning may conflict with the need to maintain canopy closure; therefore, each intervention must be evaluated in relation to the specific conditions at the sites.
Tree diversity favors growth and productivity in forests [
59;
60] by improving the overall functionality of the system and through the occupation of different ecological niches by different tree species [
61]. Crown packaging [
62], for example, is an ecological mechanism that allows tree species, with different growth potential and light requirement, to occupy the aboveground space.
An approach based on the genetic adaptation of forests aims to select the most resistant species and genotypes within a community, or to introduce genotypes selected according to assisted migration criteria. Experiences conducted in southern Germany [
63;
64] have shown that the so-called “minor species” (
Acer campestre,
Ulmus minor,
Sorbus torminalis etc.) have a higher drought resistance than the dominant species (especially
Fagus sylvatica) and can represent a valid crop alternative. This approach can favor the establishment of a more ecologically stable stand, but with lower growth and carbon sequestration potential.
Unmanaged forests
Old-growth unmanaged forests can continue to accumulate carbon, contrary to the long-standing view that they are carbon neutral [
65]. Half of the primary forests (6 × 10
8 hectares) are located in the boreal and temperate regions of the Northern Hemisphere. These forests alone sequester about 1.3 ± 0.5 Gt of carbon per year provide at least 10 per cent of the global net ecosystem productivity. The 250-years old beech forest in Hainich National Park (Germany) showed significant carbon uptake, with 494 g C m
−2 per year in 2000 and 490 g C m
−2 per year in 2001, contradicting the hypothesis that advanced forests are insignificant as carbon sinks [
66]. Unmanaged forests, at a late stage of development, can still act as significant carbon sinks.
7. Forest Disturbances
Although the forests are considered as stable carbon sinks for very long periods, there are many disturbing factors that make these formations extremely dynamic. Increases in tree mortality [
67;
68;
69;
70] and tree crown defoliation [
71] over time have been detected in forest ecosystems around the globe as direct consequence of climate change (climate extremes, such as heat, atmospheric aridity, soil drought, storms) and other related disturbances (fire severity, insect outbreaks, and spread of invasive insects and parasites). Such disturbances provoke loss of carbon storage [
72] and growth reduction [
73].
Understanding trends and causes of tree mortality and defoliation is a crucial issue, because forests have for decades been responsible for a net annual uptake of about 20% of the carbon dioxide released by human activities [
4;
74]. Yet, projections of the future of this sink diverge dramatically with tree mortality rates and defoliation emerging as one of the key uncertainties.
8. Discussion and Conclusions
Forests are an important component of the overall strategy for climate change mitigation. They are valuable and important carbon sinks and provide many other ecosystem services related to biodiversity. Transforming grasslands, shrublands, and wetlands into tree plantations, however, not only compromises the environmental values of these formations, but causes an increase in carbon emissions, through soil respiration, resulting from planting work. For these reasons the priority is to conserve and improve existing forests and natural ecosystems. Natural forests represent over 94% of the global forest area and therefore play a key role in climate regulation. The adaptation of such ecosystems to climate change and the recovery of forests degraded by fire, insects and other pathogens, or extreme climate events, are of priority importance with respect to tree plantation.
Table 3 summarizes the priority action to act in forestry to counteract climate change.
Existing forests currently store carbon below their potential. There are 287 petagrams (1 PgC = 10
15 gC) of unrealized potential, of which 78% as biomass and 22% in soil [
75]. Three-quarters of this potential can be realized through more efficient forest management, with the majority (71%) concentrated in tropical forests [
75]. According to [
76] currently carbon storage has an overall deficit of 226 Gt, of which 61% in existing forests, for which a protection and improvement management are recommended, and the remaining 39% in areas where forests have been removed or fragmented.
