Submitted:
18 March 2025
Posted:
18 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
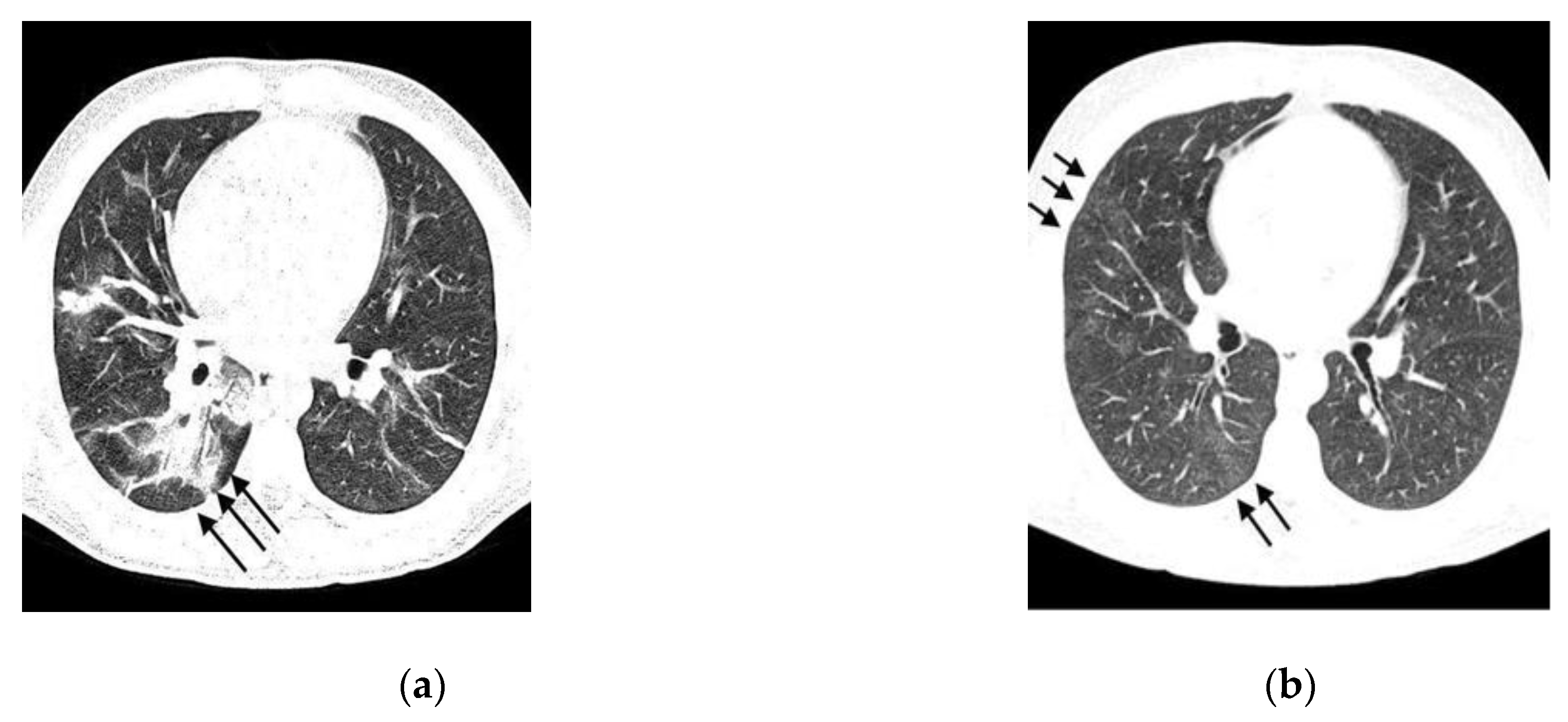
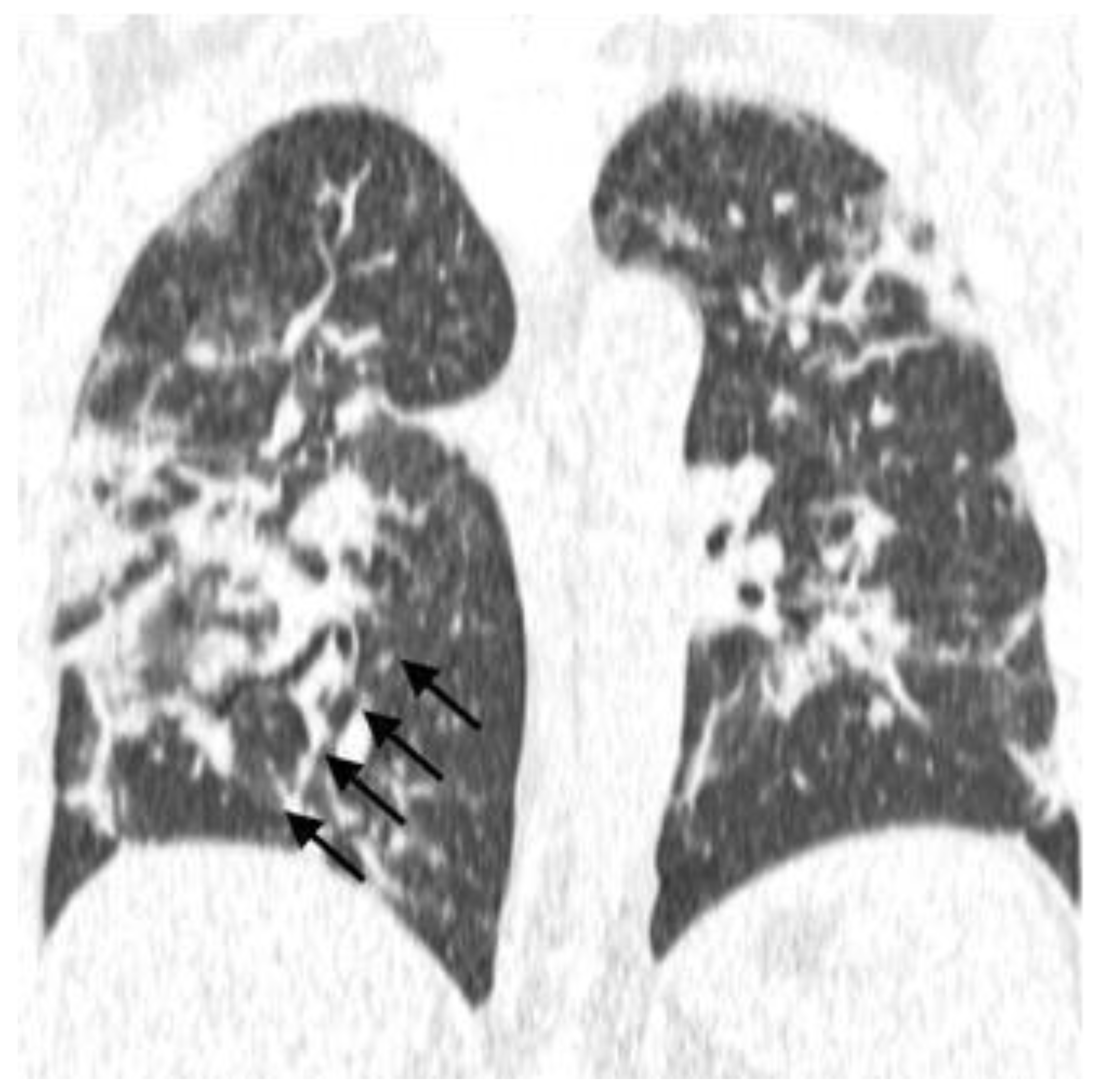
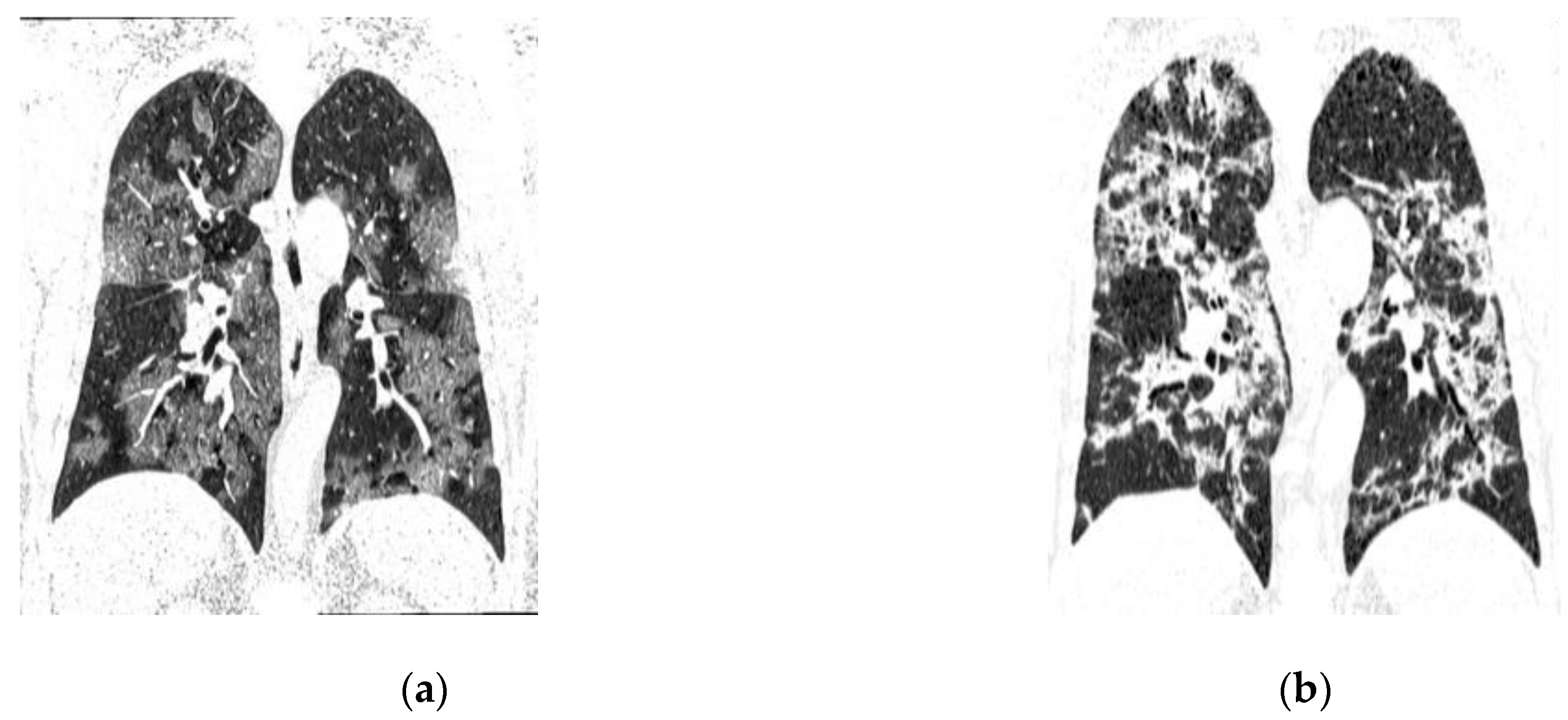
- Typical: Ground-glass opacities (peripheral, bilateral, rounded, and/or multifocal) or reversed halo sign; with or without consolidation; with or without mosaic paving;
- Possible: Absence of typical signs; unilobar, perihilar, non-peripheral, non-rounded ground-glass opacities;
- Atypical: Absence of typical and possible signs, with findings such as segmental/lobar cavitation, consolidations, micronodules, smooth septal thickening, pleural effusion, or mass;
- Negative: Absence of pulmonary parenchymal changes.
