1. Introduction
Magnesium-lithium (Mg-Li) alloys exhibit exceptional properties, including high specific strength, superior specific stiffness, excellent thermal conductivity, and remarkable electromagnetic shielding and damping performance. These characteristics offer broad application prospects in aerospace, defense equipment, electronic information, and automotive industries [
1,
2,
3]. Recognized as “green engineering materials of the 21st century” [
4,
5], Mg-Li alloys are among the most promising lightweight materials for sustainable development, addressing the growing demand for weight reduction, energy efficiency, and high integration.
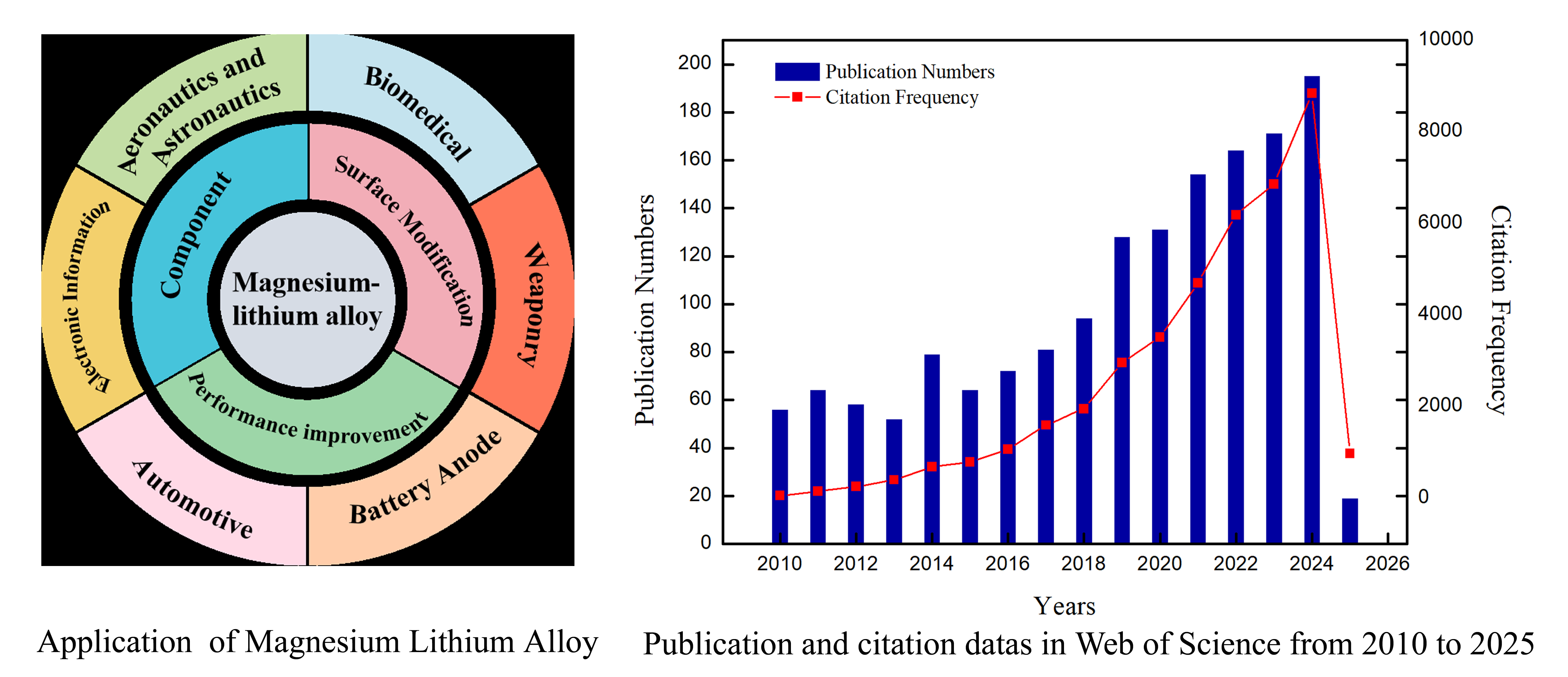
Figure 1 illustrates their diverse applications in 3C (computer, communication, and consumer electronics) products, aerospace structural components, and automotive parts.
The phase structure of Mg-Li alloys is primarily determined by their Li content. When the Li content is below 5.7 wt.%, the alloy consists of an α-Mg phase with a hexagonal close-packed (hcp) structure, where Li is dissolved in the Mg matrix. When the Li content exceeds 10.3 wt.%, the alloy is dominated by a β-Li phase with a body-centered cubic (bcc) structure, where Mg is dissolved in the Li matrix. For Li contents between 5.7 wt.% and 10.3 wt.%, the alloy exhibits a typical dual-phase structure composed of both α-Mg and β-Li phases [
6].
However, the wider application of Mg-Li alloys is hindered by their intrinsic limitations, including low strength, poor stability, and weak corrosion resistance. Particularly, the galvanic corrosion caused by the potential difference between the α-Mg and β-Li phases in biphasic Mg-Li alloys significantly compromises their performance in service environments, thereby restricting their engineering applications [
7,
8].
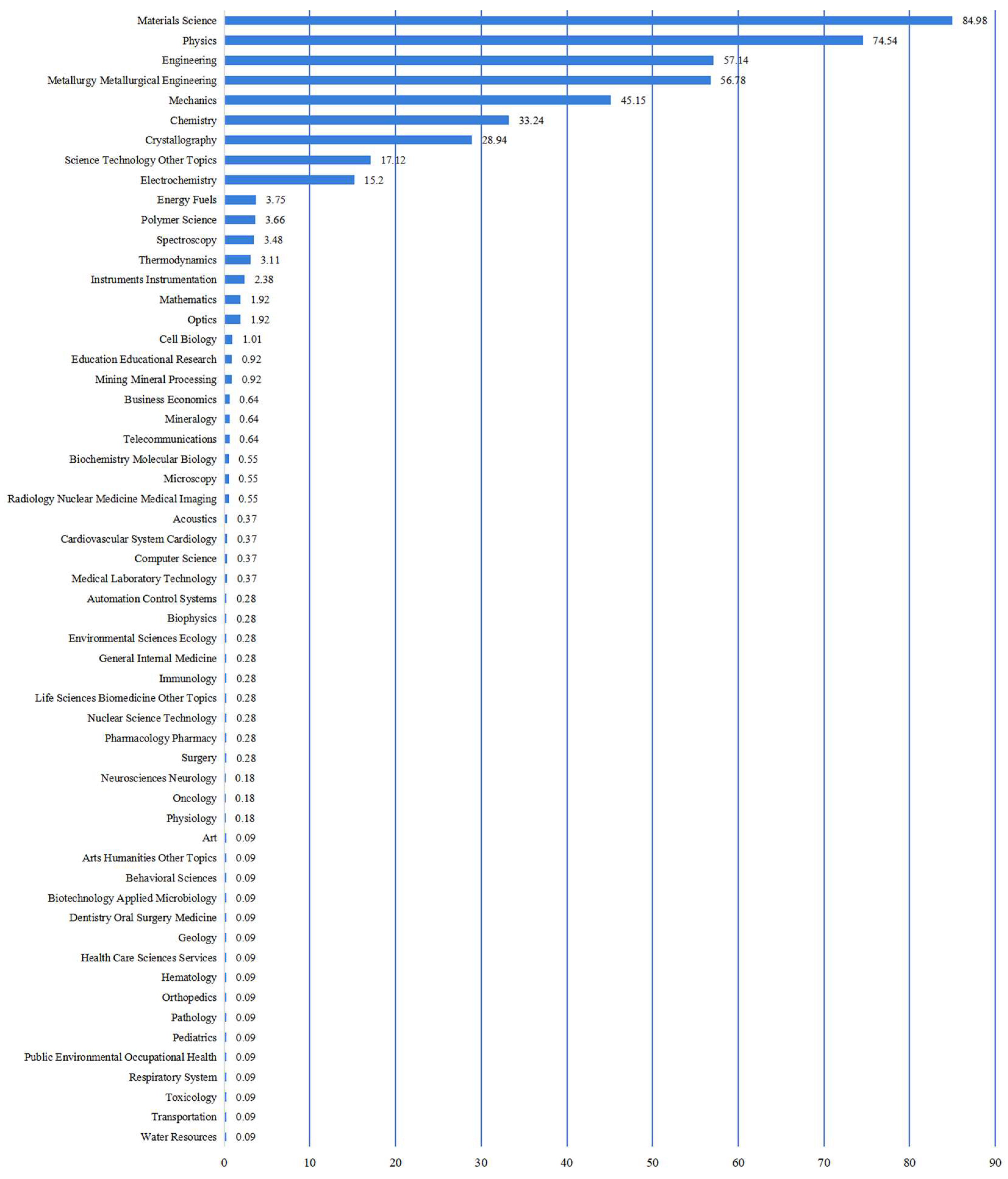
Based on the search results for “Mg-Li alloys” in the Web of Science database, over 1,600 papers published from 2010 to the present have been retrieved. The primary research areas encompass chemistry, engineering, materials science, mechanics, metallurgy, and physics. As illustrated in
Figure 2, the data analysis reveals that research on magnesium-lithium alloys is remarkably extensive, with materials science, physics, and engineering being the top three predominant fields.
Therefore, the development of surface engineering technologies aimed at enhancing the strength and corrosion resistance of magnesium-lithium alloys is of paramount importance. These technologies include chemical conversion [
9,
10,
11], electroless plating [
12,
13,
14], anodizing [
15,
16,
17], coating techniques [
18,
19,
20], and various surface modification methods [
21,
22,
23]. This article comprehensively reviews the fundamental principles and practical applications of these approaches, while also identifying their current limitations and potential development directions. The findings presented herein aim to provide valuable insights for the fabrication of high-performance magnesium-lithium alloys.
