Submitted:
17 March 2025
Posted:
17 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
2.2.1. UV–Vis–NIR and FTIR
2.2.2. TEM and HAADF-STEM
2.2.3. AFM/MFM
2.3. Methods
2.3.1. Symmetry
2.3.2. Harmony
2.3.3. Perfection
3. Results
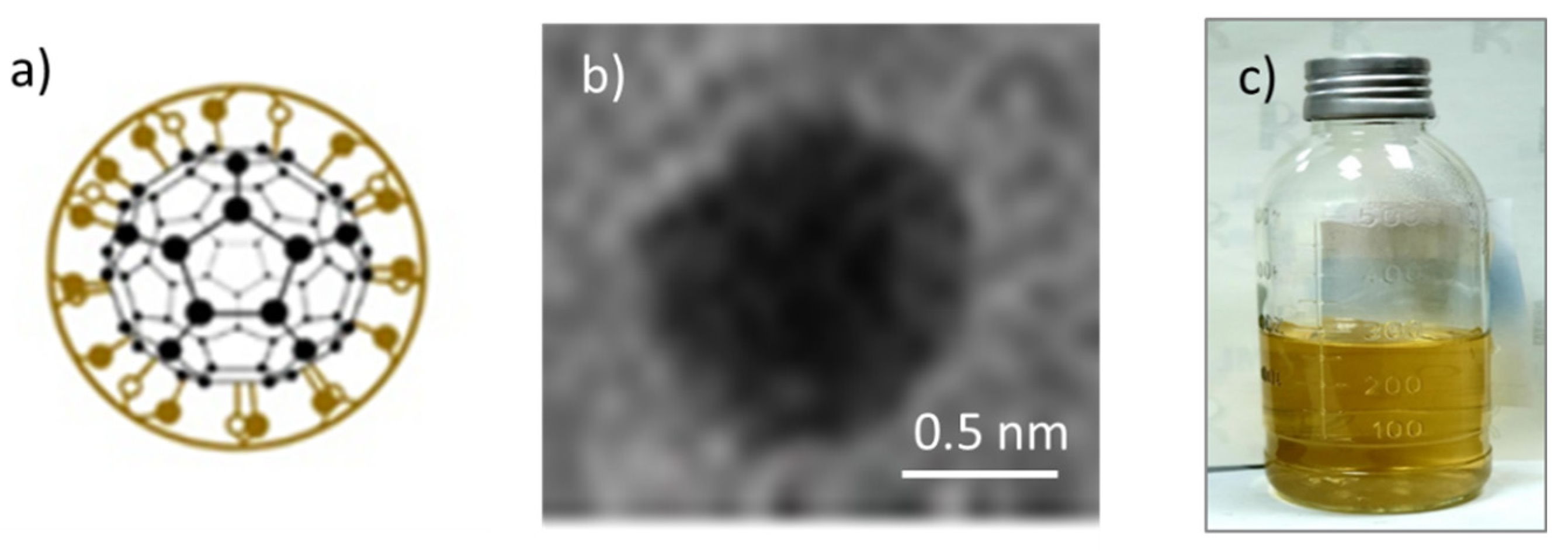
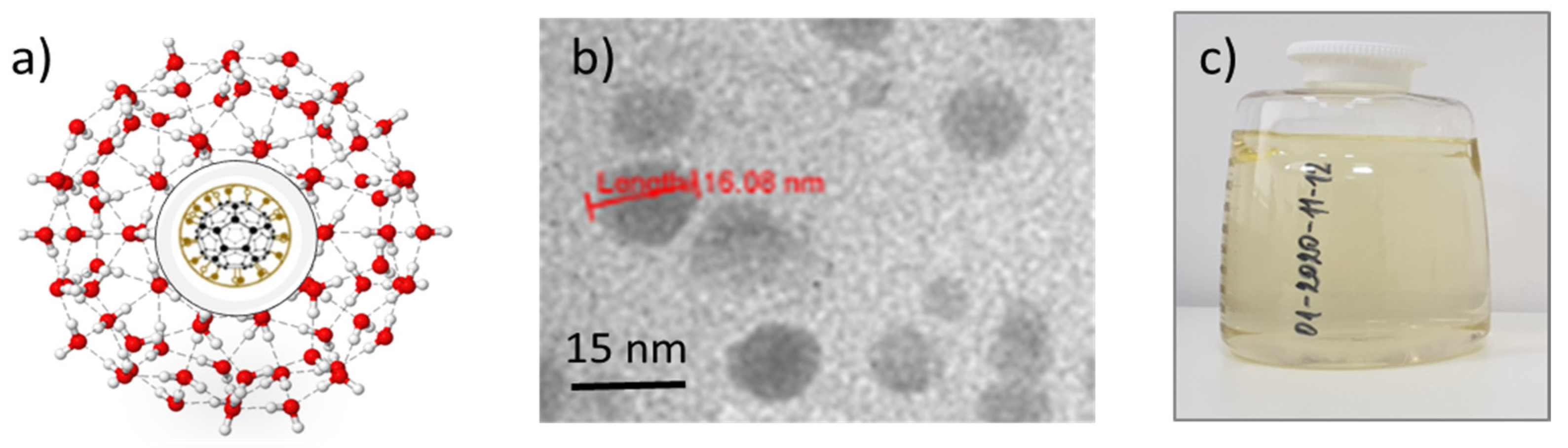
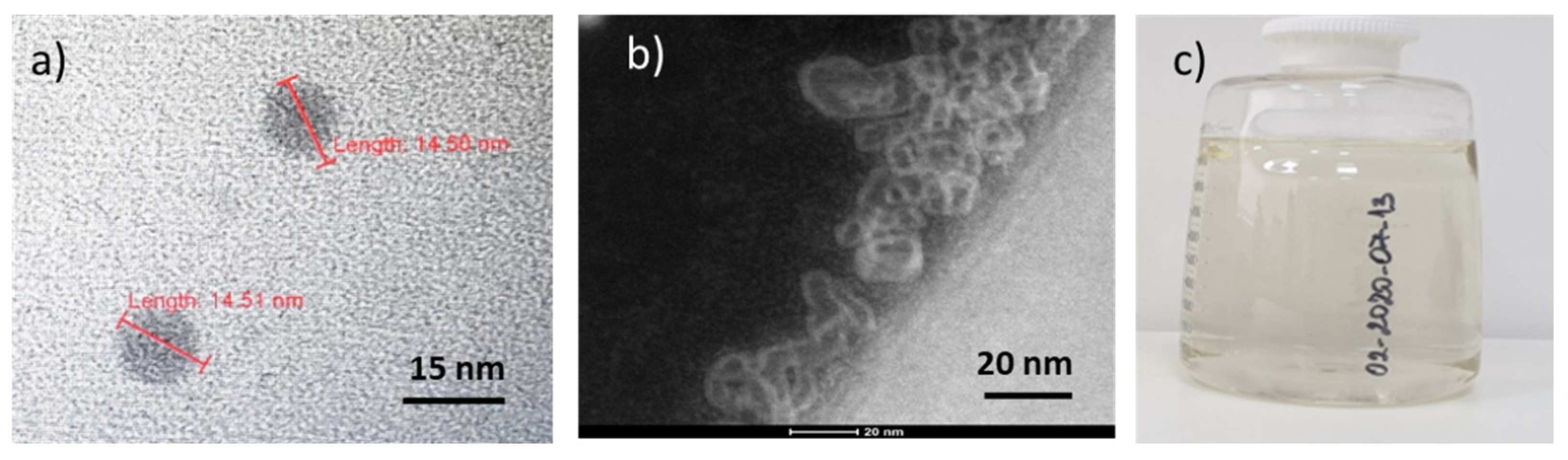
3.1. FD-C60 (the First Derivative of the C60)
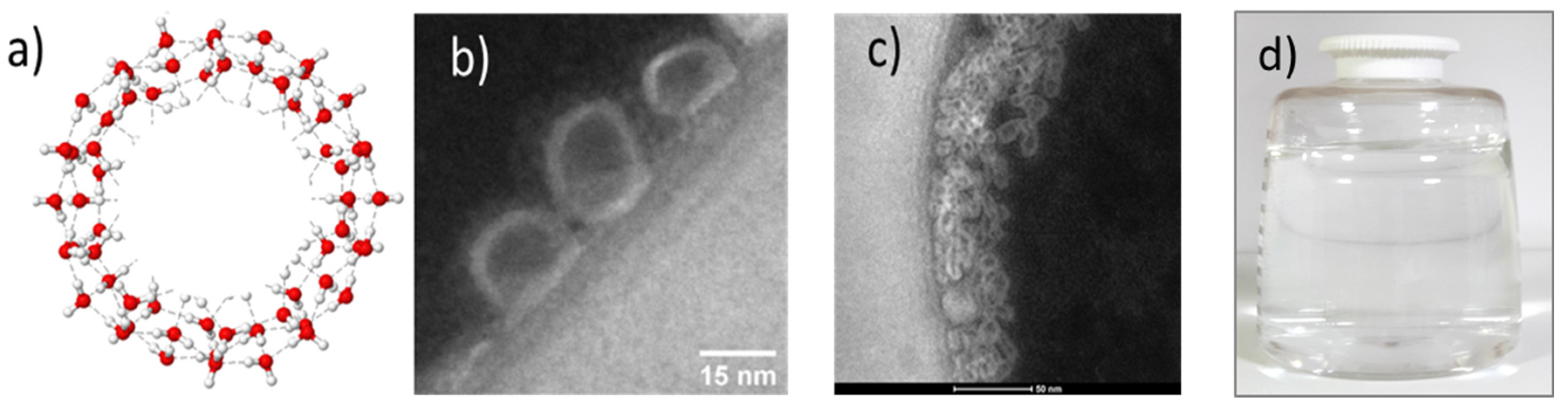
3.2. TEM Images of SD-C60 and TD-C60 Derivatives of C60
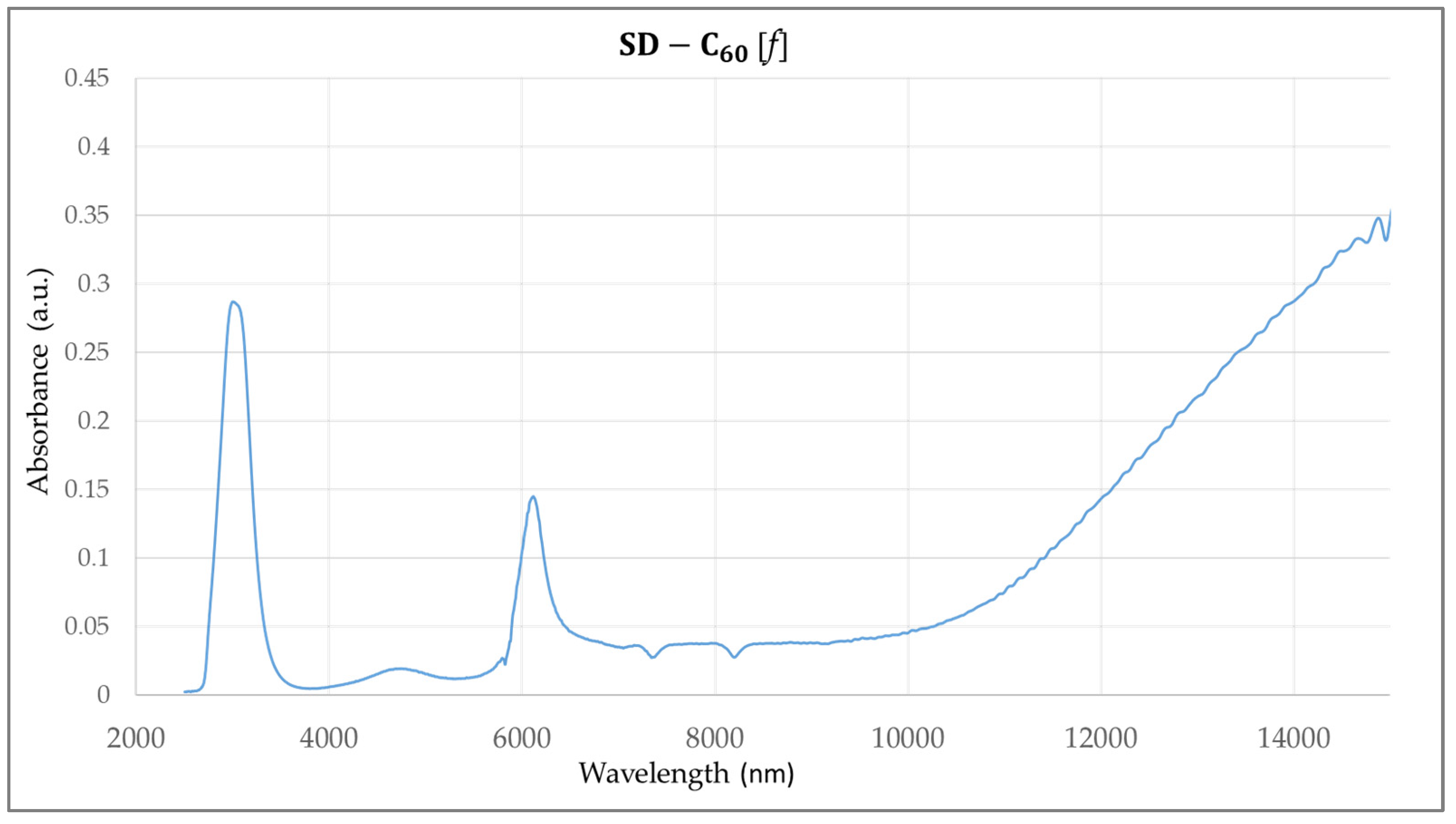
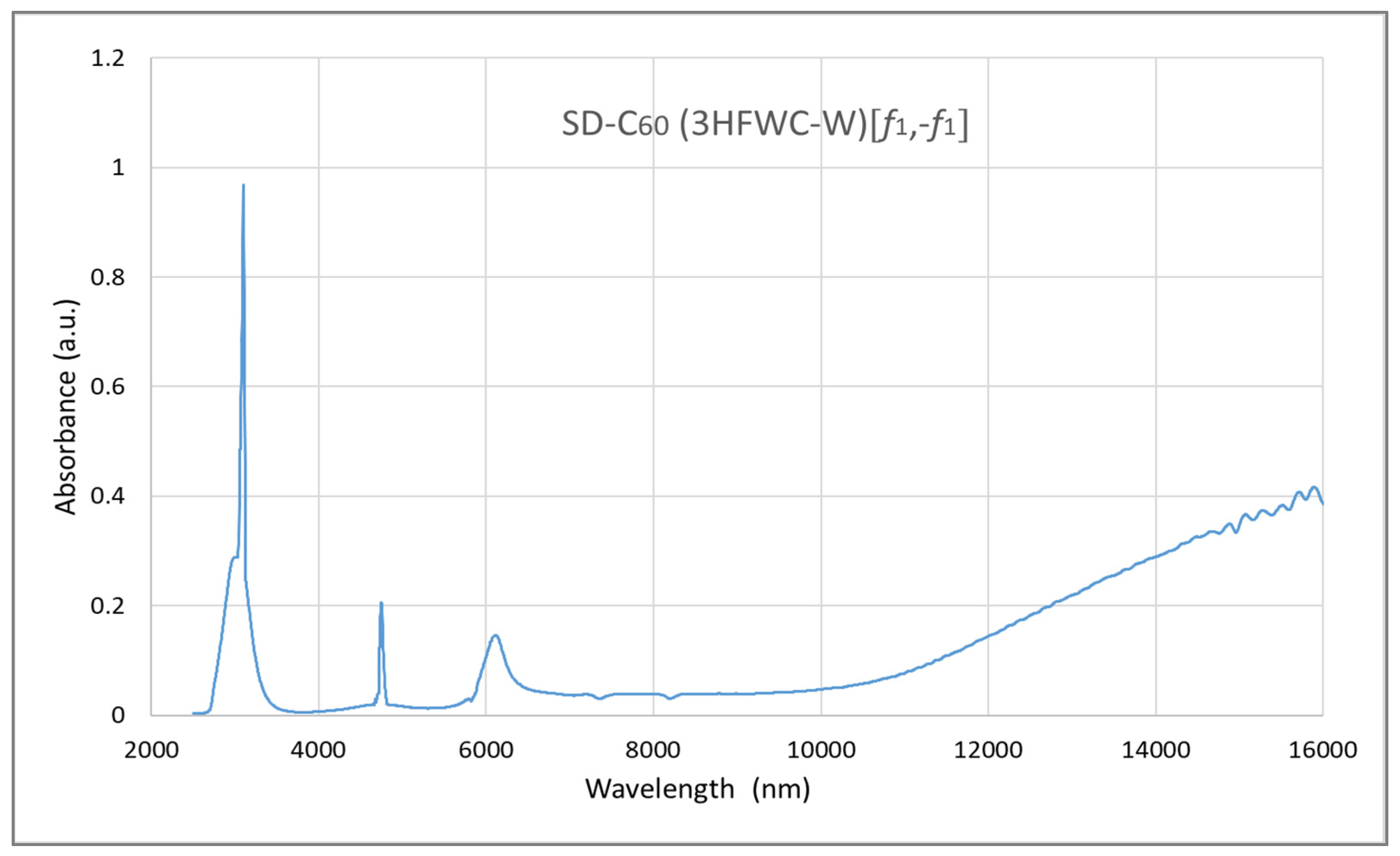
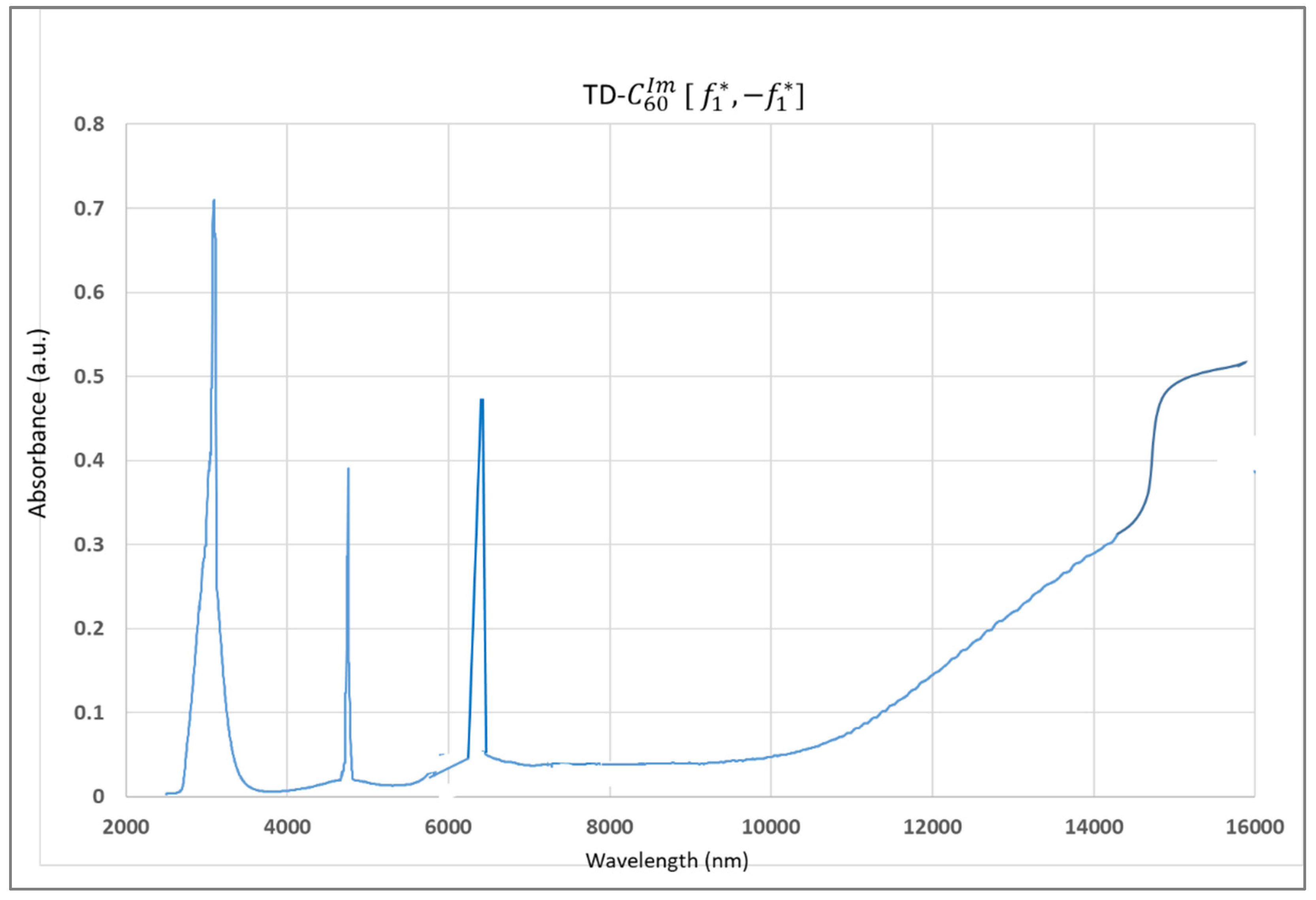
3.3. FTIR Spectra of SD-C60 and TD-C60 Derivatives of C60
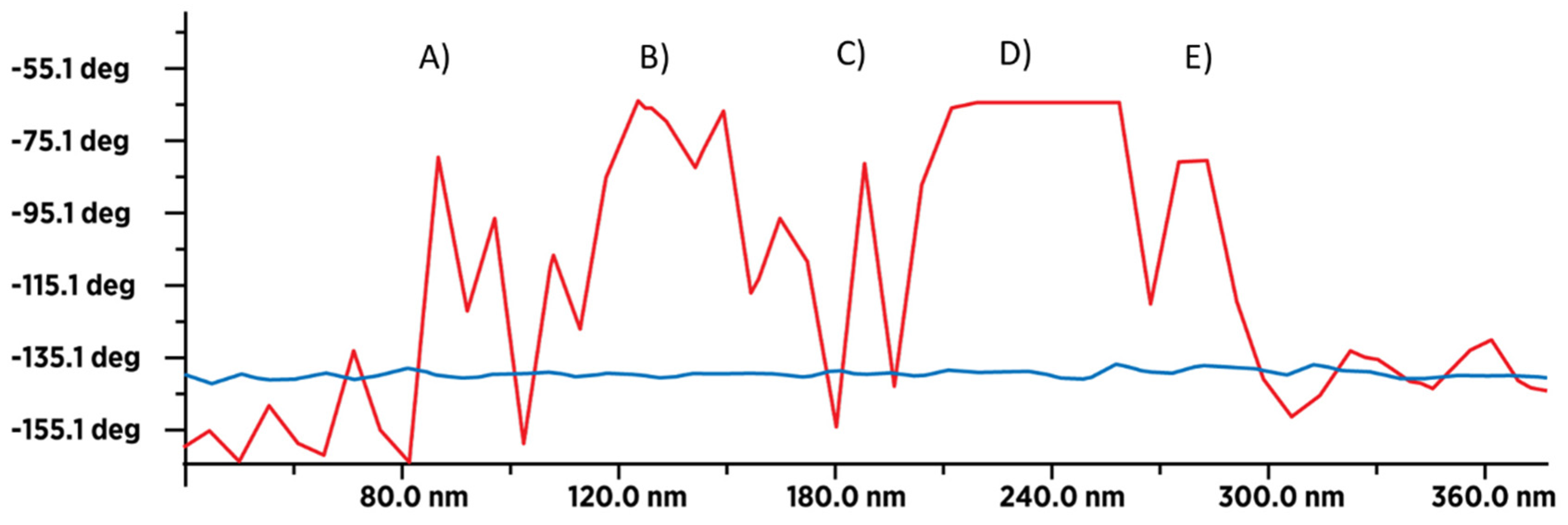
3.4. MFM Spectra of SD-C60 and TD-C60 Derivative of C60
