1. Introduction
In recent years, saponins have gained significant attention across various fields [
1,
2,
3,
4,
5,
6], particularly in nutraceutical research and food technology [
1,
7,
8,
9,
10]. These naturally occurring glycosides, characterized by their amphiphilic nature, possess a wide range of bioactivities [
11,
12,
13,
14,
15], making them ideal candidates for human health applications, both as therapeutic agents as well as functional foods and dietary supplements. Their intricate molecular structure, composed of hydrophobic aglycone backbones and hydrophilic sugar moieties, enables them to interact with biological membranes [
16,
17,
18,
19], thereby modulating physiological processes. These interactions translate into diverse pharmacological properties, including cholesterol management [
11,
20], immune modulation [
21], and cancer prevention [
20,
22]. All these uses are well-documented in both traditional medicine and modern scientific studies. However, the specific molecular mechanisms by which saponins exert their bioactivity—particularly in relation to receptor interactions and signal transduction pathways—are still areas of active research.
This review focuses on the growing potential of marine-derived saponins, particularly from species like sea cucumbers [
23] and starfish [
15], which possess distinct structural characteristics, such as sulfate groups, that clearly differentiate them from their terrestrial counterparts. These unique features also contributes to the above mentioned bioactivities and beneficial health uses. While the study of phytochemical saponins [
2,
21] has greatly advanced our understanding of saponin bioactivity, marine saponins are still underexplored, particularly at the molecular level. Potential mechanisms, such as interactions with lipid bilayers and cellular receptors, remain less characterized, and further research is needed to elucidate their structure-activity relationships. Techniques such as molecular docking [
24,
25,
26,
27,
28] and machine learning models for molecular parameter and bioactivities predictions [
29,
30,
31,
32] could provide valuable insights into these interactions, offering promising directions for future studies.
One of the primary challenges in harnessing the full potential of marine saponins lies in overcoming technical barriers related to extraction [
33], stability [
34], bioavailability [
7], and safety [
35]. Traditional extraction methods are widely used [
36,
37,
38], but advanced techniques such as supercritical fluid extraction [
39,
40], ultrasonic-assisted extraction, and microwave-assisted extraction [
33,
41,
42] are emerging as more sustainable and efficient alternatives. Additionally, the amphiphilic nature of saponins presents challenges related to their solubility and stability in biological systems, particularly in gastrointestinal environments [
7]. Recent advancements in encapsulation and nanotechnology-based delivery systems have shown promise in improving the bioavailability [
43,
44,
45,
46] and controlled release of saponins [
47], making them more viable for therapeutic and functional food applications.
Furthermore, the increasing demand for sustainable marine resources, making the best and full use of them in a circularity framework, has prompted efforts to exploit marine-derived compounds [
48,
49], such as saponins, in ways that balance ecological concerns with commercial viability. The sustainable sourcing and production of marine saponins present both opportunities and challenges for the nutraceutical and food technology sectors. Regulatory frameworks and market dynamics also play a crucial role in shaping the incorporation of these compounds into consumer products, influencing both their development and market penetration.
Through this comprehensive review, we aim to shed light on the latest advances in the application of marine saponins in nutraceuticals and functional foods, identifying key challenges and future research directions. By fostering a deeper understanding of the structural, functional, and technological attributes of marine saponins, including the potential integration of computational tools like machine learning for improved molecular parameter estimation, this review seeks to catalyze innovations that will contribute to better customers health outcomes and the sustainable development of saponin-based products.
2. Physico-Chemical, Molecular, and Biochemical Properties of Saponins
2.1. Structure and Classification
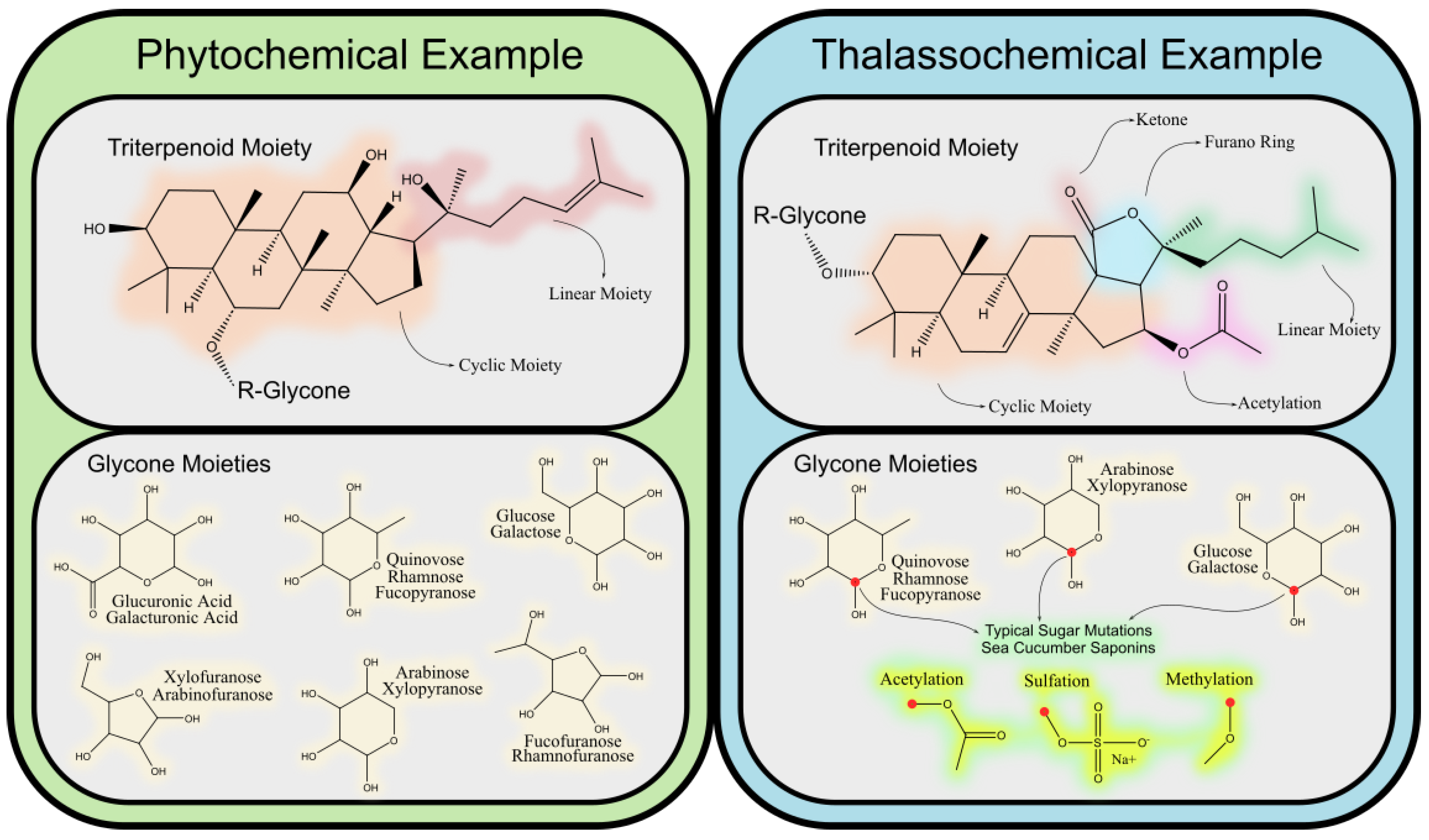
Saponins are a diverse group of glycosides characterized by their amphiphilic nature, consisting of a hydrophobic aglycone backbone (sapogenin) linked to one or more hydrophilic sugar chains. This amphiphilicity, with water- and lipid-soluble regions, is essential for their biological functionality, particularly in their interactions with cell membranes. The chemical structure of the aglycone, whether triterpenoid or steroidal, forms the basis for classifying saponins into two main groups. Both types are found in terrestrial and marine organisms.
Figure 1.
Structural classification of saponins, highlighting key differences between triterpenoid, steroidal, and marine-derived saponins.
Figure 1.
Structural classification of saponins, highlighting key differences between triterpenoid, steroidal, and marine-derived saponins.
Triterpenoid saponins, predominantly found in plant families such as
Sapindaceae [
50],
Fabaceae [
51], and
Asteraceae [
52,
53], display significant structural diversity due to variations in oxidation, glycosylation, and acylation. The oxidation of hydroxymethyl groups in the sugar moieties, often leading to uronic acid formation, enhances solubility and reactivity [
54]. These modifications play a crucial role in their interaction with biological membranes and their bioactive properties, contributing to plant defense against pathogens and clearly defining potential pharmacological capabilities, such as anti-inflammatory and cholesterol-lowering activities.
Steroidal saponins, primarily from plant families like
Liliaceae [
55,
56] and
Dioscoreaceae [
56,
57], share structural similarities with steroid hormones, owing to their 27-carbon skeleton. This resemblance to cholesterol underlies their ability to modulate cholesterol metabolism and absorption, contributing to their hypocholesterolemic [
58] and cardioprotective [
59] effects.
Marine-derived saponins, such as those from sea cucumbers and starfish, exhibit unique structural characteristics that distinguish them from plant-derived saponins. The incorporation of sulfate groups and rare sugars, such as 4-O-methyl-rhamnose and sulfated fucose, is a hallmark of marine saponins, contributing to their solubility and bioactivity. Sulfation [
60,
61], in particular, enhances their amphiphilicity and plays a critical role in their potent cytotoxic, immunomodulatory, and antimicrobial properties. For instance, holothurins from sea cucumbers have demonstrated strong cytotoxic effects [
62,
63], making them candidates for anticancer therapies.
Although saponins are mainly studied in plants and marine organisms, they have also been identified in some other kingdoms, such as fungi [
64], where they are thought to serve defensive functions. However, research into fungi-derived saponins is still underexplored.
In summary, the structural diversity of saponins, driven by factors such as glycosylation, oxidation, and sulfation, directly influences their bioactivity and functionality. Marine saponins stand out due to their unique modifications that enhance solubility and bioactivity, making them valuable for both ecological and biomedical applications.
2.2. Biosynthesis and Bioactivities
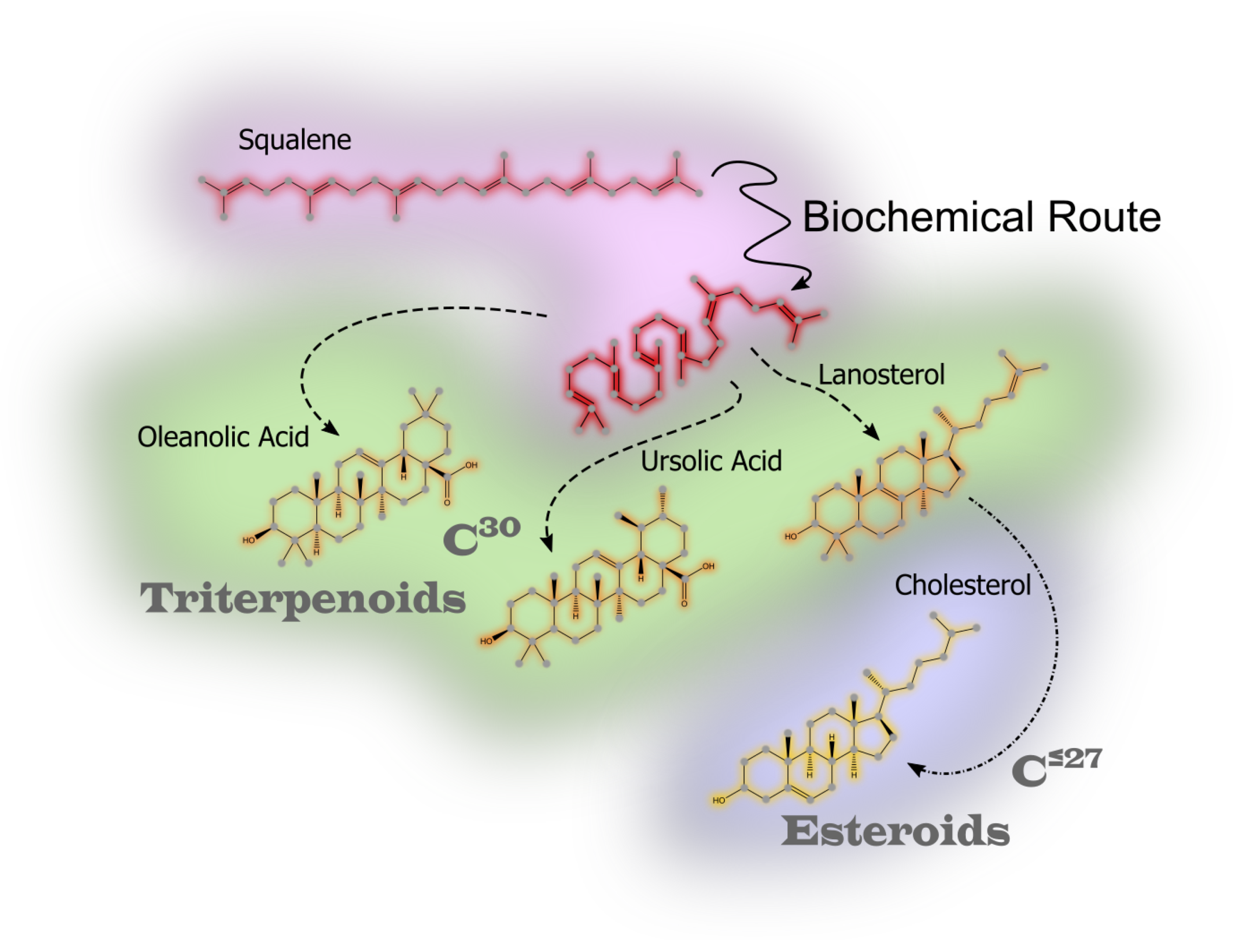
Saponins are synthesized through complex biochemical pathways, initiated by the cyclization of 2,3-oxidosqualene [
65,
66]. In plants, this process could follows the mevalonate pathway [
67,
68], leading to the formation of triterpenoid or steroidal sapogenins. Oxidation during biosynthesis, particularly in plant saponins, often results in the formation of uronic acids, enhancing solubility and enabling stronger interactions with biological targets, such as cell membranes and pathogens.
In marine organisms, saponin biosynthesis follows similar early steps but diverges significantly in later modifications, with sulfation being a key enzymatic process [
69]. This sulfation enhances the amphiphilic properties of marine saponins, allowing them to function effectively in saline environments. Sulfated marine saponins exhibit potent cytotoxic, antimicrobial, and immunomodulatory activities, likely shaped by harasing conditions of marine ecosystems.
Figure 2.
Biosynthetic pathways of saponins in terrestrial and marine organisms, illustrating enzymatic modifications that contribute to their bioactivity.
Figure 2.
Biosynthetic pathways of saponins in terrestrial and marine organisms, illustrating enzymatic modifications that contribute to their bioactivity.