2. Different Surface Engineering Techniques
Surface modification involves altering the chemical composition of the substrate surface to achieve desired changes in surface structure and properties [
24,
25,
26]. In contrast, surface treatment refers to the process of enhancing surface characteristics through microstructural modifications of the substrate material while maintaining its original chemical composition, as exemplified by surface heat treatment techniques [
27,
28,
29]. Surface coating, on the other hand, entails the deposition of a distinct film layer onto the substrate surface, where both the chemical composition and microstructure of the coating may significantly differ from those of the underlying substrate material [
30,
31,
32].
The limited corrosion resistance of Mg-Li alloys primarily stems from two factors: the low-density oxide film naturally formed on their surface and the localized galvanic corrosion induced by the presence of secondary phases and alloying elements within the matrix [
33,
34,
35]. In response to these challenges, significant research efforts have been directed toward developing effective surface protection strategies in recent years, resulting in substantial advancements in this field.
2.1. Chemical Conversion
Chemical conversion involves the formation of oxide or metallic compound passivation films on metal surfaces through chemical treatment solutions [
36,
37]. This process offers several advantages, including minimal instrumentation requirements, operational simplicity, and the ability to be completed through straightforward immersion. However, the resulting conversion films are typically thin and exhibit limited adhesion strength, which primarily serves to reduce rather than effectively prevent corrosion. Consequently, additional coating processes are often required to achieve comprehensive protection.
Currently, several surface chemical treatment techniques have been developed for Mg-Li alloys, including phytic acid conversion [
38], phosphate conversion [
39], stannate conversion [
40], and rare-earth-based conversion coatings [
41].
Phytic acid (C₆H₁₈O₂₄P₆), a novel metal surface treatment agent, forms protective films on substrate surfaces through chemical adsorption. These films effectively inhibit the penetration of corrosive anions to the metal surface, demonstrating significant corrosion protection properties [
42]. However, the quality of phytic acid conversion films is influenced by multiple parameters, including pH value, concentration, and treatment duration, making quality control challenging. Gao et al. [
43] investigated chromium-free phytic acid conversion on Mg-11Li alloy, demonstrating enhanced corrosion resistance through increased corrosion potential and reduced current density compared to conventional chromic acid conversion.
Phosphate conversion films are formed through chemical reactions between metal surfaces and phosphoric acid-based conversion solutions [
44]. Song et al. [
45] systematically analyzed the formation mechanism of phosphate conversion films on biphasic magnesium-lithium alloys using XPS and SEM. Their findings revealed a three-stage process: initial dissolution of Mg(OH)₂ and MgO from the alloy surface, subsequent dissolution of the β-phase, and finally, the establishment of dynamic equilibrium between film formation and dissolution. Stannate conversion, recognized for its environmental compatibility, offers several advantages including cost-effectiveness, rapid film formation, and uniform thickness distribution [
46,
47]. The reaction between rare earth salt solutions and magnesium-lithium alloy surfaces occurs rapidly. When combined with other salt solutions, these reactions produce protective films with enhanced corrosion resistance. Moreover, the conversion process is environmentally benign and biocompatible [
48,
49].
Xu et al. [
50] systematically investigated key parameters for coating preparation, including immersion time, temperature, pH value of the conversion solution, and curing duration. Through comprehensive optimization, they established the optimal technological parameters for RE-Si composite coating preparation, as illustrated in
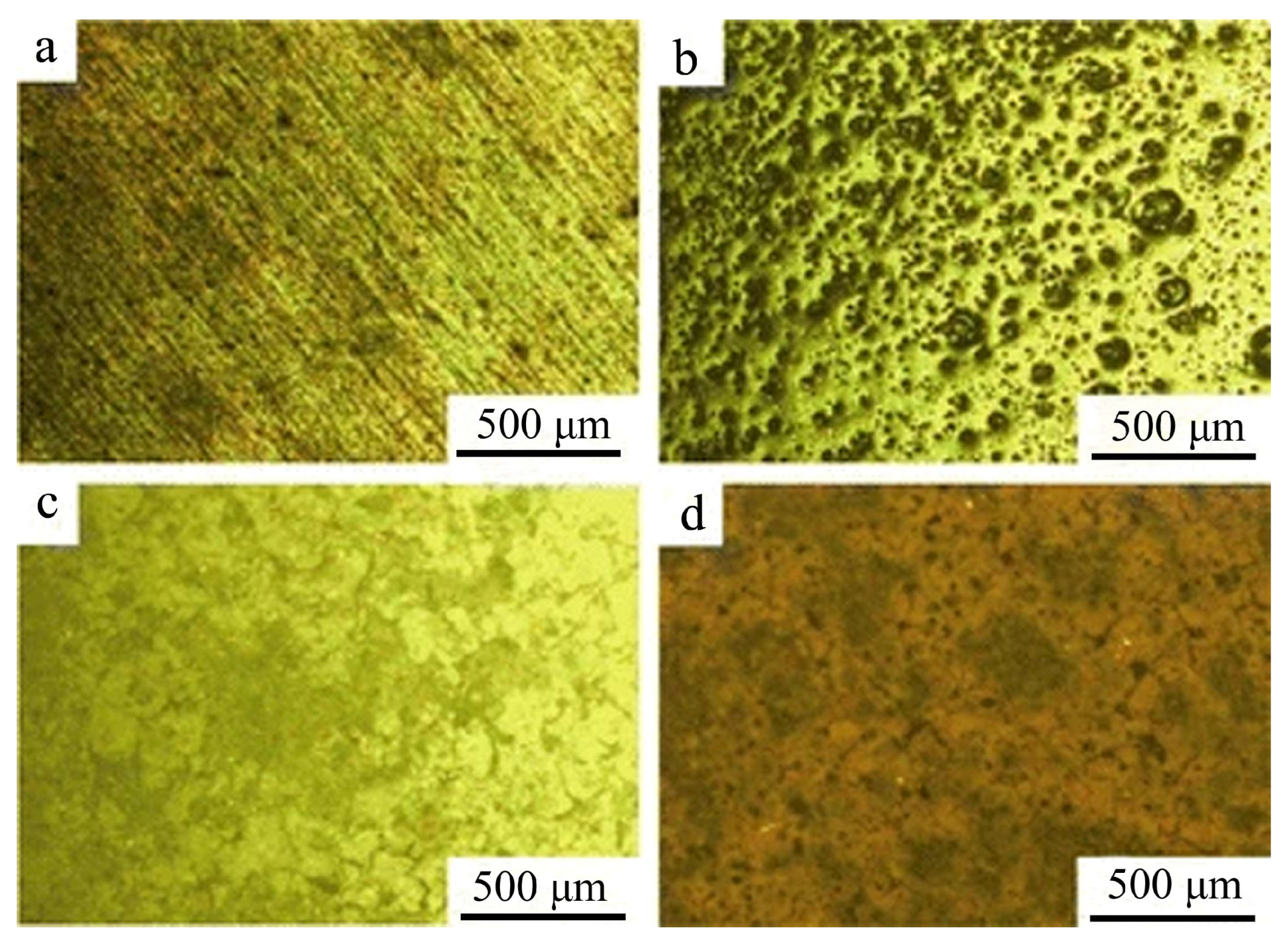
Figure 3. The study revealed four distinct morphological characteristics. The substrate surface displays numerous potholes and scratches (
Figure 3a). The silane coating, while relatively compact, contains multiple gaps and exhibits minimal thickness (
Figure 3b). The RE-Si composite coating demonstrates significantly improved compactness and uniformity compared to the silane coating, accompanied by a substantial thickness increase (
Figure 3c). However, localized fragmentation into small patches exposes the underlying silane coating and Mg-Li alloy substrate at the edges, compromising overall coating integrity and corrosion protection. The incorporation of nano-zirconium dioxide particles markedly enhances the compactness of the RE-Si composite coating while significantly reducing crack formation (
Figure 3d). These findings demonstrate that nanoparticle addition not only improves coating morphology and quality but also positively influences the conversion coating formation process.
2.2. Electroless Plating
Electroless plating is an autocatalytic deposition process that utilizes redox reactions to deposit metallic elements from solution onto substrate surfaces without external current application. This well-established technique produces coatings with excellent comprehensive performance, particularly when employing high-density nanocrystalline nickel plating, which exhibits superior wear resistance [
51,
52,
53].
However, the inherent challenge lies in the negative standard electrode potentials of Mg and Li in magnesium-lithium alloys, which renders them susceptible to corrosion by conventional Ni-P plating solutions. To address this issue, researchers have developed specialized pretreatment processes. Yang et al. [
54] investigated electroless nickel plating on Mg-8Li alloy, introducing an innovative molybdate pretreatment. Their findings revealed that the pretreatment layer serves dual functions as both an effective barrier and catalytic layer for subsequent Ni-P deposition. The molybdate layer reduces the potential difference between the Ni-P coating and substrate, thereby enhancing the alloy’s corrosion resistance.
In a separate study, Zou et al. [
55] developed a pretreatment process using Ce(NO
3)
3-KMnO
4 solution combined with ultrasonic-assisted electroless Ni-P plating for biphasic magnesium-lithium alloys. This approach resulted in coatings with improved surface smoothness, higher density, and reduced cell size, ultimately enhancing the overall coating performance.