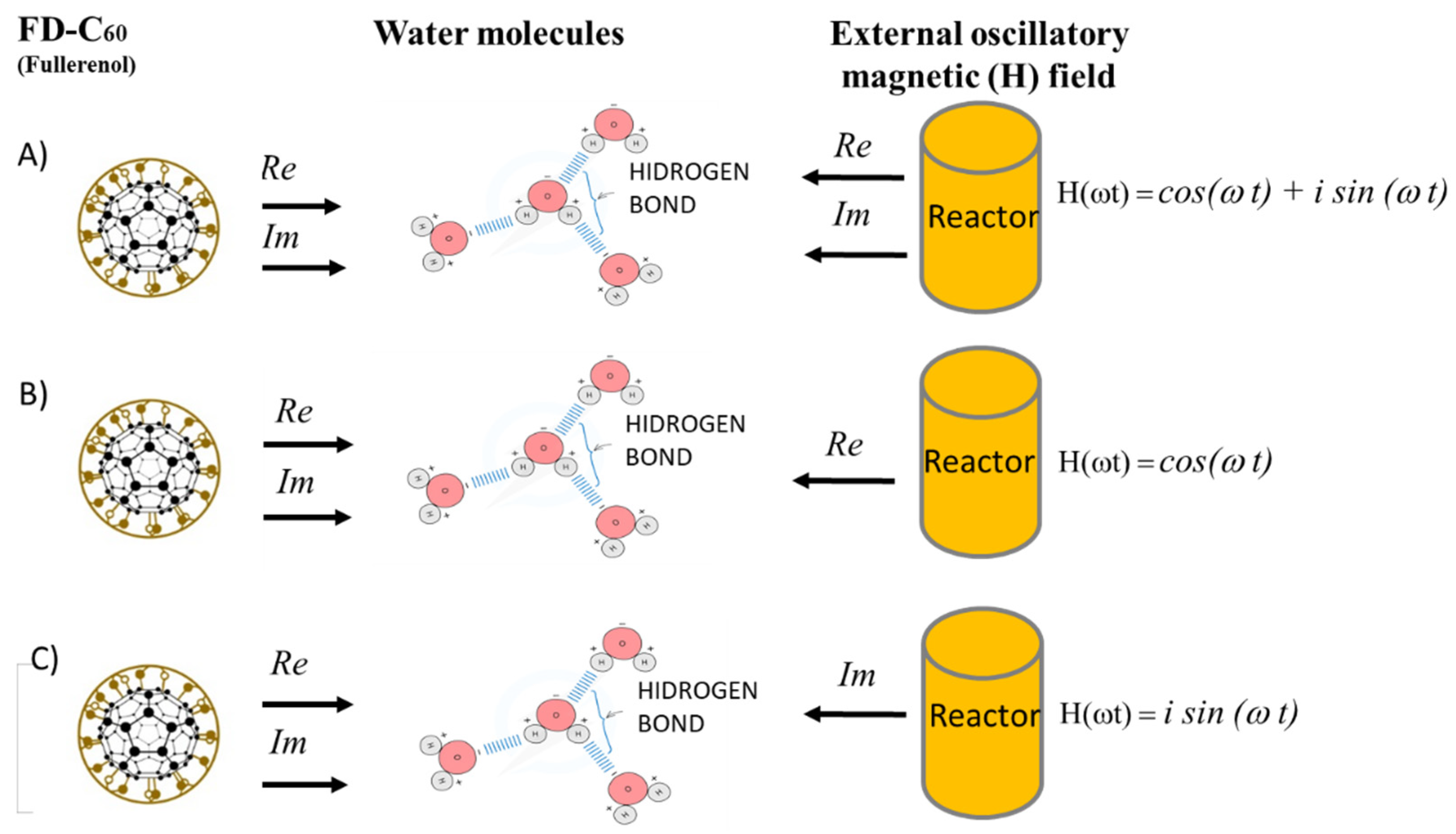
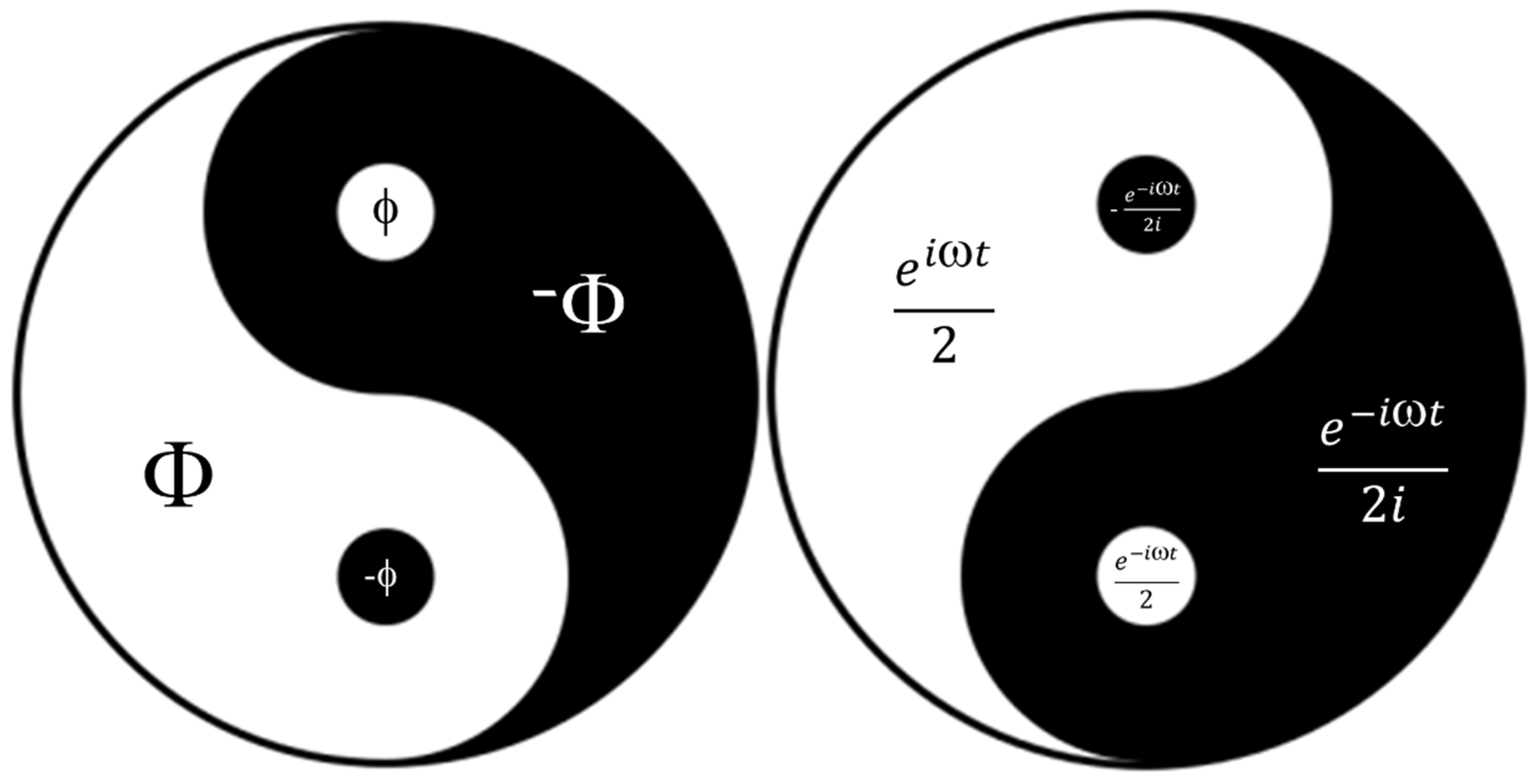
3.5. Yin-Yang Phenomenon of C60 Molecule Derivatives: Quantum-Classical Harmonic (QCH)Substance
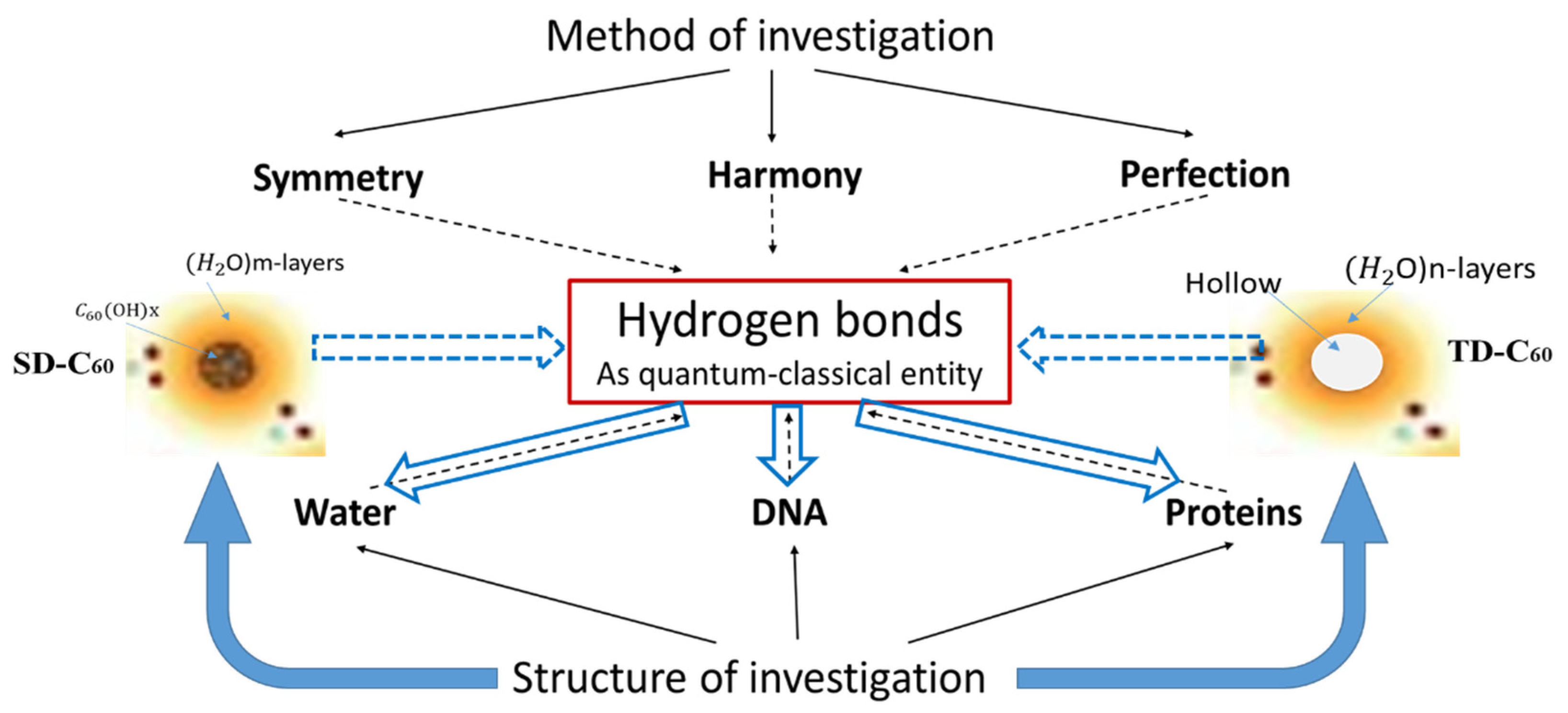
4. Discussion
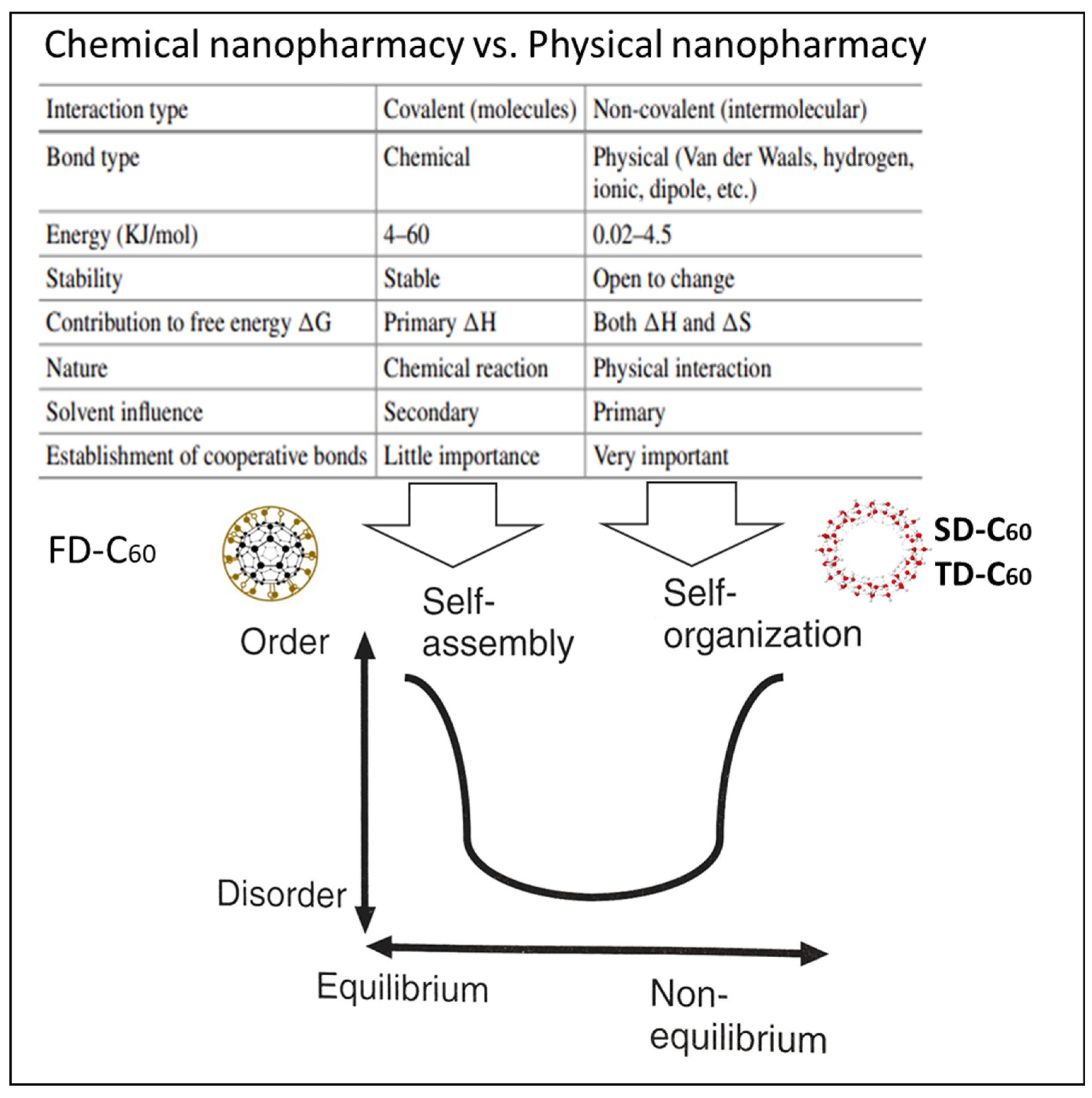
4.1. Physical Nanopharmacy vs. Chemical Nanopharmacy
4.2. Effects of C60 Molecule Derivatives in Biomedical Application
4.3. The Further Direction of Research
5. Conclusions
6. Patents
- Koruga, D. Composition of Matter Containing Harmonized Hydroxyl Modified Fullerene Substance. U.S. Patent 8,058,483 B2, 15 November 2011.
- Koruga, D. Compositions Comprising Hyper Harmonised Hydroxyl Modified Fullerene Substances. International Patent WO 2021/110234 A1, 10 June 2021.