The bioactivity of saponins stems largely from their ability to interact with cell membranes, where they disrupt lipid bilayers [
70], increasing membrane permeability and leading to cell lysis in microbial pathogens [
71]. In higher organisms, saponins modulate membrane fluidity and influence membrane protein function, contributing to cholesterol-lowering and immune-stimulating effects. In fact, also a notable role in stem cell differentiation has been shown.
The distinct modifications seen in marine saponins, such as sulfation, result in bioactivities that are less common in terrestrial saponins. These differences have significant implications for their therapeutic potential, particularly in cancer treatment, where marine saponins like holothurins have shown promising cytotoxic effects [
72]. As research continues, these unique structural modifications may unlock new avenues for biotechnological applications.
In summary, the structural complexity and diverse bioactivities of saponins are closely linked to their biosynthetic pathways and modifications. Marine saponins, with their unique sulfated structures, offer significant advantages in bioactivity and therapeutic potential, particularly in nutraceutical and pharmaceutical development.
2.3. Structural Trends and Their Implications for Computational Modeling
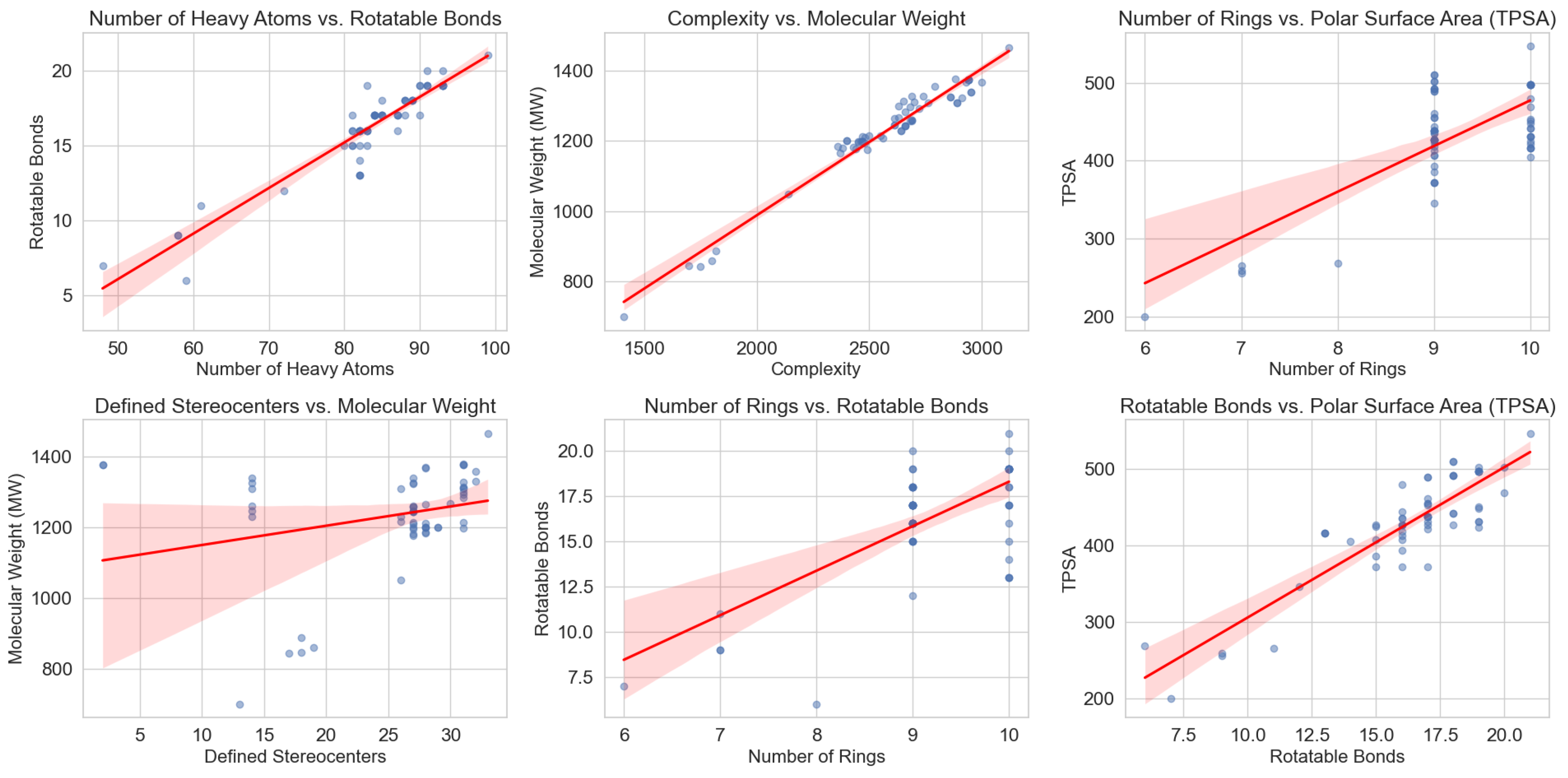
The structural complexity of saponins, particularly their glycosylation patterns and amphiphilic nature, directly influences their physicochemical properties and bioactivities. To further explore these relationships, we analyzed a curated dataset of saponins extracted from PubChem (National Center for Biotechnology Information, NCBI), applying systematic filtering criteria to ensure structural consistency. The dataset selection process prioritized molecules featuring triterpenoid or steroidal backbones, including glycosylated derivatives, and was subsequently refined to exclude multi-component entries while retaining those with specific functional moieties, such as sulfate groups or -lactone (O=C1CCCO1) motifs.
The dataset was constructed by retrieving molecular structures from PubChem that contained at least one of the following core SMILES: C12CCCC1CCC3C2CCC4C3CCCC4, C12CCCC1CCC3C2=CCC4C3CCCC4, or C12CCCC1CC=C3C2CCC4C3CCCC4, ensuring the inclusion of triterpenoid and steroidal scaffolds commonly found in saponins. Entries containing multiple molecules in a single record were excluded unless they only consisted of a primary molecule and a simple counterion (e.g., Na+ or Ca2+, thus maintaining consistency in molecular representation. Further refinement steps included filtering for molecules containing at least one sugar moiety, and in a more restrictive subset, selecting only those with at least one sulfate or oxofuranose group.
Graphical analysis of key molecular descriptors revealed notable trends among saponins. These trends were initially analyzed for the full dataset (
Figure 3), which includes both marine and terrestrial saponins, and subsequently examined in a subset restricted to marine saponins (
Figure 4). The comparison of both datasets highlights that the same structural patterns emerge in marine saponins, although with lower data density due to the limited number of marine-specific saponins currently annotated in PubChem.
One of the most evident relationships is the correlation between the number of rotatable bonds (NER) and the number of rings (NA). Initially, an increase in the number of rings leads to a greater number of flexible linkages, particularly in glycosylated derivatives where sugar moieties contribute to conformational flexibility. However, beyond a certain threshold, this trend stabilizes or even decreases, likely due to the structural constraints imposed by highly interconnected ring systems. In highly fused polycyclic systems, the molecular framework becomes more rigid, limiting additional torsional flexibility.
A similar pattern is observed in the relationship between topological polar surface area (TPSA) and the number of rings. Initially, TPSA increases linearly with ring count, reflecting the addition of polar glycosyl groups. However, at higher ring counts, this trend stabilizes or slightly declines. A plausible explanation is that steric interactions limit the exposure of polar functional groups, reducing the effective polar surface area available for solvent interactions. This behavior suggests that beyond a certain level of glycosylation, additional sugar units may not contribute proportionally to TPSA, potentially affecting solubility and membrane interactions.
From a bioactivity standpoint, TPSA plays a crucial role in determining water solubility, which directly affects both bioavailability and membrane interactions. The observed trend supports the hypothesis that an optimal degree of glycosylation may exist for achieving maximal amphiphilicity, which is critical for the detergent-like behavior of saponins. This property is directly linked to their role in disrupting lipid membranes, a mechanism of action underlying their antimicrobial and immunomodulatory effects.
As mentioned, the overall structural trends are conserved in marine saponins (
Figure 4), although the lower number of available structures results in a less pronounced representation.
Given the complexity of these structural relationships, machine learning models represent a promising avenue for predicting saponin bioactivities based on molecular descriptors. By integrating data-driven approaches with structure-activity relationship (SAR) analyses, it may be possible to refine the selection of saponins for specific therapeutic or nutraceutical applications. Future studies should focus on training graph neural networks (GNNs) to recognize structural patterns that correlate with desirable properties, leveraging the trends identified in this dataset as foundational insights for model optimization.
3. Saponins in Pharma/Nutraceuticals and Food Technology
3.1. Role of Saponins as Nutraceuticals
Saponins, with their diverse structural configurations and broad spectrum of bioactivities, have emerged as valuable compounds in the development of new nutraceuticals and functional foods. Their ability to modulate physiological processes underlies their well-documented hypocholesterolemic, anticarcinogenic, hepatoprotective, hypoglycemic, immunomodulatory, neuroprotective, anti-inflammatory, and antioxidant effects [
73,
74,
75]. These properties support their integration into formulations for both daily supplementation and targeted therapeutic interventions as well.
3.1.1. Bioactivities of Saponins
Hypocholesterolemic Activity: Saponins reduce cholesterol absorption in the digestive tract by forming insoluble saponin-cholesterol complexes, which facilitate excretion. This mechanism is well-studied in terrestrial sources such as fenugreek (
Trigonella foenum-graecum) [
73] and soybeans (
Glycine max) [
76]. Marine-derived saponins, including asterosaponins from starfish [
77], exhibit similar lipid-modulating properties, making them promising candidates for managing hypercholesterolemia and cardiovascular diseases.
Anticarcinogenic Activity: Saponins exhibit anticancer potential by inhibiting tumor growth, inducing apoptosis, and suppressing metastasis through key signaling pathways such as PI3K/AKT and NF-
B [
78]. Studies on
Weeping Pittosporum or
Pittosporum angustifolium and marine saponins from sea cucumbers highlight their strong cytotoxic effects, supporting further research into their role in cancer prevention and therapy.
Immunomodulatory and Anti-inflammatory Activities: Saponins modulate immune responses by activating antigen-presenting cells, such as dendritic cells and macrophages, enhancing their potential as vaccine adjuvants.
Quillaja saponaria-derived saponins are widely used in immunostimulating complexes (ISCOMs) [
79,
80,
81] to improve antigen delivery. Additionally, saponins suppress inflammatory pathways by inhibiting cytokines such as TNF-
and IL-6 [
82], positioning marine saponins as potential candidates for inflammatory disease treatment and vaccine development.
Hypoglycemic Activity: Saponins contribute to glucose regulation by inhibiting intestinal glucose absorption and modulating hepatic glucose metabolism. Extracts from
Gymnema sylvestre and marine saponins have demonstrated efficacy in lowering blood glucose levels, making them promising natural agents for hyperglycemia and diabetes management [
83].
Antioxidant Activity: By scavenging free radicals and enhancing endogenous antioxidant defenses, saponins help mitigate oxidative stress, a major contributor to chronic conditions such as cardiovascular and neurodegenerative diseases [
84]. These properties reinforce their role in disease prevention and overall health maintenance.
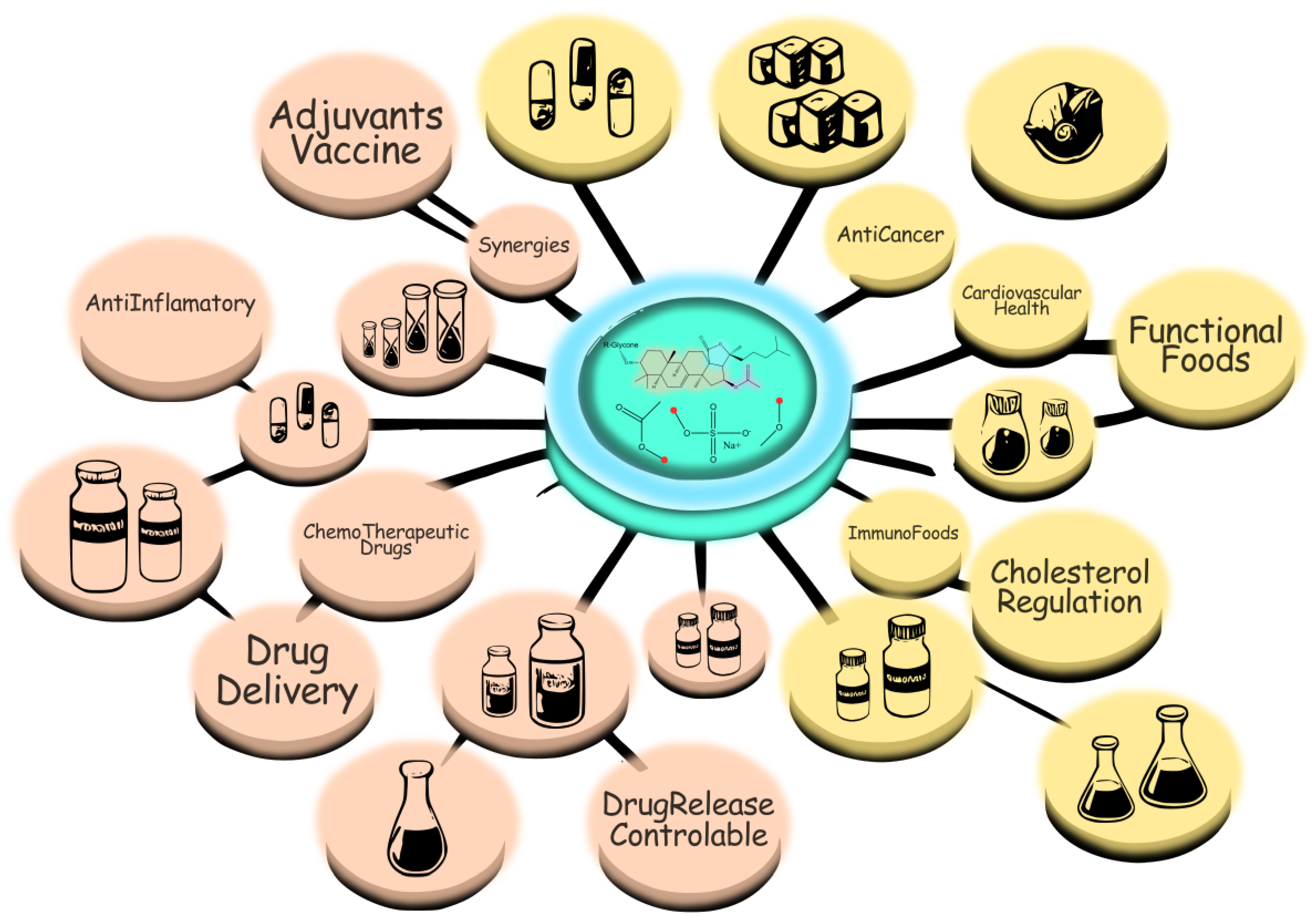
Figure 5.
Saponin structures and their bioactivities, illustrating interactions with cellular components and major therapeutic targets.
Figure 5.
Saponin structures and their bioactivities, illustrating interactions with cellular components and major therapeutic targets.
