Department of Molecular and Cellular Biosciences. Centro de Investigaciones Biológicas Margarita Salas, CSIC, Madrid, Spain
Although the versatility of vimentin assemblies has been known for long, only recently, some of these arrangements have been associated with biomolecular condensates. Disassembly of filaments occurs in a cell type dependent manner during mitosis [
16,
17], at the leading edge during cell migration [
18,
19], or as a consequence of oxidative stress [
20,
21]. The various vimentin structures observed under these conditions have been frequently described as ULF (unit length filaments), dots, or filament particles. Recently, the convergence of research on vimentin structure and biomolecular condensates has shed light on the complex organization and behavior of intermediate filaments. Thus, these cytoskeletal structures, emerge as polymers integrating alpha-helical rods with low complexity domains that could behave as intra-filament phase separated protein environments, aiding in the stability and regulation of the polymer [
12,
22]. Inspired by these findings, the vimentin membraneless compartments that arise upon oxidative stress, have been recently ascribed to biomolecular condensates [
23]. It will be interesting to assess whether other vimentin assemblies share these features, as it will be discussed in detail below.
Biomolecular Condensates
The organization of cellular material is critical for cell function, and this is achieved through various compartmentalization mechanisms, including membrane delimited organelles in eukaryotes, as well as the more recently identified membraneless compartments, present in all kinds of cells [
24,
25,
26]. These compartments allow accumulation of certain proteins and/or nucleic acids, providing an essential mechanism for the spatiotemporal regulation of their interactions. Cellular structures such as the nucleolus, Cajal bodies, P bodies, promyelocytic leukemia bodies or stress granules illustrate this particular type of molecular organization [
26].
Biomolecular condensates result from phase separation driven by multivalent interactions, such as those established by proteins with various modular interaction domains, or with regions of low sequence complexity. Nucleic acids, which can harbor sequences recognized by proteins or other nucleic acids, are often part of biomolecular condensates, and constitute key players in the positive or negative regulation of their assembly. A hallmark of biomolecular condensates is dynamics, meaning that their components remain mobile within the structure and can be exchanged with the surroundings. Consistent with a phase separation phenomenon, biomolecular condensation occurs above a threshold concentration of scaffold molecules, leading to compartments into which additional elements not required for condensation, termed clients, can selectively partition while other molecules are excluded [
25,
27]. Consequently, condensates can promote or repress biochemical reactions, they can buffer protein concentrations and provide fast adaptive and reversible responses to changes in the environment. See Fig. 1 for a summary of the features and functions of biomolecular condensates.
The formation of condensates is usually reversible, and they can be dissipated by dilution of their components, while factors modifying the threshold concentration for assembly, such as selective chemical agents, temperature, pH or ionic strength, can either favor or disfavor condensation. Among these factors, macromolecular crowding derived from the high overall concentration of macromolecules inherent to cellular environments and physiological fluids often facilitates phase separation [
28,
29]. This occurs because crowding normally promotes protein oligomerization, polymerization and complexation with other proteins or nucleic acids, through excluded volume effects and other non-specific interactions [
28]. Besides, cellular surfaces can be catalyzers of protein condensation, and in turn, condensates may influence surfaces through wetting or induction of shape changes [
30,
31]. Interestingly, PTMs are amongst the active processes that regulate phase separation, either inducing or disrupting biomolecular condensates, depending on the system and the type of modification [
24,
25,
26,
30,
32], as it will be considered in more detail below.
Biomolecular condensation has been experimentally assessed in a variety of systems, both in vitro and in cells, through the detection of approximately round droplets, concentrating mobile structures and able to undergo fusion. In cells, some of these features can be monitored by fluorescence microscopy, including time-lapse imaging, fluorescent tagging of the components involved, and fluorescence recovery after photobleaching (FRAP) assays [
33,
34]. Condensates are susceptible to disruption by certain small molecules. In particular, the aliphatic alcohol 1,6-hexanediol can provide valuable information about the material properties of phase separated compartments, due to its ability to dissolve biomolecular condensates, although there are exceptions [
35]. Nevertheless, the results obtained through the use of this compound need to be interpreted with caution because of potential non-specific or toxic effects [
36]. On the other hand, bottom up reconstitution in synthetic cell-like environments has been instrumental in dissecting the impact of elements of the intracellular complexity, such as crowding and membrane surfaces on phase separation [
37]. This approach is very useful to inspect condensates assembled by proteins and other molecules from bacteria, where in vivo measurements are hindered by the small cell size.
An impressive variety of biomolecular condensates have been so far identified, participating in multiple cellular functions, including cell signaling, division, intracellular transport, transcription regulation, RNA processing, ribosomal synthesis, and protein clearance [
32,
38]. Cytoskeletal eukaryotic and prokaryotic proteins or their modulators are key components of many of these condensates [
39,
40,
41], which, by enhancing the local protein concentration can regulate nucleation, growth and branching of cytoskeletal polymers [
41]. Moreover, biomolecular condensates can also contribute to cytoskeleton stability and regulation, transport, and interaction with membranes, therefore playing a role in the multiple cellular processes in which cytoskeletal proteins are involved [
39].
In eukaryotes, polymeric structures of actin and tubulin can be assembled inside condensates of their associated or regulatory proteins, or, in the case of tubulin, in microtubule organizing centers such as centrosomes, which exhibit phase separation and client recruitment [
42]. In turn, microtubules can promote droplet fusion, whereas filamin can modulate the phase separation behavior of actin. Moreover, the dynamics of both, actin and tubulin, is susceptible to modulation by condensates. Intermediate filaments are receiving increased attention in the fields of phase separation and biomolecular condensates since the seminal works of the McKnight group reported that certain intermediate filaments displayed a behavior reminiscent of liquid droplet-like structures, depending on their low complexity sequence N-terminal domains, or “heads” [
43]. Condensates of intermediate filament proteins will be considered in detail below.
In bacteria, the tubulin homolog FtsZ, which is the central element of the cell division machinery, assembles biomolecular condensates in cytomimetic conditions [
44]. This phase separation process is reversible, driven by crowding and controlled by GTP availability, and is enhanced by interaction with the nucleoid occlusion factor SlmA [
40]. Moreover, it could have implications for the spatiotemporal tuning of division ring assembly and for the emergence of cell subpopulations tolerant to antibiotics [
29]. In contrast, the bacterial cytoskeletal protein ParA belonging to the ParABS system, involved in DNA segregation, behaves as an ATP-fueled motor that prevents fusion of biomolecular condensates assembled by ParB, ensuring proper subcellular localization of these compartments [
45].