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, K.; Mehra, N.K.; Jain, N.K. Potentials and emerging trends in nanopharmacology. Current Opinion in Pharmacology 2014, 15, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Diaz,J.E.M., Nanotechnology in pharmacology: Advances and Applications in Drag Delivery, Joural of Pharmacology and Clinical Research, Mini Reviw, 9(5) JPCR.MS.ID.555773 (2023). [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Upasani, R.B.; Swirczewski, J.W. Process of Forming Polysubstituted. Fullerenes.U.S. Patent 5,177,248, 5 January 1993. [Google Scholar]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, .A.M.; Ausman, K.D.; Tao, .Y.J.; Sitharaman, B.; Wilson, .L.J.; Hughes, .J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Isakovic, A.; Markovic, Z.; Todorovic-Markovic, B.; Nikolic, N.; Vranjes-Djuric, S.; Mirkovic, M.; Dramicanin, M.; Harhaji, L.; Raicevic, N.; Nikolic, Z.; et al. Distinct Cytotoxic Mechanisms of Pristine versus Hydroxylated Fullerene. Toxicol. Sci. 2006, 91, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, G.; Kojić, V.; Đorđević, A.; Čanadanović-Brunet, J.; Vojinović-Miloradov, M.; Baltić, V.V. Modulating activity of fullerol C60(OH)22 on doxorubicin-induced cytotoxicity. Toxicol. Vitr. 2004, 18, 629–637. [Google Scholar] [CrossRef]
- Jiao, F.; Liu, Y.; Qu, Y.; Li, W.; Zhou, G.; Ge, C.; Li, Y.; Sun, B.; Chen, C. Studies on anti-tumor and antimetastatic activities of fullerenol in a mouse breast cancer model. Carbon 2010, 48, 2231–2243. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, F.; Qiu, Y.; Li, W.; Qu, Y.; Tian, C.; Li, Y.; Bai, R.; Lao, F.; Zhao, Y.; et al. Immunostimulatory properties and enhanced TNF- α mediated cellular immunity for tumor therapy by C60(OH)20nanoparticles. Nanotechnology 2009, 20, 415102. [Google Scholar] [CrossRef]
- Yamawaki, H.; Iwai, N. Cytotoxicity of water-soluble fullerene in vascular endothelial cells. Am. J. Physiol. Physiol. 2006, 290, C1495–C1502. [Google Scholar] [CrossRef]
- Johnson-Lyles, D.N.; Peifley, K.; Lockett, S.; Neun, B.W.; Hansen, M.; Clogston, J.; Stern, S.T.; McNeil, S.E. Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010, 248, 249–258. [Google Scholar] [CrossRef]
- Isaacs, E.D.; Shukla, A.; Platzman, P.M.; Hamann, D.R.; Barbiellini, B.; Tulk, C.A. Covalency of the Hydrogen Bond in Ice: A Direct X-Ray Measurement. Phys. Rev. Lett. 1999, 82, 600–603. [Google Scholar] [CrossRef]
- Dresselhous, M.S.; Dresselhous, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Elsevier BV: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Koruga, D. Composition of Matter Containing Harmonized Hydroxyl Modified Fullerene Substance. U.S. Patent 8,058,483 B2, 15 November 2011. [Google Scholar]
- Koruga, D. Compositions Comprising Hyper Harmonised Hydroxyl Modified Fullerene Substances. International Patent WO 2021/110234 A1, 10 June 2021. [Google Scholar]
- Matija, L.; Stanković, I.; Purić, M.; Miličić, M.; Maksimović-Ivanić, D.; Mijatovic, S.; Krajnović, T.; Koruga, D. The Second Derivative of Fullerene C60 (SD-C60) and Biomolecular Machinery of Hydrogen Bonds: Water-Based Nanomedicine. Micromachines 2023, 14, 2152. [Google Scholar] [CrossRef] [PubMed]
- Koruga, D.; Stanković, I.; Matija, L.; Kuhn, D.; Christ, B.; Dembski, S.; Jevtić, N.; Janać, J.; Pavlović, V.; De Wever, B. Comparative Studies of the Structural and Physicochemical Properties of the First Fullerene Derivative FD-C60 (Fullerenol) and Second Fullerene Derivate SD-C60 (3HFWC). Nanomaterials 2024, 14, 480. [Google Scholar] [CrossRef]
- George, J.A. An Introduction to hydrogen bonding; Oxford University Press: New York, 1997. [Google Scholar]
- Koruga, Dj., Qi Engineering: Classical-Quantum Biophysics, Acupuncture and Chaakras, Grafopen, Belgrade, 2024, ISBN 978-86-83615-44-5.