3.1.2. Saponins as Nutraceuticals
Leveraging their physiological effects, saponins have been integrated into functional food formulations to enhance health benefits, particularly in cardiovascular, metabolic, and immune-related applications.
Application in Functional Foods: The integration of saponins into functional foods offers a practical approach to leveraging their bioactive properties in daily nutrition [
85]. Advances in food technology have enabled their incorporation into diverse products such as beverages, dairy alternatives, cereals, and protein bars. These formulations contribute to cardiovascular support, immune modulation, and inflammation control, aligning with the growing demand for health-promoting dietary options.
Enhancing Bioavailability: A major challenge in saponin utilization is their low bioavailability, primarily due to poor solubility and instability in the gastrointestinal tract [
86]. To address this, novel delivery systems such as nanoparticle encapsulation, liposomes, and solid lipid nanoparticles (SLN) have been developed to protect saponins from enzymatic degradation and improve absorption, ensuring sustained bioactivity and effectiveness [
87].
Synergistic Effects with Other Nutraceuticals: Saponins enhance the efficacy of other bioactive compounds when used in combination. Their co-administration with polyphenols, flavonoids, and antioxidants in multi-component formulations has shown synergistic effects, particularly in anti-inflammatory, cardioprotective, and anticancer applications, broadening the scope of nutraceutical innovation [
88,
89].
Safety and Efficacy Studies: Despite promising preclinical findings, comprehensive clinical trials are crucial for validating saponin health benefits and establishing safety guidelines. This is particularly relevant for marine saponins, which may exhibit distinct pharmacokinetics compared to terrestrial saponins. Long-term studies and precise dosage determinations are essential for regulatory approval and consumer acceptance.
Sustainable Sourcing and Biodiversity: The growing demand for saponins necessitates sustainable sourcing strategies. Ethical, sustainable harvesting and controlled cultivation of saponin-producing plants and marine organisms are critical for preserving biodiversity. The exploration of marine biodiversity offers potential for discovering novel saponins with unique bioactivities, while biotechnological approaches could enable sustainable production, supporting global sustainability goals.
In summary, saponins are pivotal in nutraceutical and functional food applications due to their diverse bioactivities. Advances in bioavailability enhancement and sustainability practices will shape the future of saponin-based products, ensuring their continued role in promoting health and disease prevention. Future research should prioritize synergistic formulations, clinical validation, and sustainable sourcing to maximize their impact on health and nutrition.
4. Saponin Extraction, Stability, and Bioavailability
Despite their promising bioactivities, saponins industrial use face challenges related to stability and bioavailability, which must be addressed to maximize their therapeutic potential.
4.1. Extraction Methods and Challenges
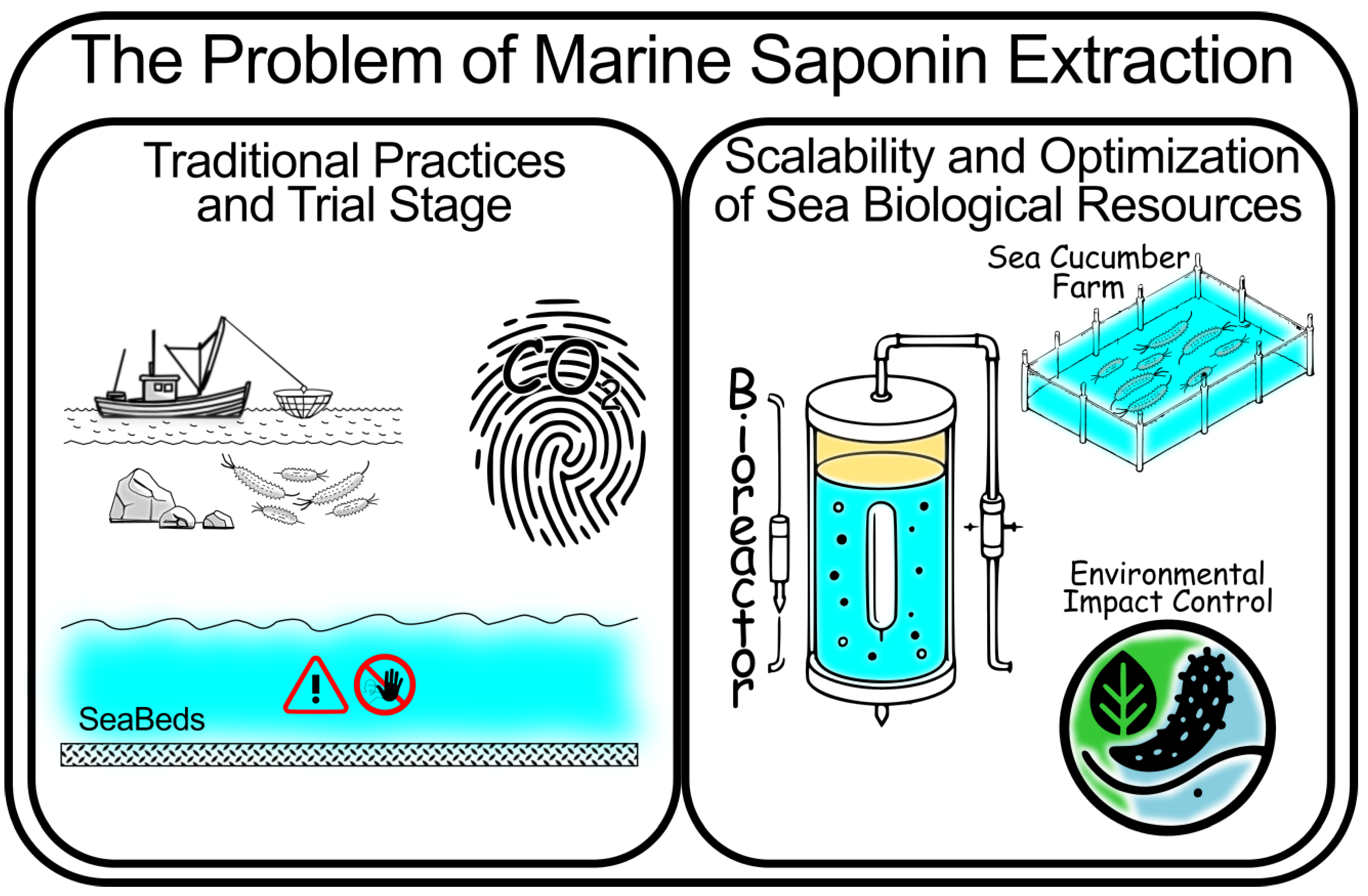
The extraction of saponins from terrestrial and marine organisms presents significant challenges, particularly regarding yield, purity, and environmental sustainability. Traditional methods such as maceration and solvent extraction have been widely used but are often hampered by long processing times, high solvent consumption, and the risk of degrading sensitive bioactive compounds. As well as problems derived from the generation and management of organic solvent wastes or sidestreams. These challenges are particularly acute for marine saponins, where maintaining the integrity of delicate structures, such as sulfated sugar moieties, is essential for preserving bioactivity.
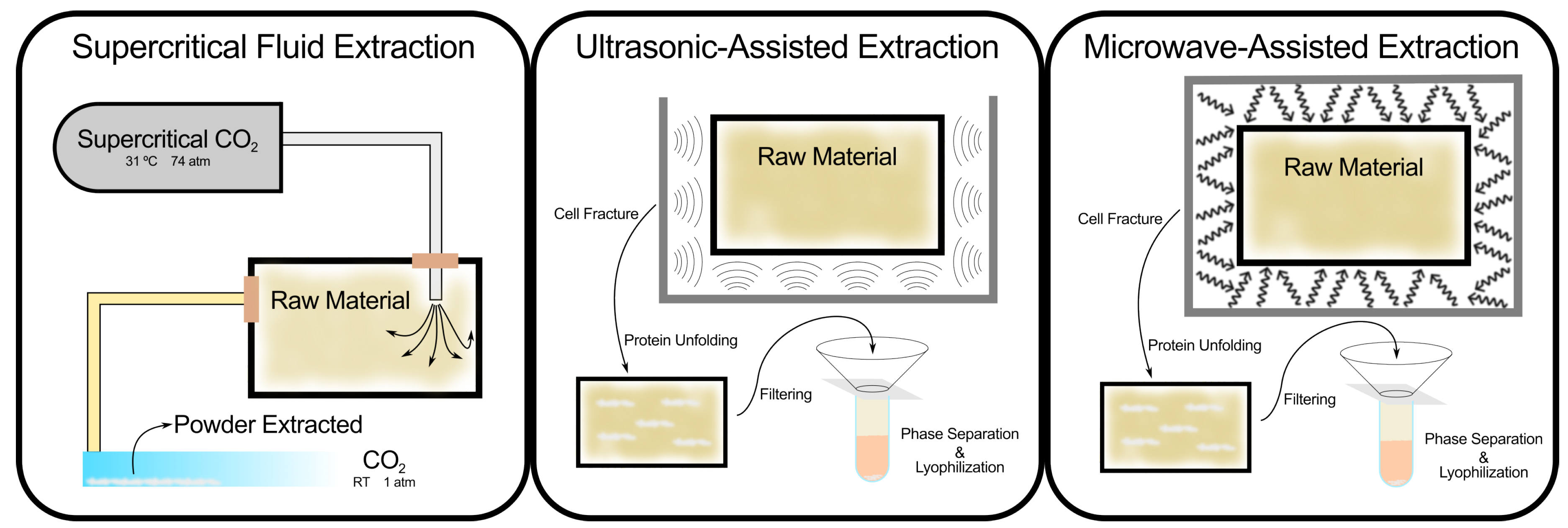
Recent advancements in extraction technologies have addressed some of these limitations. Supercritical fluid extraction (SFE) [
90,
91] using supercritical CO
2, for instance, allows for the extraction of bioactive compounds with high purity and minimal environmental impact. This method is particularly suited for marine saponins, which may be more sensitive to heat and solvents. SFE not only eliminates the need for organic solvents but also preserves the structural integrity of thermolabile compounds. Other techniques, such as ultrasonic-assisted extraction [
90,
92] (UAE) and microwave-assisted extraction [
93] (MAE), improve efficiency by reducing extraction times and increasing yields. UAE uses ultrasonic waves to disrupt cell walls, facilitating the release of saponins, while MAE accelerates solvent penetration and recovery of saponins through microwave radiation.
Figure 6.
Extraction techniques for saponins, comparing conventional and advanced methods, including supercritical fluid extraction, ultrasonic-assisted extraction, and microwave-assisted extraction.
Figure 6.
Extraction techniques for saponins, comparing conventional and advanced methods, including supercritical fluid extraction, ultrasonic-assisted extraction, and microwave-assisted extraction.
Advanced methods such as pressurized liquid extraction (PLE) and ultrahigh-pressure extraction (UPE) have shown potential in optimizing the extraction of saponins while preserving their structure [
90]. Pressurized Liquid Extraction (PLE), also known as accelerated solvent extraction (ASE), is a technique used to extract compounds from solid or semi-solid samples using solvents at high temperatures (50–200°C) and pressures (1,500–3,000 psi). The elevated conditions enhance solvent penetration, reduce extraction time, and increase efficiency by improving solubility and diffusion of saponins. Ultrahigh-pressure extraction (UHPE) is a technique that uses extremely high pressures (typically above 100 MPa) to break cell structures and enhance the extraction of bioactive compounds, such as saponins. This method improves yield, reduces solvent use, and preserves heat-sensitive compounds by minimizing thermal degradation, making it a sustainable alternative to conventional extraction techniques.
Optimizing extraction parameters—such as solvent choice, temperature, pressure, and time—is essential for maximizing yield while preserving bioactivity. Additionally, environmental sustainability must be prioritized, particularly in marine ecosystems where overexploitation could have significant ecological impacts. Future research should focus on scaling up these advanced extraction techniques while minimizing their environmental footprint, especially when sourcing marine organisms are used as raw materials to obtain saponins.
4.2. Stability and Bioavailability
The stability and bioavailability of saponins are critical factors that determine their efficacy in nutraceuticals and therapeutics. Saponins are prone to degradation under environmental factors such as light, oxygen, and heat, which can significantly reduce their bioactivity [
94]. Furthermore, their variable water solubility and limited gastrointestinal absorption present significant challenges for bioavailability.
4.2.1. Enhancing Stability
Various strategies have been employed to enhance the stability of saponins during processing and storage. The use of antioxidants, such as ascorbic acid and tocopherols, can protect saponins from oxidative degradation [
95]. Cryopreservation and lyophilization (freeze-drying) have also been adopted to stabilize saponins by removing moisture, preventing both hydrolytic and oxidative degradation. These methods are particularly valuable for saponins intended for pharmaceutical and nutraceutical applications, where maintaining bioactivity over extended periods is essential [
96?].
In addition to antioxidant protection, storing saponins in inert atmospheres (e.g. nitrogen or argon) can minimize oxidation and help preserve their stability. Advances in encapsulation technologies, discussed below, also provide a means to protect saponins from environmental degradation by creating a physical barrier that shields them from harmful light, oxygen, and temperature fluctuations.
4.2.2. Improving Bioavailability: Conflicting Evidence and Future Direction
Improving the bioavailability of saponins has been a major focus of recent research [
97,
98,
99,
100,
97]. While many saponins are inherently limited by their poor solubility in water, marine-derived saponins stand out for their notable water solubility. This increased solubility has been attributed, as already mentioned in this work, to the presence of sulfate groups or other hydrophilic moieties, which enhance their bioavailability and play a crucial role in their ecological function as toxins against predators [
101]. To this aim, micronization [
97,
98,
102], which reduces particle size, has enhanced solubility by increasing surface area, thereby improving the dissolution rate and absorption of less soluble saponins in the gastrointestinal tract.
Figure 7.
Encapsulation strategies to enhance the stability and bioavailability of saponins, including nanoemulsions, liposomes, and polymeric nanoparticles.
Figure 7.
Encapsulation strategies to enhance the stability and bioavailability of saponins, including nanoemulsions, liposomes, and polymeric nanoparticles.