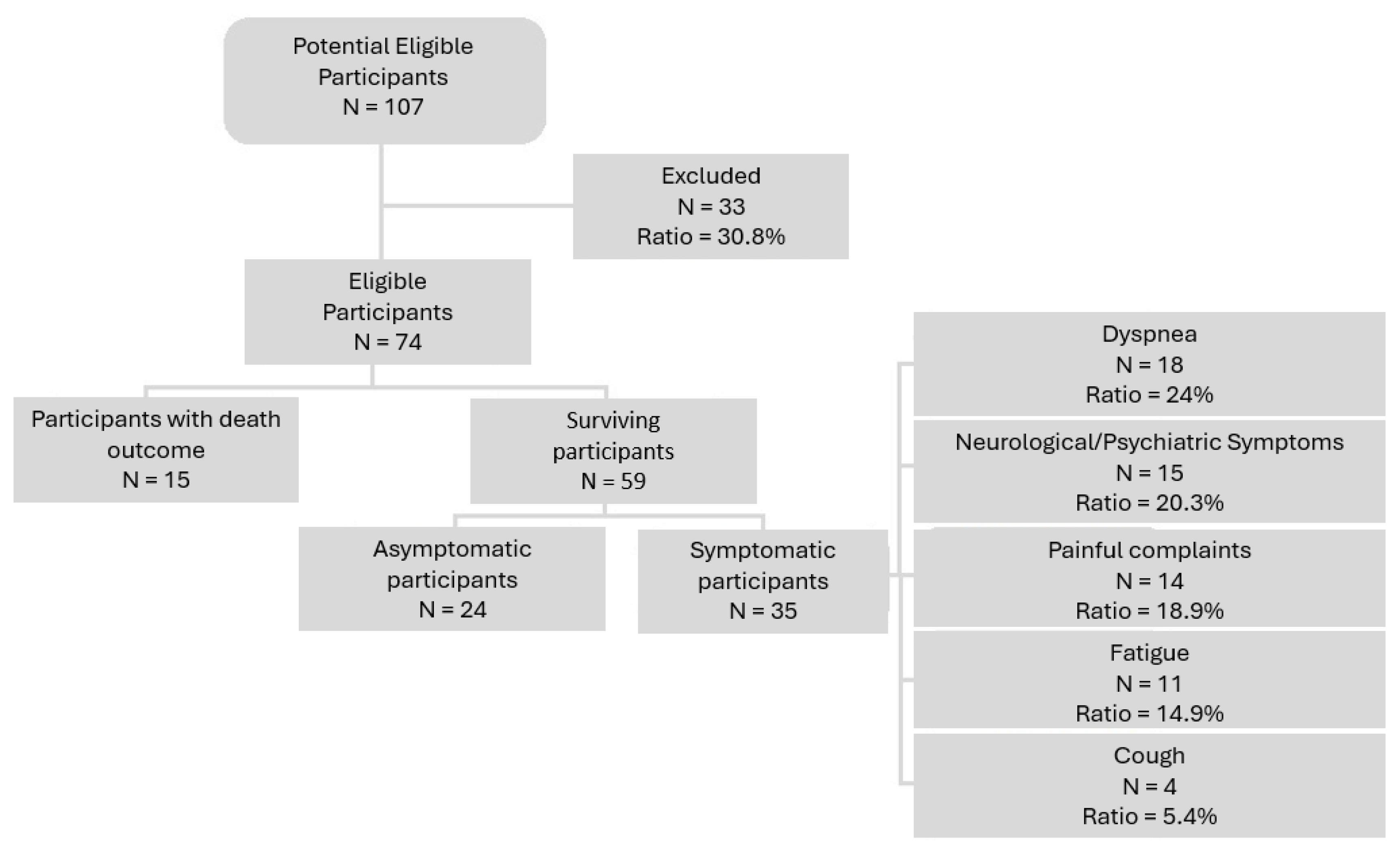
3. Results
| Symptom – COVID-19 | Total | |
|---|---|---|
| n | % | |
| S1: Cough | ||
| Yes | 57 | 77.0 |
| No | 17 | 23.0 |
| S2: Dyspnea | ||
| Yes | 50 | 67.6 |
| No | 24 | 32.4 |
| S3: Fever | ||
| Yes | 42 | 56.8 |
| No | 32 | 43.2 |
| S4: Myalgia/Fatigue | ||
| Yes | 24 | 32.4 |
| No | 50 | 67.6 |
| S5: Chest Pain | ||
| Yes | 5 | 6.8 |
| No | 69 | 93.2 |
| S6: Anosmia/Rhinorrhea | ||
| Yes | 7 | 9.5 |
| No | 67 | 90.5 |
| S7: Odynophagia | ||
| Yes | 5 | 6.8 |
| No | 69 | 93.2 |
| Symptom Onset (days) | ||
| 0-10 | 57 | 77.0 |
| 11-20 | 14 | 18.9 |
| > 20 | 3 | 4.1 |
| PCR Result | ||
| Positive | 56 | 75.7 |
| Negative | 18 | 24.3 |
| Post-COVID-19 Variable | Total | |
|---|---|---|
| n | % | |
| Outcome (1) | ||
| Asymptomatic | 24 | 32.4 |
| Symptomatic | 35 | 47.3 |
| Death | 15 | 20.3 |
| Outcome (2) | ||
| Death | 15 | 20.3 |
| Alive | 59 | 79.7 |
| Dyspnea | ||
| Yes | 18 | 24.3 |
| No | 56 | 75.7 |
| Cough | ||
| Yes | 4 | 5.4 |
| No | 70 | 94.6 |
| Fatigue | ||
| Yes | 11 | 14.9 |
| No | 63 | 85.1 |
| Pain-Related Complaints | ||
| Yes | 14 | 18.9 |
| No | 60 | 81.1 |
| Memory Loss | ||
| Yes | 7 | 9.5 |
| No | 67 | 90.5 |
| Neurological/Psychiatric Symptoms | ||
| Yes | 8 | 10.8 |
| No | 66 | 89.2 |
| Variable | Total | Death | Alive | p value | |||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| CT Pattern | |||||||
| Typical | 41 | 55.4 | 6 | 40 | 35 | 59.3 | 0.012 |
| Indeterminate | 10 | 13.5 | 5 | 33.3 | 5 | 8.5 | |
| Atypical | 12 | 16.2 | 4 | 26.7 | 8 | 13.6 | |
| Normal | 11 | 14.9 | 0 | 0 | 11 | 18.6 | |
| CT Pattern | |||||||
| Typical/Indeterminate | 51 | 68.9 | 11 | 73.3 | 40 | 67.8 | 0.47 |
| Atypical/Normal | 23 | 31.1 | 4 | 26.7 | 19 | 32.2 | |
| Ground-Glass Opacity | |||||||
| Yes | 47 | 63.5 | 9 | 60 | 38 | 64.4 | 0.75 |
| No | 27 | 36.5 | 6 | 40 | 21 | 35.6 | |
| Ground-Glass Band | |||||||
| Yes | 1 | 1.4 | 0 | 0 | 1 | 1.7 | 0.80 |
| No | 73 | 98.6 | 15 | 100 | 58 | 98.3 | |
| Mosaic Pattern | |||||||
| Yes | 19 | 25.7 | 3 | 20 | 16 | 27.1 | 0.42 |
| No | 55 | 74.3 | 12 | 80 | 43 | 72.9 | |
| Parenchymal Bands | |||||||
| Yes | 12 | 16.2 | 1 | 6.7 | 11 | 18.6 | 0.24 |
| No | 62 | 83.8 | 14 | 93.3 | 53 | 89.8 | |