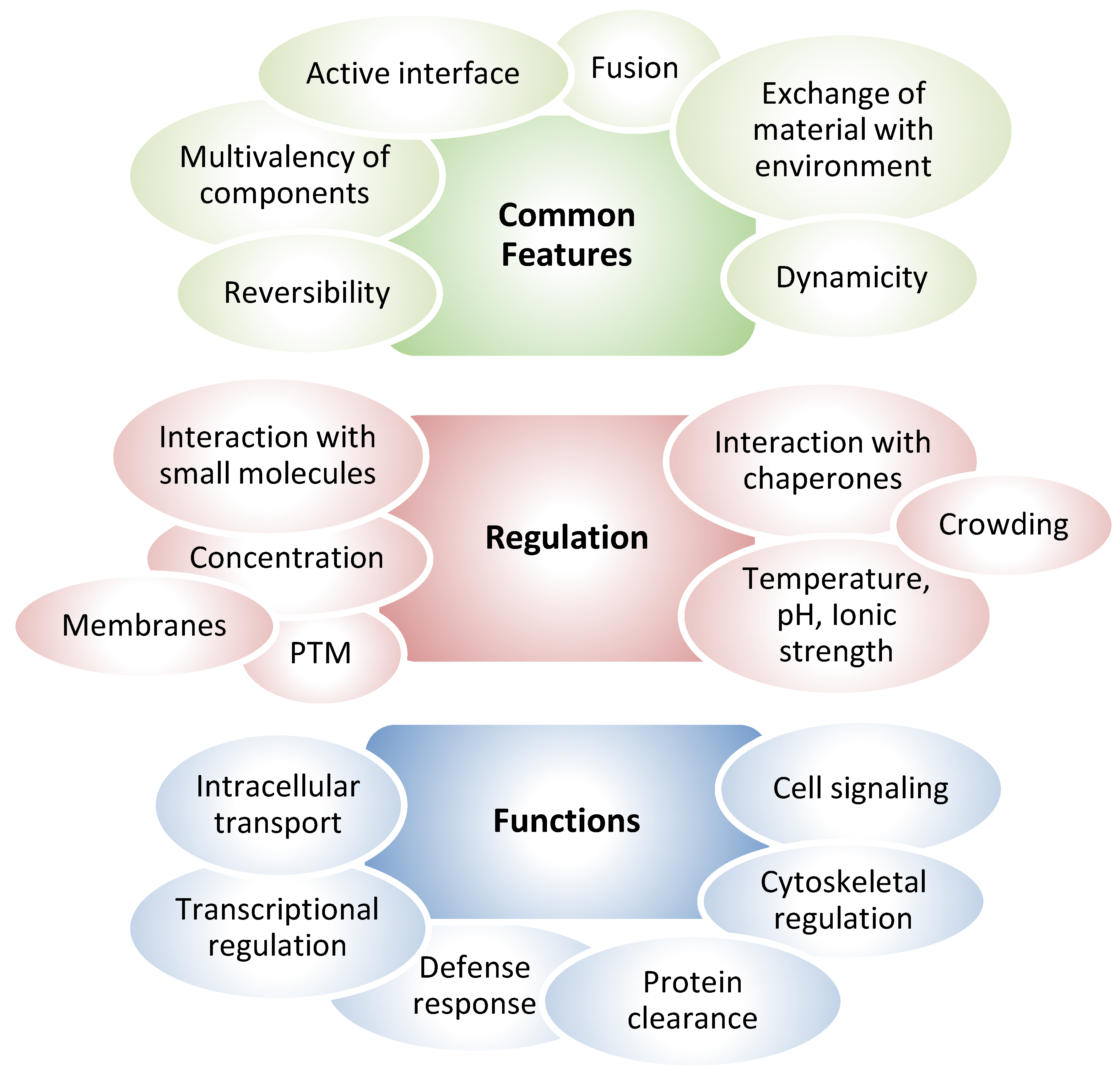
Figure 1.
Characteristics, regulation and functions of biomolecular condensates. Some common features, regulatory mechanisms and functions of biomolecular condensates are summarized. Nevertheless, knowledge
about biomolecular condensates is rapidly increasing and the concept of these structures is evolving in the light of new findings.
Figure 1.
Characteristics, regulation and functions of biomolecular condensates. Some common features, regulatory mechanisms and functions of biomolecular condensates are summarized. Nevertheless, knowledge
about biomolecular condensates is rapidly increasing and the concept of these structures is evolving in the light of new findings.
Figure 2.
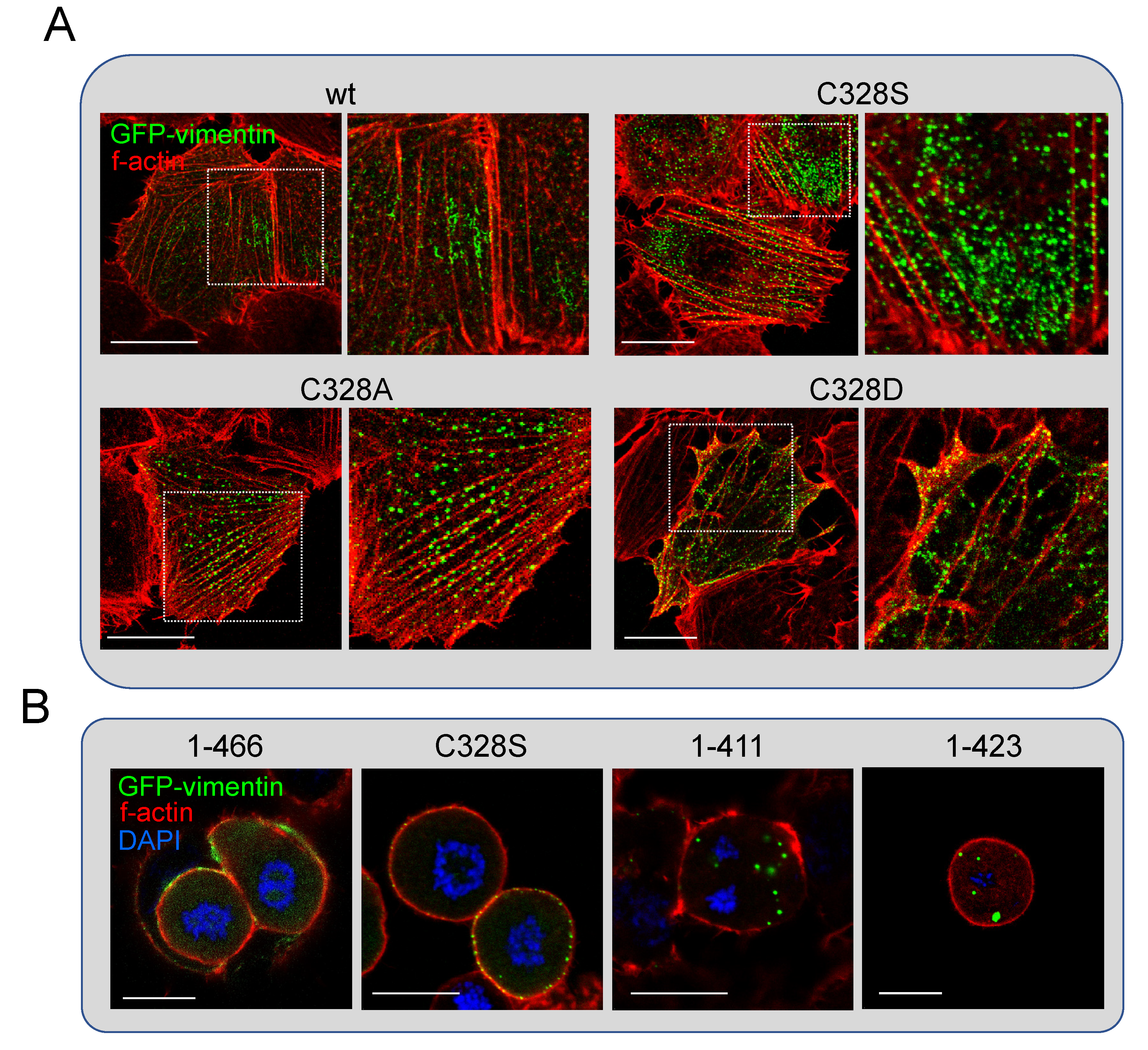
Examples of droplet-like structures of vimentin that have been reported in previous studies. (A)
Schematic representation of the vimentin monomer showing the head, rod and tail domains. The position of the single cysteine residue, C328 in human vimentin, and the Y117 residue, which is critical for elongation, are highlighted. In addition, several tail truncated mutants are schematically represented. (B) Filaments formed by
untagged vimentin yield droplet-like structures upon treatment of cells with the oxidant diamide or high
concentrations of the aliphatic alcohol 1,6-hexanediol. (C) GFP-vimentin fusion protein forms squiggles in vimentin deficient cells. Several GFP-vimentin C328 mutants, namely, GFP-vimentin C328S, C328A and C328D,
do not form squiggles but droplet-like assemblies. Confocal microscopy images shown in (B) and (C) are additional examples of the results reported in Martínez-Cenalmor et al., 2024 and González-Jiménez et al., 2023. (D) Several tail truncated mutants of GFP-vimentin, expressed in vimentin deficient cells, are not able to elongate and form condensate-like structures. Images are complementary to those shown in Duarte et al., 2019. Bars, 20 μm. (E) The mEmerald-vimentin fusion protein forms long filaments when expressed in a vimentin (-/-) HeLa clone. Nevertheless, several mutants, including S49A, S49D and Y117L only form droplets. Images are taken