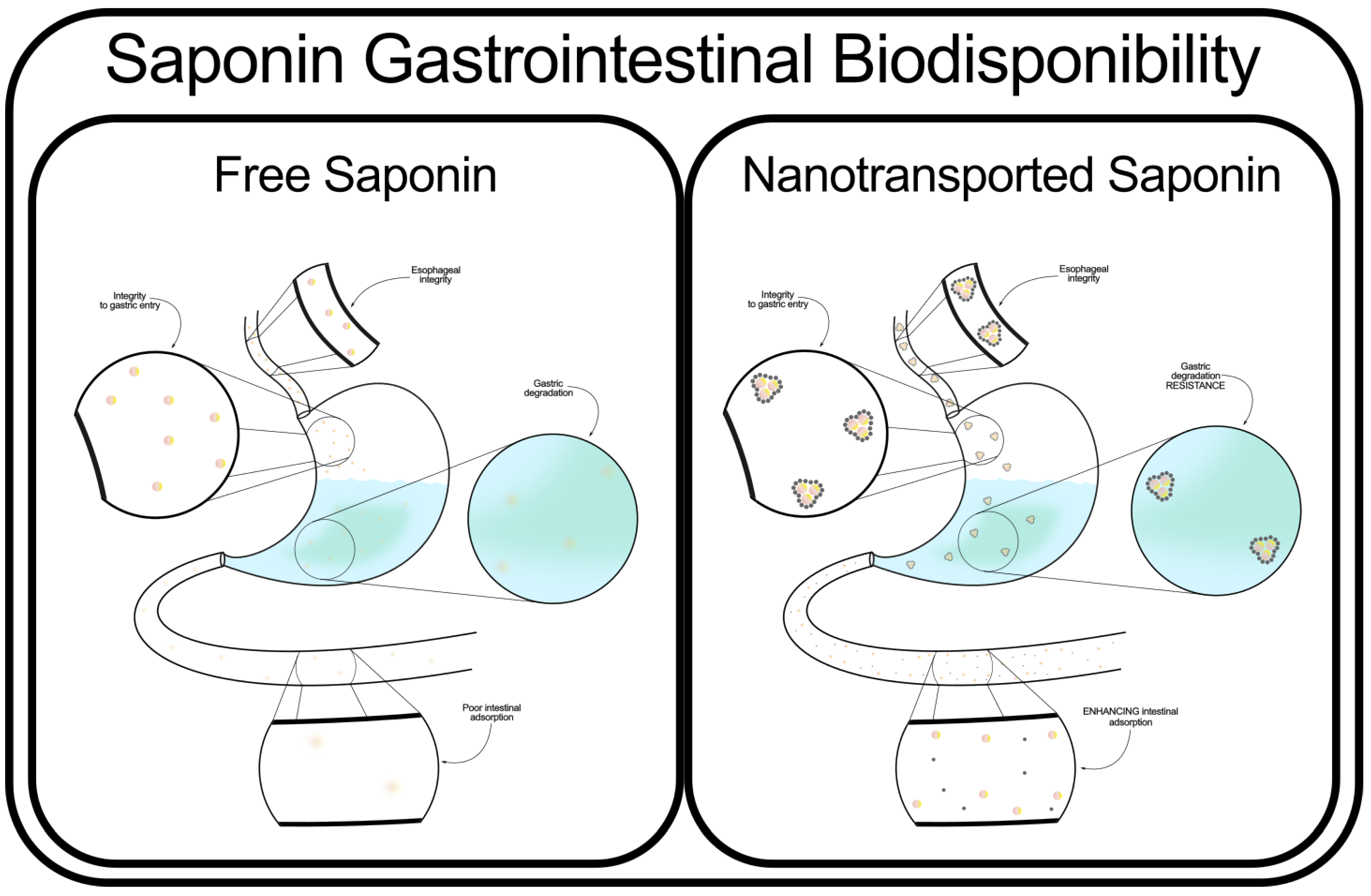
Some studies suggest that nanostructured delivery systems offer controlled release [
103] and protection from gastrointestinal degradation [
104,
105,
106], although further research is needed to establish their effectiveness for saponin transport specifically. Meanwhile, other studies demonstrated complete integrity of the chemical structure of some saponins, and zero metabolization, at least in one species of salmon [
107]. It has even been shown that gastric degradation can enhance cytotoxic activity towards cancer cells [
108].
However, paradoxically, saponins have been employed as emulsifiers and coating agents in drug delivery formulations designed to facilitate transport through the gastrointestinal tract [
109,
110,
111], as well as transdermal permeation [
112]. Some studies have shown that specific saponins enhance the solubility and absorption of co-administered compounds by modulating intestinal permeability and interacting with lipid membranes [
113]. This dual functionality raises questions regarding the underlying mechanisms governing saponin stability, transformation, and interaction with biological membranes in different formulations.
The apparent contradiction between saponins’ degradation in the gastrointestinal environment and their role as emulsifiers in drug transport suggests that their stability is highly dependent on their structural characteristics, formulation, and interaction with digestive enzymes and microbiota [
86]. Some studies suggest that specific glycosylation patterns or conjugation states determine whether saponins are hydrolyzed into inactive metabolites or act as active transport enhancers [
114].
Given these conflicting findings, further research is essential to clarify the precise conditions under which saponins degrade or act as bioavailability enhancers. Systematic studies on different saponin subclasses, their transformation pathways in the digestive system, and their interactions with pharmaceutical carriers will be crucial for optimizing their therapeutic applications and resolving existing paradoxes in the literature.
4.2.3. Recent Technological Advancements
In the last years, several innovations have further improved the extraction, stability, and bioavailability of saponins. Supercritical fluid technology [
90,
91], initially used for extraction as previously mentioned, has been adapted for encapsulation, providing a solvent-free method for delivering bioactive compounds with minimal degradation. This is especially beneficial for marine saponins, which are sensitive to solvents and thermal stress, as it preserves their bioactivity during the encapsulation process.
Additionally, the use of ionic liquids as alternative solvents has shown promise in saponin extraction. These customizable solvents can be tailored to match the polarity and thermal properties of target saponins, maximizing efficiency while minimizing degradation. This is particularly important for marine saponins, which often require delicate handling due to their complex structures.
Moreover, advances in molecular biology, including CRISPR and synthetic biology, are opening new avenues for improving the intrinsic stability and bioavailability of saponins [
115]. Genetic engineering of saponin-producing organisms could lead to novel variants with an increased amount of production, supporting more sustainable production and broader applications in nutraceuticals and pharmaceuticals [
116,
117,
118].
Machine learning (ML) can significantly enhance the bioavailability of saponins by optimizing extraction methods, predicting compound stability, and improving formulation strategies [
119,
120,
121]. By analyzing vast datasets, ML models can identify the most effective delivery systems, such as nanoencapsulation, to enhance solubility and absorption. Additionally, ML-driven molecular modeling can predict interactions between saponins and biological targets, facilitating the design of more bioavailable derivatives [
122,
123]. These advancements can lead to more efficient utilization of marine resources for pharmaceuticals, nutraceuticals, and cosmetics, maximizing their therapeutic potential.
In conclusion, addressing the challenges of saponin extraction, stability, and bioavailability is crucial for fully realizing their therapeutic and commercial potential. Technological innovations in these areas, particularly in advanced extraction and bioproduction systems, are making saponins more accessible and effective for use in nutraceuticals and pharmaceuticals. Future research should continue to focus on optimizing these processes for large-scale production, while ensuring the sustainability of natural saponin sources, especially from marine environments.
5. Practical Applications and Case Studies
Given their well-established bioactivities, saponins have been extensively studied for their therapeutic potential in clinical settings. Their ability to regulate immune responses, modulate lipid metabolism, and target cancer pathways has positioned them as promising candidates for pharmaceutical applications.
5.1. Therapeutic and Biotechnological Uses
Saponins have gained recognition for their diverse therapeutic applications, particularly in vaccine development [
124,
125,
126,
127,
128,
129], oncology [
130,
131,
132], inflammatory disease [
133,
134,
135,
136,
137], and cardiovascular [
12,
138,
139] or cerebrovascular [
99] disease management. Their immunostimulatory capacity has been leveraged in vaccine formulations, where
Quillaja saponaria-derived saponins play a crucial role as adjuvants. These compounds enhance antigen presentation by dendritic cells and macrophages, a mechanism effectively utilized in immunostimulating complexes (ISCOMs) designed for vaccines against influenza [
140], HIV [
141], malaria [
142,
143,
142], and cytomegalovirus [
144]. Their dual ability to boost humoral and cellular immune responses underpins their relevance in next-generation vaccine strategies. Furthermore, they have been successfully used as enhancer factors in human embryonic stem cell culture [
145]. Also, some reported results point to strong anti-viral activity of saponins [
146,
147,
148].
In oncology, marine saponins, such as holothurins from sea cucumbers, have shown potential as cytotoxic agents targeting cancer cells while exhibiting selective toxicity. Their role in modulating apoptotic pathways and inhibiting tumor proliferation suggests promising avenues for anticancer drug development. Notably, Holothurin A inhibits prostate cancer growth by reducing PSA expression [
149] and modulating androgen receptor (AR) activity through strong binding to the BF3 pocket, as demonstrated by
in vitro,
in silico, and molecular dynamics studies, suggesting its potential as a treatment for castration-resistant tumors.
Beyond oncology, saponins contribute to the management of chronic inflammatory disorders. Their capacity to modulate cytokine expression, particularly by inhibiting TNF-
and IL-6, offers therapeutic potential in autoimmune conditions characterized by persistent inflammation [
150]. These multifaceted properties position saponins as bioactive candidates in both immunotherapeutic and anti-inflammatory drug development.
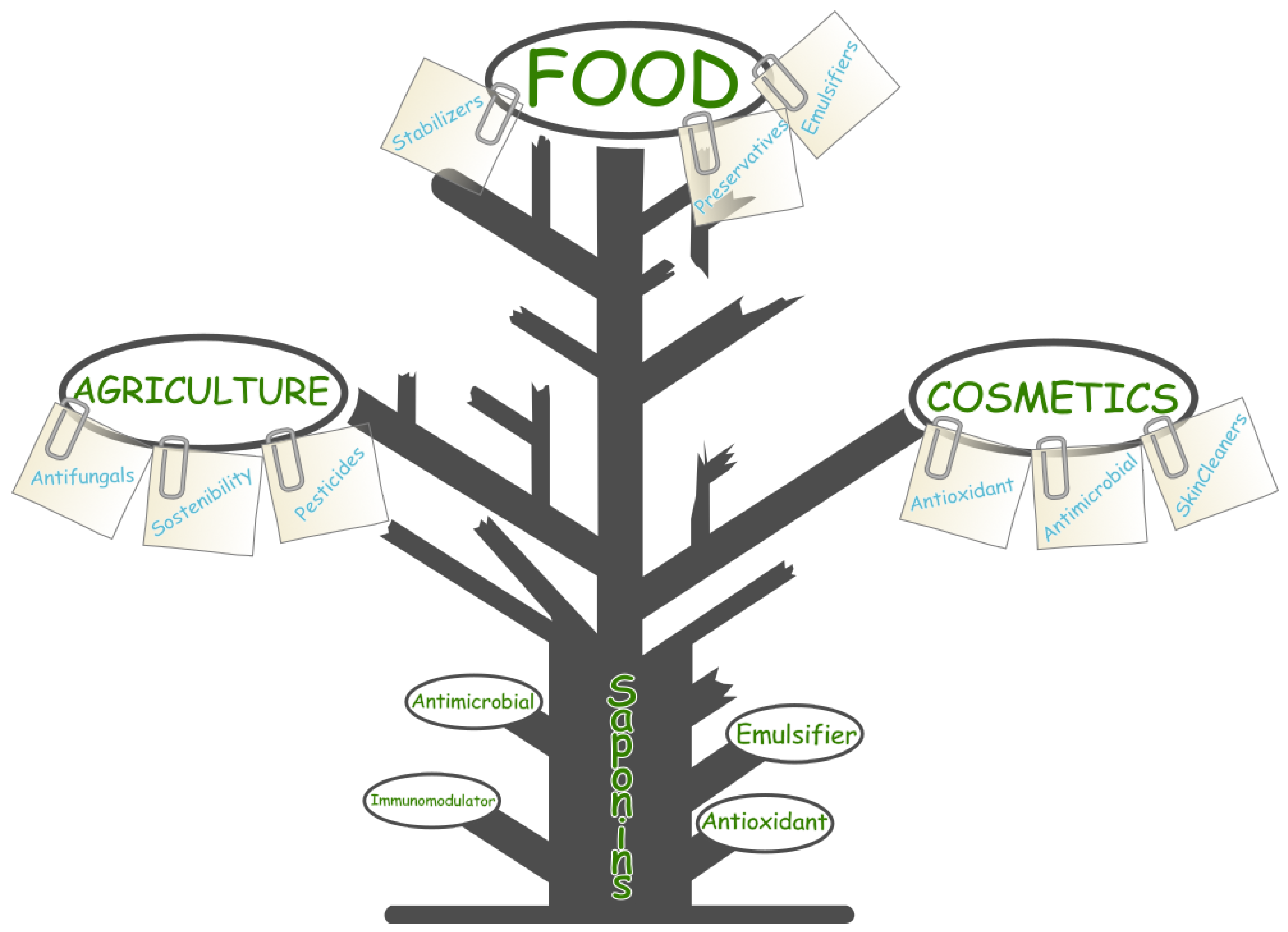
5.2. Industrial Applications
In the food industry, saponins are highly valued for their emulsifying, foaming, and stabilizing properties. These characteristics make them indispensable in formulating products such as plant-based milks and vegan emulsifiers, where texture and mouthfeel are critical to consumer acceptance. The demand for clean-label, natural ingredients has accelerated the adoption of saponins as natural emulsifiers, replacing synthetic additives in food formulations, as Tween-80. Their ability to form stable emulsions without affecting sensory properties is a significant advantage in creating consumer-friendly, high-quality products.
Saponins also extend the shelf life of food products through their antimicrobial and antioxidant properties. By inhibiting microbial growth and delaying lipid oxidation, saponins help preserve the nutritional quality and safety of food products over extended periods. This aligns with the growing market for natural preservatives, where saponins are being incorporated to reduce the reliance on synthetic preservatives, addressing consumer demand for sustainable food products.
In organic agriculture, saponins are used as natural pesticides due to their insecticidal, antifungal, and antiviral properties. These compounds offer eco-friendly alternatives to synthetic agrochemicals, supporting integrated pest management (IPM) strategies and promoting sustainable farming practices. Saponins have proven effective against a range of agricultural pests, making them valuable in reducing the environmental impact of pesticide use while maintaining crop health.
Figure 8.
Industrial applications of saponins in pharmaceuticals, functional foods, and cosmetics, emphasizing their role as emulsifiers, adjuvants, and bioactive agents.
Figure 8.
Industrial applications of saponins in pharmaceuticals, functional foods, and cosmetics, emphasizing their role as emulsifiers, adjuvants, and bioactive agents.
5.3. Cosmetic Innovations
The cosmetic industry has widely embraced saponins for their natural foaming, cleansing, and skin-conditioning properties. These compounds are commonly used in shampoos, body washes, and facial cleansers, where their gentle cleansing action provides a natural alternative to synthetic surfactants. Their ability to form stable foams and improve the sensory qualities of personal care products makes them attractive in formulations designed to meet the increasing consumer preference for natural and sustainable ingredients.
Saponins also exhibit antioxidant and anti-aging properties, making them valuable in modern skincare formulations. By scavenging free radicals and reducing oxidative stress, saponins protect the skin from environmental damage, such as UV radiation and pollution. These properties make them ideal for inclusion in anti-aging creams and serums, where they contribute to skin regeneration, improve elasticity, and reduce the appearance of wrinkles. The multifunctionality of saponins, combining cleansing, antioxidant, and anti-aging properties, positions them as key ingredients in innovative, high-performance skincare products.