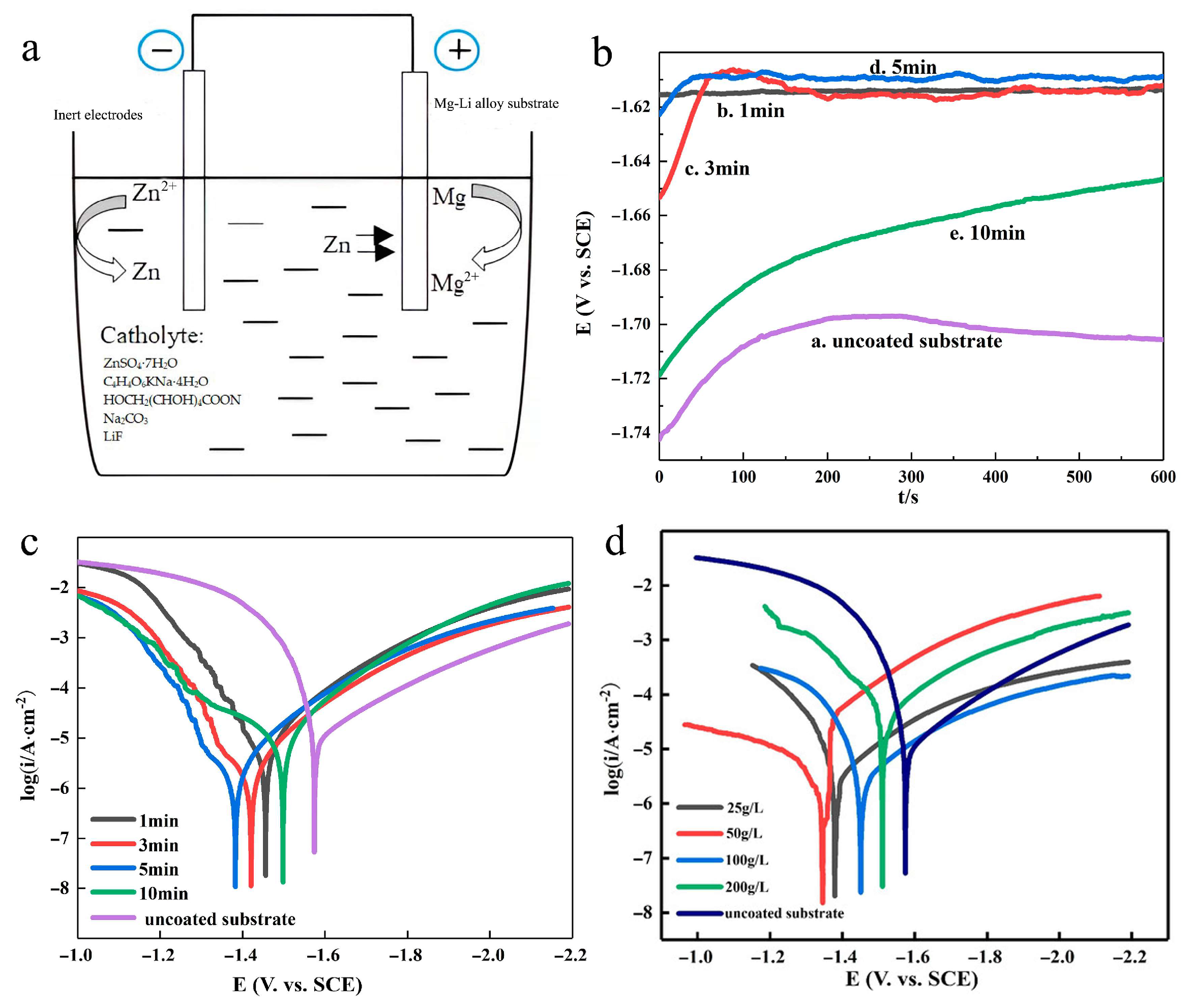
Yue et al. [
56] investigated the electroless deposition of zinc on Mg-Li alloy surfaces, as illustrated in
Figure 4. The study included a schematic representation of the electroless plating galvanic cell (
Figure 4a), open-circuit potential measurements (
Figure 4b), and tafel polarization curve analyses under various conditions (
Figure 4c,d). The experimental results demonstrated a significant positive shift in the corrosion potential of the Mg-Li alloy, reaching -1.38 V, accompanied by a substantial reduction in corrosion current density to 2.78 × 10
−6 A/cm
2. These findings indicate that the electroless zinc deposition process significantly enhances the corrosion resistance of Mg-Li alloys.
2.3. Anodic Oxidation
Anodizing represents an electrochemical process that generates a robust oxide layer on alloy surfaces through the application of specialized electrolytes and controlled electric currents [
57]. Compared to chemical conversion coatings, anodic films exhibit superior durability, enhanced wear resistance, and improved corrosion protection [
58,
59]. Furthermore, these films demonstrate exceptional characteristics, including strong adhesion, effective electrical insulation, and remarkable thermal shock resistance. Consequently, anodizing has emerged as one of the most prevalent surface treatment techniques for Mg-Li alloys [
60].
The formation of porous oxide layers during anodization primarily results from hydrogen evolution at the cathode, combined with inherent chemical and electrochemical heterogeneities on Mg-Li alloy surfaces. To address these structural limitations, the incorporation of process additives has become crucial for enhancing oxide film compactness and structural integrity. In a significant study, Chang et al. [
61] systematically investigated anodic oxidation coatings on Mg-Li alloys using various amino acids as additives. Their analysis revealed that the coatings predominantly comprised MgO, Mg(OH)
2, and LiOH. Notably, amino acid-modified coatings exhibited more uniform surface morphology and superior corrosion resistance compared to unmodified counterparts. However, the researchers observed an inverse correlation between the carbon chain length of amino acids and their beneficial effects on coating performance.
Micro-arc oxidation (MAO), alternatively referred to as plasma electrolytic oxidation or anodic spark deposition, represents an advanced surface modification technology. This process involves the formation of dense, wear-resistant, and corrosion-resistant ceramic coatings predominantly composed of metal oxides through controlled arc discharge phenomena [
62,
63,
64]. MAO technology offers numerous advantages, including operational simplicity and environmental compatibility, making it particularly suitable for applications in aerospace, electronic equipment, and marine engineering. Furthermore, its implementation on Mg-Li alloy surfaces has demonstrated significant potential [
65,
66,
67].
The MAO coating formation process is inherently complex, with multiple influencing factors that determine coating performance. These parameters include substrate composition, electrolyte formulation, additive selection, power supply configuration, voltage, current density, frequency, and duty cycle [
65,
68]. In a comprehensive study, Qian et al. [
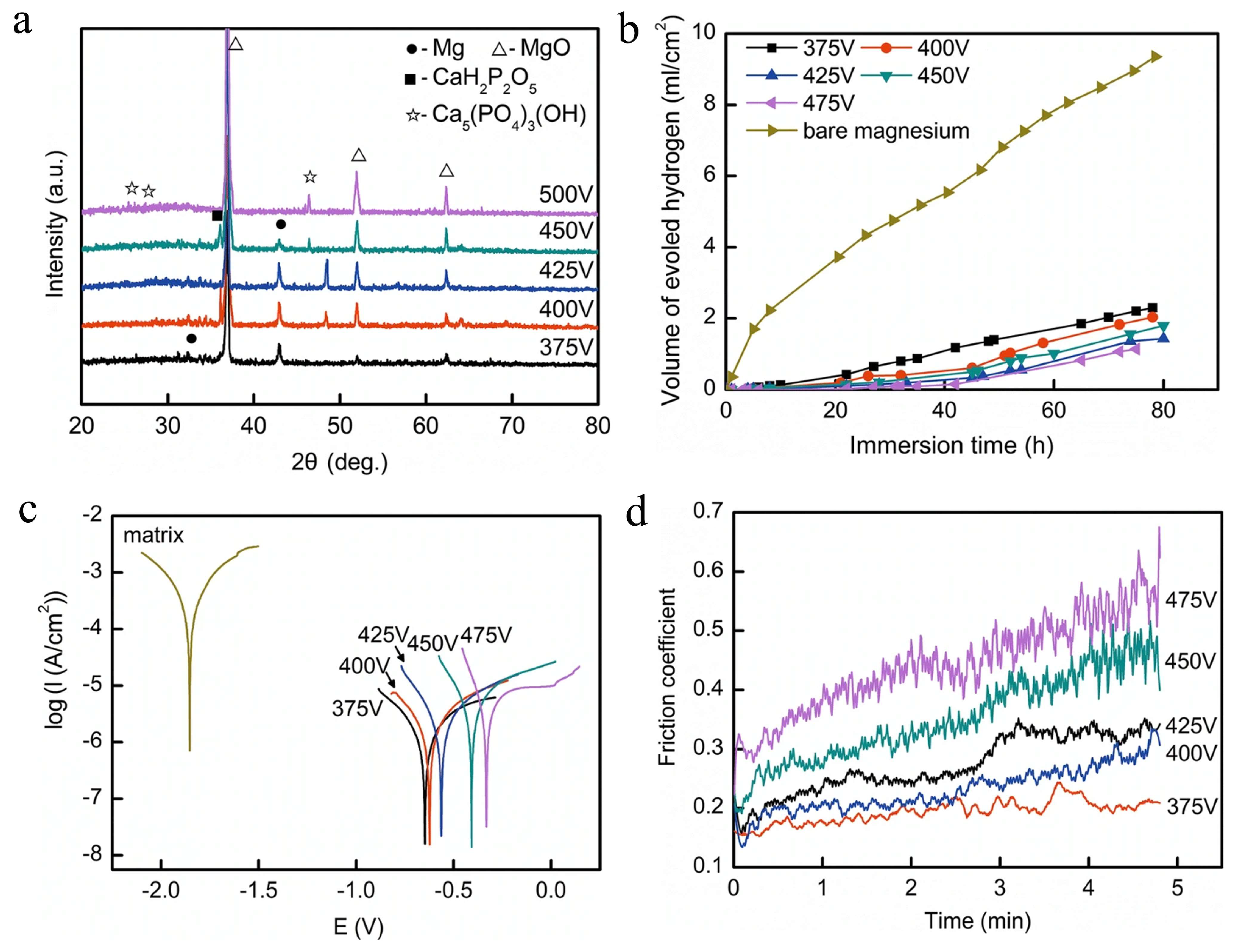
69] investigated calcium phosphate (CaP) coatings on Mg-8Li-2Ca alloy prepared through MAO in an alkaline Na₃PO₄-Ca[C₃H₇O₆P] solution at varying applied voltages, as partially illustrated in
Figure 5. The researchers employed X-ray diffraction to analyze the coating microstructure and phase composition (
Figure 5a). Electrochemical performance was evaluated through hydrogen evolution measurements and tafel plots in simulated body fluid solution (
Figure 5b,c), while tribological properties were assessed using a friction and wear testing machine (
Figure 5d). The experimental results revealed that the MAO coatings exhibited a porous structure primarily composed of MgO, Ca₅(PO₄)₃(OH), and CaH₂P₂O₅. Notably, increasing applied voltage significantly enhanced both corrosion and wear resistance properties. The corrosion current density of MAO coatings demonstrated approximately two orders of magnitude reduction compared to the untreated substrate.
