1. Introduction
Galeopsis genus comprises annual herbaceous species distributed throughout Europe and Asia but naturalized in other regions of the world (North America) [
1,
2,
3]. In Europe, nine
Galeopsis spp. are documented, of which seven occur in Romania (
G. angustifolia Ehrh.,
G. bifida Boenn.,
G. ladanum L.,
G. pubescens Besser,
G. segetum Neck.,
G. speciosa Mill.,
G. tetrahit L.) [
1,
2,
4,
5,
6].
The nomenclature of the
Galeopsis genus is linked to the helmet-like shape of the corolla and derives from the fusion of two words, one Latin (
galea) and one Greek (
opsis), with
galea meaning “helmet” and
opsis signifying “aspect” [
2].
Plants of the
Galeopsis genus feature four-sided, highly branched stems which, in certain species, may exhibit rigid, appressed trichomes and sub-nodal swellings. The leaves may be ovate, elliptical, lanceolate, or linear-lanceolate, arranged in a decussate opposite pattern. The flowers are aggregated in verticillasters positioned on the superior portions of the stems and branches. Each verticillaster comprises 6–10 (up to 16) flowers, subtended by rigid bracts that are shorter than the calyx. The flowers possess a calyx with spiny-toothed margins and a bilabiate corolla, lacking an internal ring of trichomes, with the superior lip vaulted in a helmet-like form and the inferior lip trilobed. The stamens are ascending and concealed by the superior lip, while the style bears nearly equal stigmatic lobes [
1,
2,
4,
6].
Species of the
Galeopsis genus are known by various Romanian vernacular names, including “zabră” (applied to
G. pubescens and
G. speciosa), “lungurică” (
G. tetrahit), “tapoşnic” (
G. ladanum), “cânepiţă” (
G. tetrahit), and “faţa mâţei” (
G. angustifolia). Some of these taxa are considered to be highly melliferous (notably
G. ladanum and
G. tetrahit), while others, such as
G. ladanum, are also recognized as noxious weeds in cereal crop cultivation [
2,
4,
6].
Depending on the species, plants of the
Galeopsis genus are widely distributed across extensive areas in Romania, with some taxa being particularly common (
G. ladanum,
G. speciosa,
G. tetrahit). In Romania, these species are found in ruderal habitats, forest edges, and shrubby areas (
G. bifida); in forests, forest clearings, along tracks, and within hedgerows (
G. speciosa); and along roadsides, in gardens, orchards, shrublands, forest clearings, or other ruderal sites (
G. tetrahit) [
2,
4,
6].
In the chemical composition of
Galeopsis spp., flavonoids [
3,
7,
8,
9,
10,
11,
12,
13,
14], phenolic acids [
3,
7,
8], phenylpropanoid glycosides [
3,
7,
8,
15], iridoids [
3,
7,
16,
17,
18], diterpenoids [
3,
7,
19,
20,
21], triterpenoid compounds [
3,
7], essential oil [
3,
7,
22,
23], and fatty acids [
3,
7,
24,
25,
26,
27,
28] have been identified.
Although few studies have examined the pharmacological activities of these plants, the
Galeopsis genus is noted for its sedative, anticholinesterase, neuroprotective [
3,
7,
29,
30], antioxidant [
3,
7,
31,
32,
33], anti-inflammatory [
3,
7], expectorant [
3,
7], astringent [
3,
7], diuretic [
3,
7], antianemic [
3,
7], and remineralizing [
3,
7] properties.
Some of these plants are employed in traditional medicine in certain regions.
G. ladanum is reported to be used in Italy for the treatment of respiratory disorders through an infusion prepared from its leaves or flowers [
7].
G. bifida is cited in Asian phytotherapy among various ethnic groups. In Tibet, the aerial parts of
G. bifida are utilized in the form of a decoction to treat oral afflictions (stomatitis) and gastrointestinal disorders, including gastritis, ulcers, gastroenteritis, and inflammations affecting the esophagus, stomach, or intestines, as well as conjunctivitis, cystitis, and inflammatory conditions of the genital organs. In the Far East, a tincture derived from the aerial parts of
G. bifida is employed to stimulate appetite, manage gastric ailments, and address epilepsy, while nomadic populations in northern Asia use the plant for the treatment of hepatic diseases [
3].
There is evidence suggesting the potential for intoxication—manifesting as transient limb paralysis—following the consumption of fruits (with seed oil even being implicated) from
Galeopsis spp. (
G. bifida,
G. ladanum,
G. speciosa,
G. tetrahit) [
3,
34].
Our paper aimed to investigate, for the first time, total phenolic content (TPC), total flavonoid content (TFC) and phenolic acids profile in roots, aerial parts and leaves from three wild-grown Galeopsis spp., collected from the southwestern region of Romania, along with their antioxidant and acetylcholinesterase (AChE) inhibitory potential. Also, the research provides new data for a better understanding of Galeopsis spp. in the context of therapeutic perspective.
3. Discussion
The findings of this study align closely with previous research on
Galeopsis spp., particularly regarding their phytochemical composition, antioxidant potential, and neuroprotective activity [
3,
29,
30,
31]. The high concentrations of chlorogenic acid,
p-coumaric acid, and ferulic acid observed in this study confirm the polyphenol-rich nature of these plants, supporting previous reports that identified