The ongoing processes (spontaneous or regulated) of forest adaptation to climate change favor the affirmation of thermophilic and xerophilic species, slow growing, and with a greater capacity for persistence even in the most disadvantaged sites. Examples of this are the replacement of
Pinus sylvestris with
Quercus pubescens in south of the Alps [
77], the transformation of
Quercus ilex forest into Mediterranean scrubland in coastal areas of central Italy [
78] and the expansion of
Quercus ilex in the Pyrenees to the detriment of
Fagus sylvatica [
79]. Changes in structure and species composition suggest that forest adaptation to climate change occurs at the expense of tree growth [
80], and long timescales will therefore be needed to reach or restore carbon stocks adequate for climate protection purposes.
The simplistic assumption that planting trees can immediately compensate for cutting intact forests is widespread but false [
81]. Planting new forests is a more effective measure when trees are planted in previously deforested areas [
82;
83;
84]. The largest areas for reforestation are in the intertropical regions [
82], whereas in temperate and Mediterranean zones (where the areas for new forests are limited, and a spontaneous expansion of the existing forests is happening in abandoned lands [
85], new forests can be realized mostly in peri-urban areas.
Considering the actual global potential of forestation in removing CO
2 [
43] and the ecological limitations connected, forest carbon sequestration should only be viewed as a component of a mitigation strategy, not as a substitute for the changes in energy supply, use and technology that will be required if atmospheric CO
2 concentrations are to be stabilized. Without strong reductions in emissions, this strategy holds low mitigation potential. Forest sink should be preserved to offset residual carbon emissions rather than to compensate for present emissions levels [
86].
We conclude, with Brancalion and Holl [
87], that planting trees, together with other strategies to increase forest cover in appropriate places and contexts, can make a valuable contribution to ensuring the ecological and social well-being of our planet in the coming decades, but only if these efforts are considered as one component of a multifaceted action of solutions to complex environmental problems. In addition, it should be carefully planned, implemented and monitored on a sufficiently long-time scale [
88], with stakeholder involvement and broader consideration of socio-economic complexities.
Author Contributions
FB and MP contributed in equal measure to conceptualization and writing—review and editing. Both authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support from the National Biodiversity Future Center to University of Florence, funded by the Italian Ministry of University and Research, PNRR, Missione 4 Componente 2, “Dalla ricerca all’impresa”, Investimento 1.4, Project CN00000033.
Data Availability Statement
No data available.
Acknowledgments
No acknowledgments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IPCC: Global Warming of 1.5°C. In: An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Eds: Masson-Delmotte, V., P.; Zhai, H.-O.; Pörtner, D.; Roberts, J.; Skea, P.R.; Shukla, A.; Pirani, W., Moufouma-Okia, C.; Péan, R.; Pidcock; et al. S. Cambridge University Press, Cambridge, UK and New York, NY, USA, 2018, 616 pp. [CrossRef]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.A.; Koch, A. Regenerate natural forests to store carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.W.; Thawale, P.R.; Sharma, J.K.; Gautam, R.K.; Kundargi, G.P.; Juwarkar, A.A.,Chapter 3 - Carbon Sequestration in Terrestrial Ecosystems. In: Hydrogen Production and Remediation of Carbon and Pollutants, Lichtfouse, E.; Schwarzbauer, J. Eds, Environmental Chemistry for a Sustainable World 6, Springer International Publishing Switzerland. 2015. Pages. 99-130. [CrossRef]
- Pan, Y.; Birdsey, R.A. ; Fang J. ; Houghton, R. ; Kauppi, P.E. ; Kurz, W.A. ; Phillips, O.L. ; Shvidenko, A.; Lewis ,S.L.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988-993. [CrossRef]
- FAO. State of the World Forests. Forest-sector innovations towards a more sustainable future Food and Agriculture Organization of the United Nations Rome, 2024. Pp. 122.