from Tarbet et al., 2018, published under the Creative Commons Attribution License. The image on the right shows an example of droplets formed by a vimentin-GFP Y117L mutant and has been taken from Nunes-Vicente
et al., 2021, published under a CC-BY 4.0 license, with permission from the authors. Bar, 5 μm..
Figure 2.
Examples of droplet-like structures of vimentin that have been reported in previous studies. (A)
Schematic representation of the vimentin monomer showing the head, rod and tail domains. The position of the single cysteine residue, C328 in human vimentin, and the Y117 residue, which is critical for elongation, are highlighted. In addition, several tail truncated mutants are schematically represented. (B) Filaments formed by
untagged vimentin yield droplet-like structures upon treatment of cells with the oxidant diamide or high
concentrations of the aliphatic alcohol 1,6-hexanediol. (C) GFP-vimentin fusion protein forms squiggles in vimentin deficient cells. Several GFP-vimentin C328 mutants, namely, GFP-vimentin C328S, C328A and C328D,
do not form squiggles but droplet-like assemblies. Confocal microscopy images shown in (B) and (C) are additional examples of the results reported in Martínez-Cenalmor et al., 2024 and González-Jiménez et al., 2023. (D) Several tail truncated mutants of GFP-vimentin, expressed in vimentin deficient cells, are not able to elongate and form condensate-like structures. Images are complementary to those shown in Duarte et al., 2019. Bars, 20 μm. (E) The mEmerald-vimentin fusion protein forms long filaments when expressed in a vimentin (-/-) HeLa clone. Nevertheless, several mutants, including S49A, S49D and Y117L only form droplets. Images are taken
from Tarbet et al., 2018, published under the Creative Commons Attribution License. The image on the right shows an example of droplets formed by a vimentin-GFP Y117L mutant and has been taken from Nunes-Vicente
et al., 2021, published under a CC-BY 4.0 license, with permission from the authors. Bar, 5 μm..
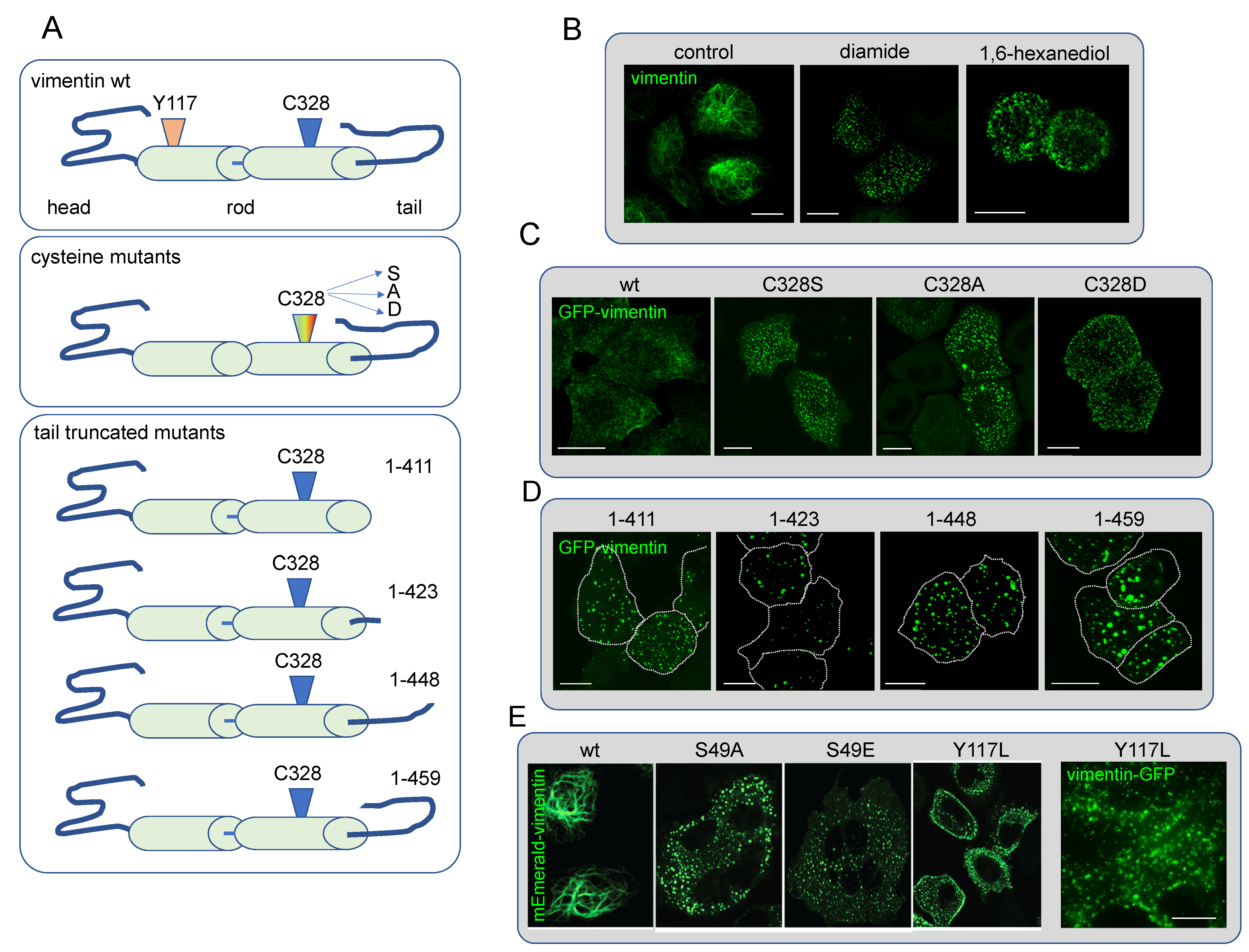
Figure 3.