- Markus Arndt, Olaf Nairz, Julian Vos-Andreae, Claudia Keller, Gerbrand van der Zouw & Anton Zeilinger, Wave-particle duality of C60 molecules, Nature, VOL 401, 680-681,1999.
- Laza Kostić, The Basic Principle, The City Library “Karlo Bjelicki”, Sombor, 2015.
- Zia, D.; Dehghan, N.; D’errico, A.; Sciarrino, F.; Karimi, E. Interferometric imaging of amplitude and phase of spatial biphoton states. Nat. Photon- 2023, 17, 1009–1016. [Google Scholar] [CrossRef]
- Mannsoori G.A, Principles of Nanotechnology: Molecular Based Study of Condensed Matter in Small Systems, World Scientific, New Jersey, 2005.
- Malsch, N.H., Biomedical Nanotechnology, CRC Press-Taylor and Francis, Boca Raton, 2005.
- Krishna, V.; Singh, A.; Sharma, P.; Iwakuma, N.; Wang, Q.; Zhang, Q.; Knapik, J.; Jiang, H.; Grobmyer, S.R.; Koopman, B.; et al. Polyhydroxy Fullerenes for Non-Invasive Cancer Imaging and Therapy. Small 2010, 6, 2236–2241. [Google Scholar] [CrossRef]
- Chen, A.; Grobmyer, S.R.; Krishna, V.B. Photothermal Response of Polyhydroxy Fullerenes. ACS Omega 2020, 5, 14444–14450. [Google Scholar] [CrossRef]
- Pickering, K.D.; Wiesner, M.R. Fullerol-Sensitized Production of Reactive Oxygen Species in Aqueous Solution. Environ. Sci. Technol. 2005, 39, 1359–1365. [Google Scholar] [CrossRef]
- Castro, E., A. Hernandez Garcia, G. Zavala, and L. Echegoyen. 2017. ’Fullerenes in Biology and Medicine’, J Mater Chem B, 5: 6523-35.
- Grebowski, J.; Konopko, A.; Krokosz, A.; DiLabio, G.A.; Litwinienko, G. Antioxidant activity of highly hydroxylated fullerene C60 and its interactions with the analogue of α-tocopherol. Free. Radic. Biol. Med. 2020, 160, 734–744. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Fernandes, N.B.; Shenoy, R.U.K.; Kajampady, M.K.; Dcruz, C.E.M.; Shirodkar, R.K.; Kumar, L.; Verma, R. Fullerenes for the treatment of cancer: an emerging tool. Environ. Sci. Pollut. Res. 2022, 29, 58607–58627. [Google Scholar] [CrossRef]
- Markelić, M.; Drača, D.; Krajnović, T.; Jović, Z.; Vuksanović, M.; Koruga, D.; Mijatović, S.; Maksimović-Ivanić, D. Combined Action of Hyper-Harmonized Hydroxylated Fullerene Water Complex and Hyperpolarized Light Leads to Melanoma Cell Reprogramming In Vitro. Nanomaterials 2022, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Markelić, M.; Mojić, M.; Bovan, D.; Jelača, S.; Jović, Z.; Purić, M.; Koruga, D.; Mijatović, S.; Maksimović-Ivanić, D. Melanoma Cell Reprogramming and Awakening of Antitumor Immunity as a Fingerprint of Hyper-Harmonized Hydroxylated Fullerene Water Complex (3HFWC) and Hyperpolarized Light Application In Vivo. Nanomaterials 2023, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Koruga,D., Hyperpolarized Light: Fundamentals of Nanobiomedical Phptonics, Zepter Book World, Belgrade 2018, ISBN:78-86-7494-136-2.
- Hernandez-Segura, A., J. Nehme, and M. Demaria. 2018. ’Hallmarks of Cellular Senescence’, Trends Cell Biol, 28: 436-53.
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013, 75, 685–705. [Google Scholar]
- Collado, M.; Serrano, M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Collado, M.; Serrano, M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Arima, Y.; Nobusue, H.; Saya, H. Targeting of cancer stem cells by differentiation therapy. Cancer Sci. 2020, 111, 2689–2695. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- El-Deiry, W. S., B. Taylor, and J. W. Neal. 2017. ’Tumor Evolution, Heterogeneity, and Therapy for Our Patients With Advanced Cancer: How Far Have We Come?’, Am Soc Clin Oncol Educ Book, 37: e8-e15.
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 1–19. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Ruscetti, M.; Leibold, J.; Bott, M.J.; Fennell, M.; Kulick, A.; Salgado, N.R.; Chen, C.-C.; Ho, Y.-J.; Sanchez-Rivera, F.J.; Feucht, J.; et al. NK cell–mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 2018, 362, 1416–1422. [Google Scholar] [CrossRef]
- Kang, T.-W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Ruscetti, M.; Morris, J.P.; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.-J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2020, 181, 424–441.e21. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Vareki, S.M. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157. [Google Scholar] [CrossRef]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef]
- Kotsafti, A.; Scarpa, M.; Castagliuolo, I.; Scarpa, M. Reactive Oxygen Species and Antitumor Immunity—From Surveillance to Evasion. Cancers 2020, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Stepwise oscillatory circuits of a DNA molecule. J. Biol. Phys. 2009, 35, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.D.; Yannoni, C.S.; Dorn, H.C.; Salem, J.R.; Bethune, D.S. C 60 Rotation in the Solid State: Dynamics of a Faceted Spherical Top. Science 1992, 255, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Matija, L.R.; Stankovic, I.M.; Puric, M.; Miličić, M.; Maksimović-Ivanić, D.; Mijatovic, S.; Krajnović, T.; Gordic, V.; Koruga, D.L. The Second Derivative of Fullerene C60 (SD-C60) and Biomolecular Machinery of Hydrogen Bonds: Water-Based Nanomedicine. Micromachines 2023, 14, 2152. [Google Scholar] [CrossRef]
- Kettle, S.F.A., Symmetry and structure, John Willey and Sons, Chichester, 1995.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



