Submitted:
14 March 2025
Posted:
17 March 2025
You are already at the latest version
Abstract
Keywords:
Introduction
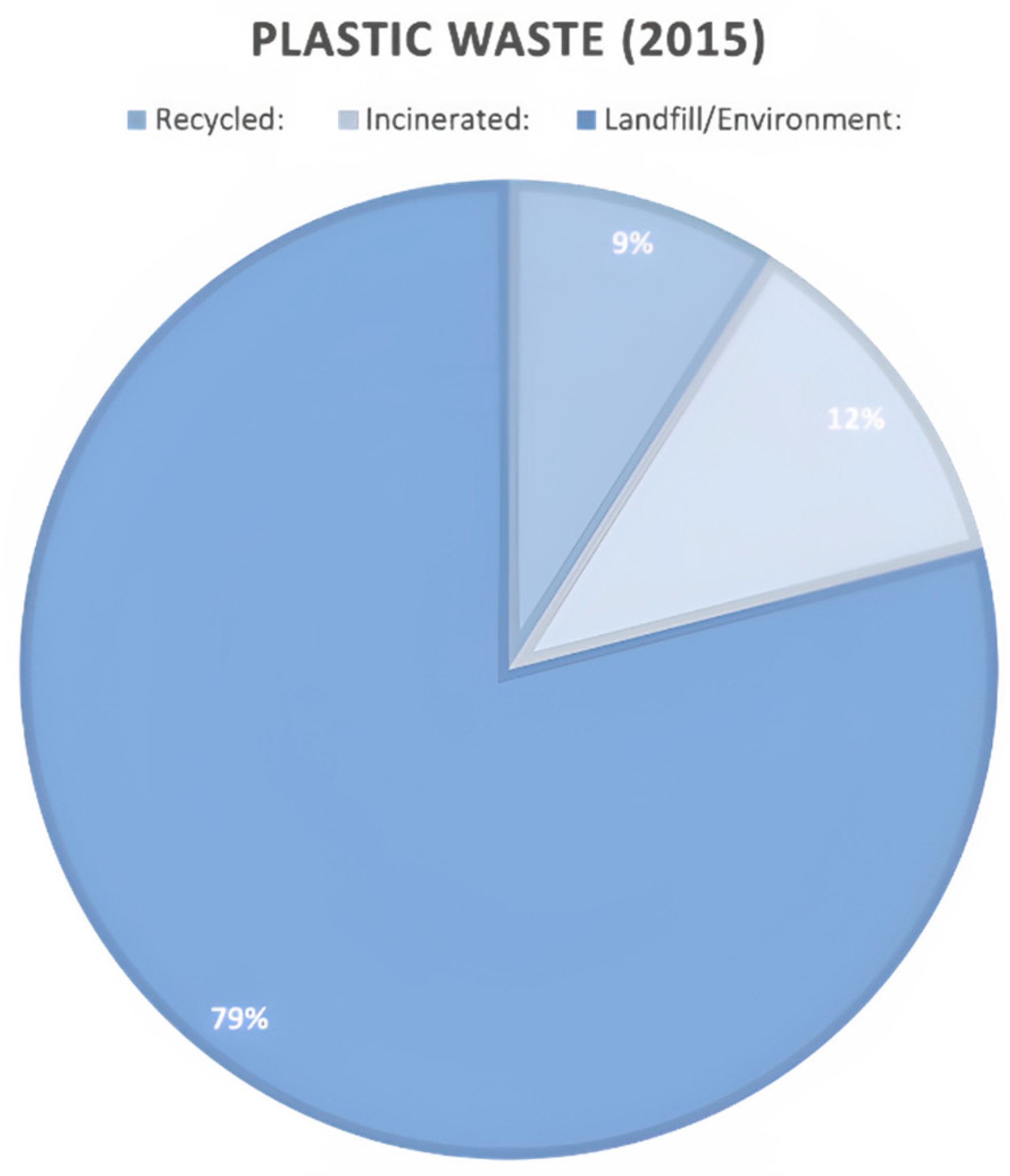
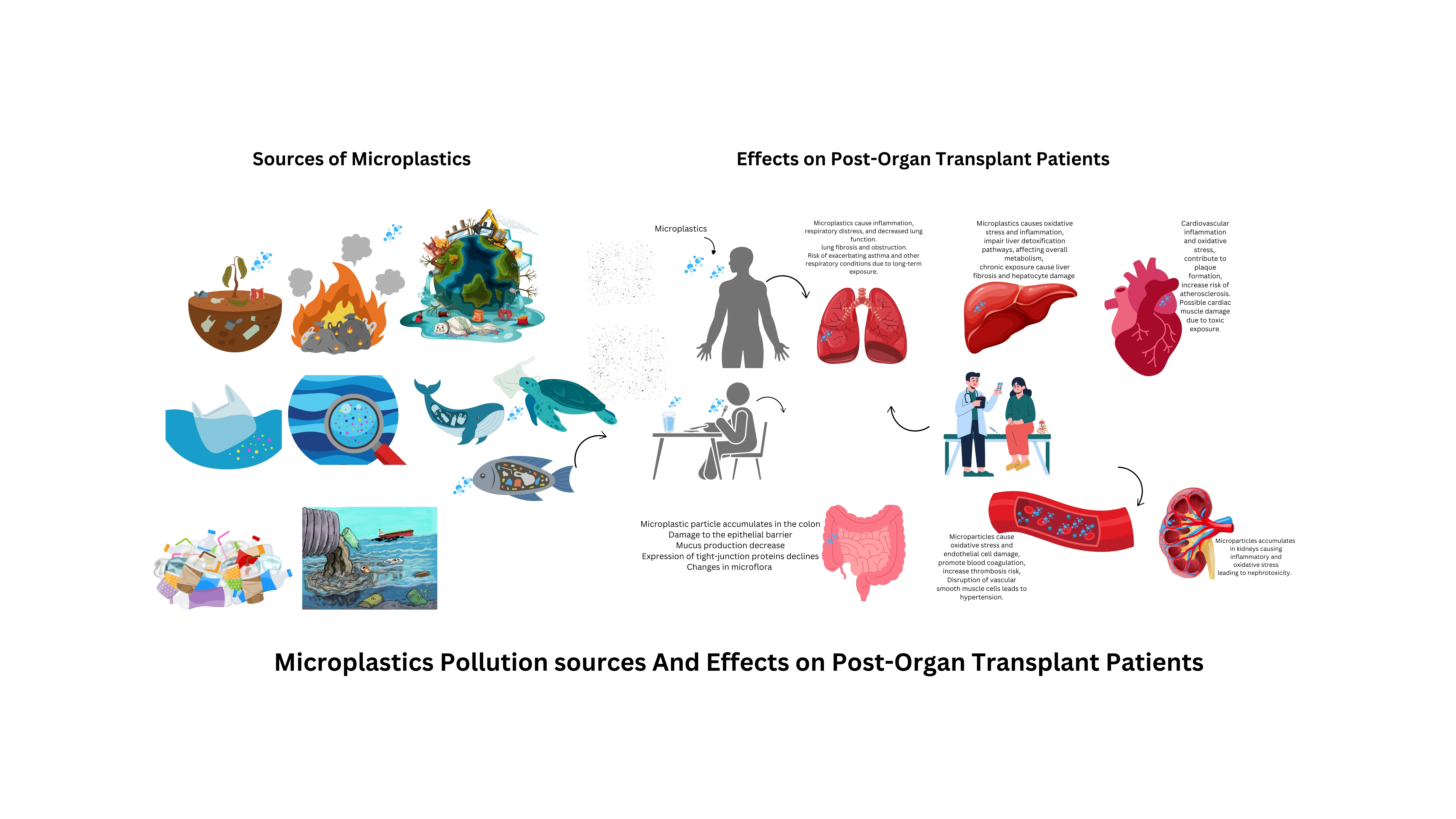
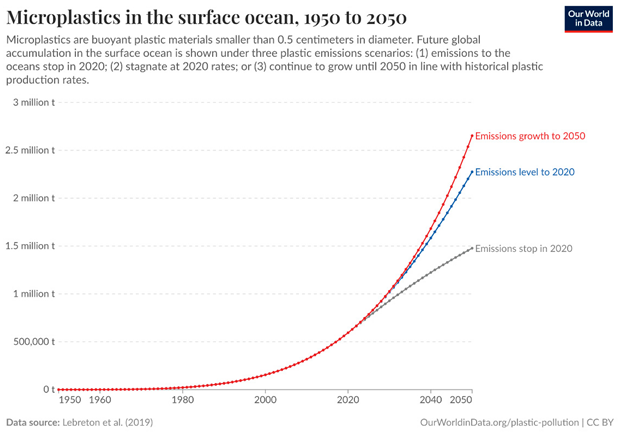
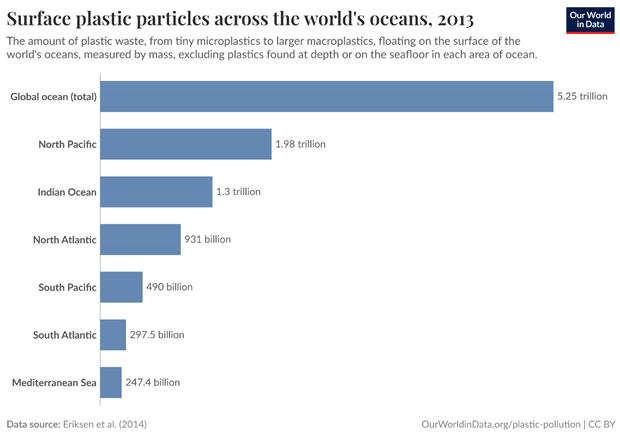
Methodology
Study Design
Ethical Considerations
Health Risk Assessment
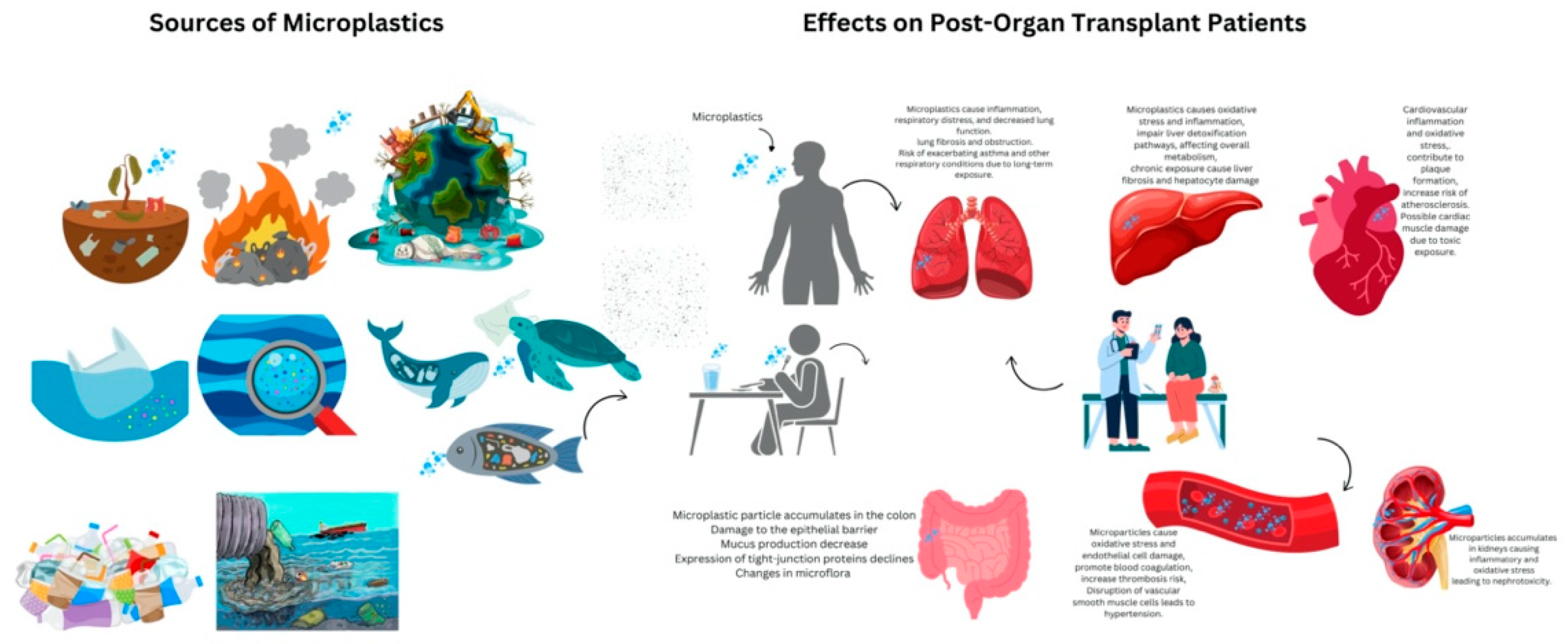
Pathways of Microplastic Exposure
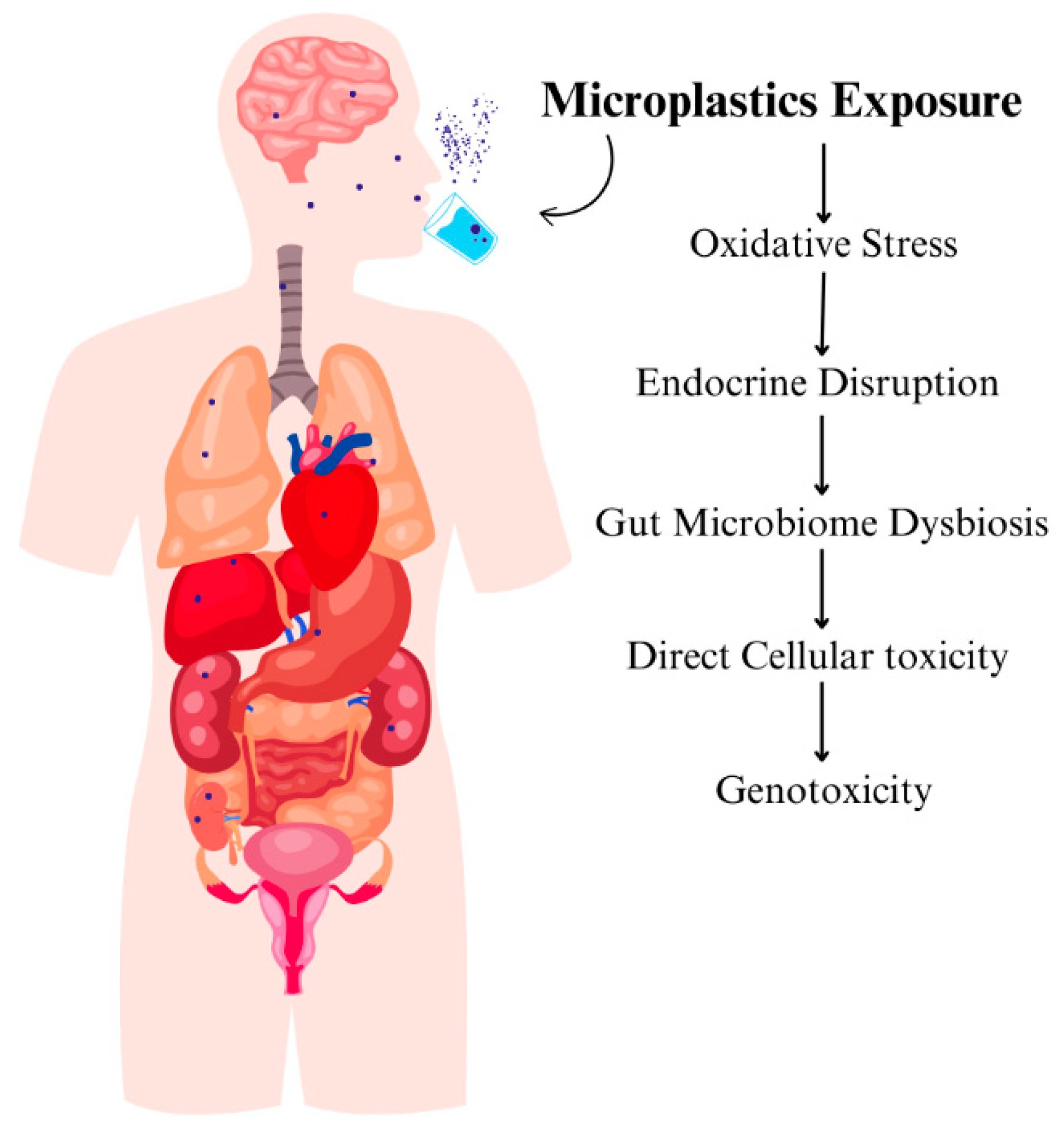
Mechanisms of Microplastic Internalization
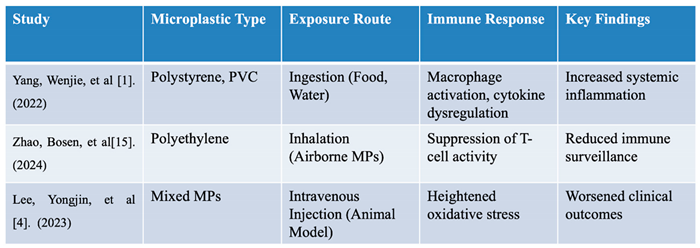
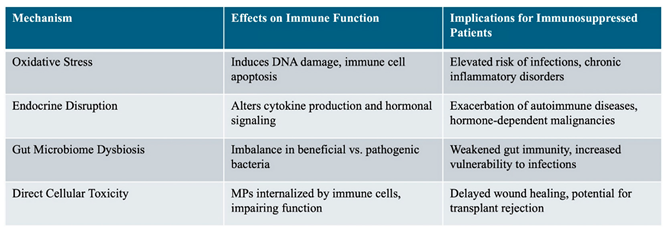
Impacts of Microplastics on Immune Function and Organ Systems
Immunotoxicity of Microplastics Through Oxidative Stress
Role of Microplastic Endocrine Disruption and Bioaccumulation
Discussion
Conclusion
Abbreviation
References
- Yang, W.; Jannatun, N.; Zeng, Y.; Liu, T.; Zhang, G.; Chen, C.; Li, Y. Impacts of microplastics on immunity. Frontiers in toxicology 2022, 4, 956885. [Google Scholar] [PubMed]
- Jambeck, J.; Geyer, R.; Wilcox, C.; Siegler, T.