- Goymer, P. A trillion trees. Nature Ecol. Evolut. 2018, 2, 208–209. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The physiological ecology of plant succession. Ann Rev. Ecol. Syst. 1979, 10, 351–371. [Google Scholar] [CrossRef]
- Büntgen, U.; Krusic, P.J.; Piermattei, A.; Coomes, D.A.; Esper, J.; Myglan, V.S.; Kirdyanov, A.V.; Camarero, J.J.; Crivellaro, A.; Körner, C. Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nature Comm. 2019, 10, 2171. [Google Scholar] [CrossRef]
- Wasternak, C. A plant’s balance of growth and defense – revisited. New Phytol. 2017, 215, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef] [PubMed]
- Issartel, J.; Coiffard, C. Extreme longevity in trees: live slow, die old? Oecologia 2011, 165, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Brienen, R.J.W.; Fan, C.; Hao, M.; Zhao, X. , Zhang; C. Tree Lifespans in a Warming World: Unravelling the Universal Trade-Off Between Growth and Lifespan in Temperate Forests. Glob Change Biol. 2025, 31, e70023. [CrossRef]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Harde, H. Understanding Increasing Atmospheric CO2. Sci. Clim. Change 2023, 3.1, 46- 67. [CrossRef]
- Teskey, R.O.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef]
- Basile-Doelsch, I.; Balesdent, J.; Pellerin, S. Reviews and syntheses: The mechanisms underlying carbon storage in soil. Biogeosciences 2020, 17, 5223–5242. [Google Scholar] [CrossRef]
- Winbourne, J.B.; Jones, T.S.; Garvey ,S.M.; Harrison, J.L.; Wang, L.; Li D.; Templer, P.H.; Hutyra, L.R.. Tree Transpiration and Urban Temperatures: Current Understanding; Implications, and Future Research Directions. BioScience 2020, 70, 576-588. [CrossRef]
- Verheyen, K.; Gillerot, L.; Blondeel, H.; De Frenne, P.; De Pauw, K.; Depauw, L.; Lorer, E.; Sanczuk, P.; Schreel, J.; Vanneste, T.; et al. Forest canopies as nature-based solutions to mitigate global change effects on people and nature. J. Ecol. 2024, 112, 2451–2461. [Google Scholar] [CrossRef]
- De Frenne, P.; Graae, B.J.; Brunet, J.; Shevtsova, A.; De Schrijver, A.; Chabrerie, O.; Cousins, S.A.O.; Decocq, G.; Diekmann, M.; Hermy, M.; et al. The response of forest plant regeneration to temperature variation along a latitudinal gradient. Ann. Bot. 2012, 109, 1037–1046. [Google Scholar] [CrossRef]
- Lintunen, J.; Rautiainen, A.; Uusivuori, J. Which Is more Important, Carbon or Albedo? Optimizing Harvest Rotations for Timber and Climate Benefits in a Changing Climate. American Journal of Agricultural Economics 2022, 104, 134–160. [Google Scholar] [CrossRef]
- Hasler, N.; Williams, C.A.; Denney, V.C.; Ellis, P.W.; Shrestha, S.; Terasaki, Hart D.E.; Wolff, N.H.; Yeo, S.; Crowther, T.; et al. Accounting for albedo change to identify climate-positive tree cover restoration. Nature Comm. 2024, 15, 2275. [CrossRef]
- Peñuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Popkin, G. How much can forests fight climate change? Nature 2019, 565, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. G.; Bergeron, Y.; Denneler, B.; Berninger, F.; Tardif, J. Response of Forest Trees to Increased Atmospheric CO2.Critical Reviews in Plant Sciences, 26, 2007, 265–283. [CrossRef]
- Keenan, T.F.; Luo, X.; Stocker, B.D; De Kauwe, M. G.; Medlyn, B. E.; Prentice, I. C.; Smith, N. G.; Terrer, C. , Wang; H., Zhang, Y.; et al. A constraint on historic growth in global photosynthesis due to rising CO2. Nat. Clim. Chang. 2023, 13, 1376–1381. [Google Scholar] [CrossRef]
- Bugmann, H.; Bigler, C. Will the CO2 fertilization effect in forests be offset by reduced tree longevity? Oecologia 2011, 165, 533–44. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. , 2004. Direct and Indirect Climate Change Effects on Photosynthesis and Transpiration. Plant Bio. 2004, 6(3), 242–253. [Google Scholar] [CrossRef]