| Subpleural Lines | |||||||
| Yes | 7 | 9.5 | 1 | 6.7 | 6 | 10.2 | 0.57 |
| No | 67 | 90.5 | 14 | 93.3 | 53 | 89.8 | |
| Consolidations | |||||||
| Yes | 22 | 29.7 | 4 | 26.7 | 18 | 30.5 | 0.52 |
| No | 52 | 70.3 | 11 | 73.3 | 41 | 69.5 | |
| Bronchial Ectasia | |||||||
| Yes | 8 | 10.8 | 1 | 6.7 | 7 | 11.9 | 0.49 |
| No | 66 | 89.2 | 14 | 93.3 | 52 | 88.1 | |
| Architectural Distortion. | 0.63 | ||||||
| Yes | 2 | 2.7 | 0 | 0 | 2 | 3.44 | |
| No | 72 | 97.3 | 15 | 100 | 57 | 96.6 | |
| Peribronchovascular Consolidation | 0.11 | ||||||
| Yes | 9 | 12,2 | 0 | 0 | 9 | 15.3 | |
| No | 65 | 87,8 | 15 | 100 | 50 | 84.7 | |
| Nodule with Ground-Glass Halo | 0.40 | ||||||
| Yes | 4 | 5,4 | 0 | 0 | 4 | 6.8 | |
| No | 70 | 94,6 | 15 | 100 | 55 | 93.2 | |
| Reversed Halo Sign | NA | ||||||
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | |
| No | 74 | 100 | 15 | 100 | 59 | 100 | |
| Percentage of Ground-Glass Opacity Involvement | |||||||
| 0-25% | 32 | 43,2 | 2 | 13.3 | 30 | 50.8 | 0.017 |
| 25-50% | 19 | 25,7 | 5 | 33.3 | 14 | 23.7 | |
| >50% | 23 | 31,1 | 8 | 53.3 | 15 | 25.4 | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus Disease 2019 |
| CO-RADS | COVID-19 Reporting and Data System |
| ACE2 | Angiotensin-converting enzyme 2 |
| AI | Artificial intelligence |
| NAAT | Nucleic acid amplification test |
| NICE | National Institute for Health and Care Excellence |
| WHO | World Health Organization |
| PACS | Picture Archiving and Communication System |
| RIS | Radiology Information System |
| RSNA | Radiological Society of North America |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| ARDS | Acute Respiratory Distress Syndrome |
| SARS-CoV-1 | Severe Acute Respiratory Syndrome Coronavirus-1 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus-2 |
| ARDS | Acute Respiratory Distress Syndrome |
| BTS | British Thoracic Society |
| STR | Society of Thoracic Radiology |
| CT | Computed tomography |
| ICU | Intensive Care Unit |
| GGO | Ground-glass opacity |
| MV | Mechanical ventilation |
References
- World Health Organization. Coronavirus disease (COVID-19) pandemic. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 19 April 2024).