G. bifida as a source of phenylethanoid glycosides and flavone derivatives with strong antioxidant properties.
3.1. Total Polyphenols and Flavonoids
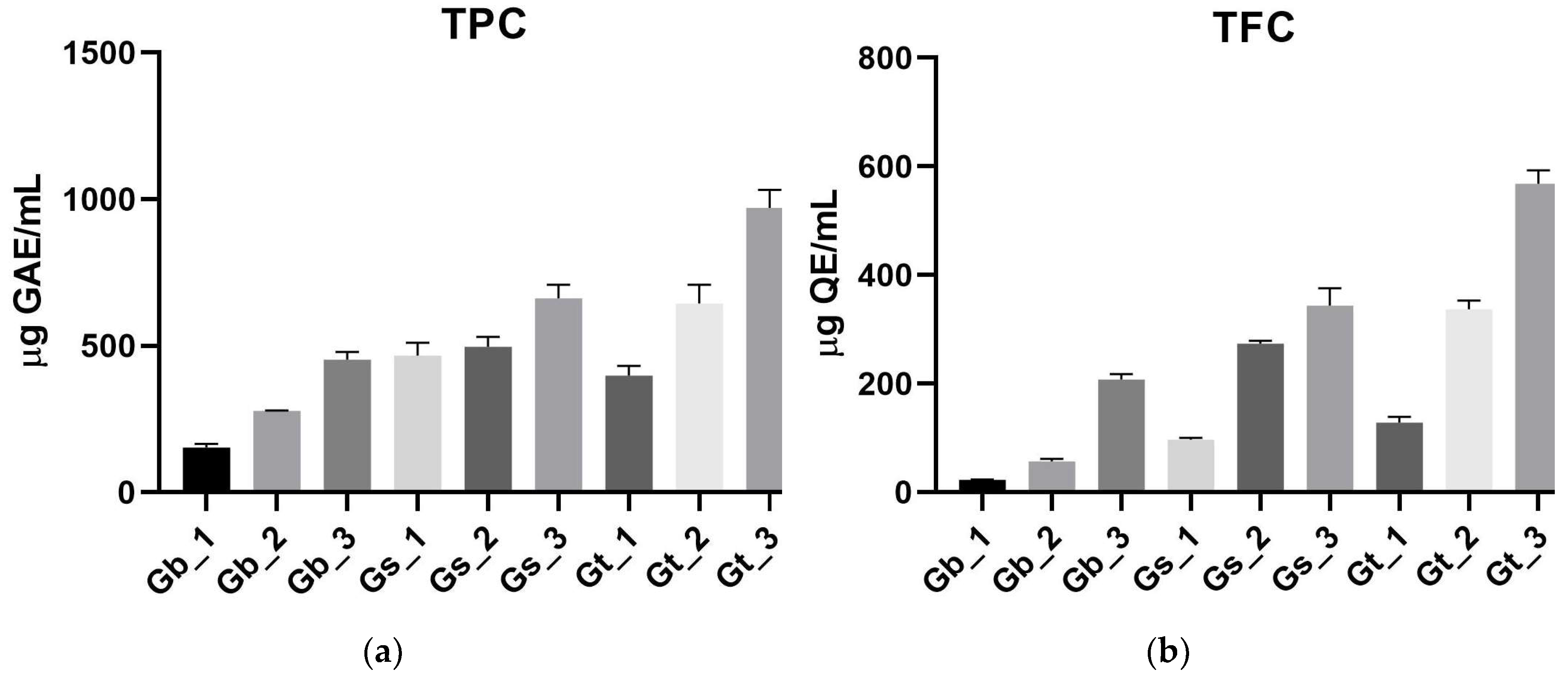
The relationship between TPC and TFC was evaluated using Pearson’s correlation analysis, following confirmation of normal data distribution via the Shapiro–Wilk test. The results indicated a strong positive correlation between TPC and TFC (r=0.9653, p<0.0001), demonstrating a significant association between these two parameters.
This finding suggests that flavonoids constitute a major portion of the total polyphenolic content in the analyzed
Galeopsis spp. The high correlation implies that an increase in TFC is accompanied by a proportional increase in TFC, reinforcing the contribution of flavonoids to the overall phytochemical profile. However, it is important to note that the aluminum chloride (AlCl
3) colorimetric method for TFC determination may lead to false-positive results due to its reaction with non-flavonoid phenolic compounds, such as certain phenolic acids, tannins, and other interfering substances, which can form complexes similar to flavonoids and overestimate flavonoid content [
35].
Given the well-documented biological activities of flavonoids, these results highlight their potential role in the antioxidant and neuroprotective effects of the extracts.
3.2. Antioxidant Activity
The antioxidant activity results are consistent with earlier studies, which demonstrated that
Galeopsis spp. possess strong DPPH and ABTS radical scavenging potential, along with FRAP assay [
3,
30,
31,
32,
33]. These effects have been previously linked to phenylethanoid glycosides and flavonoid glycosides, particularly luteolin and apigenin derivatives. The current study further supports these findings by establishing strong correlations between TPC, TFC, and antioxidant activity, indicating that these compounds are the primary contributors to the radical scavenging potential of
Galeopsis extracts. The HPTLC fingerprinting of DPPH activity further corroborated these results, showing that chlorogenic acid, along with flavonoid-related compounds, was responsible for the observed antioxidant effects.
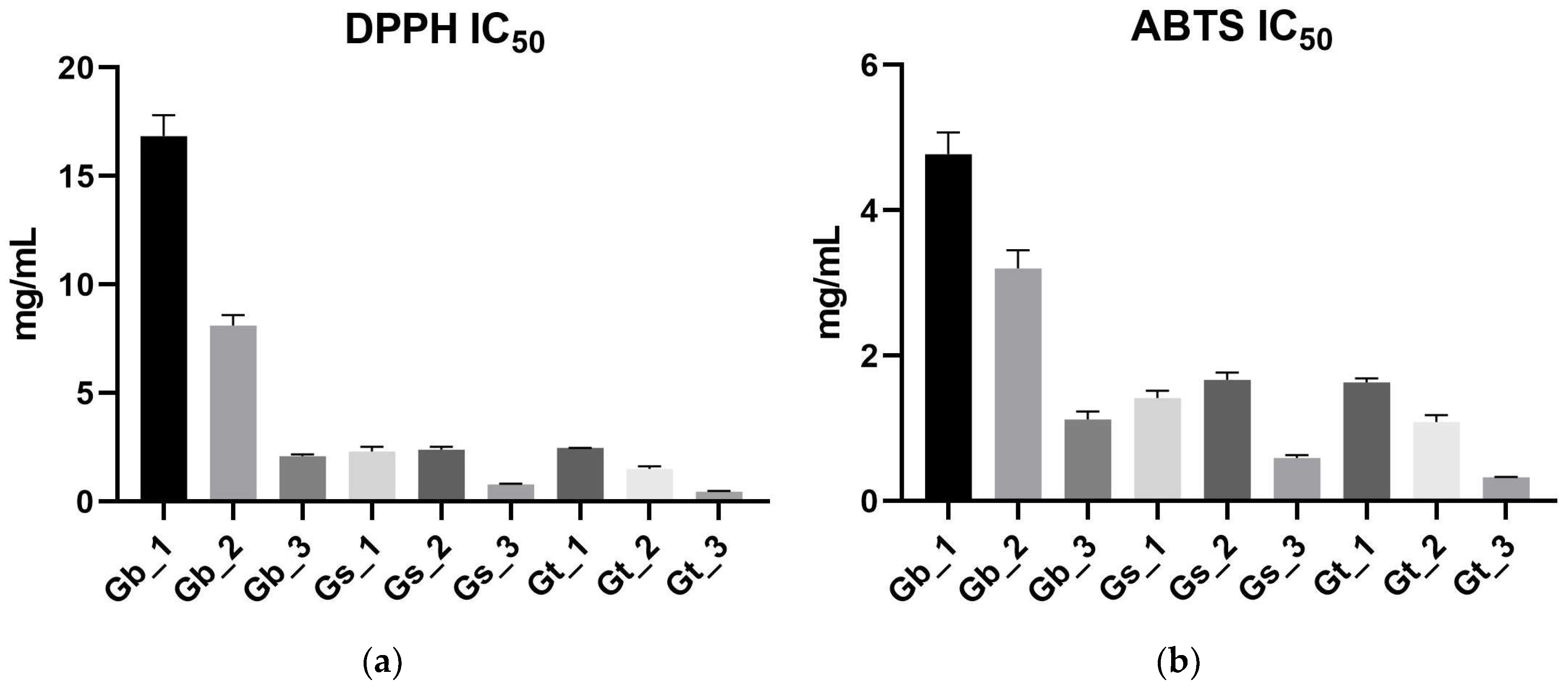
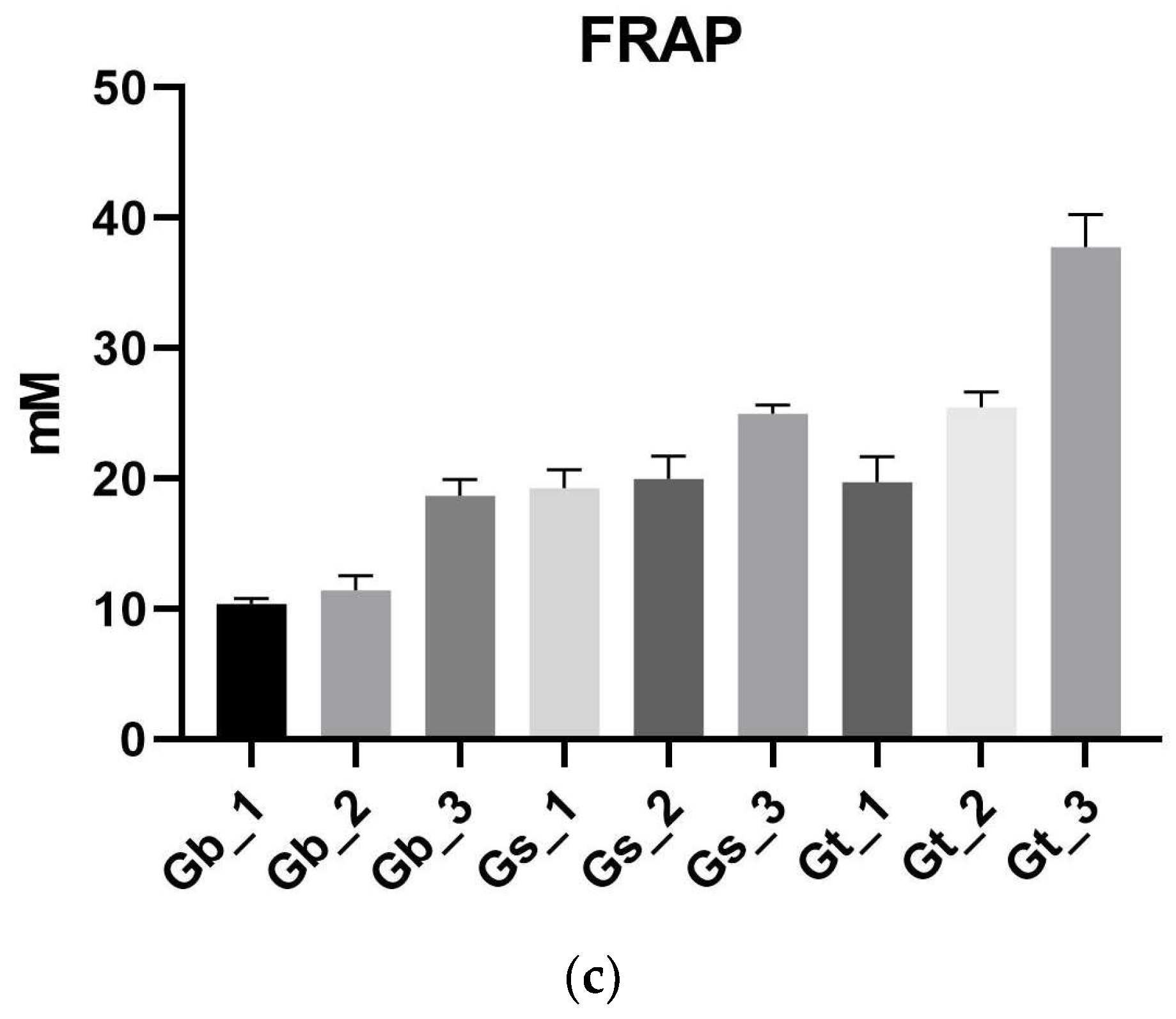
The antioxidant activities of the Galeopsis spp. were assessed using DPPH, ABTS, and FRAP assays, expressed as IC50 values (μg/mL) for DPPH and ABTS and as mM Fe2+ equivalents for FRAP. The normality of the data was evaluated using the Shapiro–Wilk test, which confirmed that ABTS and FRAP values followed a normal distribution, while DPPH values did not. Consequently, Spearman’s correlation analysis was applied to DPPH-related comparisons, while Pearson’s correlation analysis was used for ABTS vs. FRAP. The correlation analysis yielded the following results:
DPPH IC50 vs. ABTS IC50: A strong positive correlation (r=0.983, p<0.05) was observed, indicating that extracts with higher radical scavenging efficiency in the DPPH assay also exhibited strong activity in the ABTS assay;
DPPH IC50 vs. FRAP (mM Fe2+): A negative correlation (r=-0.833, p<0.05) was found, suggesting that extracts requiring higher concentrations to inhibit 50% of DPPH radicals tended to exhibit higher reducing power in the FRAP assay;