Examples of the association of GFP-vimentin droplets with actin structures. (A) GFP-vimentin wt squiggles, as well as droplets formed by several GFP-vimentin C328 mutants expressed in vimentin deficient
cells, align over actin stress fibers formed spontaneously of upon treatment with dinitroimidazole. Selected areas
of interest are enlarged at the right. Confocal microscopy images are additional examples of the experiments
reported in Pérez-Sala et al., 2015 and González-Jiménez et al., 2023. (B) Association of GFP-vimentin wt and
C328S assemblies with the actin cortex in mitosis (left), and failure of tail truncated mutants of GFP-vimentin to
relocate to the cell cortex in mitosis. Images are additional examples of the results reported in Duarte et al., 2019. Bars, 20 μm.
Figure 3.
Examples of the association of GFP-vimentin droplets with actin structures. (A) GFP-vimentin wt squiggles, as well as droplets formed by several GFP-vimentin C328 mutants expressed in vimentin deficient
cells, align over actin stress fibers formed spontaneously of upon treatment with dinitroimidazole. Selected areas
of interest are enlarged at the right. Confocal microscopy images are additional examples of the experiments
reported in Pérez-Sala et al., 2015 and González-Jiménez et al., 2023. (B) Association of GFP-vimentin wt and
C328S assemblies with the actin cortex in mitosis (left), and failure of tail truncated mutants of GFP-vimentin to
relocate to the cell cortex in mitosis. Images are additional examples of the results reported in Duarte et al., 2019. Bars, 20 μm.
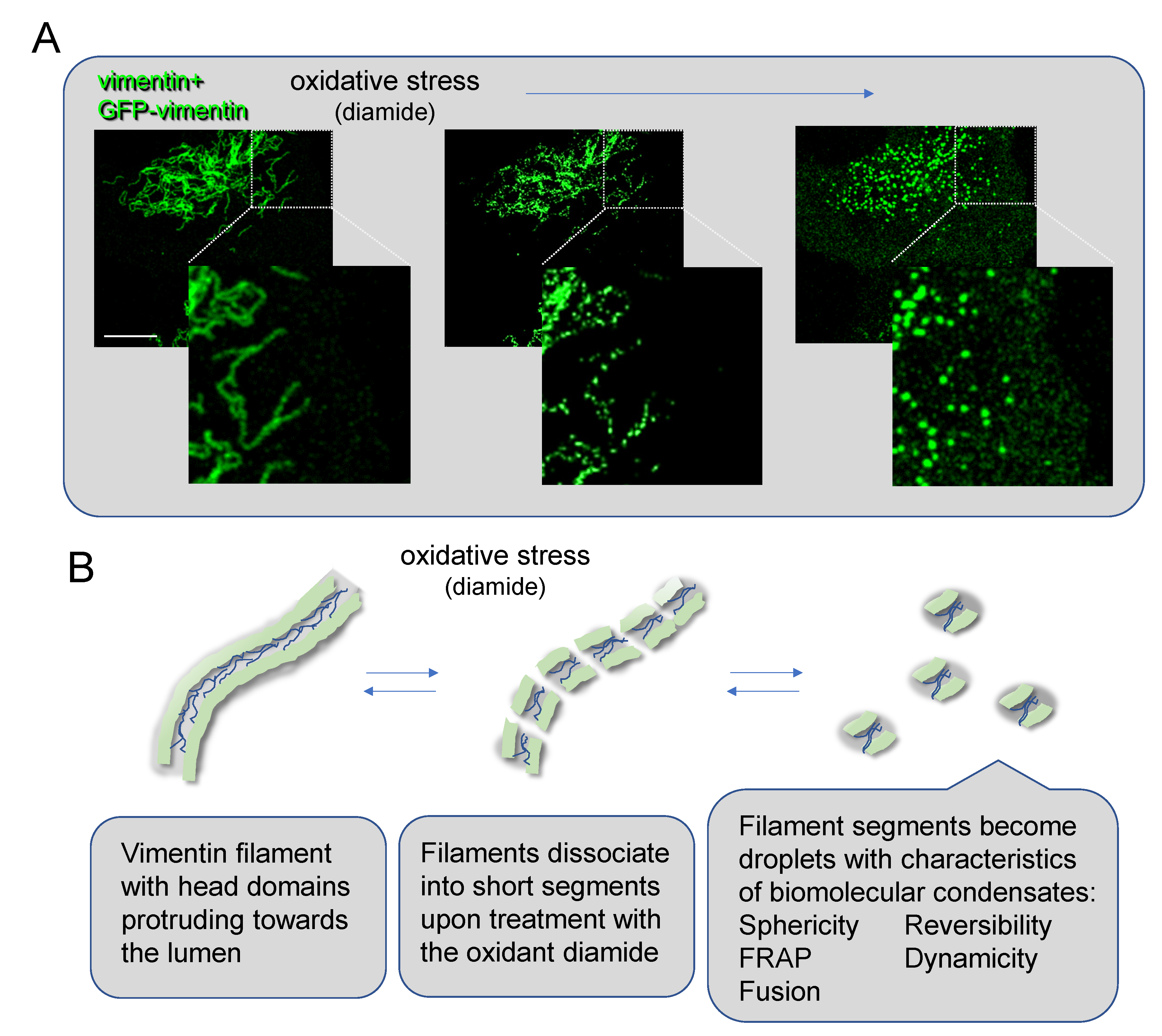
Figure 4.