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K. Marine pollution. Plastic waste inputs from land into the ocean. Sci 2015, 347, 768–771. [Google Scholar]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested–A pivotal first step towards human health risk assessment. Journal of Hazardous Materials 2021, 404, 124004. [Google Scholar] [PubMed]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health effects of microplastic exposures: current issues and perspectives in South Korea. Yonsei Medical Journal 2023, 64, 301. [Google Scholar]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic polymer contamination in bottled water. Frontiers in chemistry 2018, 6, 389699. [Google Scholar]
- Alfaro-Núñez, A.; Astorga, D.; Cáceres-Farías, L.; Bastidas, L.; Soto Villegas, C.; Macay, K.; Christensen, J.H. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Scientific reports 2021, 11, 6424. [Google Scholar]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Scientific reports 2019, 9, 15775. [Google Scholar] [CrossRef]
- Li, L.a.; Xu, R.; Jiang, L.; Xu, E.G.; Wang, M.; Wang, J.; Li, B.; Hu, M.; Zhang, L.; Wang, Y. Effects of microplastics on immune responses of the yellow catfish Pelteobagrus fulvidraco under hypoxia. Frontiers in physiology 2021, 12, 753999. [Google Scholar] [PubMed]
- Lu, K.; Lai, K.P.; Stoeger, T.; Ji, S.; Lin, Z.; Lin, X.; Chan, T.F.; Fang, J.K.-H.; Lo, M.; Gao, L. Detrimental effects of microplastic exposure on normal and asthmatic pulmonary physiology. Journal of hazardous materials 2021, 416, 126069. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nature Reviews Immunology 2020, 20, 95–112. [Google Scholar]
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Shao, L.; Ye, J.; He, P. Is incineration the terminator of plastics and microplastics? Journal of Hazardous Materials 2021, 401, 123429. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environmental pollution 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in seafood and the implications for human health. Current environmental health reports 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Raveendran, S.; Singh, S.; Pillai, S. Environmental impacts of microplastics and nanoplastics: A current overview. Frontiers in Microbiology 2021, 12, 768297. [Google Scholar] [CrossRef]
- Sykes, E.A.; Dai, Q.; Tsoi, K.M.; Hwang, D.M.; Chan, W.C. Nanoparticle exposure in animals can be visualized in the skin and analysed via skin biopsy. Nature communications 2014, 5, 3796. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and human health: a micro issue? Environmental science & technology 2017, 51, 6634–6647. [Google Scholar]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The potential toxicity of microplastics on human health. Science of The Total Environment 2024, 912, 168946. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharmaceutica Sinica B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicology in Vitro 2016, 31, 126–136. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Science of the Total Environment 2019, 694, 133794. [Google Scholar] [CrossRef]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nature Reviews Immunology 2003, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environmental Pollution 2022, 300, 118986. [Google Scholar] [PubMed]
- McKechnie, J.L.; Blish, C.A. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell host & microbe 2020, 27, 863–869. [Google Scholar]
- Greven, A.C.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environmental toxicology and chemistry 2016, 35, 3093–3100. [Google Scholar]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environmental Pollution 2018, 235, 322–329. [Google Scholar]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: a prospective case series. Annals of internal medicine 2019, 171, 453–457. [Google Scholar]
- Mohana, A.A.; Islam, M.M.; Rahman, M.; Pramanik, S.K.; Haque, N.; Gao, L.; Pramanik, B.K. Generation and consequence of nano/microplastics from medical waste and household plastic during the COVID-19 pandemic. Chemosphere 2023, 311, 137014. [Google Scholar]
- Djouina, M.; Vignal, C.; Dehaut, A.; Caboche, S.; Hirt, N.; Waxin, C.; Himber, C.; Beury, D.; Hot, D.; Dubuquoy, L. Oral exposure to polyethylene microplastics alters gut morphology, immune response, and microbiota composition in mice. Environmental Research 2022, 212, 113230. [Google Scholar]
- Chartres, N.; Cooper, C.B.; Bland, G.; Pelch, K.E.; Gandhi, S.A.; BakenRa, A.; Woodruff, T.J. Effects of Microplastic Exposure on Human Digestive, Reproductive, and Respiratory Health: A Rapid Systematic Review. Environmental Science & Technology 2024, 58, 22843–22864. [Google Scholar]
- Auguste, M.; Balbi, T.; Ciacci, C.; Canonico, B.; Papa, S.; Borello, A.; Vezzulli, L.; Canesi, L. Shift in immune parameters after repeated exposure to nanoplastics in the marine bivalve Mytilus. Frontiers in immunology 2020, 11, 426. [Google Scholar] [PubMed]
- Espinosa, C.; Beltrán, J.M.G.; Esteban, M.A.; Cuesta, A. In vitro effects of virgin microplastics on fish head-kidney leucocyte activities. Environmental Pollution 2018, 235, 30–38. [Google Scholar]
- Park, E.-J.; Han, J.-S.; Park, E.-J.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicology letters 2020, 324, 75–85. [Google Scholar]
- Han, S.; Bang, J.; Choi, D.; Hwang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. Surface pattern analysis of microplastics and their impact on human-derived cells. ACS Applied Polymer Materials 2020, 2, 4541–4550. [Google Scholar]
- AshaRani, P.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-J.; Li, J.-W.; Xu, E.G.; Sun, X.-D.; Zhu, F.-P.; Ding, Z.; Tian, H.; Dong, S.-S.; Xia, P.-F.; Yuan, X.-Z. Short-term exposure to positively charged polystyrene nanoparticles causes oxidative stress and membrane destruction in cyanobacteria. Environmental Science: Nano 2019, 6, 3072–3079. [Google Scholar]
- Pathak, D. Enemies of the hormones: microplastics and endocrine disruptors impacting public health. In Health and Climate Change, Elsevier: 2025; pp. 119-150.