ABTS IC50 vs. FRAP (mM Fe2+): A negative correlation (r=-0.817, p<0.05) was also observed, indicating an inverse relationship between radical scavenging capacity and ferric-reducing ability.
The strong correlation between DPPH and ABTS IC50 values suggests that both assays measure similar radical scavenging mechanisms, likely driven by polyphenolic compounds. Since lower IC50 values indicate higher antioxidant activity, the negative correlations suggest that extracts requiring lower concentrations for DPPH and ABTS inhibition also tend to exhibit stronger reducing power in the FRAP assay.
These findings highlight the complexity of antioxidant mechanisms and reinforce the necessity of using multiple assays to obtain a comprehensive understanding of antioxidant potential.
To further examine the relationship between polyphenolic content and antioxidant activity, the correlation between TPC and the three antioxidant assays (DPPH IC50, ABTS IC50, and FRAP in mM Fe2+ equivalents) was assessed. The results revealed the following correlations:
TPC vs. DPPH IC50: A strong negative correlation (r=-0.9333, p=0.0007), indicating that extracts with higher polyphenol content required lower concentrations to inhibit 50% of DPPH radicals, thus demonstrating stronger radical scavenging activity;
TPC vs. ABTS IC50: A moderate negative correlation (r=-0.8833, p=0.0031), suggesting that higher polyphenol levels were associated with greater ABTS radical scavenging efficiency;
TPC vs. FRAP (mM Fe2+ equivalents): A strong positive correlation (r=0.9333, p=0.0007), indicating that extracts with higher polyphenol content exhibited greater ferric-reducing power.
These findings confirm that polyphenols play a key role in the antioxidant activity of the Galeopsis spp., contributing both to radical scavenging (DPPH, ABTS) and reducing power (FRAP). The observed negative correlations with DPPH and ABTS IC50 values indicate that extracts with higher TPC exhibited lower IC50 values, confirming their enhanced ability to neutralize free radicals. Conversely, the strong positive correlation between TPC and FRAP implies that polyphenols are also effective electron donors, reinforcing their reducing capacity.
To evaluate the contribution of flavonoids to antioxidant activity, the correlation between TFC and the three antioxidant assays (DPPH IC50, ABTS IC50, and FRAP in mM Fe2+ equivalents) was analyzed. The correlation analysis revealed the following relationships:
TFC vs. DPPH IC50: A strong negative correlation (r=-0.9167, p=0.0013), indicating that extracts with higher flavonoid content required lower concentrations to inhibit 50% of DPPH radicals, confirming their potent radical scavenging capacity;
TFC vs. ABTS IC50: A moderate negative correlation (r=-0.8833, p=0.0031), suggesting that an increase in flavonoid content was associated with improved ABTS radical scavenging efficiency;
TFC vs. FRAP (mM Fe2+ equivalents): A strong positive correlation (r=0.9333, p=0.0007), indicating that extracts with higher flavonoid content exhibited greater ferric-reducing power.