Process of vimentin filament transition into biomolecular condensates by diamide treatment. (A)
The effect of diamide on the integrity of vimentin filaments was monitored by confocal microscopy in live cells as described in Martínez-Cenalmor et al., 2024. Scale bar, 10 μm. Selected areas of interest are enlarged at the
bottom. (B) Cartoon representation of the process of vimentin filament dissociation into short segments and
subsequently into droplets with the characteristics of biomolecular condensates. The image on the left represents
a filament open longitudinally showing the disordered head domains protruding into the lumen of the hollow
tube formed by the rod domains (green). The central and right images represent a speculative view of the process
of filament severing and final dissociation into droplets. Please note that the composition and structure of vimentin condensates is not known.
Figure 4.
Process of vimentin filament transition into biomolecular condensates by diamide treatment. (A)
The effect of diamide on the integrity of vimentin filaments was monitored by confocal microscopy in live cells as described in Martínez-Cenalmor et al., 2024. Scale bar, 10 μm. Selected areas of interest are enlarged at the
bottom. (B) Cartoon representation of the process of vimentin filament dissociation into short segments and
subsequently into droplets with the characteristics of biomolecular condensates. The image on the left represents
a filament open longitudinally showing the disordered head domains protruding into the lumen of the hollow
tube formed by the rod domains (green). The central and right images represent a speculative view of the process
of filament severing and final dissociation into droplets. Please note that the composition and structure of vimentin condensates is not known.
Formation of Protein Condensates. Regulation by Posttranslational Modifications with Focus on Redox Processes
Although there are themes common to various biomolecular condensates, their precise regulation will depend on their nature and composition. The formation of some condensates appears to occur in response to somehow non specific processes; nevertheless, detailed research is showing that they are governed by distinct sophisticated mechanisms, and that generalization of their behavior and regulation is not possible. Biomolecular condensates are in many instances triggered in response to stresses like heat shock, nutrient starvation, osmotic and oxidative stress. This is the case of stress granules, which sequester damaged proteins and RNA, thus conferring protection through mechanisms still incompletely understood [
46]. Interestingly, depending on the intensity and type of stress triggering stress granules, the material properties of these condensates can vary, and chaperones may be required for their dissolution [
47]. Understanding the mechanisms that control condensate assembly, interconversion with other structures and material properties may provide opportunities for new therapeutic strategies to fight pathological processes such as neurodegeneration, cancer and infections, for which links with failures in condensate regulation have been established [
38].
Involvement of proteins in phase separation processes is tightly regulated by the wide variety of PTMs that they can undergo. These modifications can significantly change the valency and intrinsic solubility of scaffold proteins forming condensates, as well as modify the partitioning of client proteins in these compartments, hence playing a pivotal role in the regulation of condensate composition, dynamics and stability [
25,
48,
49].
Multiple examples are currently available of eukaryotic proteins the phase separation of which is regulated by PTMs including acetylation, glycosylation, poly-ADP-ribosylation, phosphorylation and redox modifications [
48,
49]. Phosphorylation is one of the best studied PTMs influencing biomolecular condensation by rapidly promoting or suppressing phase separation in response to different cues [
30]. Lysine acetylation has also been shown to modify phase separation inhibiting or enhancing it depending on the context. In fact, given the great structural variety of PTMs, more than mere switches, they can act as fine tuners of phase separation [
30].
Among PTMs affecting proteins in condensates and/or condensate formation, redox modifications play a significant role. In fact, there seems to be a bidirectional interplay between protein condensation or aggregation and oxidative modifications. Redox modifications, in particular of thiol residues in proteins, comprise a plethora of structurally diverse modifications that can occur and be regulated both enzymatically and non-enzymatically, and can have structure specific effects [
50,
51]. Moreover, certain redox modifications can be dynamic and interexchange or evolve from reversible to more stable or irreversible modifications when oxidative stress persists [
52]. Disulfide bonding, either through formation of mixed disulfides, as in protein glutathionylation, or intramolecular or intermolecular protein disulfides, has been reported to promote phase separation [
49,
53]. In aging, increases in cysteine sulfenylation and sulfonylation have been found to promote liquid-liquid phase separation, and eventually, evolution of condensates to aggregates, whereas persulfidation prevents this transition by dissolving condensates [
54]. In turn, the concentration of proteins within condensates can affect the rate and mechanisms of oxidation, as observed upon macromolecular crowding or in nanodomains [
55,
56]. Importantly, condensates can influence cellular redox status [
57]. In fact, biomolecular condensates are themselves redox active structures [
58]. An electric field is generated at the interface between the condensate and the environment, at which spontaneous redox reactions can occur, with transfer of electrons, generation of H
2O
2 and electroactive species, which may contribute to the modulation of cellular redox balance [
58].
Although PTMs have been less frequently studied in bacteria, growing evidence supports their importance in the regulation of bacterial protein features [
59], including the ability to phase separate [
60]. Along this line, phosphorylation dramatically enhances phase separation of a membrane transporter important for
Mycobacterium tuberculosis infection of hosts [
61], whereas lysine acetylation may alter the phase separation of the histone-like protein HU [
62], at the same time reducing the DNA binding and DNA compaction ability of the protein [
60]. PTMs also play a role in the regulation of phase separation of viral proteins [
63]. Understanding the PTMs-dependent regulation of biomolecular condensation of proteins important for proliferation of microorganisms in infected hosts may be relevant to develop novel antiviral and antibacterial strategies. Importantly, this includes assemblies of proteins from the microorganism and/or the host. In fact, cytoskeletal proteins and, in particular intermediate filament proteins, such as vimentin, play a key role in the interactions of pathogens with the host and often in their replication mechanisms [
14,
64,
65], for which their study entails special interest.