Additionally, the antimicrobial properties of saponins enhance their utility in cosmetics by protecting against microbial contamination, improving product safety and shelf life without the need for synthetic preservatives.
5.4. Research and Development
Ongoing research has driven the development of advanced extraction and purification technologies aimed at improving the yield, purity, and bioactivity of saponin-rich extracts. Techniques such as enzymatic extraction, supercritical fluid extraction, and microwave-assisted extraction have proven effective in isolating saponins with higher purity and reduced environmental impact, minimizing solvent use and energy consumption. These technologies are essential to meet the growing demand for high-quality saponins in both nutraceutical and pharmaceutical industries.
Figure 9.
Nanotechnology-based delivery systems for saponins, including nanoemulsions, liposomes, and polymeric nanoparticles, which enhance bioavailability and controlled release.
Figure 9.
Nanotechnology-based delivery systems for saponins, including nanoemulsions, liposomes, and polymeric nanoparticles, which enhance bioavailability and controlled release.
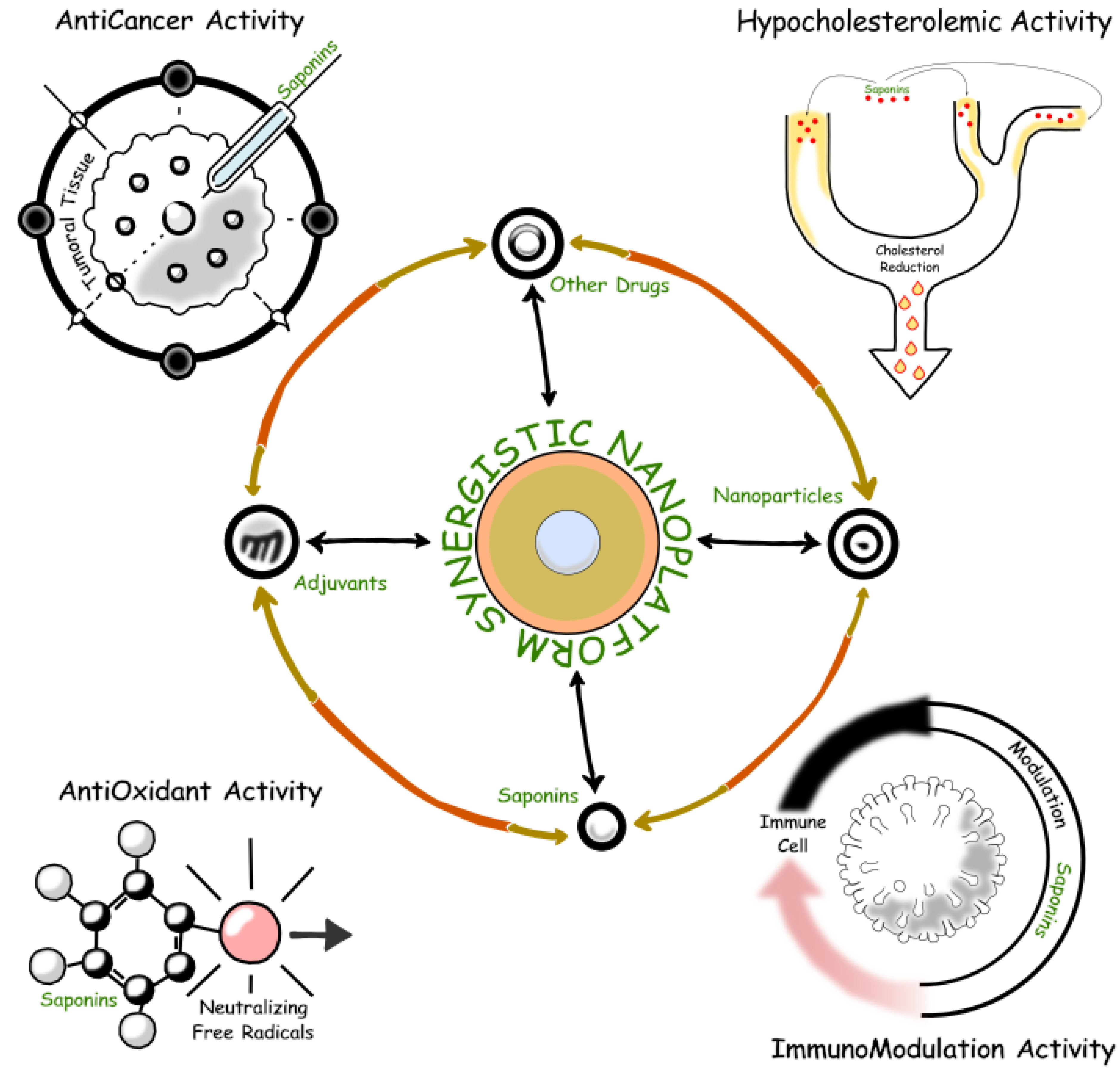
Research has also explored the synergistic effects of saponins with other bioactive compounds, such as polyphenols and flavonoids [
151], leading to multi-component nutraceutical products offering enhanced antioxidant and anti-inflammatory benefits. For example, the combination of saponins with polyphenols has been shown to synergistically enhance bioactivity, offering a holistic approach to managing cardiovascular health and reducing inflammation [
152]. Such synergistic formulations represent a new frontier in functional foods and nutraceuticals targeting specific health conditions.
Advances in nanotechnology have revolutionized saponin delivery in food and pharmaceutical applications. Encapsulation techniques, such as nanoemulsions, liposomes, and polymeric nanoparticles, have significantly improved the bioavailability of saponins, ensuring more efficient absorption and enhanced therapeutic efficacy [
152,
153,
154,
155]. These technologies not only enhance saponin delivery but also provide controlled release mechanisms, prolonging bioactivity and improving consumer outcomes.
5.5. The Potential of Thalassochemicals: Marine Saponins
Marine-derived saponins, particularly those from sea cucumbers and starfish, hold significant potential for nutraceutical and pharmaceutical applications due to their unique structures and bioactivities. These marine saponins, characterized by sulfated sugar residues and rare sugar moieties, exhibit a broad range of bioactivities, including cytotoxic, immunomodulatory, and anti-inflammatory effects, making them attractive candidates for health-promoting products.
Holothurinosides, a group of marine saponins, have demonstrated the ability to modulate lipid metabolism by inhibiting pancreatic lipase, thereby reducing fat absorption and promoting weight management [
156,
157]. These properties make them valuable in the development of nutraceuticals targeting obesity and hyperlipidemia. Furthermore, their potent cytotoxic effects against various cancer cell lines highlight their potential as novel anticancer agents, with studies demonstrating their ability to induce apoptosis and inhibit tumor proliferation in aggressive cancers [
149,
158,
159]. The structural uniqueness of marine saponins, particularly their sulfation patterns, plays a crucial role in enhancing their bioactivity and therapeutic potential.
5.6. Conclusion on Practical Applications
Saponins offer a diverse array of practical applications across multiple industries, ranging from therapeutics and food technology to cosmetics and agriculture. Their bioactivities, including immunomodulatory, anticancer, and antioxidant properties, make them invaluable in developing innovative products that cater to the growing demand for natural, sustainable, and health-promoting ingredients.
As research continues to uncover the full potential of saponins, new applications and formulations will undoubtedly emerge. Advances in extraction methods, synergistic interactions with other bioactives, and improvements in bioavailability will shape the future of saponin-based products. In particular, marine saponins represent an exciting frontier in thalassochemicals, offering unique bioactivities that could revolutionize the nutraceutical and pharmaceutical industries.
Figure 10.
Proposed future directions for marine saponin research, emphasizing sustainability, regulatory challenges, and potential applications in next-generation nutraceuticals.
Figure 10.
Proposed future directions for marine saponin research, emphasizing sustainability, regulatory challenges, and potential applications in next-generation nutraceuticals.
6. Conclusions and Outlook
While terrestrial saponins have been extensively studied, marine saponins remain underexplored, particularly regarding their molecular interactions, bioavailability challenges, and technological applications. This review compiles the latest advances in marine saponin research, addressing key gaps in extraction techniques, stability enhancement, and pharmaceutical potential. This review has highlighted the significant progress made in understanding the structure, bioactivities, and practical applications of saponins from both terrestrial and marine sources. Saponins have emerged as versatile bioactive compounds with a wide range of health-promoting properties, including hypocholesterolemic effects, immune modulation, anticancer activity, and antioxidant potential. Their unique molecular structures, particularly those of marine-derived saponins, underpin their diverse biological functions and their increasing relevance in nutraceuticals, functional foods, and pharmaceuticals.
The potential of saponins is particularly evident in their incorporation into nutraceutical and functional food products, where they can contribute to the management of chronic diseases such as cardiovascular disorders, diabetes, and cancer. Their ability to interact with cellular membranes, modulate lipid metabolism, and stimulate immune responses positions them as promising candidates for the development of innovative health products. Additionally, the synergistic effects between saponins and other bioactives, such as polyphenols and flavonoids, open new avenues for creating more effective nutraceutical formulations that harness these combined bioactivities.
Despite these promising developments, challenges remain. The extraction and purification of saponins, particularly from marine sources, continue to present technical difficulties. While advanced extraction methods like supercritical fluid extraction and ultrasonic-assisted extraction offer solutions, further refinement is required to meet the demands of large-scale industrial applications. Ensuring that these methods are scalable, efficient, and environmentally sustainable will be crucial for the broader commercialization of saponins.
Another significant challenge is the limited bioavailability of saponins, primarily due to their poor solubility and stability in the gastrointestinal tract. Advances in delivery systems, such as nanoparticle encapsulation, liposomal formulations, and solid lipid nanoparticles (SLN), show great promise in enhancing saponin absorption and bioefficacy. Future research should focus on optimizing these technologies to improve therapeutic outcomes, particularly in oral bioavailability and disease-targeted applications.
The continued exploration of saponins in nutraceuticals and functional foods will require overcoming bioavailability challenges while navigating the evolving regulatory landscape to ensure safe and effective applications. This regulatory landscape for saponin-containing products also presents obstacles that must be addressed. While preclinical studies have demonstrated the health benefits of saponins, robust clinical trials are essential to validate these findings in human populations and ensure the long-term safety of saponin consumption. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) will require comprehensive data to support health claims before approving saponins for use in nutraceuticals and functional foods. Detailed safety assessments and rigorous clinical trials will be crucial for establishing the credibility of saponins as functional ingredients.
Sustainability is another key factor, especially for marine-derived saponins. As demand grows, it is essential to implement ethical sourcing practices that protect marine ecosystems. Sustainable aquaculture and synthetic biology approaches to saponin production could mitigate the environmental impact of extraction, ensuring that the increased use of saponins does not contribute to ecological degradation. Focusing on sustainable practices will be critical for preserving biodiversity and maintaining the long-term viability of saponin resources.
In conclusion, saponins represent a highly promising class of bioactive compounds with vast potential to improve public health and advance the fields of nutraceuticals and pharmaceuticals. The future of saponins depends on overcoming current challenges related to extraction, bioavailability, regulatory compliance, and sustainability. Continued innovation in these areas will unlock the full potential of saponins, ensuring their lasting impact on human health and the global nutraceutical industry. As new applications emerge and existing technologies improve, saponins are poised to play a pivotal role in the development of next-generation health-promoting products, driving innovation in both food and pharmaceutical sciences.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, V.D-A., T.H. and L.T.A; methodology, V.D-A., T.H. and L.T.A; investigation, , V.D-A., T.H. and L.T.A; writing—original draft preparation, V.D-A., T.H. and L.T.A; writing—review and editing, V.D-A., T.H. and L.T.A; visualization, V.D-A., T.H. and L.T.A; supervision, T.H. and L.T.A.; project administration, X.X.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
Financial support by Xunta de Galicia through grant IN606B-2023/006 is gratefully acknowledged. Facilities provided by the Galician Supercomputing Centre (CESGA) are also acknowledged..