2.4. Coating Technology
Surface engineering technologies for Mg-Li alloys encompass a diverse array of advanced methodologies, including vapor deposition, thermal spraying, and the development of superhydrophobic coatings through organic-inorganic hybridization. Vapor deposition techniques are primarily classified into physical vapor deposition (PVD) [
70,
71] and chemical vapor deposition (CVD) [
72], with plasma-enhanced chemical vapor deposition (PECVD) emerging as a particularly noteworthy technique [
73]. PECVD employs plasma to activate reactive gases, thereby facilitating chemical reactions on or near the substrate surface to form durable solid films.
Thermal spraying techniques, including plasma spraying [
74,
75] and cold spraying [
76,
77,
78], involve the deposition of metallic or ceramic particles onto alloy surfaces to create protective coatings. These coatings effectively isolate the substrate from corrosive environments, significantly enhancing both corrosion and wear resistance. For instance, Al₂O₃ ceramic coatings have demonstrated substantial improvements in surface hardness and corrosion resistance of Mg-Li alloys [
78]. However, challenges persist in optimizing the interfacial bonding strength and coating uniformity.
Superhydrophobic coatings represent an innovative approach through the construction of micro-nano structured surfaces that induce high water contact angles, thereby minimizing corrosive media adhesion [
79,
80,
81]. These coatings not only enhance corrosion resistance but also impart self-cleaning properties to Mg-Li alloys [
82,
83,
84]. Significant progress has been made in this field, with researchers achieving promising results through chemical modification and laser processing techniques for superhydrophobic coating preparation on Mg-Li alloys [
85]. These advancements demonstrate considerable potential for expanding the practical applications of Mg-Li alloys in demanding environments.
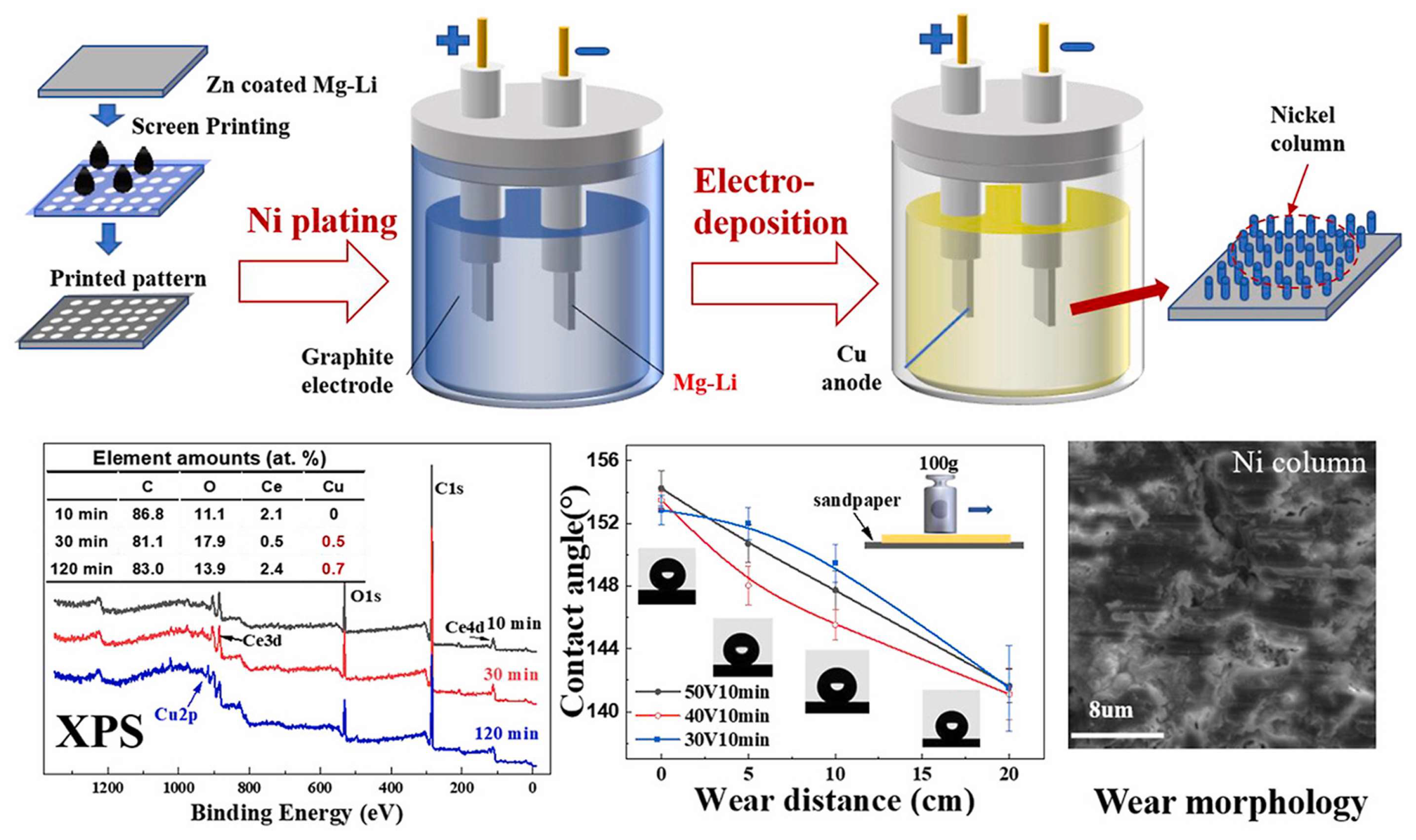
In a pioneering study, He et al. [
86] developed a novel approach combining screen-printed mask electroplating with electrodeposition to fabricate dense micro/nano-scale papillary-structured superhydrophobic surfaces on Mg-Li alloys. The research revealed that extended deposition times induced copper anode dissolution, resulting in the formation of copper compounds within the superhydrophobic coating. This process generated dense, needle-like structures on the papillae, as illustrated in
Figure 6. Notably, the armor-like nickel columns effectively maintained high contact angles despite structural wear, demonstrating remarkable surface properties including low adhesion, self-cleaning capability, chemical stability, and exceptional corrosion resistance.
2.5. Other Surface Modification Techniques
In addition to the aforementioned methods, surface engineering for Mg-Li alloys also encompasses surface nanocrystallization and composite surface engineering technologies, which provide advanced performance enhancements [
23,
87]. Surface nanocrystallization represents an innovative approach that significantly improves surface properties through grain refinement at the nanoscale [
88]. Composite surface engineering, defined as the strategic integration of two or more surface treatment techniques, creates composite modified layers with gradient or multilayer structures on material surfaces, thereby enhancing their functional characteristics [
89].
Surface nanocrystallization of Mg-Li alloys can be accomplished through advanced techniques such as surface mechanical attrition treatment (SMAT) [
90] and ultrasonic nanocrystal surface modification (UNSM) [
24]. Zhang et al. [
90] conducted a comprehensive investigation of Mg-3wt.% Li-6wt.% Al alloy using SMAT. Their findings revealed that grain refinement primarily occurs through dislocation slip, with twinning playing a significant role during the initial stages when grain sizes are larger. This process resulted in substantial surface hardness enhancement.