These results suggest that flavonoids significantly contribute to both radical scavenging activity and reducing power in the tested Galeopsis spp. The negative correlations with DPPH and ABTS IC50 values indicate that flavonoid-rich extracts exhibited stronger antioxidant activity, requiring lower concentrations to achieve 50% inhibition. The strong positive correlation between TFC and FRAP further supports the role of flavonoids as efficient electron donors, reinforcing their involvement in redox reactions.
3.3. Neuroprotective Activity
Regarding neuroprotective activity, the results of this study reinforce previous findings on the AChE inhibitory potential of
Galeopsis spp. Earlier research identified several bioactive metabolites with AChE inhibitory properties, including iridoid glycosides (harpagide, harpagide 8-O-acetate, ajugoside), phenylethanoid glycosides (verbascoside, isoverbascoside), flavonoid glycosides (luteolin and apigenin derivatives), and hydroxycinnamic acids (caffeoylquinic acids, e.g., chlorogenic acid) [
3,
7,
8,
29,
30]. The current study confirmed that chlorogenic acid was present in all samples and exhibited moderate AChE inhibition, supporting its role as a neuroprotective agent. Additionally, an unknown compound at the same R
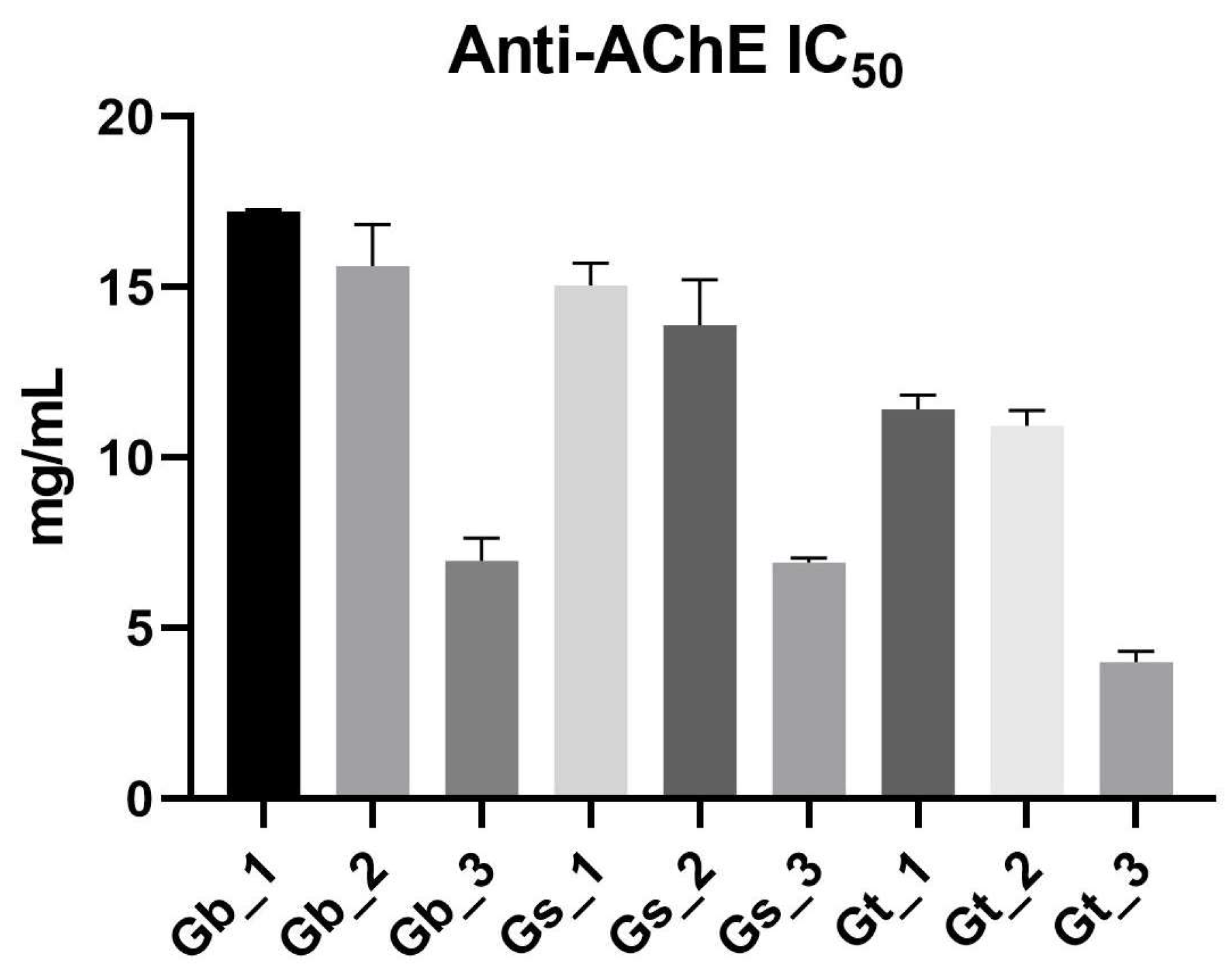
f as caffeic acid exhibited inhibitory activity in the AChE HPTLC assay, suggesting the presence of another bioactive metabolite contributing to neuroprotection. The strongest AChE inhibition zones were observed in
G. tetrahit leaves, which also exhibited a unique orange-fluorescent flavonoid does not present in the other species. These results suggest that
G. tetrahit may contain distinct neuroactive flavonoids that warrant further investigation.
The neuroprotective potential of the Galeopsis spp. was assessed through AChE inhibition activity, and its relationship with TPC and TFC was analyzed. The correlation analysis yielded the following results:
AChE inhibition vs. TPC: A moderate negative correlation (r=-0.8266, p=0.0060), suggesting that extracts with higher total polyphenol content exhibited greater AChE inhibition. The 95% confidence interval (CI) ranged from -0.9624 to -0.3603, supporting the statistical robustness of this relationship;
AChE inhibition vs. TFC: A moderate negative correlation (r=-0.8335, p=0.0053), indicating that an increase in flavonoid content was associated with stronger AChE inhibition. The 95% CI ranged from -0.9640 to -0.3793, reinforcing the reliability of the association.
Both correlations were statistically significant (p<0.05) and suggest that polyphenols, particularly flavonoids, may play a role in the neuroprotective activity of these extracts. The negative correlation indicates that extracts with higher levels of polyphenols and flavonoids required lower concentrations to inhibit AChE, highlighting their potential as natural AChE inhibitors.
These findings align with previous research suggesting that polyphenolic compounds, including flavonoids, can modulate cholinergic activity and contribute to neuroprotective effects. Further investigations into specific bioactive compounds responsible for AChE inhibition could provide deeper insights into their potential application in managing neurodegenerative conditions.