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Ceraolo, C.; Giorgi, F. M. Genomic variance of the 2019-nCoV coronavirus. J. Med Virol. 2020, 92, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S. R.; Leibowitz, J. L. Coronavirus pathogenesis. Adv. Virus Res. 2011, 81, 85–164. [Google Scholar] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Saheb Sharif-Askari, N.; Sharif-Askari, F.S.; Alabed, M.; Temsah, M.H.; Al Heialy, S.; Hamid, Q.; et al. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Ward, I. L.; Bermingham, C.; Ayoubkhani, D.; Gethings, O.J.; Pouwels, K.B.; Yates, T.; et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ 2022, 378, e070695. [Google Scholar] [CrossRef]
- Shen, Y.; Li, C.; Dong, H.; Wang, Z.; Martinez, L.; Sun, Z.; et al. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in Eastern China. JAMA Intern. Med. 2020, 180, 1665–1671. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z. L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 9, 141–154. [Google Scholar] [CrossRef]
- Siddiqi, H. K.; Mehra, M. R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Ferguson, N.; Laydon, D.; Nedjati Gilani, G.; Imai, N.; Ainslie, K.; Baguelin, M.; Bhatia, S.; et al. Report 9 - Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College, London, 2020.
- Brazil, M. S. Diagnóstico e Tratamento da COVID-19 Tratamento. Secretaria De Ciência, Tecnologia, Inovação e Insumos Estratégicos em Saúde, 1, 1–398, 2020.
- Brazil. Painel coronavirus. Coronavirus Brasil, 2022. Available online: https://covid.saude.gov.br/ (accessed on 27 September 2022).
- Fonseca, E.K.U.N.; Ferreira, L.C.; Loureiro, B.M.C.; Strabelli, D.G.; de Farias, L.P.G.; de Queiroz, G.A.; et al. Tomografia computadorizada de tórax no diagnóstico de COVID-19 em pacientes com resultado falso-negativo na RT-PCR. Einstein 2021, 19, AO6363. [Google Scholar] [CrossRef]
- Silva, F. Imaging findings of COVID-19 on computed tomography: a narrative review. Scientific Article Roentgen. 2023, 4. [Google Scholar]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Vanesa, Y.C.L.; et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic testing for SARS-CoV-2: interim guidance. 2022. Available online: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2 (accessed on 19 April 2024).