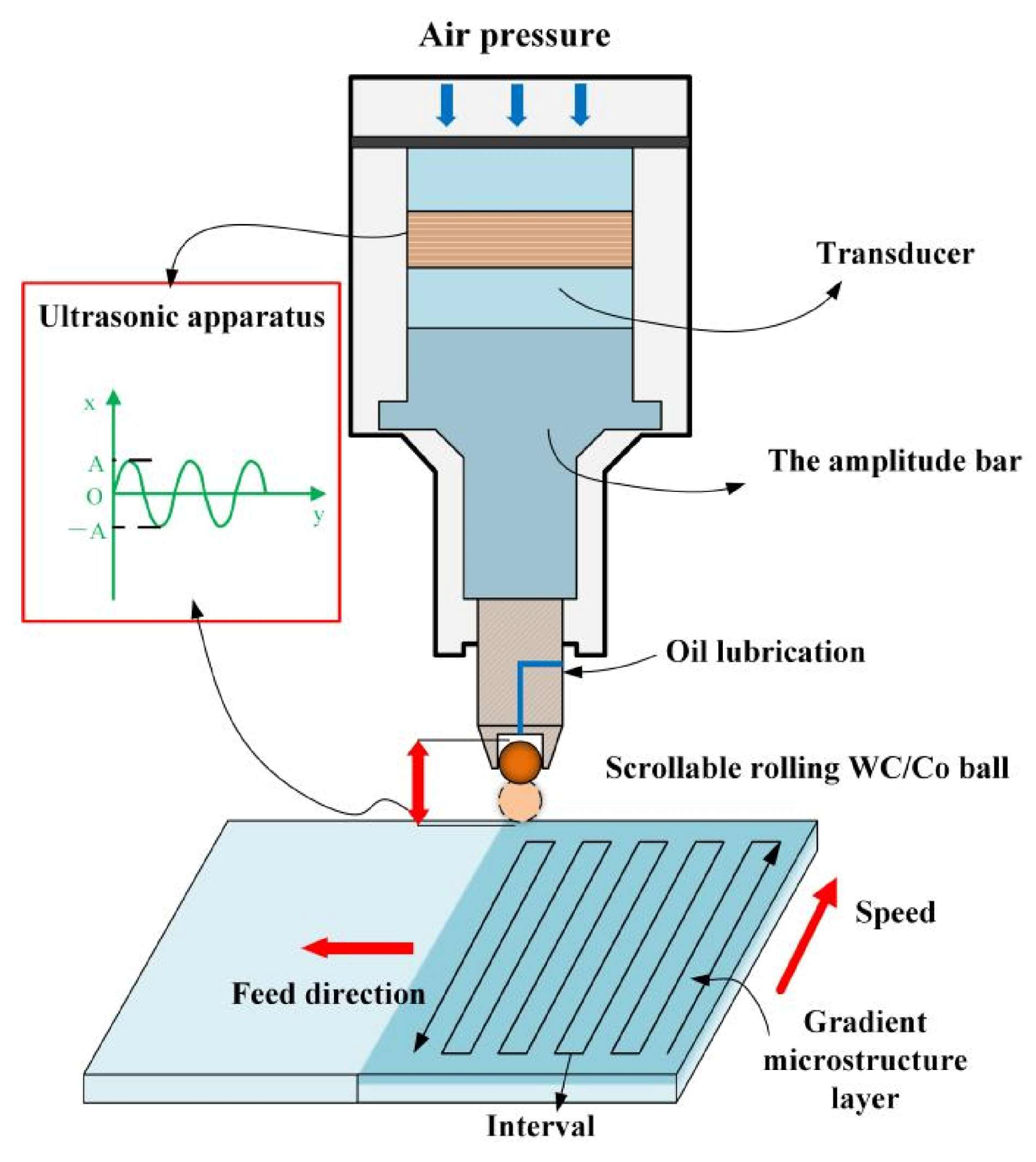
Figure 7 presents the schematic diagram of the UNSM instrument, based on research by Zou et al. [
91]. Their systematic study examined the effects of UNSM treatment on hot-rolled Mg-Li alloys (LAE361 and LA106), focusing on microstructure evolution, mechanical properties, deformation mechanisms, and corrosion resistance. The treatment demonstrated remarkable improvements in surface hardness and significant reductions in surface roughness. Notably, UNSM treatment achieved simultaneous enhancements in both strength and plasticity [
91]. However, the underlying mechanisms responsible for these improvements warrant further in-depth investigation.
Composite coating technologies for Mg-Li alloys have evolved to include various innovative combinations, such as plasma electrolysis with chemical conversion [
87], sol-gel methods [
92], superhydrophobic coatings integrated with electroless plating [
93], micro-arc oxidation [
94,
95], and micro-arc oxidation combined with chitosan (CS) [
96].
Li et al. [
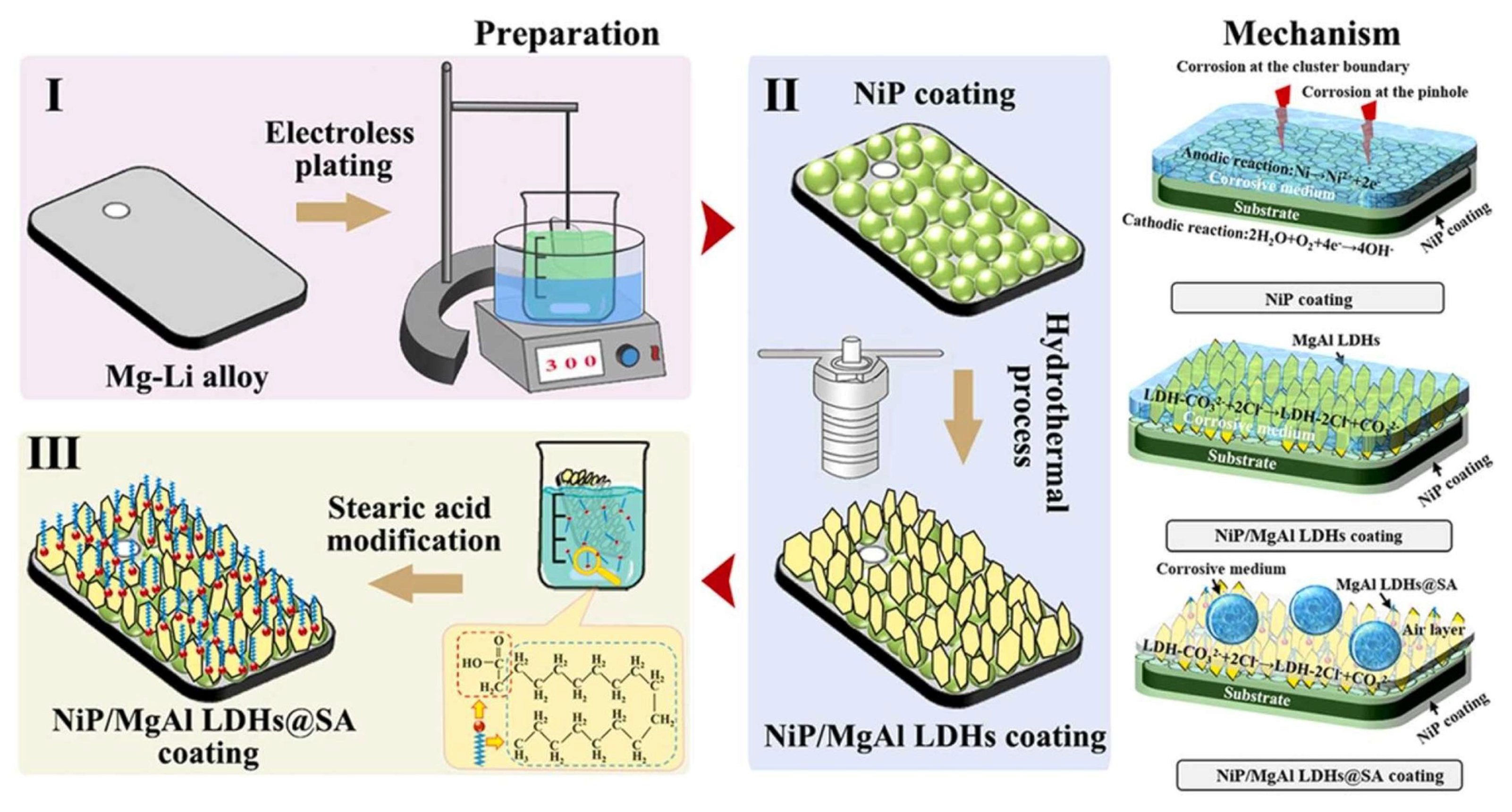
93] successfully developed an innovative superhydrophobic composite coating system consisting of nickel-based metal coupled with layered double hydroxides (LDHs) on Mg-Li alloy substrates.
Figure 8 illustrates the schematic diagram of the preparation process and underlying mechanisms, which primarily involve three sequential steps: (1) deposition of Ni-P coating, (2) in-situ growth of MgAl LDHs, and (3) surface modification with stearic acid. The study also presented a comprehensive analysis of the corrosion mechanisms for different coating systems. Experimental results demonstrated that MgAl LDHs uniformly grew in situ on the electroless-plated Ni-P coating through hydrothermal treatment. The resulting MgAl LDHs exhibited a complex honeycomb-like micro-nano structure, increasing the composite coating’s surface roughness to 126 nm. This superhydrophobic composite coating represents a significant advancement in corrosion protection for Mg-Li alloys, substantially enhancing their durability and performance in demanding environments [
93].