- Linderholm, H.W. Growing season changes in the last century. Agricultural and Forest Meteorology 2006, 137, 1–14. [Google Scholar] [CrossRef]
- Duffy, K.A.; Schwalm, C.R.; Arcus, V.L.; Koch, G.W.; Liang, L.L.; Schipper, L.A. How close are we to the temperature tipping point of the terrestrial biosphere? Sci. Adv. 2021, 7, eaay1052. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Campioli, M.; Deckmyn, G.; Janssens, I.A. The Impact of Winter and Spring Temperatures on Temperate Tree Budburst Dates: Results from an Experimental Climate Manipulation. Plos One 2012, 7, e47324. [Google Scholar] [CrossRef]
- Rahmati, M.; Graf, A.; Poppe Terán, C.; Amelung, W. , Dorigo W.; Hendricks Franssen H-J.; Montzka C.; Or D.; Sprenger M.; Vanderborgh J.; et al. Continuous increase in evaporative demand shortened the growing season of European ecosystems in the last decade. Commun Earth Environ 2023, 4, 236. [Google Scholar] [CrossRef]
- Singh, A.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Secondary metabolites responses of plants exposed to ozone: an update. Environ Sci Pollut Res 2023, 30, 88281–88312. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropspheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Glob. Change Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Schmitz, A.; Sanders, T.G.M.; Bolte, A.; Bussotti, F.; Dirnböck, T.; Johnson, J.; Peñuelas, J.; Pollastrini, M.; Prescher, A.-K.; Sardans, J.; Verstraeten, A.; et al. Responses of forest ecosystems in Europe to decreasing nitrogen deposition. Environ. Pollut. 2019, 244, 980–994. [Google Scholar] [CrossRef]
- Braun, S.; Thomas, V.F.D.; Quiring, R.; Flückiger, W. Does nitrogen deposition increase forest production? The role of phosphorus. Environ. Pollut. 2010, 158, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Jonard, M.; Fürst, A.; Verstraeten, A.; Thimonier, A.; Timmermann, V.; Potočić, N.; Waldner, P.; Benham, S.; Hansen, K.; Merilä, P.; et al. Tree mineral nutrition is deteriorating in Europe. Glob. Change Biol. 2015, 21, 418–430. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J. M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I. A.; Wu, M.; Berry J., A.; et al. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef]
- Beedlow, P.A.; Tingey, D.T.; Phillips, D.L.; Hogsett, W.E. and Olszyk; D.M. Rising atmospheric CO2 and carbon sequestration in forests. Fr. Ecol. Environ. 2004, 2, 315–322. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Forzieri, G.; Cescatti, A. Transition from positive to negative indirect CO2 effects on the vegetation carbon uptake. Nature Comm. 2024, 15, 1500. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Palmero-Iniesta, M.; Pino, J.; Pesque, L.; Espelta, J.M. Recent forest area increase in Europe: expanding and regenerating forests differ in their regional patterns, drivers and productivity trends. Eur. J. For. Res 2021, 140, 793–805. [Google Scholar] [CrossRef]
- Francini, S.; Chirici, G.; Chiesi, L.; Costa, P.; Caldarelli, G.; Mancuso, S. Global spatial assessment of potential for new peri-urban forests to combat climate change. Nature Cities 2014, 1, 286–294. [Google Scholar] [CrossRef]
- Veldman, J.W.; Overbeck, G.E.; Negreiros, D.; Mahy, G.; Le Stradic, S. .; Fernandes, G.W.; Durigan, G.; Buisson, E.; Putz, F.E.; Bond, W.J. Where Tree Planting and Forest Expansion are Bad for Biodiversity and Ecosystem Services. BioScience 2015, 65, 1011–1018. [Google Scholar] [CrossRef]
- Green, J.K.; Keenan, T.F. The limits of forest carbon sequestration. Science 2022, 376, 692–693. [Google Scholar] [CrossRef]