- Bhalla, S.; Kay, F.U.; Abbara, S.; Bhalla, S.; Chung, J.H.; Chung, M.; et al. Radiological Society of North America Expert Consensus Statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J. Thorac. Imaging 2020, 35, 219–227. [Google Scholar]
- Prokop, M.; Van Everdingen, W.; Van Rees Vellinga, T.; Quarles Van Ufford, H.; Stöger, L.; Beenen, L.; et al. CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology 2020, 296, 97–104. [Google Scholar] [CrossRef]
- Lieveld, A.W.E.; Azijli, K.; Teunissen, B.P.; Van Haaften, R.M.; Kootte, R.S.; Van Den Berk, I.A.H.; Van Der Horst, S.F.B.; et al. Chest CT in COVID-19 at the ED: validation of the COVID-19 Reporting and Data System (CO-RADS) and CT severity score: a prospective, multicenter, observational study. Chest 2021, 159, 1126–1135. [Google Scholar] [CrossRef]
- Kim, H.; Hong, H.; Yoon, S.H. Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology 2020, 296, E145–E155. [Google Scholar] [CrossRef]
- Mogami, R.; Lopes, A.J.; Araújo Filho, R.C.; Almeida, F.C.S.; Messeder, A.M.C.; Koifman, A.C.B.; et al. Chest computed tomography in COVID-19 pneumonia: a retrospective study of 155 patients at a university hospital in Rio de Janeiro, Brazil. Radiol Bras. 2021, 54, 1–8. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Ciccarese, F.; Coppola, F.; Spinelli, D.; Galletta, G. L.; Lucidi, V.; Paccapelo, A.; et al. Diagnostic accuracy of North America Expert Consensus Statement on reporting CT findings in patients suspected of having COVID-19 infection: an Italian single-center experience. Radiol. Cardiothorac. Imaging 2020, 2, e200312. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef]
- Adams, H.J.A.; Kwee, T.C.; Yakar, D.; Hope, M.D.; Kwee, R.M. Systematic review and meta-analysis on the value of chest CT in the diagnosis of coronavirus disease (COVID-19): Sol Scientiae, Illustra Nos. Am. J. Roentgenol. 2020, 215, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Campos, C. Imaging findings of COVID-19 on computed tomography: A Narrative Review. Scientific Article ROENTGEN. 2023, 4. [Google Scholar]
- Amorim, A. Imaging findings of COVID-19 on computed tomography: a narrative review. Scientific Article ROENTGEN. 2023, 4. [Google Scholar]
- Xie, X.; Zhong, Z.; Zhao, W.; Zheng, C.; Wang, F.; Liu, J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 2020, 296, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Raptis, C.A.; Hammer, M.M.; Short, R.G.; Shah, A.; Bhalla, S.; Bierhals, A.J.; et al. Chest CT and coronavirus disease (COVID-19): a critical review of the literature to date. Am. J. Roentgenol. 2020, 215, 839–842. [Google Scholar] [CrossRef]
- Simpson, S.; Kay, F. U.; Abbara, S.; Bhalla, S.; Chung, J.H.; Chung, M.; et al. Radiological Society of North America Expert Consensus Statement on reporting Chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020, 35, 219–227. 35.