- Puri, M.; Gandhi, K.; Suresh Kumar, M. A global overview of endocrine disrupting chemicals in the environment: Occurrence, effects, and treatment methods. International Journal of Environmental Science and Technology 2023, 20, 12875–12902. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Ortega, D.E.; Miranda-Rojas, S. Mechanistic insights into the adsorption of endocrine disruptors onto polystyrene microplastics in water. Environmental Pollution 2023, 319, 121017. [Google Scholar]
- Solleiro-Villavicencio, H.; Gomez-De León, C.T.; Del Río-Araiza, V.H.; Morales-Montor, J. The detrimental effect of microplastics on critical periods of development in the neuroendocrine system. Birth Defects Research 2020, 112, 1326–1340. [Google Scholar]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proceedings of the National Academy of Sciences 2016, 113, E5062–E5071. [Google Scholar]
- Espinosa, C.; Cuesta, A.; Esteban, M.Á. Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L.). Fish & shellfish immunology 2017, 68, 251–259. [Google Scholar]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environmental science & technology 2019, 53, 7068–7074. [Google Scholar]
- Cordier, M.; Uehara, T. Will innovation solve the global plastic contamination: how much innovation is needed for that? 2024.
- Huang, W.; Wang, X.; Chen, D.; Xu, E.G.; Luo, X.; Zeng, J.; Huan, T.; Li, L.; Wang, Y. Toxicity mechanisms of polystyrene microplastics in marine mussels revealed by high-coverage quantitative metabolomics using chemical isotope labeling liquid chromatography mass spectrometry. Journal of hazardous materials 2021, 417, 126003. [Google Scholar] [PubMed]
- Rochman, C.; Tahir, A.; Williams, S.; Baxa, D.; Lam, R.; Miller, J. SJ 2015. Anthropogenic debris in seafood: Plastik debris and fibers from textiles in 59fish and bivalves sold for human consumption. Scientific reports 5.
- Ambrosini, R.; Azzoni, R.S.; Pittino, F.; Diolaiuti, G.; Franzetti, A.; Parolini, M. First evidence of microplastic contamination in the supraglacial debris of an alpine glacier. Environmental pollution 2019, 253, 297–301. [Google Scholar]
- Assas, M.; Qiu, X.; Chen, K.; Ogawa, H.; Xu, H.; Shimasaki, Y.; Oshima, Y. Bioaccumulation and reproductive effects of fluorescent microplastics in medaka fish. Marine Pollution Bulletin 2020, 158, 111446. [Google Scholar]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PloS one 2014, 9, e111913. [Google Scholar]
- Abihssira-García, I.S.; Park, Y.; Kiron, V.; Olsvik, P.A. Fluorescent microplastic uptake by immune cells of atlantic salmon (Salmo salar L.). Frontiers in Environmental Science 2020, 8, 560206. [Google Scholar]
- Watson-Wright, W.M.; Wells, P.G.; Duce, R.A.; Gilardi, K.V.; Girvan, A.S.; Huber, M.E.; Kershaw, P.J.; Linders, J.B.; Luit, R.J.; Vivian, C.M. The UN Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP)—An ocean science-policy interface standing the test of time. Marine Pollution Bulletin 2024, 199, 115917. [Google Scholar]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth-Science Reviews 2020, 203, 103118. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