- Payn, T.; Carnus, J.-M.; Freer-Smith, P.; Kimberle,y M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L. N.; Wingfield, M.J., Changes in planted forests and future global implications. For. Ecol. Manage. 2015, 352, 57–67. [CrossRef]
- Malkamäki, A.; D’Amato, D.; Hogarth, N.J.; Kanninen, M.; Pirard, R.; Toppinen, A.; Zhou, W. A systematic review of the socio-economic impacts of large-scale tree plantations, worldwide. Glob. Environ. Chang 2018, 53, 90–103. [Google Scholar] [CrossRef]
- Forster, E.J.; Healey, J.R.; Dymond, C.; Styles, D. Commercial afforestation can deliver effective climate change mitigation under multiple decarbonisation pathways. Nature Comm. 2021, 12, 831. [Google Scholar] [CrossRef]
- Bukoski, J.J.; Cook-Patton, S.C.; Melikov, C.; Ban, H.; Chen, J.L.; Goldman, E.D.; Harris, N.L.; Potts, M.D. Rates and drivers of aboveground carbon accumulation in global monoculture plantation forests. Nature Comm. 2022, 13, 4206. [Google Scholar] [CrossRef]
- Surya Prabha, A.C.; Velumani, R.; Senthivelu, M.; Pragadeesh, S. Carbon Sequestration in Plantations and Agriculture Systems: A Review. Environ.Ecol. 2022, 40, 893–898. [Google Scholar]
- Wessely, J.; Essl, F.; Fiedler, K.; Gattringer, A.; Hülber, B.; Ignateva, O.; Moser, D.; Rammer, W.; Dullinger, W.; Seidl, R. A climate-induced tree species bottleneck for forest management in Europe. Nature Ecol. Evolut. 2024, 8, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Streit, K.; Brang, P.; Frei, E.R. The Swiss common garden network: testing assisted migration of tree species in Europe. Front. For. Glob. Change 2024, 7, 1396798. [Google Scholar] [CrossRef]
- Koralewski, T.E.; Wang, H.H.; Grant, W.E.; Byram, T.D. Plants on the move: Assisted migration of forest trees in the face of climate change. For. Ecol. Manage. 2015, 344, 30–37. [Google Scholar] [CrossRef]
- Bantis, F.; Graap, J. , Früchtenicht, E.; Bussotti, F.; Radoglou, K.; Brüggemann, W. Field Performances of Mediterranean Oaks in Replicate Common Gardens for Future Reforestation Under Climate Change in Central and Southern Europe: First Results from a Four-Year Study. Forests 2021, 678, 1–16. [Google Scholar] [CrossRef]
- MacKenzie, W.H.; Mahony, C.R. An ecological approach to climate change-informed tree species selection for reforestation. For. Ecol. Manage. 2021, 481, 118705. [Google Scholar] [CrossRef]
- Nuñez, M.A.; Davis, K.T.; Dimarco, R.D.; Peltzer, D.A: Paritsis, J.; Maxwell, B.D.; Pauchard, A. Should tree invasions be used in treeless ecosystems to mitigate climate change? Front. Ecol. Environ 2021, 19, 334–341. [CrossRef]
- Noormets, A.; Epron, D.; Domec, J.C.; McNulty, S.G.; Fox, T.; Sun, G.; King, J.S. Effects of forest management on productivity and carbon sequestration: A review and hypothesis. For. Ecol. Manage. 2015, 355, 124–140. [Google Scholar] [CrossRef]
- Brang, P.; Spathelf, P.; Bo Larsen, J.; Bauhus, J.; Boncčìna, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; Lexer, M.L.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Vilà-Cabrera, A.; Coll, L.; Martínez-Vilalta, J.; Retana. Forest management for adaptation to climate change in the Mediterranean basin: A synthesis of evidence. For. Ecol. Manage. 2018, 407, 16–22. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; de Miguel, S.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef] [PubMed]
- Beugnon, R.; Ladouceur, E.; Sünnemann, M.; Cesarz, S.; Eisenhauer, N. Diverse forests are cool: promoting diverse forests to mitigate carbon emissions and climate change. J. Sustain. Agric. Enviro. 2022, 1, 5–8. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M. The functional role of biodiversity in the context of global change. In: Forests and Global Change, Coomes, D.D.; Burslem, D.F.R.P.; Simonson, W.D. Eds. Published by Cambridge University Press. British Ecological Society 2014. Pages 195-237.