- Chaganti, S.; Grenier, P.; Balachandran, A.; Chabin, G.; Cohen, S.; Flohr, T.; et al. Automated quantification of CT patterns associated with COVID-19 from chest CT. Radiol Artif Intell. 2020, 2, e200048. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zhang, J.S.; Zhang, H.H.; Nan, Y.D.; Zhao, Y.; Fu, E.Q.; et al. Automated detection and quantification of COVID-19 pneumonia: CT imaging analysis by a deep learning-based software. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2525–2532. [Google Scholar] [CrossRef]
- Constantine, A. Imaging findings of COVID-19 on computed tomography: a narrative review. Scientific Article ROENTGEN. 2023, 4. [Google Scholar]
- Yu, M.; Liu, Y.; Xu, D.; Zhang, R.; Lan, L.; Xu, H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 Pneumonia. Korean J. Radiol. 2020, 21, 746–755. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021, 299, 177–186. [Google Scholar] [CrossRef]
- Wells, A.U.; Sujal, A.D.; Desai, R.D. Interstitial lung disease after COVID-19 infection: a catalog of uncertainties. Radiology 2021, 299, 216–218. [Google Scholar] [CrossRef]
- D’Cruz, R.F.D.; Waller, M.D.; Perrin, F.; Periselneris, J.; Norton, S.; Smith, L.J.; et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021, 7, 00655. [Google Scholar] [CrossRef]
- Li, L.Q.; Huang, T.; Wang, Y.Q.; Wang, Z.P.; Liang, Y.; Huang, T.B.; et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef]
- Galvão, M.R.H.; Roncalli, A.G. Fatores associados a maior risco de ocorrência de óbito por COVID-19: análise de sobrevivência com base em casos confirmados. Rev. Bras. Epidemiol. 2020, 23. [Google Scholar] [CrossRef]
- Fang, X.; Ming, C.; Cen, Y.; Lin, H.; Zhan, K.; Yang, S.; et al. Post-sequelae one year after hospital discharge among older COVID-19 patients: A multi-center prospective cohort study. J. Infect. 2022, 84, 179–186. [Google Scholar] [CrossRef]
- COVID-19 Epidemiological Surveillance Guide. Ministério da Saúde, Brasil, 2022. Available online: https://www.gov.br/saude/pt-br (accessed on 19 April 2024).
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z. A.; Zhang, N.; et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef]
| Variable | Total | Death | Alive | p value | |||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| Gender | |||||||
| Male | 30 | 40.5 | 6 | 40 | 24 | 40.7 | 0.96 |
| Female | 44 | 59.5 | 9 | 60 | 35 | 59.3 | |
| Age Group | |||||||
| ≥ 60 years | 30 | 41.1 | 12 | 85.7 | 18 | 30.5 | 0.0001 |
| < 60 years | 43 | 58.9 | 2 | 14.3 | 41 | 69.5 | |
| Location | |||||||
| Inpatient | 32 | 43.2 | 6 | 40 | 26 | 44.1 | 0.78 |
| Outpatient | 42 | 56.8 | 9 | 60 | 33 | 55.9 | |
| Comorbidities | |||||||
| Yes | 41 | 55.4 | 14 | 93.3 | 27 | 45.8 | 0.0009 |
| No | 33 | 44.6 | 1 | 6.7 | 32 | 54.2 | |
| Diabetes mellitus | |||||||
| Yes | 12 | 16.2 | 2 | 13.3 | 10 | 16.9 | 0.54 |
| No | 62 | 83.8 | 13 | 86.7 | 49 | 83.1 | |
| Hypertension | |||||||
| Yes | 15 | 20.3 | 5 | 33.3 | 10 | 16.9 | 0.14 |
| No | 59 | 79.7 | 10 | 66.7 | 49 | 83.1 | |
| Obesity | |||||||
| Yes | 2 | 2.7 | 1 | 6.7 | 1 | 1.7 | 0.37 |
| No | 72 | 97.3 | 14 | 93.3 | 58 | 98.3 | |
| Smoking/ex-smoking | |||||||
| Yes | 0 | 0,0 | 0 | 0 | 0 | 0 | NA |
| No | 74 | 100,0 | 15 | 100 | 59 | 100 | |
| Mechanical ventilation | |||||||
| Yes | 5 | 6,8 | 2 | 13,3 | 3 | 5,1 | 0.27 |
| No | 69 | 93,2 | 13 | 86,7 | 56 | 94,9 | |
| Sample | n | mean | SD | Median | IQR | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| Total | 73 | 54.5 | 17.41 | 54 | 40-67 | 15 | 94 |
| Evolution | |||||||
| Death | 14 | 71.4 | 13.4 | 70 | 63-83 | 48 | 94 |
| Alive | 59 | 50.5 | 15.4 | 50 | 40-62 | 15 | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