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Timilsena, Y.P.; Phosanam, A.; Stockmann, R. Perspectives on Saponins: Food Functionality and Applications. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, X.; Liu, F.; Mao, B.; Zhang, Q.; Zhao, J.; Chen, W.; Cui, S. Sources, metabolism, health benefits and future development of saponins from plants. FOOD RESEARCH INTERNATIONAL 2024, 197. [Google Scholar] [CrossRef]
- Jolly, A.; Hour, Y.; Lee, Y.C. An outlook on the versatility of plant saponins: A review. FITOTERAPIA 2024, 174. [Google Scholar] [CrossRef]
- Kholif, A.E. A Review of Effect of Saponins on Ruminal Fermentation, Health and Performance of Ruminants. VETERINARY SCIENCES 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Jolly, A.; Kim, H.; Moon, J.Y.; Mohan, A.; Lee, Y.C. Exploring the imminent trends of saponins in personal care product development: A review. INDUSTRIAL CROPS AND PRODUCTS 2023, 205. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Mersni, D.; Lourith, N. Plant-derived saponins and their prospective for cosmetic and personal care products. BOTANICAL STUDIES 2024, 65. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, R.; Chen, J.; Li, S.; Huang, K.; Cao, H.; Farag, M.A.A.; Battino, M.; Daglia, M.; Capanoglu, E.; et al. Health benefits of saponins and its mechanisms: perspectives from absorption, metabolism, and interaction with gut. CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 2024, 64, 9311–9332. [Google Scholar] [CrossRef]
- Geng, X.; Wang, J.; Liu, Y.; Liu, L.; Liu, X.; Zhao, Y.; Wang, C.; Liu, J. Research progress on chemical diversity of saponins in Panax ginseng. CHINESE HERBAL MEDICINES 2024, 16, 529–547. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Nakamura, S.; Muraoka, O.; Yoshikawa, M. New biofunctional effects of oleanane-type triterpene saponins. JOURNAL OF NATURAL MEDICINES 2023, 77, 644–664. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.; Zhang, J.; Thakur, K.; Battino, M.; Cao, H.; Farag, M.A.; Xiao, J.; Wei, Z. Asparagus saponins: effective natural beneficial ingredient in functional foods, from preparation to applications. CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 2024, 64, 12284–12302. [Google Scholar] [CrossRef]
- Xiao, M.Y.; Li, S.; Pei, W.J.; Gu, Y.L.; Piao, X.L. Natural Saponins on Cholesterol-Related Diseases: Treatment and Mechanism. PHYTOTHERAPY RESEARCH 2025. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Wang, L.; Zeng, M.; Wang, Y.; Yu, B.; Zeng, W.; Jiang, X.; Suo, Y. Saponins as therapeutic candidates for atherosclerosis. PHYTOTHERAPY RESEARCH 2024, 38, 1651–1680. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Fakhri, S.; Ghanadian, S.M.; Bahrami, G.; Mansouri, K.; Iranpanah, A.; Farzaei, M.H.; Mojarrab, M. Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways. METABOLITES 2023, 13. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, M.; Zhang, X.; Zheng, Y.; Lv, N.; Wang, L.; Gan, J.; Li, Y.; Jiang, X.; Yang, L. Therapeutic Candidates for Alzheimer’s Disease: Saponins. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES 2023, 24. [Google Scholar] [CrossRef]
- Smith, S.J.; Wang, T.; Cummins, S.F. Asteroid Saponins: A Review of Their Bioactivity and Selective Cytotoxicity. MARINE DRUGS 2024, 22. [Google Scholar] [CrossRef]
- Chen, M.; Balhara, V.; Castillo, A.M.J.; Balsevich, J.; Johnston, L.J. Interaction of saponin 1688 with phase separated lipid bilayers. BIOCHIMICA ET BIOPHYSICA ACTA-BIOMEMBRANES 2017, 1859, 1263–1272. [Google Scholar] [CrossRef]
- Li, J.; Monje-Galvan, V. Effect of Glycone Diversity on the Interaction of Triterpenoid Saponins and Lipid Bilayers. ACS APPLIED BIO MATERIALS 2023, 7, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Ondevilla, J.C.; Hanashima, S.; Mukogawa, A.; Miyazato, D.G.; Umegawa, Y.; Murata, M. Effect of the number of sugar units on the interaction between diosgenyl saponin and membrane lipids. BIOCHIMICA ET BIOPHYSICA ACTA-BIOMEMBRANES 2023, 1865. [Google Scholar] [CrossRef]
- Sreij, R.; Prevost, S.; Dargel, C.; Dattani, R.; Hertle, Y.; Wrede, O.; Hellweg, T. Interaction of the Saponin Aescin with Ibuprofen in DMPC Model Membranes. MOLECULAR PHARMACEUTICS 2018, 15, 4446–4461. [Google Scholar] [CrossRef]
- Jiang, M.; Hong, C.; Zou, W.; Ye, Z.; Lu, L.; Liu, Y.; Zhang, T.; Ding, Y. Recent advances in the anti-tumor activities of saponins through cholesterol regulation. FRONTIERS IN PHARMACOLOGY 2025, 15. [Google Scholar] [CrossRef]
- Shen, L.; Luo, H.; Fan, L.; Tian, X.; Tang, A.; Wu, X.; Dong, K.; Su, Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. MOLECULES 2024, 29. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Y.; Bai, H.; Wang, Y.; Yang, B.; Wang, Q.; Kuang, H. Effects of saponins from Chinese herbal medicines on signal transduction pathways in cancer: A review. FRONTIERS IN PHARMACOLOGY 2023, 14. [Google Scholar] [CrossRef]
- Fagbohun, O.F.; Joseph, J.S.; Oriyomi, O.V.; Rupasinghe, H.P.V. Saponins of North Atlantic Sea Cucumber: Chemistry, Health Benefits, and Future Prospectives. MARINE DRUGS 2023, 21. [Google Scholar] [CrossRef]
- Li, J.; Monje-Galvan, V. In Vitro and In Silico Studies of Antimicrobial Saponins: A Review. PROCESSES 2023, 11. [Google Scholar] [CrossRef]
- Stitou, M.; Toufik, H.; Bouachrine, M.; Lamchouri, F. Quantitative structure-activity relationships analysis, homology modeling, docking and molecular dynamics studies of triterpenoid saponins as Kirsten rat sarcoma inhibitors. JOURNAL OF BIOMOLECULAR STRUCTURE & DYNAMICS 2021, 39, 152–170. [Google Scholar] [CrossRef]
- Khoa, N.M.; Phong, N.V.; Yang, S.Y.; Min, B.S.; Kim, J.A. Spectroscopic analysis, kinetic mechanism, computational docking, and molecular dynamics of active metabolites from the aerial parts of Astragalus membranaceus Bunge as tyrosinase inhibitors. BIOORGANIC CHEMISTRY 2023, 134. [Google Scholar] [CrossRef]
- Taiwo, B.J.; Olubiyi, O.O.; Wang, X.; Fisusi, F.A.; Akinniyi, G.A.; Van Heerden, F.R.; Strodel, B. Schistosomiasis: Snail-vector control, molecular modelling and dynamic studies of bioactive N-acetylglycoside saponins from Tetrapleura tetraptera. COMPUTATIONAL BIOLOGY AND CHEMISTRY 2018, 77, 363–372. [Google Scholar] [CrossRef]
- Iksen, I.; Witayateeraporn, W.; Wirojwongchai, T.; Suraphan, C.; Pornputtapong, N.; Singharajkomron, N.; Nguyen, H.M.; Pongrakhananon, V. Identifying molecular targets of Aspiletrein-derived steroidal saponins in lung cancer using network pharmacology and molecular docking-based assessments. SCIENTIFIC REPORTS 2023, 13. [Google Scholar] [CrossRef]
- Drewe, J.; Schoning, V.; Danton, O.; Schenk, A.; Boonen, G. Machine Learning-Based Analysis Reveals Triterpene Saponins and Their Aglycones in Cimicifuga racemosa as Critical Mediators of AMPK Activation. PHARMACEUTICS 2024, 16. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Liu, H.; Chang, W.; Xu, Y.; Lin, F. Prediction of Hemolytic Toxicity for Saponins by Machine-Learning Methods. CHEMICAL RESEARCH IN TOXICOLOGY 2019, 32, 1014–1026. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Li, X.; Sun, M.; Jiang, M.; Shi, X.; Xu, X.; Ding, M.; Chen, B.; Yu, H.; et al. Machine learning prediction for constructing a universal multidimensional information library of Panax saponins (ginsenosides). FOOD CHEMISTRY 2024, 439. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Xian, B.; Jiang, H.; Zhou, T.; Chen, S.; Wen, F.; Pei, J. Machine learning and bioinformatics-based insights into the potential targets of saponins in Paris polyphylla smith against non-small cell lung cancer. FRONTIERS IN GENETICS 2022, 13. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Huo, Y.; Yang, W.; Chen, J.; Gao, Z.; Yang, Z. Ultrasonic extraction and antioxidant evaluation of oat saponins. ULTRASONICS SONOCHEMISTRY 2024, 109. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, H.; Zheng, T.; Jiang, J.; Xu, Y.; Jia, F.; He, K.; Yang, Y. Quantitative Changes and Transformation Mechanisms of Saponin Components in Chinese Herbal Medicines during Storage and Processing: A Review. MOLECULES 2024, 29. [Google Scholar] [CrossRef]
- Wang, Y.H. Naturally Occurring Polyhydroxylated Spirostanol Saponins, A Review of the Classification, Sources, Biosynthesis, Biological Activities, and Toxicity. CHEMISTRY & BIODIVERSITY 2024. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Huang, J.; Qiu, Q.; Liang, Y.; Meng, J.; Park, R.Y.; Li, P.C.H.; Sun, Y. Development of organic three-phase laminar flow microfluidic chip for extraction of ginsenosides from Panax ginseng. JOURNAL OF PHARMACEUTICAL AND BIOMEDICAL ANALYSIS 2023, 236. [Google Scholar] [CrossRef]
- Landa-Cansigno, C.; Serviere-Zaragoza, E.; Morales-Martinez, T.K.; Ascacio-Valdes, J.A.; Morreeuw, Z.P.; Gauyat, C.; Stiger-Pouvreau, V.; Reyes, A.G. The antioxidant and anti-elastase activity of the brown seaweed Sargassum horridum (Fucales, Phaeophyceae) and their early phenolics and saponins profiling for green cosmetic applications. ALGAL RESEARCH-BIOMASS BIOFUELS AND BIOPRODUCTS 2023, 75. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Zheng, Y.; Liu, M.; Wang, K. Effects of Different Culture Times Genes Expression on Ginsenoside Biosynthesis of the Ginseng Adventitious Roots in Panax ginseng. HORTICULTURAE 2023, 9. [Google Scholar] [CrossRef]
- Zakharenko, A.; Romanchenko, D.; Thinh, P.D.; Pikula, K.; Hang, C.T.T.; Yuan, W.; Xia, X.; Chaika, V.; Chernyshev, V.; Zakharenko, S.; et al. Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica Semper, 1868. MOLECULES 2020, 25. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Tao, L.; Zhang, X.; Hao, F.; Zhao, S.; Han, L.; Bai, C. Recent Advances in Separation and Analysis of Saponins in Natural Products. SEPARATIONS 2022, 9. [Google Scholar] [CrossRef]
- Hou, Y.J.; Wang, P.w.; Zhang, H.; Fan, Y.Y.; Cao, X.; Luo, Y.Q.; Li, Q.; Njolibimi, M.; Li, W.j.; Hong, B.; et al. A high-permeability method for extracting purple yam saponins based on ultrasonic-assisted natural deep eutectic solvent. FOOD CHEMISTRY 2024, 457. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, X.; Zhang, C.; Xie, P.; Huang, L. Enhanced and Green Extraction of Saponins from Gleditsia sinensis Lam. Pods by Ultrasound-Assisted Deep Eutectic Solvents: Optimization and Comprehensive Characterization. FOOD AND BIOPROCESS TECHNOLOGY 2025, 18, 1919–1938. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Li, H.; You, L.; Pedisic, S.; Shao, P. Saponins Based on Medicinal and Edible Homologous Plants: Biological Activity, Delivery Systems and Its Application in Healthy Foods. FOOD BIOENGINEERING 2024, 3, 464–481. [Google Scholar] [CrossRef]
- Luo, J.; Jia, M.; Yang, X.; Chai, Y.; Bao, Y. Interaction between lactic acid bacteria and Polygonatum sibiricum saponins and its application to microencapsulated co-delivery. FOOD CHEMISTRY 2024, 448. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Li, X.; Zhao, H.; Li, H.; Kuang, J.; Li, J.; Guo, J.; Huang, T.; Li, J. Tea saponin-Zein binary complex as a quercetin delivery vehicle: preparation, characterization, and functional evaluation. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2024, 279. [Google Scholar] [CrossRef]
- Jo, S.; El-Demerdash, A.; Owen, C.; Srivastava, V.; Wu, D.; Kikuchi, S.; Reed, J.; Hodgson, H.; Harkess, A.; Shu, S.; et al. Unlocking saponin biosynthesis in soapwort. NATURE CHEMICAL BIOLOGY 2024. [Google Scholar] [CrossRef]
- Kaminski, J.; Bujak, P.; Dlugosz, M. Permeabilization of Calendula officinalis L. hairy root cultures for the release of accumulated triterpenoid saponins. PLANT CELL TISSUE AND ORGAN CULTURE 2024, 159. [Google Scholar] [CrossRef]
- Han, Y.; Kim, D.H.; Pack, S.P. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. MARINE DRUGS 2024, 22. [Google Scholar] [CrossRef]
- Yosri, N.; Khalifa, S.A.M.; Attia, N.F.; Du, M.; Yin, L.; Abolibda, T.Z.; Zhai, K.; Guo, Z.; El-Seedi, H.R. Advancing sustainability in the green engineering of nanocomposites based on marine-derived polymers and their applications. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2024, 274. [Google Scholar] [CrossRef]
- Sundar, S.S.S.S.; Rajamanickam, C.; Saraswathy, S.; Venkatesan, K.; Balakumbahan, R.; Vijayasamundeeswari, A.; Sankar, C. Sapindaceae fruits: A comprehensive overview on phytochemicals, nutraceuticals and health benefits application. PLANT SCIENCE TODAY 2024, 11, 14. [Google Scholar] [CrossRef]
- Guillen-Sanchez, J.S.; Rojas-Villacorta, W.; de Albuquerque, R.D.D.G. Andean Fabaceae Species with Pharmacological Potential: Exploration of Antioxidant, Anticarcinogenic, and Antimicrobial Properties. AGRICULTURE-BASEL 2024, 14. [Google Scholar] [CrossRef]
- Yaoita, Y.; Kikuchi, M.; Machida, K. Terpenoids and Related Compounds from Plants of the Family Compositae (Asteraceae). NATURAL PRODUCT COMMUNICATIONS 2012, 7, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES 2021, 22. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Akhtar, N.; Alsayegh, A.A.; Abusudah, W.F.; Almohmadi, N.H.; Shaheen, H.M.; Singh, T.G.; De Waard, M. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Genus Acacia. MOLECULES 2022, 27. [Google Scholar] [CrossRef]
- Xu, C.; Xia, B.; Zhang, Z.; Lin, Y.; Li, C.; Lin, L. Research progress in steroidal saponins from the genus Polygonatum: Chemical components, biosynthetic pathways and pharmacological effects. PHYTOCHEMISTRY 2023, 213. [Google Scholar] [CrossRef]