- Morin, X.; Toigo, M.; Fahse, L.; Guillemot, J.; Cailleret, M.; Bertrand, R.; Cateau, E.; de Coligny, F.; García-Valdés, R.; Ratcliffe, S.; et al. More species, more trees: The role of tree packing in promoting forest productivity. J. Ecol. 2025, 113, 371–386. [Google Scholar] [CrossRef]
- Walentowski, H.; Falk, W.; Mette, T.; Kunz, J.; Bräuning, A.; Meinardus, C.; Zang, Ch.; Sutcliffe, L.; Leuschner, Ch., 2017. Assessing future suitability of tree species under climate change by multiple methods: a case study in southern Germany. Ann. For. Res. 2017, 60, 101–126. [CrossRef]
- Kunz, J.; Löffler, G.; Bauhus, J. Minor European broadleaved tree species are more drought-tolerant than Fagus sylvatica but not more tolerant than Quercus petraea. For. Ecol. Manage. 2018, 414, 15–27. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Knohl, A.; Schulze, E.D.; Kolle, O.; Buchmann, N. ; Large carbon uptake by an unmanaged 250-year-old deciduous forest in Central Germany. Agric. For. Meteorol. 2003, 118, 151–167. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier,M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 2010, 259, 660–684. [CrossRef]
- McDowell N.G.; Allen ,.CD.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [CrossRef]
- Senf, C.; Sebald, J.; Seidl, R. Increasing canopy mortality impacts the future demographic structure of Europe’s forests. One Earth 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; Lopez, R.; Saenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nature Comm. 2022, 13, 1761. [Google Scholar] [CrossRef]
- Michel, A.; Haggenmüller, K.; Kirchner, T.; Prescher, A.-K.; Schwärzel, K.; Wohlgemuth, L. Eds. Forest Condition in Europe: The 2024 Assessment. ICP Forests Technical Report under the UNECE Convention on Long-range Transboundary Air Pollution (Air Convention). Eberswalde: Thünen Institute 2024. 96 pages. [CrossRef]
- Senf, C.; Esquivel-Muelbert, A.; Pugh, T. A.M.; Anderegg, W.R.L.; Anderson-Teixeira, K.J.; Arellano, G.; Beloiu Schwenke, M.; Bentz, B.J.; Boehmer, H.J.; Bond-Lamberty, B.; et al. Towards a global understanding of tree mortality. New Phytol. 2025, 245, 2377–2392. [Google Scholar] [CrossRef]
- Bussotti, F.; Potočić, N.; Timmermann, V.; Lehmann, M.M.; Pollastrini, M. Tree crown defoliation in forest monitoring: concepts, findings, and new perspectives for a physiological approach in the face of climate change, Forestry 2024. cpad066. [CrossRef]
- Pugh, T.A.M.; Lindeskog. M.; Smith. B.; Poulte.r B.; Arneth. A.; Haverd. V.; Calle. L. Role of forest regrowth in global carbon sink dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382. [CrossRef]
- Walker, W.S.; Gorelik, S.R.; Cook-Patton, S.C.; Baccini, A.; Farina, M.K.; Solvik, K.K.; Ellis, P.W.; Sanderman, J.; Houghton, R.A.; Leavitt, S.M.; et al. The global potential for increased storage of carbon on land. Proc. Natl. Acad. Sci. USA 2022, 119, e2111312119. [Google Scholar] [CrossRef]
- Mo, L.; Zohner, C.M.; Reich, P.B.; Liang, J.; de Miguel, S.; Nabuurs, G.-J.; Renner, S.S.; van den Hoogen, J.; Araza, A.; Herold, M.; Mirzagholi, L.; et al. Integrated global assessment of the natural forest carbon potential. Nature 2013, 624, 92–101. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Maye,r P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Change Biol. 2013, 19, 229–240. [CrossRef]
- Bussotti, F.; Bettini, D.; Carrari, E.; Selvi, F.; Pollastrini, M. Health condition of forests in central Italy (Tuscany) after recurrent droughts and heat events. Ecol. Medit. 2023, 49, 37–47. [Google Scholar]