To further explore the relationship between AChE inhibition and antioxidant activity, the correlation between AChE inhibition and DPPH IC50, ABTS IC50, and FRAP (mM Fe2+ equivalents) was analyzed. The normality of the data was confirmed using the Shapiro–Wilk test, and Spearman’s correlation analysis was performed to assess statistical associations. The results of the correlation analysis revealed the following:
AChE inhibition vs. DPPH IC50: A moderate positive correlation (r=0.6887, p=0.0402), indicating that extracts with higher AChE inhibition also tended to require lower concentrations to scavenge 50% of DPPH radicals. However, the correlation was weaker compared to other parameters, as reflected by the 95% CI ranging from 0.04535 to 0.9283;
AChE inhibition vs. ABTS IC50: A strong positive correlation (r=0.8085, p=0.0083), suggesting that extracts with greater AChE inhibition demonstrated enhanced ABTS radical scavenging activity. The 95% CI (0.3117 to 0.9581) reinforces the statistical robustness of this relationship;
AChE inhibition vs. FRAP (mM Fe2+ equivalents): A moderate negative correlation (r=-0.8238, p=0.0063), showing that extracts with higher AChE inhibition exhibited stronger reducing power. The negative correlation suggests that extracts with high AChE inhibition had greater ferric-reducing capacity, a trend supported by the 95% CI of -0.9617 to -0.3526.
These findings indicate a clear link between neuroprotective and antioxidant activities in the Galeopsis spp. analyzed extracts. The positive correlations with DPPH and ABTS IC50 values suggest that extracts with stronger radical scavenging properties may also have neuroprotective potential. Meanwhile, the negative correlation with FRAP highlights that reducing power may play an independent or complementary role in AChE inhibition.
The strong association between AChE inhibition and antioxidant activity aligns with existing research suggesting that oxidative stress is closely linked to neurodegenerative diseases and that antioxidants may exert neuroprotective effects by reducing oxidative damage and modulating cholinergic activity. Future studies focusing on specific bioactive compounds with dual antioxidant and neuroprotective activities could provide further insights into their mechanisms of action and potential therapeutic applications.
Overall, the findings of this study confirm and expand upon previous research, reinforcing the high polyphenol and flavonoid content of Galeopsis spp., particularly in leaves. The strong antioxidant and neuroprotective activity observed in G. tetrahit and G. speciosa leaves suggests that these plants contain valuable bioactive compounds with potential therapeutic applications in oxidative stress-related and neurodegenerative diseases. Future research should focus on isolating and characterizing the specific compounds responsible for these effects to better understand their pharmacological potential.
3.4. Study Limitations
While this study provides valuable insights into the phytochemical composition, antioxidant potential, and neuroprotective activity of Galeopsis spp., several limitations should be considered when interpreting the results.
3.4.1. Variability in Plant Material and Environmental Influence
The chemical composition of plant extracts is highly influenced by environmental factors such as soil composition, climate, altitude, and harvesting conditions. Since the Galeopsis spp. studied were collected from specific locations, the results may not be entirely generalizable to plants grown in different geographical regions. A more comprehensive study incorporating plants from diverse habitats would strengthen the findings.
3.4.2. Extraction Method and Solvent Specificity
The study employed ultrasound-assisted extraction (UAE) using 70% ethanol, a method chosen for its efficiency in extracting polyphenols and flavonoids. However, other bioactive compounds, such as alkaloids, lipophilic terpenes, or polysaccharides, may have been underrepresented due to the solvent selectivity. Future studies should explore multiple extraction methods to capture a broader range of secondary metabolites.
3.4.3. HPTLC and UHPLC Identification Constraints
Although HPTLC fingerprinting and UHPLC quantification provided valuable insights into the phenolic composition, the study relied on reference standards for compound identification. Unknown compounds detected at similar Rf values to known standards (e.g., the compound observed at the same Rf as caffeic acid in AChE inhibition assays) were not structurally characterized. Advanced analytical techniques such as liquid chromatography–tandem mass spectrometry (LC–MS/MS) or nuclear magnetic resonance (NMR) spectroscopy should be employed in future research to confirm compound identities and detect novel bioactive molecules.
3.4.4. Lack of In Vivo Validation
The study focused on in vitro tests, including antioxidant (DPPH, ABTS, FRAP) and neuroprotective (AChE inhibition) assays, to assess the bioactivity of Galeopsis spp. extracts. While these assays are reliable indicators of biological potential, in vitro results do not always translate into in vivo efficacy due to differences in bioavailability, metabolism, and cellular interactions. Future studies should incorporate cell-based models, animal studies, and pharmacokinetic analyses to better understand the bioavailability and in vivo effectiveness of the identified compounds.
3.4.5. Potential Interference in Quantification Assays
The AlCl₃ colorimetric assay used for TFC is known to produce false positives, as certain phenolic acids and other non-flavonoid compounds can react with AlCl₃, leading to an overestimation of flavonoid content. This limitation suggests that more specific flavonoid quantification techniques, such as UHPLC–MS/MS, should be employed to validate the results.
Despite these limitations, this study provides novel and valuable insights into the bioactivity of Galeopsis spp., a group of plants that has been largely overlooked in phytochemical and pharmacological research. Currently, few studies have systematically investigated the chemical composition and biological activity of these species, making this research a significant contribution to the understanding of their medicinal potential. Addressing the outlined limitations in future studies would enable a more comprehensive understanding of their pharmacological properties, enhance compound identification, and validate in vivo relevance for potential therapeutic applications.
4. Materials and Methods
4.1. Plant Material
The roots, aerial parts and leaves of wild-grown Galeopsis spp. were harvested during the flowering period (July–August 2024) from Oltenia Region, southwest Romanian flora. The plant material for analysis was stored in the Herbarium of the Department of Pharmaceutical Botany, Faculty of Pharmacy, University of Medicine and Pharmacy of Craiova. The plant material was air-dried and deposited in brown paper bags, at room temperature (RT), in a cool and dark area, 24 hours before processing for extraction and analysis. Our research did not involve endangered or protected plant species.
A systematic notation representing different
Galeopsis spp., their respective vegetal products, date/site of collection and voucher specimens was used in this study. A clear and organized reference to the specific plant species and parts analyzed in the experiments was facilitated by this notation (
Table 2).
4.2. Chemicals and Reagents
The solvents used in this study included ethanol, methanol, acetonitrile and ethyl acetate (Merck, Darmstadt, Germany). Ultrapure water was obtained using a HALIOS 6 lab water system (Neptec, Montabaur, Germany) to ensure the required purity for aqueous solutions and dilutions. For UHPLC analysis, formic acid (Merck) was used as an additive to enhance the performance of the mobile phases.
The reagents selected to support the experimental assays included Folin-Ciocalteu reagent, sodium carbonate, DPPH, ABTS, potassium persulfate, sodium acetate, acetic acid, 2,4,6-tris(2-pyridyl)-1,3,5-triazine (TPTZ), quercetin, natural products–polyethylene glycol (NP–PEG) reagent, ferric chloride (FeCl3), ferrous sulfate heptahydrate (FeSO4·7H2O), and hydrochloric acid (HCl) (Sigma-Aldrich, Taufkirchen, Germany). These reagents were used for the determination of TPC, antioxidant activity, and enzymatic assays. For TPC, Folin–Ciocalteu reagent was used together with sodium bicarbonate. AlCl3 from Sigma-Aldrich was specifically used for the TFC assay.
For the AChE inhibition assay, the primary reagents included AChE from Electrophorus electricus, 1-naphthyl acetate, Fast Blue B salt, Tris-HCl buffer solution (pH 7.8, 0.05 M) and rivastigmine as a positive control (Sigma-Aldrich).