- Porte, S.; Joshi, V.; Shah, K.; Chauhan, N.S. Plants’ steroidal saponins - A review on its pharmacology properties and analytical techniques. WORLD JOURNAL OF TRADITIONAL CHINESE MEDICINE 2022, 8, 350–385. [Google Scholar] [CrossRef]
- Sharma, S.; Kaul, S.; Dhar, M.K. A systematic review on ethnobotany, phytochemistry and pharmacology of Dioscorea bulbifera L. (Dioscoreaceae). SOUTH AFRICAN JOURNAL OF BOTANY 2024, 170, 367–393. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, B.; Gutierrez-Uribe, J.A.; Guajardo-Flores, D.; Santos-Zea, L. Microencapsulation of steroidal saponins from agave sap concentrate using different carriers in spray drying. FOOD SCIENCE AND TECHNOLOGY INTERNATIONAL 2022, 28, 622–633. [Google Scholar] [CrossRef]
- Singh, D.; Chaudhuri, P.K. Structural characteristics, bioavailability and cardioprotective potential of saponins. INTEGRATIVE MEDICINE RESEARCH 2018, 7, 33–43. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Zhi, Z.; Hu, Y.; Ge, J.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. 4-O-Sulfation in sea cucumber fucodians contribute to reversing dyslipidiaemia caused by HFD. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2017, 99, 96–104. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Sulfated polysaccharides in sea cucumbers and their biological properties: A review. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2023, 253. [Google Scholar] [CrossRef]
- Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.M.; Ghandourah, M.; Al-Farawati, R. Sulfated Triterpene Glycosides from the Saudi Red Sea Cucumber Holothuria atra with Antioxidant and Cytotoxic Activities. THALASSAS 2021, 37, 817–824. [Google Scholar] [CrossRef]
- Grauso, L.; Yegdaneh, A.; Sharifi, M.; Mangoni, A.; Zolfaghari, B.; Lanzotti, V. Molecular Networking-Based Analysis of Cytotoxic Saponins from Sea Cucumber Holothuria atra. MARINE DRUGS 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Nayak, H.; Kushwaha, A.; Behera, P.C.; Shahi, N.C.; Kushwaha, K.P.S.; Kumar, A.; Mishra, K.K. The Pink Oyster Mushroom, Pleurotus djamor (Agaricomycetes): A Potent Antioxidant and Hypoglycemic Agent. INTERNATIONAL JOURNAL OF MEDICINAL MUSHROOMS 2021, 23, 29–36. [Google Scholar]
- Yu, H.; Chen, B.; Li, J.; Dong, N.; Chang, X.; Wang, J.; Peng, H.; Zha, L.; Gui, S. Identification and functional characterization of two trans-isopentenyl diphosphate synthases and one squalene synthase involved in triterpenoid biosynthesis in Platycodon grandiflorus. PLANTA 2023, 258. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Zhou, Y.; Lu, Z.; Pu, Y.; Zhang, H. Cloning and functional characterization of the oxidative squalene cyclase gene in the deep-sea holothurian Chiridota sp. GENE 2024, 894. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, L.; Liu, L.; Liang, Y.; Sun, Y.; Wu, J. Both the mevalonate and the non-mevalonate pathways are involved in ginsenoside biosynthesis. PLANT CELL REPORTS 2014, 33, 393–400. [Google Scholar] [CrossRef]
- Mohanan, P.; Yang, T.J.; Song, Y.H. Genes and Regulatory Mechanisms for Ginsenoside Biosynthesis. JOURNAL OF PLANT BIOLOGY 2023, 66, 87–97. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Sun, L. Triterpenoid saponin biosynthesis genes and their expression patterns during the development of sea cucumber Apostichopus japonicus. JOURNAL OF OCEANOLOGY AND LIMNOLOGY 2021, 39, 2295–2308. [Google Scholar] [CrossRef]
- Geisler, R.; Pedersen, M.C.; Hannappel, Y.; Schweins, R.; Prevost, S.; Dattani, R.; Arleth, L.; Hellweg, T. Aescin-Induced Conversion of Gel-Phase Lipid Membranes into Bicelle-like Lipid Nanoparticles. LANGMUIR 2019, 35, 16244–16255. [Google Scholar] [CrossRef]
- Vo, N.N.Q.; Fukushima, E.O.; Muranaka, T. Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. JOURNAL OF NATURAL MEDICINES 2017, 71, 50–58. [Google Scholar] [CrossRef]
- Sheng, F.; Yang, S.; Li, M.; Wang, J.; Liu, L.; Zhang, L. Research Progress on the Anti-Cancer Effects of Astragalus membranaceus Saponins and Their Mechanisms of Action. MOLECULES 2024, 29. [Google Scholar] [CrossRef]
- Singh, V.I.; Sharma, R.K.; Kumar, Y.; Saqulain, S. Pharmacological aspects & medicinal uses of Trigonella foenum-graecum: A Current Review. INTERNATIONAL JOURNAL OF AYURVEDIC MEDICINE 2021, 12, 776–786. [Google Scholar]
- Yoshikawa, M.; Murakami, T.; Matsuda, H. Medicinal foodstuffs.: X.: Structures of new triterpene glycosides, gymnemosides-c, -d, -e, and -f, from the leaves of Gymnema sylvestre R. BR.:: Influence of gymnema glycosides on glucose uptake in rat small intestinal fragments. CHEMICAL & PHARMACEUTICAL BULLETIN 1997, 45, 2034–2038. [Google Scholar]
- Ji, Y.J.; Kim, H.D.; Lee, E.S.; Jang, G.Y.; Seong, H.A. Heat Treatment Enhances the Neuroprotective Effects of Crude Ginseng Saponin by Increasing Minor Ginsenosides. INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- SIDHU, G.; OAKENFULL, D. A MECHANISM FOR THE HYPOCHOLESTEROLEMIC ACTIVITY OF SAPONINS. BRITISH JOURNAL OF NUTRITION 1986, 55, 643+. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsieh, H.J.; Hsieh, C.H.; Hwang, D.F. Antioxidative and anticancer activities of various ethanolic extract fractions from crown-of-thorns starfish (Acanthaster planci). ENVIRONMENTAL TOXICOLOGY AND PHARMACOLOGY 2014, 38, 761–773. [Google Scholar] [CrossRef]
- Baecker, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Lindequist, U. Cytotoxic Saponins from the Seeds of Pittosporum angustifolium. ZEITSCHRIFT FUR NATURFORSCHUNG SECTION C-A JOURNAL OF BIOSCIENCES 2014, 69, 191–198. [Google Scholar] [CrossRef]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. VACCINE 2016, 34, 1162–1171. [Google Scholar] [CrossRef]
- Fleck, J.D.; Betti, A.H.; da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. MOLECULES 2019, 24. [Google Scholar] [CrossRef]
- de Groot, C.; Mueller-Goymann, C.C. Saponin Interactions with Model Membrane Systems Langmuir Monolayer Studies, Hemolysis and Formation of ISCOMs. PLANTA MEDICA 2016, 82, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lin, J.; Huang, Q.; Liang, P.; Huang, J.; Jian, C.; Lin, C.; Li, X. Panax notoginseng Saponins Attenuate Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury in Human SH-SY5Y Cells by Regulating the Expression of Inflammatory Factors through miR-155. BIOLOGICAL & PHARMACEUTICAL BULLETIN 2019, 42, 462–467. [Google Scholar] [CrossRef]
- Reddy, R.M.I.; Latha, P.B.; Vijaya, T.; Rao, D.S. The Saponin-Rich Fraction of a Gymnema sylvestre R. Br. Aqueous Leaf Extract Reduces Cafeteria and High-Fat Diet-Induced Obesity. ZEITSCHRIFT FUR NATURFORSCHUNG SECTION C-A JOURNAL OF BIOSCIENCES 2012, 67, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Ma, J.; Jin, S.; Wang, T.; Sui, Y.; Chen, L. Effects of saponins Rb<sub>1</sub> and Re in American ginseng combined intervention on immune system of aging model. FRONTIERS IN MOLECULAR BIOSCIENCES 2024, 11. [Google Scholar] [CrossRef]
- Ruan, W.; Liu, J.; Zhang, S.; Huang, Y.; Zhang, Y.; Wang, Z. Sour Jujube (Ziziphus jujuba var. spinosa): A Bibliometric Review of Its Bioactive Profile, Health Benefits and Trends in Food and Medicine Applications. FOODS 2024, 13. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Su, W.W.; Liu, Y.L.; Zhang, W.J.; Zeng, X. Gut microbiota-mediated metabolism of Panax notoginseng saponins and its role in pharmacokinetics and pharmacodynamics. TRADITIONAL MEDICINE RESEARCH 2024, 9. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Liu, R.; Liu, R.; Sui, W.; Geng, J.; Zhu, Q.; Wu, T.; Zhang, M. Sodium alginate-sodium hyaluronate-hydrolyzed silk for microencapsulation and sustained release of kidney tea saponin: The regulation of human intestinal flora in vitro. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2023, 249. [Google Scholar] [CrossRef]
- Fang, M.; Meng, Y.; Du, Z.; Guo, M.; Jiang, Y.; Tu, P.; Hua, K.; Lu, Y.; Guo, X. The Synergistic Mechanism of Total Saponins and Flavonoids in Notoginseng-Safflower against Myocardial Infarction Using a Comprehensive Metabolomics Strategy. MOLECULES 2022, 27. [Google Scholar] [CrossRef]
- Li, L.; Song, W.; Chang, Q.; Sun, Y.; Fang, D.; Qiao, W. The Synergistic Antidepressant Effect: Compatibility of Alkaloids with Saponins from Ziziphi Spinosae Semen. EVIDENCE-BASED COMPLEMENTARY AND ALTERNATIVE MEDICINE 2022, 2022. [Google Scholar] [CrossRef]
- Jegal, J.; Jeong, E.J.; Yang, M.H. A Review of the Different Methods Applied in Ginsenoside Extraction From Panax ginseng and Panax quinquefolius Roots. NATURAL PRODUCT COMMUNICATIONS 2019, 14. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Kalenik, T.K.; Nosyrev, A.E.; Stratidakis, A.K.; Mezhuev, Y.O.; Burykina, I.T.; Nicolae, A.C.; Arsene, A.L.; Tsatsakis, A.M.; et al. SUPERCRITICAL FLUID TECHNOLOGY AND SUPERCRITICAL FLUID CHROMATOGRAPHY FOR APPLICATION IN GINSENG EXTRACTS. FARMACIA 2019, 67, 202–212. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Ultrasound-assisted extraction of Catharanthus roseus (L.) G. Don (Patricia White cultivar) stem for maximizing saponin yield and antioxidant capacity. JOURNAL OF FOOD PROCESSING AND PRESERVATION 2018, 42. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, M.D.; Nguyen, T.T.T.; Khong, T.T.; Nguyen, V.H.; Nguyen, H.N.; Huynh, B.N.D.; Tran, H.T.M.; Trang, T.S. Microwave-assisted extraction for optimizing saponin yield and antioxidant capacity from cacao pod husk (Theobroma cacao L.). JOURNAL OF FOOD PROCESSING AND PRESERVATION 2021, 45. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shim, S.L.; Jang, E.S.; Choi, S.G. Ginsenoside stability and antioxidant activity of Korean red ginseng ( Panax ginseng CA meyer) extract as affected by temperature and time. LWT-FOOD SCIENCE AND TECHNOLOGY 2024, 200. [Google Scholar] [CrossRef]
- Weigel, F.; Weiss, J.; Decker, E.A.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of emulsifiers and antioxidants on physical and chemical stability. FOOD CHEMISTRY 2018, 242, 395–403. [Google Scholar] [CrossRef]
- Phrompittayarat, W.; Wittaya-Areekul, S.; Jetiyanon, K.; Putalun, W.; Tanaka, H.; Ingkaninan, K. Stability Studies of Saponins in Bacopla monnieri Dried Ethanolic Extracts. PLANTA MEDICA 2008, 74, 1756–1763. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Li, X.; Song, Y.; Li, W.; Liu, K.; Su, D.; Feng, Y.; Yang, S. Comparative pharmacokinetic profiles of five poorly soluble pulchinenosides in different formulations from Pulsatilla chinensis saponins extracts for enhanced bioavailability. BIOMEDICAL CHROMATOGRAPHY 2015, 29, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Yang, X.L.; Xiao, W.; Wang, Z.Z.; Ding, G.; Huang, W.Z.; Yang, Z.L.; Zhang, C.F. Microcrystalline Preparation of Akebia Saponin D for its Bioavailability Enhancement in Rats. AMERICAN JOURNAL OF CHINESE MEDICINE 2015, 43, 513–528. [Google Scholar] [CrossRef]
- Zeng, M.; Pan, L.; Qi, S.; Cao, Y.; Zhu, H.; Guo, L.; Zhou, J. Systematic review of recent advances in pharmacokinetics of four classical Chinese medicines used for the treatment of cerebrovascular disease. FITOTERAPIA 2013, 88, 50–75. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Reglero, G.; Martin, D. Chemical Characterization and Bioaccessibility of Bioactive Compounds from Saponin-Rich Extracts and Their Acid-Hydrolysates Obtained from Fenugreek and Quinoa. FOODS 2020, 9. [Google Scholar] [CrossRef]
- Thimmappa, R.; Wang, S.; Zheng, M.; Misra, R.C.; Huang, A.C.; Saalbach, G.; Chang, Y.; Zhou, Z.; Hinman, V.; Bao, Z.; et al. Biosynthesis of saponin defensive compounds in sea cucumbers. NATURE CHEMICAL BIOLOGY 2022, 18, 774+. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.H.; Kim, J.E.; Kim, Y.S.; Ryu, C.H.; Lee, H.J.; Kim, H.M.; Jeon, H.; Won, H.J.; Lee, J.Y.; et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. JOURNAL OF GINSENG RESEARCH 2018, 42, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Boskov, I.A.; Savic, I.M.; Stanisavljevic, N.D.G.; Kundakovic-Vasovic, T.D.; Selgrad, J.S.R.; Gajic, I.M.S. Stabilization of Black Locust Flower Extract via Encapsulation Using Alginate and Alginate-Chitosan Microparticles. POLYMERS 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xue, B.; Yang, C.; Miao, L.; Zhou, L.; Chen, Q.; Cai, Q.; Liu, Y.; Liu, D.; He, H.; et al. Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. FITOTERAPIA 2018, 129, 272–282. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Zhang, J.; Deng, M.; Yang, L. Influence of ginsenoside Rh<sub>1</sub> and F<sub>1</sub> on human cytochrome P450 enzymes. PLANTA MEDICA 2006, 72, 126–131. [Google Scholar] [CrossRef]
- Xie, J.; Luo, Y.; Chen, Y.; Ma, Y.; Yue, P.; Yang, M. Novel breviscapine nanocrystals modified by panax notoginseng saponins for enhancing bioavailability and synergistic anti-platelet aggregation effect. COLLOIDS AND SURFACES B-BIOINTERFACES 2019, 175, 333–342. [Google Scholar] [CrossRef]
- Knudsen, D.; Ron, O.; Baardsen, G.; Smedsgaard, J.; Koppe, W.; Froklaer, H. Soyasaponins resist extrusion cooking and are not degraded during gut passage in Atlantic salmon (Salmo salar L.). JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY 2006, 54, 6428–6435. [Google Scholar] [CrossRef]