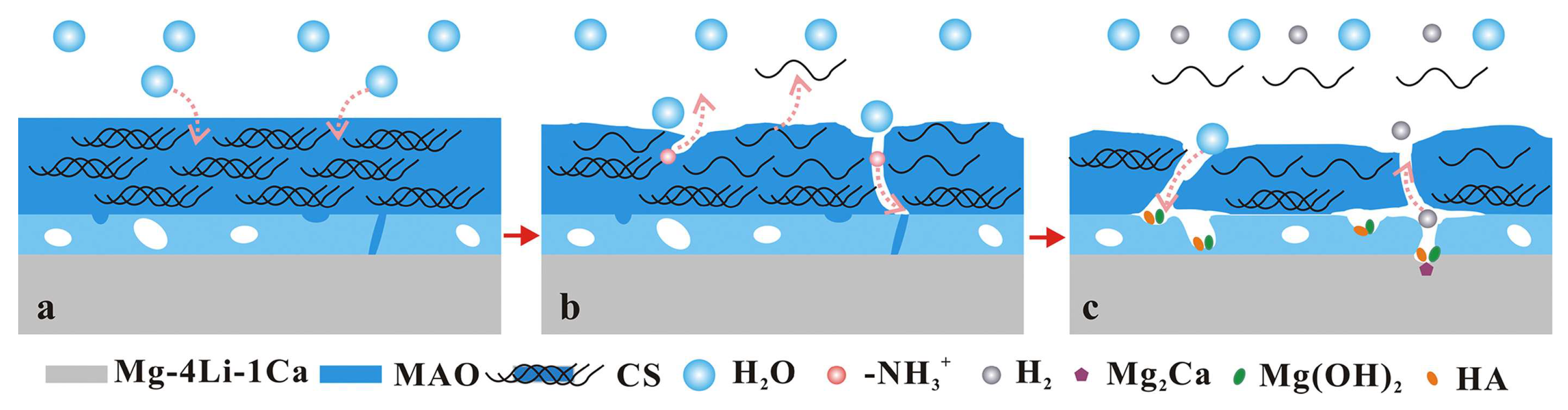
Figure 9 presents the schematic illustration of the self-degradation mechanism of MAO/CS composite coatings on Mg-4Li-1Ca alloys in Hank’s solution [
96]. The degradation process of CS on the Mg-4Li-1Ca alloy proceeds through five distinct stages: (1) water diffusion through the CS film onto the MAO coating (
Figure 9a); (2) release of R-NH₃⁺ through CS self-degradation (
Figure 9b); (3) chemical corrosion of the MAO coating through existing cracks and pores; (4) electrochemical corrosion of the substrate; and (5) eventual peeling of the CS film (
Figure 9c).
3. Conclusions
With the rapid advancement of materials science, nanotechnology, and intelligent manufacturing, surface treatment technologies for magnesium-lithium alloys are undergoing transformative developments. Future research in this field will concentrate on four key dimensions: performance enhancement, environmental sustainability, intelligent innovation, and multi-scenario adaptability:
(1) Collaborative Design of High-Precision Multifunctional Composite Coatings
Future research will emphasize the development of intelligent coatings that integrate multiple functionalities, including corrosion resistance, wear resistance, and thermal/electrical conductivity. By incorporating nanomaterials into ceramic or polymer matrices, gradient structures can be engineered at the micro-scale to achieve a balance between mechanical performance and environmental responsiveness. For instance, self-healing coatings utilizing microcapsule technology or dynamic chemical bonds can autonomously repair surface damage, significantly extending service life. Furthermore, bio-inspired coatings, fabricated with precision, will enhance the stability of magnesium-lithium alloys in humid or corrosive environments. Computational materials science simulations will play a critical role in optimizing composition distribution and interfacial bonding strength, ensuring long-term compatibility with the substrate.
(2) Iterative Upgrading of Green and Low-Carbon Surface Treatment Processes
Chromium-free conversion coatings, low-temperature plasma deposition, and bio-based coating technologies are poised to replace traditional electroplating and chemical oxidation methods. For example, micro-arc oxidation employing water-soluble ionic liquid electrolytes can substantially reduce toxic byproducts while improving coating density. Energy-efficient technologies such as photocuring and laser-induced deposition will streamline processing steps and minimize carbon emissions. Under the principles of the circular economy, recyclable coating designs and regeneration techniques will emerge as key research areas, fostering sustainable development across the industry chain.
(3) Full-Chain Integration of Intelligent and Digital Surface Engineering
Artificial intelligence and digital twin technologies will revolutionize coating development and application. Machine learning-driven high-throughput experimental platforms will enable rapid screening of optimal coating formulations and process parameters, significantly reducing trial-and-error costs. The integration of 3D printing with topology optimization algorithms will facilitate the creation of customized functional surface structures for complex components. Online monitoring systems will provide real-time tracking of coating deposition processes, ensuring consistent quality. Digital twin models will simulate coating failure mechanisms under extreme conditions, offering theoretical support for lifespan prediction. This integration will drive a paradigm shift from “experience-driven” to “data-driven” approaches.
(4) Innovative Breakthroughs in Extreme Environment-Adaptive Coatings
For applications in extreme environments—such as aerospace cryogenic conditions, deep-sea high-pressure zones, or nuclear radiation settings—coatings must demonstrate exceptional environmental tolerance. High-temperature oxidation-resistant coatings could unlock new applications for magnesium-lithium alloys in engine components. Nanoscale oxide protective layers, fabricated via atomic layer deposition (ALD), can effectively mitigate hydrogen permeation, addressing hydrogen embrittlement in acidic or irradiated environments. Additionally, adaptive color-changing coatings (e.g., thermochromic or mechanochromic materials) may incorporate sensing capabilities to provide real-time structural health monitoring. The successful development of such coatings will enable breakthrough applications of magnesium-lithium alloys in defense, energy, and other high-tech sectors.
Author Contributions
Writing—original draft preparation, N.N. L.; conceptualization, N.N. L, Z.J. H., L. X. and Y.P. T; methodology, N.N. L., Z.J. H, Y. S., Y.P. T., and S. L.; writing—review and editing, L. X. and J. P.; investigation, N.N. L., Z.J. H, Y. F., Y. S., X. C, and J. P; formal analysis, N.N. L, Z.J. H, Y. F., Y.P. T, X. C, and S. L.; data curation, Y. F., S. L., L. X., and J. P.; resources, Y. F., Y. S., X. C, S. L. and Y.P. T. ; supervision L. X. and J. P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Key R & D and Promotion Project of the Henan Province (Science and Technology Research) (No. 252102220078, 252102231064, and 252102220064), the fund of Henan Province Science and Technology Research Project (Nos. 232102220055 and 242102220072).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The data presented in this study are available on request from the corresponding author
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, B.-J.; Luan, J.-Y.; Xu, D.-K.; Sun, J.; Li, C.-Q.; Han, E.-H. Research Progress on the Corrosion Behavior of Magnesium–Lithium-Based Alloys: A Review. Acta Met. Sin. (English Lett. 2018, 32, 1–9. [CrossRef]
- Peng, X.; Shihao, X.; Ding, D.; Liao, G.; Guohua, W.; Liu, W.; Ding, W. Microstructural evolution, mechanical properties and corrosion behavior of as-cast Mg-5Li-3Al-2Zn alloy with different Sn and Y addition. J. Mater. Sci. Technol. 2021, 72, 16–22. [CrossRef]
- Cain, T.W.; Labukas, J.P. The development of β phase Mg–Li alloys for ultralight corrosion resistant applications. npj Mater. Degrad. 2020, 4, 1–10. [CrossRef]
- Tang, H.; Yan, Y.D.; Zhang, M. L.; Li, X.; Han, W.; Xue, Y.; Zhang, Z. J.; He, H. Fabrication of Mg-Pr and Mg-Li-Pr alloys by electrochemical co-reduction from their molten chlorides. Electrochim. Acta. 2013, 107, 209−215.
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloy. 2021, 9, 41–56. [CrossRef]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mater. Process. Tech. 2001, 117, 276-281.
- Song, Y.; Shan, D.; Chen, R.; Han, E.-H. Corrosion characterization of Mg–8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [CrossRef]
- Zhang, C.; Huang, X.; Zhang, M.; Gao, L.; Wu, R. Electrochemical characterization of the corrosion of a Mg–Li Alloy. Mater. Lett. 2008, 62, 2177–2180. [CrossRef]
- Ma, Y.; Li, N.; Li, D.; Zhang, M.; Huang, X. Characteristics and corrosion studies of vanadate conversion coating formed on Mg–14 wt% Li–1 wt% Al–0.1 wt% Ce alloy. Appl. Surf. Sci. 2012, 261, 59-67.
- Yang, L.; Li, J.; Yu, X.; Zhang, M.; Huang, X. Lanthanum-based conversion coating on Mg–8Li alloy. Appl. Surf. Sci. 2008, 255, 2338–2341. [CrossRef]
- Zeng, R.-C.; Sun, X.-X.; Song, Y.-W.; Zhang, F.; Li, S.-Q.; Cui, H.-Z.; Han, E.-H. Influence of solution temperature on corrosion resistance of Zn-Ca phosphate conversion coating on biomedical Mg-Li-Ca alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 3293–3299. [CrossRef]
- Luo, H.-J.; Song, B.-N.; Liu, Y.-H.; Yao, G.-C. Electroless Ni-P plating on Mg-Li alloy by two-step method. Trans. Nonferrous Met. Soc. China 2011, 21, 2225–2230. [CrossRef]
- Yin, T.; Wu, R.; Leng, Z.; Du, G.; Guo, X.; Zhang, M.; Zhang, J. The process of electroplating with Cu on the surface of Mg–Li alloy. Surf. Coatings Technol. 2013, 225, 119–125. [CrossRef]
- Pei, S.-F.; Li, S.-Q.; Zhong, L.; Cui, K.-F.; Yang, J.; Yang, Z.-G. Analysis of the Causes of Differences between the Upper and Lower Surfaces of Electroless Ni–P Coating on LZ91 Magnesium–Lithium Alloy. Coatings 2022, 12, 1157. [CrossRef]
- Wang, L.; Wu, T.; Cao, D.; Yin, J.; Wang, G.; Zhang, M. Self-growth of micro-and nano-structured Mg(OH)2 on electrochemically anodised Mg–Li alloy surface. J. Exp. Nanosci. 2015, 10, 56-65.