Vimentin Filament Structure and Assembly
Similar to other intermediate filament proteins, vimentin monomers are constituted by three main segments. The head (N-terminal) and tail (C-terminal) domains, totaling 140 residues, possess mainly a low complexity sequence with intrinsically disordered structure, whereas the central rod domain is predominantly α-helical, comprising 326 residues. Vimentin monomers form parallel coiled-coil dimers and subsequently staggered antiparallel tetramers, or even higher order polymeric assemblies, under non-denaturing conditions. Due to its complexity, the structure of this protein has been elusive. Nevertheless, numerous excellent studies employing diverse approaches have yielded models that have contributed to the understanding of its assembly. These models proposed an intricate and interlaced structure, with multiple interactions being involved in the formation of tetramers, which is supported by the presence of stretches of hydrophobic amino acids and electrostatic interactions. Association of several tetramers, from 5 to 8 depending on the models [
12,
66], would constitute the basic or minimal units for filament assembly known as “unit length filament” or ULF. The size of ULF in cells is heterogeneous and can range between approximately 60 and 120 nm [
11,
12]. Elongation of the filaments is believed to occur through connection of these units at either end, which also implies an important overlap of C- and N-terminal domains of adjacent units. Indeed, high resolution microscopy studies in cells have been able to detect an approximately 10 nm overlap between consecutive ULF in filaments [
67].
Interestingly, recent insight obtained by cryo-electron microscopy approaches [
12] is consistent with mature vimentin filaments being constituted by 40 polypeptide chains in cross-section, assembled into five protofibrils, and reveals that certain highly conserved regions of the protein fall within close proximity, providing a molecular interlock mechanism important for filament structure. Moreover, this model unveils an unforeseen disposition of the unstructured N- and C-terminal segments. Whereas tail domains appear to dispose between the protofibrils providing lateral contact sites, the head domains occupy the center of the hollow filament, where they converge to form a central fiber which appears as a density in electron microscopy. These putatively interlaced head domains would help filament assembly and contribute to keep the filament together. Therefore, intermediate filaments arise as unique polymers that are “secured” not only by the tight “rope-like” interactions between dimers and tetramers in the protofibrils, but also by the “thread-like” head domains that hold the filament together from the inside. These interactions would also be operative in ULF [
12]. Importantly, before this model was available, studies from the McKnight group had revealed that the isolated head domains of several intermediate filament proteins, including vimentin, could phase separate forming gel-like condensates [
43]. These studies described the capacity of these N-terminal domains to engage in labile cross-beta interactions, providing a transient structural order that would underlie the dynamics of intermediate filament assembly. Moreover, their studies provided insight into the mechanism of filament disassembly by PTMs of the head domains, in particular by phosphorylation (reviewed in [
22]).
Vimentin filaments are highly dynamic structures, both in vitro and in cells. Recent work challenges the classical view of the relative stability of vimentin filaments in vitro [
68]. Indeed, vimentin tetramers are continuously being exchanged between filaments and the environment, which contains a soluble pool of vimentin, over the course of hours. Impairment of this exchange (considered a “filament self repair” mechanism), causes filament breakage. In cells, information on filament dynamics has been obtained through FRAP experiments in which recovery of fluorescence has been shown to occur within seconds or minutes [
20,
69]. Moreover, in sharp contrast to other cytoskeletal polymers, vimentin filaments can exchange subunits at any point along the filament length, and grow by end-to-end annealing of assembled filament segments at either end [
70].
Posttranslational Modifications of Vimentin. Critical Residues
Vimentin assembly and behavior are tightly and finely regulated by PTMs in a spatiotemporal manner. Perhaps the best characterized PTM-regulated processes are vimentin dynamics in mitosis and during cell migration. Vimentin is a target for a plethora of modifications. Recent proteomic studies together with evidence collected in databases, e.g. Phosphosite (
https://www.phosphosite.org), point to the existence of hundreds of sites for PTMs throughout its sequence. These modifications can occur in various combinations, each of which would yield a functional entity or proteoform. As most of the information on PTMs comes from bottom-up studies [
71], there is little information on the precise features of the relevant vimentin proteoforms. In theory, diverse proteoforms can be present at different places and proportions along vimentin filaments and specifically influence their assembly, shape, subcellular location and/or interactions (for a more detailed discussion please see [
3]).
The vimentin sequence presents certain “hot spots or regions” for PTMs. The head domain is the site for numerous PTMs that affect vimentin assembly. Vimentin head phosphorylation is involved in filament reorganization/disassembly during cell division. In fact, there are at least 37 phosphorylation sites in the N-terminal vimentin segment comprising residues 1-117, but also, several sites for acetylation, methylation, ubiquitination, glycosylation and even proteolytic cleavage (see references at Phosphosite). In contrast to phosphorylation, glycosylation by O-linked b-N-acetylglucosamine (O-GlcNAc) of several sites in the head domain, and particularly of serine 49, promotes interactions between vimentin molecules that appear to be required for filament assembly in cells [
72]. In light of the recent models positioning the head domains forming a fiber in the lumen of the filament, PTM of these domains could greatly influence their interactions either stabilizing (glycosylation) or destabilizing the filament (phosphorylation). Nevertheless, the question arises on how enzymes catalyzing some of these PTMs gain access to the head domain in the intact filament. In turn, tail domains are accessible on the filament surface providing contacts between protofibrils [
12], and can harbor several modifications, although their functional importance is less known. Vimentin tails from the monomers in the parallel dimer are believed to organize in pairs. These partially disordered domains have been proposed to adopt different dispositions, either projecting forward from the end of the helical rods or folding back on them. Moreover, they have been reported to be present in several conformations in cells that differ in the accessibility of precise segments to binding of specific antibodies and appear to correlate with the degree of filament packing [
73]. Interestingly, the predominance of the accessible or packed conformation could be determined by PTMs.
The single cysteine residue of vimentin, C328, constitutes a critical hot spot for modification. This residue is conserved in all species and members of the type III intermediate filament class [
3], and is the target for a plethora of modifications with distinct structure-dependent consequences on vimentin assembly and remodeling, both in vitro and in cells [
3,
21,
74,
75]. Modification of C328 is critical for redox regulation of the network, as well as for its response to oxidative and electrophilic stress [
15,
21]. Moreover, C328 can bind zinc, which influences its susceptibility to modification, as well as filament bundling and network organization [
20,
76]. Of note, C328 can be found modified by certain moieties under control conditions in several systems, and increases in the extent or changes in the nature of the modification occur upon exposure to stress or in pathophysiology, in association with vimentin reorganization [
71].
Importantly, the regions known as molecular interlocks [
12] can also be sites of PTMs, some of which could have an important impact on filament stability. For instance, vimentin Y117, located at the interlock, can be phosphorylated by Src leading to filament disassembly during cell migration [
77]. Also, Y117 mutation results in impaired assembly. Indeed, a Y117L vimentin mutant was early reported to be unable to elongate, forming only ULF, as suggested by its in vitro properties and its appearance as small dots in cells [
78]. Indeed, constructs of the vimentin Y117L mutant have become widely used tools as models for the study of ULF behavior [
11,
79].