In UHPLC analysis, a set of phenolic acid standards – including caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, gallic acid, protocatechuic acid, syringic acid, and vanillic acid (Merck Millipore, Darmstadt, Germany) – was used for calibration and compound identification.
For HPTLC analysis, Silica gel 60 F254 glass plates (20×10 cm) were obtained from Merck.
4.3. Extraction Procedure
The extraction of plant material was carried out using a UAE method, with 70% ethanol as the solvent. A measured quantity of 1 g of finely ground plant material was combined with 10 mL of the ethanol solution in an appropriate container. The mixture underwent ultrasonic treatment in a Bandelin Sonorex Digiplus DL 102H ultrasound bath (Bandelin electronic GmbH & Co. KG, Berlin, Germany) operating at 100 W power and a frequency of 35 kHz for 20 min at a controlled temperature of 50°C. The application of ultrasonic waves facilitated the breakdown of plant cell walls, enhancing the release of bioactive compounds into the solvent.
Following extraction, the solution was filtered through a 0.22 μm syringe filter equipped with a water wettable polytetrafluoroethylene (WWPTFE) membrane (Acrodisc, Pall Corporation, Port Washington, NY, USA) to separate the liquid extract from any residual solid material. The obtained extract was subsequently used for TPC and TFC determination, as well as antioxidant and neuroprotection assays.
For UHPLC analysis, 1 mL of the extract was carefully evaporated under a gentle nitrogen stream to eliminate the solvent. The dried residue was then reconstituted in a mixture of water and acetonitrile (9:1, v/v) to ensure compatibility with the UHPLC mobile phase system. This step was essential for optimizing the dissolution of bioactive compounds prior to chromatographic separation and detection. Before injection into the UHPLC system, the reconstituted solution was filtered through a 0.22 μm syringe filter to remove any particulate matter.
4.4. Standards Preparation
Caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, gallic acid, protocatechuic acid, syringic acid, and vanillic acid were used as standards for the UHPLC analysis. Stock solution of each standard was prepared at 1 mg/mL concentration using methanol. To achieve calibration concentrations ranging from 0.1 μg/mL to 50 μg/mL, serial dilutions were made. For both standards and samples, a volume of 10 μL was injected into the UHPLC system.
4.5. Total Polyphenols and Flavonoids
4.5.1. TPC Assay
The TPC was quantified using the Folin–Ciocalteu method, in a 96-well microplate format. Twenty microliters (20 μL) of the plant extract were pipetted into each well, followed by the addition of 100 μL of Folin–Ciocalteu reagent. The mixture was allowed to react for three minutes, after which 80 μL of a 4% sodium carbonate solution was added. The microplate was stirred for another three minutes to ensure homogeneity. To facilitate color development, the reaction mixture was incubated in the dark for two hours. Following incubation, absorbance was measured at 620 nm using a FLUOstar Optima microplate reader (BMG Labtech, Ortenberg, Germany). A gallic acid standard curve was prepared, with calibration solutions ranging from 5 mg/mL to 625 μg/mL, enabling the quantification of phenolic compounds in the extracts, expressed as mg gallic acid equivalents (GAE) per g of plant extract. Each measurement was performed in triplicate to ensure accuracy and reproducibility [
36].
4.5.2. TFC Assay
The TFC was assessed using the AlCl
3 colorimetric assay. A quercetin standard curve was prepared in 96% ethanol, with concentrations ranging from 30 to 100 μg/mL. For each assay, 50 μL of plant extract or quercetin standard solution was added to a 96-well microplate, followed by the addition of 10 μL of 10% AlCl
3 solution. To this mixture, 150 μL of 96% ethanol was added, followed by 10 μL of 1 M sodium acetate. A blank control was prepared using 96% ethanol in place of the sample. After thorough mixing, the reaction was incubated for 40 min at RT in the dark. Absorbance was recorded at 410 nm using a FLUOstar Optima microplate reader (BMG Labtech, Ortenberg, Germany). The results were expressed as mg quercetin equivalents (QE) per g of plant extract. Each sample was analyzed in triplicate to ensure reproducibility [
36,
37].
4.6. Antioxidant Activity Assays
4.6.1. DPPH Antioxidant Assay
The DPPH radical scavenging assay was conducted by adding 50 μL of each sample to a 96-well microplate, followed by serial dilutions to obtain a gradient of decreasing concentrations. Next, 150 μL of a 0.2 mM DPPH solution in ethanol was added into each well. The reaction mixtures were incubated in the dark for 30 min at RT, after which the absorbance was measured at 517 nm using a FLUOstar Optima microplate reader (BMG Labtech). The antioxidant potential was evaluated by calculating the half-maximal inhibitory concentration (IC
50), which represents the concentration required to scavenge 50% of the DPPH radicals. Each sample was analyzed in triplicate to ensure accuracy [
36].
4.6.2. ABTS Antioxidant Assay
In the ABTS radical scavenging assay, 50 μL of each sample was added to a 96-well microplate, followed by serial dilutions in the same manner as the DPPH assay. Then, 150 μL of ABTS reagent, prepared by mixing 7.4 mM ABTS with 2.6 mM potassium persulfate, was added to each well. After a reaction time of six minutes, the absorbance was measured at 620 nm using a FLUOstar Optima microplate reader (BMG Labtech). The IC
50 value, representing the sample concentration necessary to inhibit 50% of the ABTS radicals, was determined from a dose–response curve. Each sample was tested in triplicate [
38].
4.6.3. FRAP Antioxidant Assay
The FRAP assay was performed by preparing a fresh FRAP reagent consisting of acetate buffer (pH 3.6), 10 mM TPTZ solution in 40 mM HCl, and 20 mM FeCl
3 solution. A calibration curve was established using Fe
2+ standards in the range of 65 to 500 μM. In each assay, 10 μL of the sample or standard was added to a 96-well microplate, followed by 190 μL of freshly prepared FRAP reagent. The reaction mixtures were incubated for 30 min at RT, after which the absorbance was recorded at 595 nm. The results were expressed as μmol Fe
2+ equivalents, and all analyses were carried out in triplicate to ensure reliability [
38].
4.7. Neuroprotective Activity Assay
The AChE inhibitory activity was assessed using a microplate-based assay, with each sample tested in triplicate to ensure reliability. The assay aimed to evaluate the ability of the test samples to inhibit AChE activity across a range of concentrations. Each sample underwent serial dilution directly on a 96-well microplate, starting from the stock extract solution, to generate a concentration gradient. To initiate the reaction, 50 μL of 1-naphthyl acetate solution (3 mg/mL in ethanol) was added to each well, serving as the enzymatic substrate. This was followed by the addition of 150 μL of AChE solution (3.33 U/mL) to catalyze the reaction, leading to the formation of measurable enzymatic products. To facilitate the detection of enzyme activity, 50 μL of Fast Blue B salt solution (3 mg/mL in water) was introduced into each well. This reagent reacts with the enzymatic products, producing a distinct color change that correlates with AChE activity. Rivastigmine (1 mg/mL in methanol), a known AChE inhibitor, was included as a positive control to establish a reference for the inhibitory potential of the test samples. Absorbance was recorded at 595 nm using a FLUOstar Optima microplate reader (BMG Labtech), and the collected data were analyzed to determine the IC
50 value for each sample, indicating the concentration required to inhibit 50% of AChE activity [
38].