- Peñuelas, J.; Boada, M. A global change-induced biome shift in the Montseny mountains (NE Spain). Glob. Change Biol. 2003, 9, 131–140. [Google Scholar]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, .SM.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar Pedro, A.; et.al. Forest resilience to drought varies across biomes. Glob. Change Biol. 2018, 24, 2143–2158. [CrossRef]
- Holl, K.D.; Brancalion, P.H.S. Tree planting is not a simple solution. Science 2020, 368, 580–581. [Google Scholar] [CrossRef]
- Waring, B.; Neumann, M.; Prentice, I.C.; Adams, M.; Smith, P.; Siegert, M. Forests and Decarbonization – Roles of Natural and Planted Forests. Front. For. Glob. Change 2020, 3, 58. [Google Scholar] [CrossRef]
- Warner, E.; Cook-Patton, S.C.; Lewis, O.T.; Brown, N.; Koricheva, J.; Eisenhauer, N.; Ferlian, O.; Gravel, D.; Hall, J.S.; Jactel, H.; et al. Young mixed planted forests store more carbon than monocultures—a meta-analysis. Front. For. Glob. Change 2023, 6, 1226514. [Google Scholar] [CrossRef]
- MacKenzie, A R; Ullah, S; Foyer, C. H. Building forests for the future. Food and Energy Security 2014, 13, e518. [CrossRef]
- Martín-Forés, I.; Magro, S.; Bravo-Oviedo, A.; Alfaro-Sánchez, R.; Espelta, J.M.; Frei, T.; Valdés-Correcher, E.; Rodríguez Fernández-Blanco, C.; Winkel, G.; Gerzabek, G.; et al. Spontaneous forest regrowth in South-West Europe: Consequences for nature's contributions to people. People Nat 2020, 2, 980–994. [Google Scholar] [CrossRef]
- Roebroek Caspar, T. J.; Duveiller, G.; Seneviratne, S.I.; Davin, E.L.; Cescatti, A. Releasing global forests from human management: How much more carbon could be stored? Science 2023, 380, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Brancalion, P.H.S.; Holl, K.D. Guidance for successful tree planting initiatives. J. App. Ecol. 2020, 57, 2349–2361. [Google Scholar] [CrossRef]
- Di Sacco, A.; Hardwick, K. A.; Blakesley, D.; Brancalion, P.H.S.; Breman, E.; Rebola, L.C.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G.; Shaw, K.; et al. Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob. Change Biol. 2021, 27, 1328–1348. [Google Scholar] [CrossRef]
Table 1.
Carbon stored in different ecosystems ([
3]. GtC gigatons of carbon.
Table 1.
Carbon stored in different ecosystems ([
3]. GtC gigatons of carbon.
| |
Area |
Vegetation |
Soil |
Total |
GtC |
| Biome |
Million Km2
|
(GtC) |
(GtC) |
(GtC) |
|
| Tropical forests |
17.6 |
212 |
216 |
428 |
24.32 |
| Temperate forests |
10.4 |
59 |
100 |
159 |
15.29 |
| Boreal forests |
13.7 |
88 |
471 |
559 |
40.80 |
| Tropical savannas |
22.5 |
66 |
264 |
330 |
14.67 |
| Temperate grasslands |
12.5 |
9 |
295 |
304 |
24.32 |
| Deserts and semideserts |
45.5 |
8 |
191 |
199 |
4.37 |
| Tundra |
9.5 |
6 |
121 |
127 |
13.37 |
| Wetlands |
3.5 |
15 |
225 |
240 |
68.57 |
| Croplands |
16 |
3 |
128 |
131 |
8.19 |
| Total |
151.2 |
466 |
2011 |
2477 |
16.38 |
Table 3.
priority action to act in forestry to counteract climate change.
Table 3.
priority action to act in forestry to counteract climate change.
| Defense and conservation of existing natural ecosystems, even if treeless. |
| Restoration of natural forests threatened by disturbances. |
| Selection of species and genotypes adapted to future climatic conditions for reforestation purposes. |
| Individuation of adequate mixtures of tree species to promote biodiversity and resilience. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).