Vimentin Has Been Detected in a Variety of Assemblies Reminiscent of Biomolecular Condensates
Among the diversity of assemblies that vimentin can form in cells, droplets or dots of variable sizes have been frequently observed as a consequence of filament disassembly or when monitoring certain mutants. Although precise information on their composition and structure is often lacking, they have been frequently assumed to correspond to ULF.
Particles or droplets formed by endogenous untagged vimentin were early observed by immunofluorescence at peripheral regions of spreading cells [
80], in lamellipodia of migrating fibroblasts [
18], and in some cell types during mitosis [
81]. More recently, it has been reported that treatment of cells with relatively high concentrations (from 5 to 8%) of 1,6-hexanediol, an aliphatic alcohol that disrupts labile cross-ꞵ interactions, disassembles vimentin filaments into particles [
23,
43] (Fig. 2). This is consistent with the behavior of vimentin head domains as structures forming a phase separated “compartment” within the filament that would be disrupted by this compound. Interestingly, exposure of cells to various oxidants leads to the partial or full conversion of the vimentin network into droplets with the characteristics of biomolecular condensates [
20,
23] (Fig. 2). However, the vimentin particles observed in these various studies may not necessarily correspond to the same entities, and further characterization is needed. The size of vimentin droplets may be variable (estimated <0.05 µm
2 in [
23]), as can be their composition, since they have been reported to associate with kinesin [
80] or nestin [
17]). Also, vimentin in droplets could bear different PTMs (phosphorylation [
17] or oxidation [
23]). Interestingly, vimentin dots were early proposed to serve as precursors of squiggle, and subsequently filament assembly [
80]. Recently, the work by Sivagurunatham et al., using a cell line with inducible vimentin expression has illustrated that, upon induction, vimentin is first detected as small particles which appear to evolve towards short filaments or squiggles and later form filaments [
82]. Notably, in this vein, oxidant elicited vimentin condensates are reversible and can reassemble into filaments [
23].
Information on the dynamics of vimentin wild type (wt) particles or droplets has arisen from the use of fluorescent constructs, either alone or in combination with endogenous or untagged vimentin. These studies have allowed to evidence their fast movement [
80], rapid recovery of fluorescence after photobleaching, and ability to fuse, as demonstrated by the coalescence of particles bearing vimentin with different fluorescent tags [
23]. Several of these features are consistent with the behavior of biomolecular condensates. The convergence of vimentin particles could indicate their potential to assemble into full filaments [
83]. Importantly, the observation that condensates generated after oxidative stress can fuse and reassemble into filaments upon removal of the oxidant [
23], suggests that this could be the case under certain settings. Nevertheless, further efforts are needed to monitor this process in detail in live cells.
Ideally, when using fluorescent constructs, it is necessary to keep a low proportion of tagged versus untagged vimentin in order to illuminate the network without significantly altering its properties. In general, tags located at the amino terminal of the protein could alter its assembly. This is especially obvious when expressing fusion constructs of vimentin and fluorescent proteins in vimentin deficient cells. GFP-vimentin, for instance, cannot form full filaments, but only short filaments or squiggles in SW13/cl.2 adrenal carcinoma cells [
20], and a mixture of thin filaments and particles in MCF7 breast cancer cells [
15], since both cell types lack endogenous vimentin. Interestingly, short filaments formed by these vimentin fusion proteins are highly susceptible to disruption by treatment with a variety of oxidants and electrophiles, leading to droplets, whereas untagged filaments are more resistant to these agents. For instance, various electrophilic lipids induce bundling and juxtanuclear accumulation of untagged vimentin, with partial disassembly at the cell periphery, but provoke a nearly full conversion of GFP-tagged vimentin into droplets [
15,
20]. Indeed, this higher susceptibility has been long recognized and exploited for monitoring agents potentially forming adducts with vimentin [
15,
20].
Moreover, several vimentin mutants that otherwise would form various kinds of filamentous assemblies, display defective elongation when expressed as tagged proteins in vimentin deficient cells, leading to the formation of droplets. This is the case of several mutants of the single cysteine residue, including GFP-vimentin C328S, C328A and C328D [
15] (Fig. 2). Particularly interesting are the truncated mutants of the tail domain (GFP-vimentin(1-411), (1-423), (1-448) and (1-459). The untagged versions of these mutants form abnormal filament networks in vimentin deficient cells. Nevertheless, the GFP-tagged versions form droplets of variable size, reminiscent of condensates [
16] (Fig. 2). Another example of this behavior is mEmerald-vimentin wt, which is able to form long filaments in vimentin deficient HeLa cells. However, the S49A and S49E mutants of this construct form only droplets [
72] (Fig. 2). Taken together, these observations suggest that disruption or mutation of certain residues, e.g. C328 or S49, as well as truncation of the tail domain, can lead to droplets of vimentin fusion proteins. Nevertheless, additional evidence of the behavior of these droplets as biomolecular condensates is required.
As mentioned above, the elongation deficient Y117L mutant, considered to form only ULF, poses a special case. Fluorescent constructs of this mutant (Fig. 2) have been used in numerous studies to generate a wealth of information on the behavior of vimentin particles, including their dynamics, movement in cells, coalescence [
79,
83], exchange of subunits with the environment evidenced through FRAP assays [
84], precise dimensions of ULF in cells determined by superresolution studies [
11], and interaction with other cytoskeletal structures, including microtubules and f-actin [
79,
83]. Therefore, this accumulated evidence supports the behavior of Y117L ULF as biomolecular condensates. In addition, these studies have demonstrated that the movement of Y117L ULF requires metabolic energy [
84], and that their size in cells displays a heterogeneous distribution, with an average length of approximately 59 nm and a “tilted” orientation, determined by their connections with the microtubule network [
11].
Precisely, the potential interactions of vimentin ULF with other cytoskeletal structures constitute an interesting aspect. Robert et al., found that vimentin ULF could move along microtubules, whereas interaction with f-actin would restrict this transport [
79]. ULF movement could be mediated by molecular motors, as suggested by their colocalization with kinesin [
80]. In turn, interaction with actin could influence the properties of actin fibers, as it has been reported that vimentin Y117L ULF lining these fibers produce a wetting effect that influences actin susceptibility to depolymerizing agents [
83]. Interestingly, GFP-vimentin wt squiggles as well as droplets formed by several GFP-vimentin mutants (GFP-vimentin C328S, C328A and C328D) effectively align on actin stress fibers, either spontaneously or after treatment with certain electrophilic compounds such as dinitroimidazole [
15] (Fig. 3A). On the other hand, vimentin droplets display a random distribution without a particular association with actin or tubulin structures in cells treated with other electrophiles, such as diamide, which can also disrupt the actin cytoskeleton [
23]. Interestingly, droplets formed by GFP-vimentin C328S effectively associate with the actin cortex in mitosis, whereas truncated tail mutants (GFP-vimentin (1-411 and 1-423) fail to do so [
16], consistent with the role of vimentin tail in cortical relocation of vimentin during cell division [
16] (Fig 3B). Therefore, the interaction of vimentin droplets with cytoskeletal structures would be determined by a complex interplay in which structural factors from the protein components, including PTM, as well as cellular context factors will play an important role.