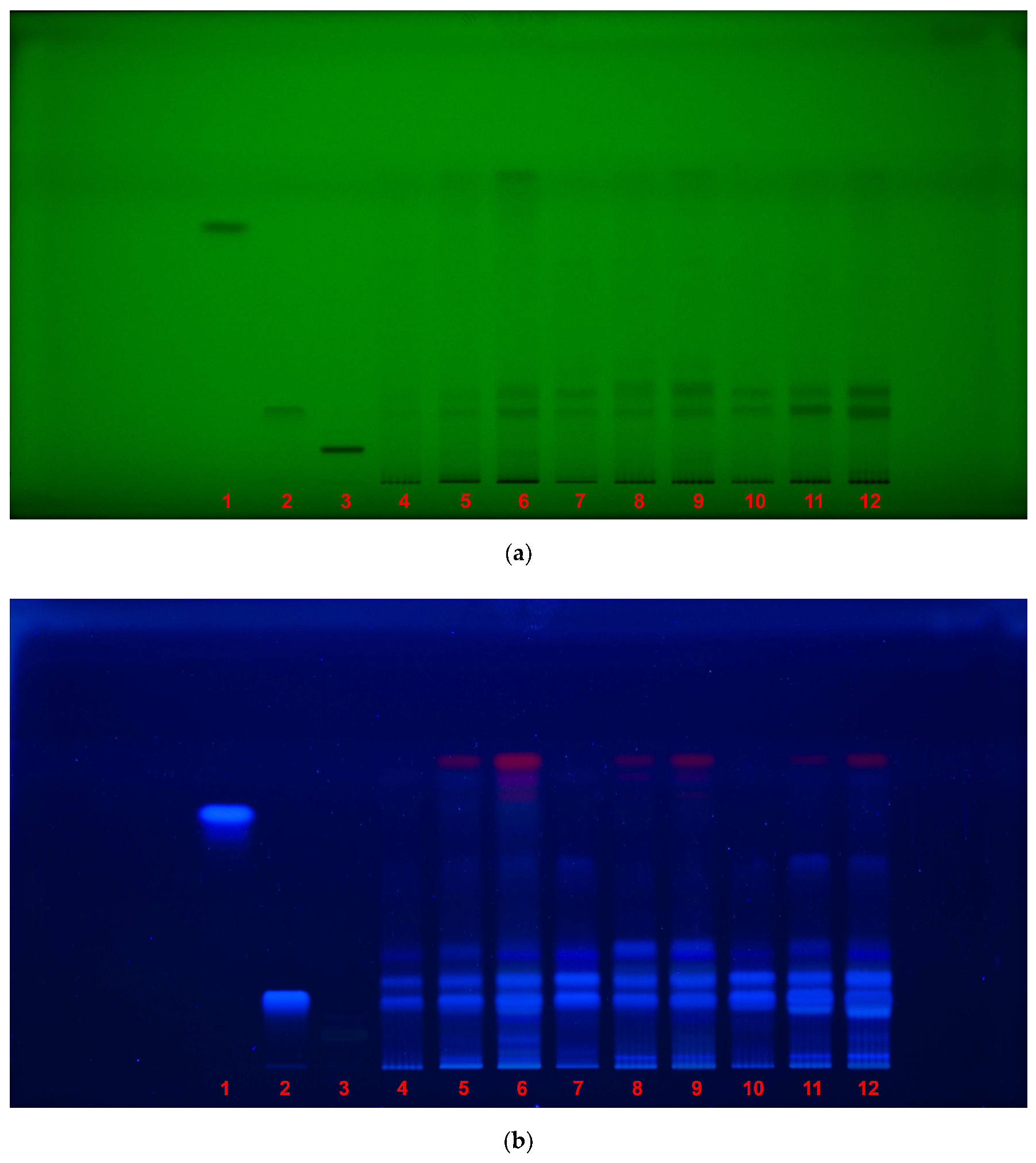
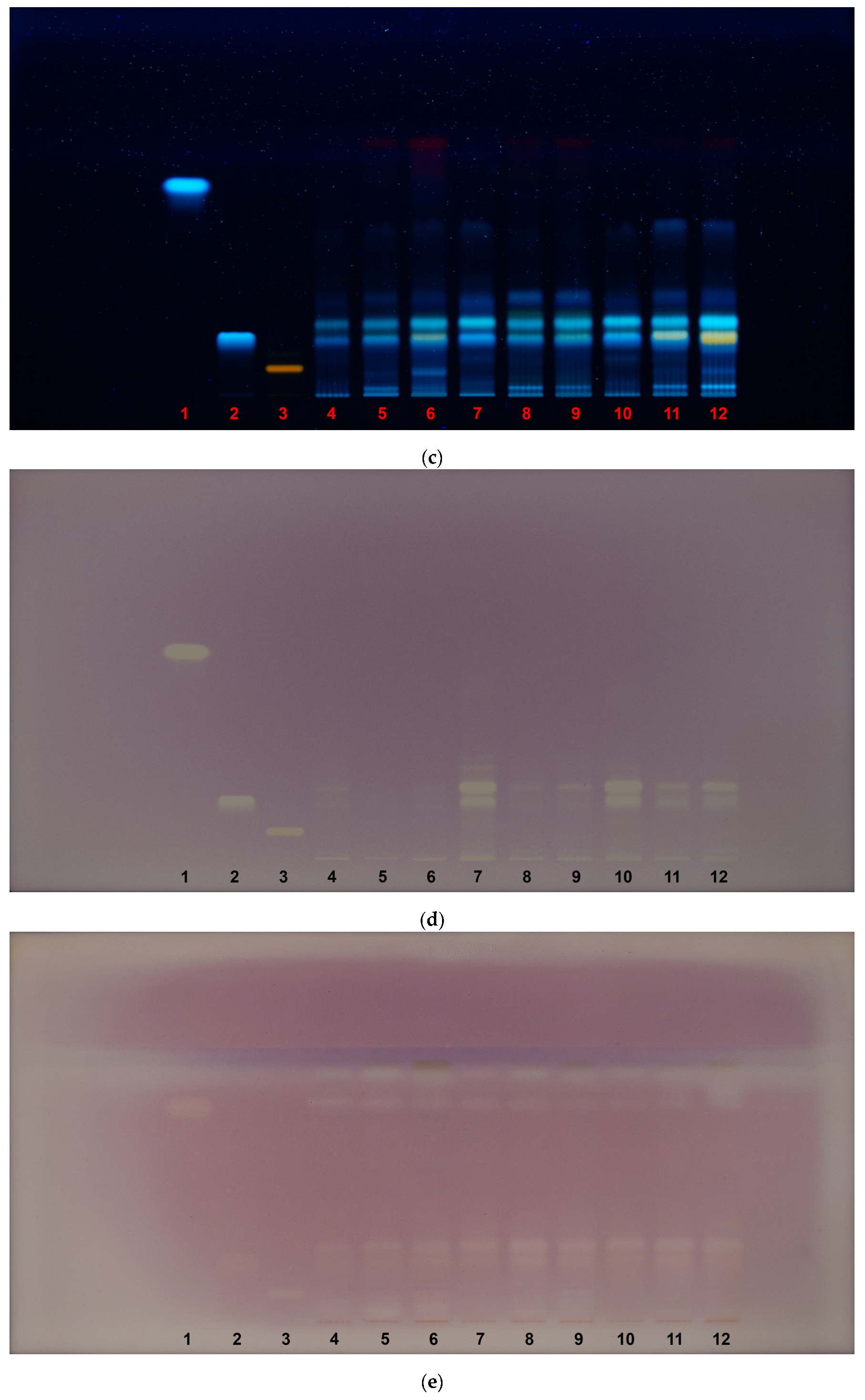
4.8. HPTLC Fingerprinting for Antioxidant and Neuroprotective Activity
HPTLC fingerprinting was performed to assess the antioxidant potential (DPPH assay) and AChE inhibitory activity of the plant extracts [
39]. Caffeic acid, chlorogenic acid, and rutin were used as reference standards.