- Sharma, A.K.; Rani, R.U.; Bhojaraj, H.; Narayanamurthy, H. Galvanic black anodizing on Mg-Li alloys. J. Appl. Electrochem. 1993, 23, 500–507. [CrossRef]
- Wang, J.-Y.; Liu, C.-M.; Chen, W.-K.; Liu, Y.-M.; Ger, M.-D. Microstructure and Corrosion Resistance of Anodized Mg-9 mass%Li-1 mass%Zn Alloy. Mater. Trans. 2008, 49, 1355–1358. [CrossRef]
- Ji, P.; Long, R.; Hou, L.; Wu, R.; Zhang, J.; Zhang, M. Study on hydrophobicity and wettability transition of Ni-Cu-SiC coating on Mg-Li alloy. Surf. Coatings Technol. 2018, 350, 428–435. [CrossRef]
- Yamauchi, N.; Ueda, N.; Okamoto, A.; Sone, T.; Tsujikawa, M.; Oki, S. D. L. C. DLC coating on Mg-Li alloy. Surf. Coat. Tech. 2007, 201, 4913-4918.
- Yao, Z.; Ju, P.; Xia, Q.; Wang, J.; Su, P.; Wei, H.; Li, D.; Jiang, Z. Preparation of thermal control coatings on Mg–Li alloys by plasma electrolytic oxidation. Surf. Coatings Technol. 2016, 307, 1236–1240. [CrossRef]
- Ma, Y.; Li, N.; Li, D.; Zhang, M.; Huang, X. A two-step surface treatment, combining fluoride pretreatment and anodic electrophoresis deposition of waterborne acrylic resin, for Mg–Li–Al–Ce alloy. Mater. Lett. 2013, 90, 11–13. [CrossRef]
- Zhang, L.; Zou, Y.; Wang, H.; Meng, L.; Liu, J.; Zhang, Z. Surface nanocrystallization of Mg-3 wt.% Li-6 wt.% Al alloy by surface mechanical attrition treatment. Mater. Charact. 2016, 120, 124–128. [CrossRef]
- Li, Y.; Zhu, X.C.; Sun, J.; Yao, Q.T.; Du, X.D.; Wu, Y.C. Nitriding behaviour of Mg-Li alloy with surface mechanical nano-alloying pretreatment. Surf. Eng. 2020, 37, 1075–1083. [CrossRef]
- Zou, Y.; Shen, R.; Lu, Z.; Zhou, Y.; Li, Y. Enhanced low-cycle fatigue behavior LZ91 Mg–Li alloy with ultrasonic nanocrystal surface modification. Fatigue Fract. Eng. Mater. Struct. 2023, 46, 2485–2495. [CrossRef]
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [CrossRef]
- Prakash, S.; Karacor, M.; Banerjee, S. Surface modification in microsystems and nanosystems. Surf. Sci. Rep. 2009, 64, 233–254. [CrossRef]
- Montealegre, M.A.; Castro, G.; Rey, P.; Arias, J.L.; Vázquez, P.; González, M. Surface treatments by laser technology. Contemp. Mater. 2010, 1, 19-30.
- Wagner, H.-E.; Brandenburg, R.; Kozlov, K.; Sonnenfeld, A.; Michel, P.; Behnke, J. The barrier discharge: basic properties and applications to surface treatment. Vacuum 2003, 71, 417–436. [CrossRef]
- Sun, Y.-H.; Wang, R.-C.; Peng, C.-Q.; Feng, Y.; Yang, M. Corrosion behavior and surface treatment of superlight Mg–Li alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 1455–1475. [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Energy Chem. 2020, 43, 220–235. [CrossRef]
- Rickerby, D.; Bull, S. Engineering with surface coatings: The role of coating microstructure. Surf. Coatings Technol. 1989, 39-40, 315–328. [CrossRef]
- Ma, X.C.; Jin, S.Y.; Wu, R.Z.; Wang, J.X.; Wang, G.X.; KRIT, B.; Betsofen, S. Corrosion behavior of Mg− Li alloys: A review. T. Nonferr. Metal. Soc. 2021, 31, 3228-3254.
- Chang, T.-C.; Wang, J.-Y.; Chu, C.-L.; Lee, S. Mechanical properties and microstructures of various Mg–Li alloys. 2006, 60, 3272–3276. [CrossRef]
- Li, C.; Xu, D.; Chen, X.-B.; Wang, B.; Wu, R.; Han, E.; Birbilis, N. Composition and microstructure dependent corrosion behaviour of Mg-Li alloys. Electrochimica Acta 2018, 260, 55–64. [CrossRef]
- Cheng, D.; Negreiros, F.R.; Aprà, E.; Fortunelli, A. Computational Approaches to the Chemical Conversion of Carbon Dioxide. ChemSusChem 2013, 6, 944–965. [CrossRef]
- Jiang, C.-C.; Cao, Y.-K.; Xiao, G.-Y.; Zhu, R.-F.; Lu, Y.-P. A review on the application of inorganic nanoparticles in chemical surface coatings on metallic substrates. RSC Adv. 2017, 7, 7531–7539. [CrossRef]
- Zhang, C.H.; Li, R.M.; Gao, L.L.; Zhang, M.L. Corrosion protection of Mg–11Li–3Al–0·5RE alloy using hybrid epoxy/silica conversion coatings. Corros. Eng. Sci. Techn. 2013, 48, 276-281.
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E. H. A novel phosphate conversion film on Mg-8.8 Li alloy. Surf. Coat. Tech. 2009, 203, 1107-1113.
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of Corrosion-Resistant Conversion Coatings for Magnesium and Its Alloys. Corrosion 2011, 67, 035005–1. [CrossRef]
- Yang, X.; Wang, G.; Dong, G.; Gong, F.; Zhang, M. Rare earth conversion coating on Mg-8.5 Li alloys. J. Alloy. Compd. 2009, 487, 64-68.
- Shi, W.; Song, Z.; Wang, J.; Li, Q.; An, Q. Phytic acid conversion film interfacial engineering for stabilizing zinc metal anode. Chem. Eng. J. 2022, 446. [CrossRef]
- Gao, L.; Zhang, C.; Zhang, M.; Huang, X.; Jiang, X. Phytic acid conversion coating on Mg–Li alloy. J. Alloy. Compd. 2009, 485, 789–793. [CrossRef]
- Zhou, W.; Shan, D.; Han, E.-H.; Ke, W. Structure and formation mechanism of phosphate conversion coating on die-cast AZ91D magnesium alloy. Corros. Sci. 2008, 50, 329–337. [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E. H. Formation mechanism of phosphate conversion film on Mg-8.8 Li alloy. Corros. Sci. 2009, 51, 62-69.
- Yang, L.; Zhang, M.; Li, J.; Yu, X.; Niu, Z. Stannate conversion coatings on Mg–8Li alloy. J. Alloy. Compd. 2009, 471, 197–200. [CrossRef]
- Hung, S.-M.; Lin, H.; Chen, H.-W.; Chen, S.-Y.; Lin, C.-S. Corrosion resistance and electrical contact resistance of a thin permanganate conversion coating on dual-phase LZ91 Mg–Li alloy. J. Mater. Res. Technol. 2021, 11, 1953–1968. [CrossRef]
- Saji, V.S. Review of rare-earth-based conversion coatings for magnesium and its alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [CrossRef]
- Song, D.; Jing, X.; Wang, J.; Lu, S.; Yang, P.; Wang, Y.; Zhang, M. Microwave-assisted synthesis of lanthanum conversion coating on Mg–Li alloy and its corrosion resistance. Corros. Sci. 2011, 53, 3651–3656. [CrossRef]
- Xu, F.-F.; Zhao, Y.; Xu, Y. Preparation and corrosion resistance of rare earth–silane composite conversion coatings on magnesium–lithium alloy surface. Rare Met. 2015, 42, 1011–1017. [CrossRef]
- Mazur, K.; Stefańska, A.; Hebda, M. Analysis of Chemical Nickel-Plating Process. Mater. Sci. 2018, 54, 387–394. [CrossRef]
- Bremner, J.G.M. Nickel Plating by Chemical Reduction. Nature 1948, 162, 183–184. [CrossRef]
- Huang, Z.; Nguyen, T.T.; Zhou, Y.; Qi, G. A low temperature electroless nickel plating chemistry. Surf. Coatings Technol. 2019, 372, 160–165. [CrossRef]
- Yang, L.; Li, J.; Zheng, Y.; Jiang, W.; Zhang, M. Electroless Ni-P plating with molybdate pretreatment on Mg-8Li alloy. J. Alloy. Compd. 2009, 467, 562-566.