Among other intermediate filaments, keratin also forms droplets upon cell treatment with 8% hexanediol [
43], whereas the fusion construct mEmerald-desmin C333S forms droplets when expressed in SW13/cl.2 cells, which are deficient in cytoplasmic intermediate filaments [
85]. Interestingly, fluorescent constructs of desmin and neurofilament head domains phase separate forming hydrogels in vitro [
22].
Together, these observations raise the question of the functional importance of intermediate filament condensates, and of vimentin in particular. In addition to the modulation of cytoskeletal structures or facilitating traffic of filament precursors, vimentin “ULF” have been reported to support some of the functions of filamentous vimentin, including the modulation of transcription of certain genes [
82]. Moreover, as we will consider in detail below, they could constitute intermediates in filament assembly, and/or transient protective structures in the response to certain types of stress.
Oxidative Stress Induces Vimentin Condensates
Vimentin is highly responsive to redox changes and oxidative stress. The remodeling of vimentin filaments or squiggles into dots or droplets in response to oxidants has been known for a decade [
20]. In some cases, this remodeling affects the whole cell, whereas in others it occurs preferentially at peripheral areas [
23]. In fact, although the most obvious effect of some electrophilic compounds is often the compaction of filaments at the juxtanuclear region, careful observation of the cellular periphery from which filaments have retracted, allows the observation of vimentin particles [
15].
Interestingly, treatment of cells with the oxidant diamide elicits a full remodeling of vimentin filaments, which can be monitored in time, revealing a progressive severing of filaments into shorter segments, that finally appear as droplets (Fig. 4). These droplets provide an interesting model for study since they fulfil several criteria to be considered biomolecular condensates, including shape, FRAP and fusion [
23] (Fig. 4). This process would imply a double novelty. First, it could represent a primary evidence of condensate formation by one of the major cytoskeletal proteins
per se. Second, whereas most condensates described arise by recruitment or concentration of proteins into a phase separated compartment, diamide-elicited vimentin condensates would result from the dissociation of a “phase separated” polymer into smaller units, which could constitute, as yet, a unique mechanism. Importantly, this remodeling is completely dependent on the presence of the single vimentin cysteine, C328, which acts as a sensor for oxidants and electrophiles [
20]. However, other modifications could contribute to this effect, as suggested by the observation that a vimentin mutant in which several phosphorylation sites from the head domain have been suppressed by serine to alanine mutation [
86], is partially protected from diamide-induced transition into dots [
21]. This observation supports the protein-dependent modulation of phase separation by phosphorylation, which has been reported to decrease or prevent phase separation of other proteins [
49]. In the case of vimentin, phosphorylation of certain residues aids in filament disassembly, and this could play a positive role in the remodeling of filaments into discrete condensates. If we consider that the mature filament is a “hollow tube” with a phase separated “filling” constituted by the head domains, a certain degree of phosphorylation could contribute to dissociate this tube into smaller phase separated units or droplets, leading to the formation of more typical biomolecular condensates. Nevertheless, hyperphosphorylation could have an apparently opposite effect, dissolving the phase separated droplets into completely diffuse protein. This hypothesis would be in agreement with observations indicating that the oxidant diamide elicits filament dissociation into droplets, while combination of diamide with the phosphatase inhibitor calyculin A, resulting in vimentin hyperphosphorylation, leads to dissolution of droplets into a diffuse protein pattern [
21]. Interestingly, opposite effects of phosphorylation and hyperphosphorylation have been previously reported for other proteins, such as TDP43 [
49].
Thus, vimentin droplets could be an intermediate state between filaments and full protein disassembly. In fact, assays with aliphatic alcohols also support this interpretation. A variety of aliphatic alcohols with defined spacings between the hydroxyl groups differentially influence phase separation mediated by labile hydrophobic interactions. Whereas 1,6-hexanediol selectively disrupts condensates bound by these interactions, 2,5-hexanediol is not effective [
87]. Interestingly, vimentin structures display a concentration-dependent response to dissolution by 1,6-hexanediol. Intact filaments undergo a separation into fragments or particles upon exposure to “high” concentrations of the alcohol (≥5% in cells) [
23,
87], whereas oxidant-induced droplets are more susceptible and become solubilized by treatment of cells with ~ 3% 1,6-hexanediol [
23]. In turn, 2,5-hexanediol has no effect on either structure. This suggests that both, filaments and droplets, may be hold together by phase separated domains, but the latter are more susceptible to disruption, potentially due to the higher accessibility of the domains involved, likely the head domains.
An important feature of diamide-elicited vimentin biomolecular condensates is their reversibility, that is, the condensates can readily fuse and reconnect into filaments when oxidative stress is over [
23]. This recovery process does not appear to require new protein synthesis. Therefore, these observations also support the contention that vimentin condensates can serve as filament precursors. Whether this is the case upon de novo synthesis of vimentin in cells remains to be confirmed. Nevertheless, condensate formation could play a role in reversible disassembly/assembly processes, such as that occurring during mitosis or under certain kinds of stress.
It has long been known that vimentin in both filaments and ULF can be exchanged with “soluble precursors”, as revealed by FRAP experiments [
20,
69,
84]. Interestingly, vimentin filament fragmentation into droplets and subunit exchange in cells are an energy requiring process, as indicated by the observation that they are blocked by ATP-depleting strategies [
21,
84]. The “units” that are exchanged are not completely characterized, although a recent work suggests that in vitro, these subunits would be vimentin tetramers [
68]. Interestingly, in vitro experiments also reveal that vimentin filaments are more resistant to disruption by oxidants than the non polymerized protein, that is, oxidation of soluble vimentin can drastically alter subsequent polymerization, whereas preformed filaments appear to be less affected by similar modifications [
21]. This suggests that, in cells, oxidation of soluble precursors could hamper their incorporation into filaments or lead to abnormal structures. Alternatively, dissociated subunits could interact with cellular proteins precluding their reincorporation.
Interestingly, prolonged incubation with oxidants, which is associated with increased irreversible oxidative modifications, hampers the reversibility of vimentin condensates. This indicates that accumulation of deleterious PTM can block fusion and reassembly. Indeed, dissolving condensates with aliphatic alcohols also greatly delays vimentin reassembly [
23], thus indicating that the presence of vimentin in condensates facilitates this process.