Sample application was carried out using a Linomat 5 applicator, where 2 μL of each extract and standard were applied to the HPTLC plates. The chromatographic separation was conducted in a twin trough chamber using a mobile phase consisting of ethyl acetate, formic acid, and water (90:6:9, v/v/v). Prior to development, the chamber was saturated for 20 min to ensure optimal separation conditions. The plates were developed up to a solvent front position of 7 cm.
For the AChE inhibition assay, the plate was sprayed using the CAMAG Derivatizer (CAMAG, Muttenz, Switzerland) with 0.5 mL Tris-HCl buffer solution (pH 7.8, 0.05 M) used for prewetting and then 1.5 mL AChE solution (6.66 U/mL), after which the plate was sprayed with 0.5 mL of the 1:1 substrate/chromogenic reagent mixture (ethanolic 1-naphthyl acetate solution and aqueous Fast Blue B salt solution, 3 mg/mL each) and dried (three min).
Following development, the plates were air-dried at RT for 10 min before analysis. Visualization was conducted at 254 nm and 366 nm without derivatization, as well as post-derivatization using NP–PEG reagent at 366 nm and for DPPH and AChE in white light.
This method enabled the identification of bioactive compounds within the extracts based on their retention factor (Rf) values and corresponding color changes indicative of antioxidant and neuroprotective properties.
4.9. UHPLC Analysis of Phenolic Acids
UHPLC analysis was performed using a Waters Acquity Arc system, equipped with a photodiode array (PDA) detector and a QDa mass detector (Waters, Milford, Massachusetts, USA). Chromatographic separation was achieved using a CORTECS C18 column (4.6×50 mm, 2.7 μm particle size), which was maintained at a temperature of 30°C.
The mobile phase consisted of water with 0.01% formic acid (A) and acetonitrile with 0.01% formic acid (B). The gradient elution program was initiated with 99% A at a constant flow rate of 0.8 mL/min, held for one minute. Between 1 and 13 min, the proportion of mobile phase A was gradually reduced to 70%, which remained unchanged until 13.10 min. From 13.60 to 17.60 min, the composition shifted to 20% A, allowing for column cleaning and the removal of strongly retained compounds. The mobile phase then returned to its initial condition of 99% A at 18.10 min, and this was maintained until 21.10 min for system re-equilibration before the next injection. To ensure analytical stability and reproducibility, the column was equilibrated for 10 min between injections. Throughout the analysis, samples were kept at 8°C to preserve their integrity.
For quantification, absorbance detection was set at 265 nm for gallic acid, protocatechuic acid, vanillic acid, and syringic acid, while 325 nm was used for chlorogenic acid, caffeic acid,
p-coumaric acid, and ferulic acid. Mass confirmation was carried out in negative ion mode, targeting specific mass-to-charge (
m/z) ratios: 153 (protocatechuic acid), 163 (
p-coumaric acid), 167 (vanillic acid), 169 (gallic acid), 179 (caffeic acid), 193 (ferulic acid), 197 (syringic acid), and 353 (chlorogenic acid) [
38,
39].
4.10. Statistical Analysis
All experimental data were analyzed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). The results were expressed as mean±standard deviation (SD), with all experiments performed in triplicate (n=3). The normality of the data was assessed using the Shapiro–Wilk test, and based on the results, appropriate statistical tests were applied.
For comparisons between Galeopsis spp. (G. bifida, G. speciosa, and G. tetrahit) and plant parts (roots, aerial parts, and leaves), a two-way ANOVA was performed to determine the influence of these factors on TPC, TFC, antioxidant activity (DPPH, ABTS, FRAP assays), and AChE inhibition. Post-hoc Tukey’s multiple comparisons test was conducted to identify statistically significant differences between groups.
For correlation analyses, Pearson’s correlation coefficient (r) was used when the data followed a normal distribution, while Spearman’s correlation test was applied for non-normally distributed data. Correlations were evaluated between TPC, TFC, and bioactivity assays (antioxidant and AChE inhibition tests) to determine potential relationships between polyphenolic content and biological activity. The significance threshold was set at α=0.05, with results considered statistically significant at p<0.05, highly significant at p<0.01, very highly significant at p<0.001, and extremely significant at p<0.0001.
All statistical tests were conducted in accordance with standard biostatistical methodologies, ensuring robust and reproducible data interpretation.
5. Conclusions
This study provides a comprehensive analysis of the phytochemical composition, antioxidant activity, and neuroprotective potential of three Galeopsis spp. (G. bifida, G. speciosa, and G. tetrahit). The results confirm that leaves contain the highest concentrations of phenolic acids and flavonoids, particularly chlorogenic acid, p-coumaric acid, and ferulic acid, which were identified as major bioactive compounds. Strong antioxidant activity was demonstrated through DPPH, ABTS, and FRAP assays, with leaves, particularly those of G. tetrahit, exhibiting the greatest radical scavenging potential. Additionally, AChE inhibition assay revealed that G. tetrahit leaves exhibited the strongest neuroprotective effects, which may be attributed to their high phenolic acids and flavonoid content.
These findings align with previous research on Galeopsis spp., reinforcing their potential as natural sources of antioxidants and neuroprotective agents. However, this study represents one of the few in-depth investigations into the phytochemistry and bioactivity of these species, highlighting the need for further research on compound isolation, structural characterization, and in vivo validation. The presence of an unknown neuroactive compound at the same Rf as caffeic acid in AChE inhibition assay suggests that Galeopsis spp. may contain previously unidentified bioactive molecules, warranting additional pharmacological exploration.
Overall, this study supports the medicinal relevance of Galeopsis spp., particularly in applications related to oxidative stress and neurodegenerative disorders. Future work should focus on elucidating the mechanisms of action, exploring clinical relevance, and assessing the safety profile of these bioactive compounds to unlock their full therapeutic potential.
Author Contributions
Conceptualization, R.M.G., C.B., L.E.B., A.B. and J.N.; methodology, R.M.G., L.E.B., A.B. and G.D.M.; validation, A.-E.S. and O.E.N.; investigation, A.-E.S., A.B., A.R., A.C.T., M.V.C. and O.E.N.; data curation, M.V.C. and G.D.M.; writing—original draft preparation, L.E.B., A.B. and G.D.M.; writing—review and editing, A.B. and G.D.M.; supervision, C.B., L.E.B. and J.N. All authors have read and agreed to the published version of the manuscript.