- Zou, Y.; Zhang, Z.; Liu, S.; Chen, D.; Wang, G.; Wang, Y.; Zhang, M.; Chen, Y. Ultrasonic-Assisted Electroless Ni-P Plating on Dual Phase Mg-Li Alloy. J. Electrochem. Soc. 2014, 162, C64–C70. [CrossRef]
- Yue, A.; Cao, Y.; Zhang, Y.; Zhou, S. Study of Electroless-Deposited Zn on the Surface of Mg-Li Alloy. Materials 2023, 16, 5511. [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing Treatments for Magnesium Alloys and Their Effect on Corrosion Resistance in Various Environments. Adv. Eng. Mater. 2006, 8, 511–533. [CrossRef]
- Zhu, X.; Song, Y.; Yu, D.; Zhang, C.; Yao, W. A novel nanostructure fabricated by an improved two-step anodizing technology. Electrochem. Commun. 2013, 29, 71–74. [CrossRef]
- Li, J.; Zheng, Z.; Li, S.; Ren, W.; Zhang, Z. Preparation and galvanic anodizing of a Mg–Li alloy. Mater. Sci. Eng. A 2006, 433, 233–240. [CrossRef]
- Chang, L.M.; Wang, P.; Liu, W. Effect of Amino Acids on the Structure and Corrosion Resistance of Mg-Li Alloy Anodic Oxide Film. Adv. Mater. Res. 2010, 146-147, 785–788. [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloy. Compd. 2023, 948. [CrossRef]
- Dou, J.; Chen, Y.; Yu, H.; Chen, C. Research status of magnesium alloys by micro-arc oxidation: a review. Surf. Eng. 2017, 33, 731–738. [CrossRef]
- Jin, S.; Ma, X.; Wu, R.; Wang, G.; Zhang, J.; Krit, B.; Betsofen, S.; Liu, B. Advances in micro-arc oxidation coatings on Mg-Li alloys. Appl. Surf. Sci. Adv. 2022, 8. [CrossRef]
- Shi, L.; Xu, Y.; Li, K.; Yao, Z.; Wu, S. Effect of additives on structure and corrosion resistance of ceramic coatings on Mg–Li alloy by micro-arc oxidation. Curr. Appl. Phys. 2010, 10, 719–723. [CrossRef]
- Chen, F.; Zhang, Y.; Zhang, Y. Effect of graphene on micro-structure and properties of MAO coating prepared on Mg-Li alloy. Int. J. Electrochem. Sci. 2017, 12, 6081-6091.
- Song, L.; Kou, Y.; Song, Y.; Shan, D.; Zhu, G.; Han, E. H. Fabrication and characterization of micro-arc oxidation (MAO) coatings on Mg-Li alloy in alkaline polyphosphate electrolytes without and with the addition of K2TiF6. Mater. Corros. 2011, 62, 1124-1132.
- Qian, B.-Y.; Miao, W.; Qiu, M.; Gao, F.; Hu, D.-H.; Sun, J.-F.; Wu, R.-Z.; Krit, B.; Betsofen, S. Influence of Voltage on the Corrosion and Wear Resistance of Micro-Arc Oxidation Coating on Mg–8Li–2Ca Alloy. Acta Met. Sin. (English Lett. 2018, 32, 194–204. [CrossRef]
- Wang, P.; Shih, Y.; Lin, M.; Lin, H.; Chen, M.; Lin, K. A study of atomic layer deposited LiAlxOy films on Mg–Li alloys. Thin Solid Films 2010, 518, 7501–7504. [CrossRef]
- Xavier, R.; Sivaperuman, K. Review on the of physical vapor deposition on imminent chemiresistive metal oxide gas sensors and their future scope. Mater. Today Commun. 2023, 38. [CrossRef]
- Li, S.Q.; Zhang, L.; Liu, T.T.; Zhang, Y.W.; Guo, C.; Wang, Y.; Du, F.H. A dendrite-free lithium-metal anode enabled by designed ultrathin MgF2 nanosheets encapsulated inside nitrogen-doped graphene-like hollow nanospheres. Adv. Mater. 2022, 34, 2201801.
- Yang, X.; Tian, B.; Jian, M.; Wu, M.; Li, W.; Jiang, J.; Guo, Z.; Yang, L. Molecular dynamics simulation of uniaxial stretching for silicon nitride deposited by PECVD. Appl. Surf. Sci. 2024, 682. [CrossRef]
- Tsujikawa, M.; Adachi, S.-I.; Abe, Y.; Oki, S.; Nakata, K.; Kamita, M. Corrosion Protection of Mg-Li Alloy by Plasma Thermal Spraying of Aluminum. Plasma Process. Polym. 2007, 4, S593–S596. [CrossRef]
- Oki, S.; Tsujikawa, M.; Morishige, T.; Kamita, M. Thin Protective Aluminum Layer on Mg-Li Alloy by Plasma Spraying and Cold Rolling. Plasma Process. Polym. 2009, 6, S954–S957. [CrossRef]
- Bao, Y.; Fu, B.; Jiao, Y.; Dong, T.; Li, J.; Li, G. Study of Wear and Corrosion Resistance of Cold Sprayed TC4 Coating on the Surface of Mg-Li Alloy. Coatings 2023, 13, 988. [CrossRef]
- Feng, K.; Wang, S.; Zhang, K.; Huo, L.; Zhou, H. Microstructure and Properties of Cold-Sprayed Al-x%Al2O3 Composite Coatings on LA43M Mg-Li Alloy. J. Therm. Spray Technol. 2023, 33, 1–13. [CrossRef]
- Wan, S.; Cui, X.; Jin, Q.; Ma, J.; Wen, X.; Su, W.; Zhang, X.; Jin, G.; Tian, H. Microstructure and properties of cold sprayed aluminum bronze coating on MBLS10A-200 magnesium-lithium alloy. Mater. Chem. Phys. 2022, 281. [CrossRef]
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent Progresses of Superhydrophobic Coatings in Different Application Fields: An Overview. Coatings 2021, 11, 116. [CrossRef]
- Hooda, A.; Goyat, M.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coatings 2020, 142. [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7. [CrossRef]
- Ouyang, Y.; Chen, Z.; Guo, E.; Qiu, R.; Wang, X.; Kang, H.; Wang, T. Bioinspired superhydrophobic surface via one-step electrodeposition and its corrosion inhibition for Mg-Li alloy. Colloids Surfaces A: Physicochem. Eng. Asp. 2022, 648. [CrossRef]
- Li, Z.; Yuan, Y. Preparation and characterization of superhydrophobic composite coatings on a magnesium–lithium alloy. RSC Adv. 2016, 6, 90587–90596. [CrossRef]
- Zhang, Y.; Yao, J. Fabrication of biodegradable superhydrophobic Zn-Fe coating on ultra-light Mg-Li alloy. Surf. Coatings Technol. 2024, 486. [CrossRef]
- He, H.; Zhou, S.; Du, J.; Yang, H.; Chen, D. Anti-icing and corrosion resistance of superhydrophobic coatings by precision machining and one-step electrodeposition on Mg-Li alloy. Colloids Surfaces A: Physicochem. Eng. Asp. 2024, 685. [CrossRef]
- He, H.; Du, J.; Sang, J.; Hirahara, H.; Aisawa, S.; Chen, D. Superhydrophobic coatings by electrodeposition on Mg–Li alloys: Attempt of armor-like Ni patterns to improve the robustness. Mater. Chem. Phys. 2023, 304. [CrossRef]
- Li, Z.; Yuan, Y.; Jing, X. Composite coatings prepared by combined plasma electrolytic oxidation and chemical conversion routes on magnesium-lithium alloy. J. Alloy. Compd. 2017, 706, 419–429. [CrossRef]
- Wang, Q.; Li, Y.; Lu, Z.; Zhang, Y.; Zou, Y. Effects of Ultrasonic Nanocrystal Surface Modification on Mechanical and Corrosion Behavior of LZ91 Mg–Li Alloy. Mater. Trans. 2020, 61, 1258–1264. [CrossRef]
- Zhang, J.M.; Lian, D.D.; Hou, A.R.; Wang, Z.H.; Zhang, M.C.; Wu, J.W.; Wang, C.C. Comparative study on microstructure, corrosion morphology, and friction wear properties of layered double hydroxide/steam coating composite coatings on Mg–Li Alloy. Adv. Eng. Mater. 2024, 26, 2400058.
- Zhang, L.; Zou, Y.; Wang, H.; Meng, L.; Liu, J.; Zhang, Z. Surface nanocrystallization of Mg-3 wt.% Li-6 wt.% Al alloy by surface mechanical attrition treatment. Mater. Charact. 2016, 120, 124–128. [CrossRef]
- Zou, Y.; Liu, S.; Wang, Q.; Li, Y. A Comparative Study on Mechanical and Corrosion Behaviours of α/(α + β) Mg-Li Alloys Subjected to Ultrasonic Nanocrystal Surface Modification. Metals 2022, 12, 681. [CrossRef]
- Li, Z.; Jing, X.; Yuan, Y.; Zhang, M. Composite coatings on a Mg–Li alloy prepared by combined plasma electrolytic oxidation and sol–gel techniques. Corros. Sci. 2012, 63, 358–366. [CrossRef]
- Li, D.; Cui, X.; Wen, X.; Li, Y.; Liu, E.; Jin, G.; Zheng, W. Improved electrochemical behavior of Mg-Li alloys by superhydrophobic layered double hydroxides/Ni-based composite coatings. J. Alloy. Compd. 2023, 947. [CrossRef]
- Ouyang, Y.; Zhou, Z.; Guo, E.; Qiu, R.; Chen, Z.; Kang, H.; Wang, T. Boosting corrosion resistance of Mg-Li alloy: Implanting bioinspired superhydrophobic surfaces into MAO matrix for enhanced protection. Ceram. Int. 2024, 50, 48425–48439. [CrossRef]
- Zhang, C.; Zhang, F.; Song, L.; Zeng, R.; Li, S.; Han, E. Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloy. Compd. 2017, 728, 815–826. [CrossRef]
- Yu, C.; Cui, L.-Y.; Zhou, Y.-F.; Han, Z.-Z.; Chen, X.-B.; Zeng, R.-C.; Zou, Y.-H.; Li, S.-Q.; Zhang, F.; Han, E.-H.; et al. Self-degradation of micro-arc oxidation/chitosan composite coating on Mg-4Li-1Ca alloy. Surf. Coatings Technol. 2018, 344, 1–11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).