- Wang, J.R.; Yau, L.F.; Zhang, R.; Xia, Y.; Ma, J.; Ho, H.M.; Hu, P.; Hu, M.; Liu, L.; Jiang, Z.H. Transformation of Ginsenosides from Notoginseng by Artificial Gastric Juice Can Increase Cytotoxicity toward Cancer Cells. JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY 2014, 62, 2558–2573. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Lee, Y.Y.; Chen, N.; Wang, Y.; Qiu, C. Physicochemical and In Vitro Digestion Properties of Curcumin-Loaded Solid Lipid Nanoparticles with Different Solid Lipids and Emulsifiers. FOODS 2023, 12. [Google Scholar] [CrossRef]
- Fu, W.; Liang, Y.; Xie, Z.; Wu, H.; Zhang, Z.; Lv, H. Preparation and evaluation of lecithin/zein hybrid nanoparticles for the oral delivery of Panax notoginseng saponins. EUROPEAN JOURNAL OF PHARMACEUTICAL SCIENCES 2021, 164. [Google Scholar] [CrossRef]
- Gonzalez, P.J.; Sorensen, P.M. Characterization of saponin foam from Saponaria officinalis for food applications. FOOD HYDROCOLLOIDS 2020, 101. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Santamaria-Echart, A.; Colucci, G.; Plasencia, P.; Costa, P.S.; Dias, M.M.; Pinho, S.P.; Barreiro, M.F. Saponin-based natural nanoemulsions as alpha-tocopherol delivery systems for dermal applications. JOURNAL OF MOLECULAR LIQUIDS 2023, 391. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Nanostructured lipid carriers (NLCs) stabilized by natural or synthetic emulsifiers for lutein delivery: Improved physicochemical stability, antioxidant activity, and bioaccessibility. FOOD CHEMISTRY 2023, 403. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Peng, J.; Li, Y.; Gong, L.; Lv, Y.; Liu, H.; Zhang, T.; Yang, S.; Liu, H.; Li, J.; et al. Pharmacokinetics, Bioavailability, Excretion and Metabolism Studies of Akebia Saponin D in Rats: Causes of the Ultra-Low Oral Bioavailability and Metabolic Pathway. FRONTIERS IN PHARMACOLOGY 2021, 12. [Google Scholar] [CrossRef]
- Choi, H.S.; Koo, H.B.; Jeon, S.W.; Han, J.Y.; Kim, J.S.; Jun, K.M.; Choi, Y.E. Modification of ginsenoside saponin composition via the CRISPR/Cas9-mediated knockout of protopanaxadiol 6-hydroxylase gene in Panax ginseng. JOURNAL OF GINSENG RESEARCH 2022, 46, 505–514. [Google Scholar] [CrossRef]
- Confalonieri, M.; Carelli, M.; Gianoglio, S.; Moglia, A.; Biazzi, E.; Tava, A. CRISPR/Cas9-Mediated Targeted Mutagenesis of CYP93E2 Modulates the Triterpene Saponin Biosynthesis in Medicago truncatula. FRONTIERS IN PLANT SCIENCE 2021, 12. [Google Scholar] [CrossRef]
- Takagi, K.; Yano, R.; Tochigi, S.; Fujisawa, Y.; Tsuchinaga, H.; Takahashi, Y.; Takada, Y.; Kaga, A.; Anai, T.; Tsukamoto, C.; et al. Genetic and functional characterization of Sg-4 glycosyltransferase involved in the formation of sugar chain structure at the C-3 position of soybean saponins. PHYTOCHEMISTRY 2018, 156, 96–105. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, P.; Ge, F.; Liu, D.Q.; Chen, C.Y. Enhancement of triterpenoid saponins biosynthesis in Panax notoginseng cells by co-overexpressions of 3-hydroxy-3-methylglutaryl CoA reductase and squalene synthase genes. BIOCHEMICAL ENGINEERING JOURNAL 2017, 122, 38–46. [Google Scholar] [CrossRef]
- Naha, S.; Kaur, S.; Bhattacharya, R.; Cheemanapalli, S.; Iyyappan, Y. ANPS: machine learning based server for identification of anti-nutritional proteins in plants. FUNCTIONAL & INTEGRATIVE GENOMICS 2024, 24. [Google Scholar] [CrossRef]
- Zheng, S.; Li, F.; Luo, J.; Wang, Y.; Bao, J.; Lin, J.; Jiang, W. Optimizing the extraction process of total saponins from Panax vietnamensis based on response surface analysis and backpropagation neural network-genetic algorithm. INDUSTRIAL CROPS AND PRODUCTS 2024, 220. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Li, X.; Sun, M.; Jiang, M.; Shi, X.; Xu, X.; Ding, M.; Chen, B.; Yu, H.; et al. Machine learning prediction for constructing a universal multidimensional information library of Panax saponins (ginsenosides). Food Chemistry 2024, 439, 138106–138106. [Google Scholar] [CrossRef] [PubMed]
- Drewe, J.; Schoning, V.; Danton, O.; Schenk, A.; Boonen, G. Machine Learning-Based Analysis Reveals Triterpene Saponins and Their Aglycones in Cimicifuga racemosa as Critical Mediators of AMPK Activation. PHARMACEUTICS 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Y.; Liu, H.; Chang, W.; Xu, Y.; Lin, F. Prediction of Hemolytic Toxicity for Saponins by Machine-Learning Methods. CHEMICAL RESEARCH IN TOXICOLOGY, 1014; 32. [Google Scholar] [CrossRef]
- `t Veld, L.G.M.H.I.; Cornelissen, L.A.M.; van den Bogaard, L.; Ansems, M.; Ho, I.N.; Adema, G.J. Saponin-based adjuvant uptake and induction of antigen cross-presentation by CD11b+dendritic cells and macrophages. NPJ VACCINES 2025, 10. [Google Scholar] [CrossRef]
- Ahmed, M.; Farris, E.; Swanson, R.V.; Das, S.; Yang, Y.; Martin, T.; Khader, S.A. Saponin TQL1055 adjuvant-containing vaccine confers protection upon Mycobacterium tuberculosis challenge in mice. HUMAN VACCINES & IMMUNOTHERAPEUTICS 2024, 20. [Google Scholar] [CrossRef]
- Yousefpour, P.; Zhang, Y.J.; Maiorino, L.; Melo, M.B.; Arainga Ramirez, M.A.; Kumarapperuma, S.C.; Xiao, P.; Silva, M.; Li, N.; Michaels, K.K.; et al. Modulation of antigen delivery and lymph node activation in nonhuman primates by saponin adjuvant saponin/monophosphoryl lipid A nanoparticle. PNAS NEXUS 2024, 3. [Google Scholar] [CrossRef]
- Swart, M.; Allen, J.; Reed, B.; Gil, A.I.; Verspuij, J.; Schmit-Tillemans, S.; Chakkumkal, A.; Findeis, M.; Hafner, A.V.; Harjivan, C.; et al. Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate. VACCINES 2024, 12. [Google Scholar] [CrossRef]
- Bai, D.; Kim, H.; Wang, P. Development of semisynthetic saponin immunostimulants. MEDICINAL CHEMISTRY RESEARCH 2024, 33, 1292–1306. [Google Scholar] [CrossRef]
- Chang, C.C.; Algaissi, A.; Lai, C.C.; Chang, C.K.; Lin, J.S.; Wang, Y.S.; Chang, B.H.; Chang, Y.C.; Chen, W.T.; Fan, Y.Q.; et al. Subunit vaccines with a saponin-based adjuvant boost humoral and cellular immunity to MERS coronavirus. VACCINE 2023, 41, 3337–3346. [Google Scholar] [CrossRef]
- Cui, A.; Liu, H.; Liu, X.; Zhang, M.; Xiao, B.; Wang, B.; Yang, J. Steroidal saponins: Natural compounds with the potential to reverse tumor drug resistance (Review). ONCOLOGY LETTERS 2024, 28. [Google Scholar] [CrossRef]
- Prithviraj, T. Plant-derived glycosides in cancer treatment: diverse strategies for tumor suppression. NATURAL PRODUCT RESEARCH 2024. [Google Scholar] [CrossRef]
- Teymouri, F.; Karimi, E. Development of chitosan-folate modified PLGA nanoparticles for targeted delivery of diosgenin as an anticancer agent. DISCOVER ONCOLOGY 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.L.d.O.; Marinho, P.A.F.; de Oliveira, A.M.; de Souza, T.A.; Cibulski, S.P.; Alves, H.d.S. Apodanthera glaziovii (Cucurbitaceae) Shows Strong Anti-Inflammatory Activity in Murine Models of Acute Inflammation. PHARMACEUTICS 2024, 16. [Google Scholar] [CrossRef]
- Zhang, W.; Li, F.; Cheng, J.; Wang, Y.; Zheng, Y.; Li, H.; Lin, M.; Ruan, J.; Zhang, Y.; Wang, T. Saponins from Dolichos lablab seeds with anti-inflammatory activity. BIOORGANIC CHEMISTRY 2024, 151. [Google Scholar] [CrossRef] [PubMed]
- Hieu, N.V.; Vinh, L.B.; Phong, N.V.; Cong, P.V.; Dat, N.T.; Dan, N.V.; Duc, N.V.; Tao, H.M.; Tam, L.T.; Anh, L.T.; et al. Two New Steroidal Saponins with Potential Anti-Inflammatory Effects from the Aerial Parts of Gnetum formosum Markgr. PLANTS-BASEL 2024, 13. [Google Scholar] [CrossRef]
- Liang, Z.W.; Guan, Y.H.; Lv, Z.; Yang, S.C.; Zhang, G.H.; Zhao, Y.H.; Zhao, M.; Chen, J.W. Optimization of saponin extraction from the leaves of Panax notoginseng and Panax quinquefolium and evaluation of their antioxidant, antihypertensive, hypoglycemic and anti-inflammatory activities. FOOD CHEMISTRY-X 2024, 23. [Google Scholar] [CrossRef]
- Tao, H.H.; Zhou, Y.Q.; Wei, X.; Yin, X.; Zhao, C.; Zhou, Y. Anti-inflammatory activity of a new lactone isolated from the leaves of Ardisia crenata Sims. CHEMISTRY & BIODIVERSITY 2024, 21. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, J.; Cao, Y.; Shen, Y.; Liu, J.; Zhao, J. Extraction and biological activities of polysaccharides and saponins from Aralia elata: a review. NATURAL PRODUCT RESEARCH 2024. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Diao, M.; Li, J.; Xie, N. Production and pharmaceutical research of minor saponins in Panax notoginseng (Sanqi): Current status and future prospects. PHYTOCHEMISTRY 2024, 223. [Google Scholar] [CrossRef]
- Silveira, F.; Garcia, F.; Garcia, G.; Chabalgoity, J.A.; Rossi, S.; Baz, M. Intranasal Delivery of Quillaja brasiliensis Saponin-Based Nanoadjuvants Improve Humoral Immune Response of Influenza Vaccine in Aged Mice. VACCINES 2024, 12. [Google Scholar] [CrossRef]
- Bhagchandani, S.H.; Yang, L.; Lam, J.H.; Maiorino, L.; Ben-Akiva, E.; Rodrigues, K.A.; Romanov, A.; Suh, H.; Aung, A.; Wu, S.; et al. Two-dose priming immunization amplifies humoral immunity by synchronizing vaccine delivery with the germinal center response. SCIENCE IMMUNOLOGY 2024, 9. [Google Scholar] [CrossRef]
- Plieskatt, J.; Bang, P.; Wood, G.K.; Naghizadeh, M.; Singh, S.K.; Jore, M.M.; Theisen, M. Clinical formulation development of Plasmodium falciparum malaria vaccine candidates based on Pfs48/45, Pfs230, and PfCSP. VACCINE 2024, 42, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Min, H.; Yao, S.; Yao, G.; Zhang, D.; Zhang, B.; Chen, M.; Liu, F.; Cui, L.; Zheng, L.; et al. Evaluation of different types of adjuvants in a malaria transmission-blocking vaccine. INTERNATIONAL IMMUNOPHARMACOLOGY 2024, 131. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Ruiz, S.; Garinot, M.; Chavagnac, C.; Agrawal, P.; Escobar, J.; Revet, L.; Asensio, M.J.; Piras, F.; Fang, F.G.; et al. SPA14 liposomes combining saponin with fully synthetic TLR4 agonist provide adjuvanticity to hCMV vaccine candidate. NPJ VACCINES 2024, 9. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Fang, S.; Liu, Z.; Liu, H.; Zhao, Y.; Liu, L.; Shao, Z. Saponins enhance the stability and cost-efficiency of human embryonic stem cell culture. CELL REGENERATION 2025, 14. [Google Scholar] [CrossRef]
- Pu, X.; Ren, J.; Ma, X.; Liu, L.; Yu, S.; Li, X.; Li, H. Polyphylla saponin I has antiviral activity against influenza A virus. INTERNATIONAL JOURNAL OF CLINICAL AND EXPERIMENTAL MEDICINE 2015, 8, 18963–18971. [Google Scholar]
- Karpova, E.A.; Kostikova, V.A.; Khramova, E.P.; Shaldaeva, T.M.; Vasil’eva, O.Y.; Mazurkova, N.A.; Filippova, E.I.; Mazurkov, O.Y.; Makarevich, E.V. Roots of Rosa majalis Herrm. as a source of antioxidants and anti-influenza agents. RENDICONTI LINCEI-SCIENZE FISICHE E NATURALI 2024. [Google Scholar] [CrossRef]
- Dai, Q.; Wu, S.T.; Zheng, X.; You, P.T.; Liu, Y.W.; Zhao, Y.; Zhang, X.Q. Chikusetsusaponin IVa Targets Nrf2 to Inhibit H9N2 Avian Influenza Virus Infection. PHARMACOGNOSY MAGAZINE 2024. [Google Scholar] [CrossRef]
- Pranweerapaiboon, K.; Garon, A.; Seidel, T.; Janta, S.; Plubrukarn, A.; Chaithirayanon, K.; Langer, T. In vitro and in silico studies of holothurin A on androgen receptor in prostate cancer. JOURNAL OF BIOMOLECULAR STRUCTURE & DYNAMICS 2022, 40, 12674–12682. [Google Scholar] [CrossRef]
- Pothiaraj, G.; Manoranjani, M.; Pitchaikani, S.; Seker, G.K.; Saravanan, K.M.; Rajan, M.; Shakila, H. Investigation of therapeutic and immunomodulatory activity of Bacopa saponin from Bacopa monnieri. SOUTH AFRICAN JOURNAL OF BOTANY 2022, 151, 639–650. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Li, X.; Zhao, H.; Li, H.; Kuang, J.; Li, J.; Guo, J.; Huang, T.; Li, J. Tea saponin-Zein binary complex as a quercetin delivery vehicle: preparation, characterization, and functional evaluation. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2024, 279. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Shao, P.; Kai, D.; Yang, L.; Sun, P.; Feng, S. Novel Nanoliposomes Synergistically Modulated by Sitogluside and Dioscin: Stability, Bioavailability, and Capacity To Alleviate Hyperuricaemia. JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY 2024, 73, 2596–2612. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Yu, Y.; Lv, D.; Ma, S.; Gao, M.; Yang, Y.; Yuan, C.; Liu, Y.; Wang, C. Panax notoginseng saponins and acetylsalicylic acid co-delivered liposomes for targeted treatment of ischemic stroke. INTERNATIONAL JOURNAL OF PHARMACEUTICS 2024, 667. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zhang, J.; Hou, J.; Feng, R.; Yin, J. Pharmacokinetics study of ginsenoside Rg1 liposome by pulmonary administration. HELIYON 2024, 10. [Google Scholar] [CrossRef] [PubMed]
- COLLINS, N. ADVANCED DEVELOPMENT OF LQ A LIPOSOME-BASED SAPONIN-CONTAINING ADJUVANT FOR USE IN PANSARBECOVIRUS VACCINES 2023. Awarded Grant.
- Guo, L.; Gao, Z.; Zhang, L.; Guo, F.; Chen, Y.; Li, Y.; Huang, C. Saponin-enriched sea cucumber extracts exhibit an antiobesity effect through inhibition of pancreatic lipase activity and upregulation of LXR-β signaling. PHARMACEUTICAL BIOLOGY 2016, 54, 1312–1325. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yanagita, R.C.; Liu, C.; Hu, X.; Dong, P.; Xue, C.; Xue, Y. Effects of two sulfated triterpene saponins echinoside A and holothurin A on the inhibition of dietary fat absorption and obesity reduction. BIOSCIENCE BIOTECHNOLOGY AND BIOCHEMISTRY 2014, 78, 139–146. [Google Scholar] [CrossRef]
- SANTHAKUMARI, G.; STEPHEN, J. ANTIMITOTIC EFFECTS OF HOLOTHURIN. CYTOLOGIA 1988, 53, 163–168. [Google Scholar] [CrossRef]
- Wargasetia, T.L.; Ratnawati, H.; Widodo, N. Anticancer Potential of Holothurin A, Holothurin B, and Holothurin B3 from the Sea Cucumber Holothuria scabra, 2020. Proceedings Paper. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).