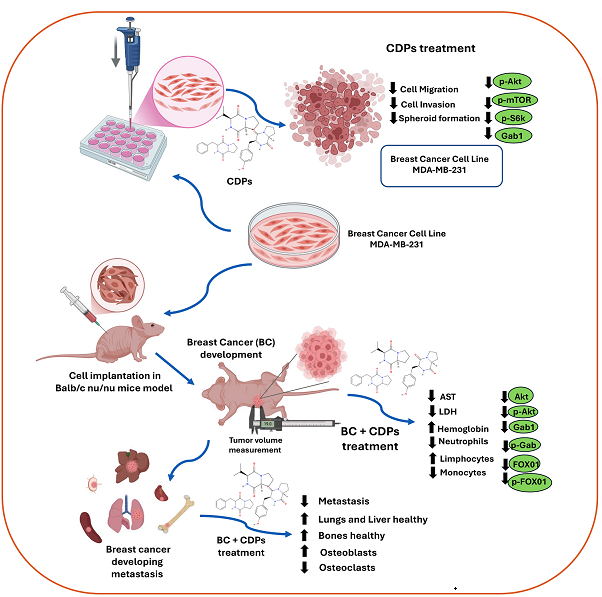
1. Introduction
Breast cancer is the principal cause of death in women worldwide; it is a clinically heterogeneous disease, and around 10-15% of patients present aggressive phenotypes and develop metastasis [
1]. Triple-negative breast cancer (TNBC) comprises a group of diseases with different histological, genomic, and immunological profiles, characterized by lacking hormonal estrogen and progesterone receptors, as well as human epidermal growth factor receptor 2 (HER2). According to postulate in the “soil and seed” theory, tumor cells thrive in those tissues with genetic and metabolic characteristics similar to their needs. For example, breast cancer cells tend to metastasize the bone, lung, and liver [
2]. Other factors in developing breast cancer, such as obesity, represent a poor prognosis for patients since the interaction of adipocytes with tumor cells provides an optimal microenvironment that favors proliferation, invasion, metastasis, or resistance to cell death [
3]. The tumor microenvironment also recruits the malignant cells with other types of cells, such as adipocytes, mesenchymal-type cells, such as fibroblasts, and the cells of the immune system, such as macrophages, T-lymphocytes, and neutrophils. These interactions are mediated by the secretion of chemokines, interleukins, or growth factors, which promote proliferation, invasion, and resistance to cell death, positioning these components as possible new therapeutic strategies [
4].
Among the first-line therapeutic options for treating metastatic breast cancer are taxanes, anthracyclines, methotrexate, carboplatin, PD-1 or PD-L1 inhibitors, PARP inhibitors, and their combination with immunotherapy, radiotherapy, and surgery [
5]. However, a significant percentage of patients negatively respond to these treatments, resulting in low survival and poor quality of life. Therefore, the search for new therapeutic options that improve the prognosis for patients with metastatic breast cancer is of great importance.
Bone metastasis is a common complication in advanced-stage breast and prostate cancer, disrupting the bone remodeling cycle mediated by osteoblasts and osteoclasts [
6]. Currently, available T cell-based immunotherapies show promise in the treatment of cancer; they are often ineffective in patients with advanced stages of cancer that include bone metastases [
2]. Metastases predominantly occur in trabecular and red marrow-rich bones, suggesting that specific bone environments favor metastatic growth [
7]. Once bone metastases develop, the five-year survival rate drops to approximately 20% [
8]. Bone metastases significantly impact patient health, yet current treatments, including bisphosphonates and antiresorptive antibodies, remain insufficient; therefore, develop of novel therapies that target both tumor cells and the bone microenvironment with dual antiresorptive (reducing bone remodeling) and anticancer properties are required [
9].
Cyclodipeptides (CDPs) are a class of molecules synthesized by a broad type of organisms, which possess interesting biological activities such as cytotoxic effects in different cancer cell lines [
10,
11,
12]. Previously, we described that CDPs, cyclo(L-Pro-L-Tyr), cyclo(L-Pro-L-Val), and cyclo(L-Pro-L-Phe) isolated from the
Pseudomonas aeruginosa PAO1 bacterium induce apoptosis in cervical, colon, leukemia, and melanoma cancer cells by inhibiting phosphorylation of multiple kinases from the PI3K/Akt/mTOR pathway, including mTORC1/C2 complexes [
11]. Furthermore, a significant decrease in tumors in mice treated with CDPs at 0.1 mg/g of weight was found in a mouse murine melanoma model. Additionally, treatment with CDPs led to the restoration of hematological parameters and a decrease in tissue damage markers AST and ALT, as well as a marked reduction in the expression of proteins that mediate pathways involved in energy metabolism, lipid synthesis, epithelial-mesenchymal transition, invasion, and metastasis [
13]. Interestingly, transcriptomic analysis in HeLa cells found that CDPs inhibit gene expression in the mevalonate pathway and lipid synthesis [
14]. Given this background, we sought to determine whether CDPs could have a promising effect on breast cancer and whether this effect could be observed in advanced stages of tumorigenesis [
15].
In this work, we first used monolayer culture to evaluate the migratory and invasive potential of the MDA-MB-231 human triple-negative breast cancer cell line treated with CDPs and combined with Methotrexate (MTX). Further, the effect of the CDPs on advanced-stage mammary tumors developed, and their ability to prevent the appearance of metastatic foci through the implantation of the MDA-MB-231 cells in the mammary tissue of mice model was evaluated.
2. Materials and Methods
2.1. Chemicals, Reagents, and Cell Culture
Chemicals and reagents included are Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich), fetal bovine serum (FBS; Gibco Life Technology), and trypsin solution (Sigma Life Science). Cyclodipeptides mixture composed mainly by cyclo(L-Pro-L-Tyr), cyclo(L-Pro-L-Val), and cyclo(L-Pro-L-Phe) are isolated from the
Pseudomonas aeruginosa PAO1 bacterium cells-free supernatant with a purity > 90% was used as therapeutic bioactive compounds [
16,
17]. The CDPs mixture was dissolved in a DMSO-water ratio 1:3 to prepare stock solutions (100 mg/mL). The MDA-MB-231 cell line was obtained from invasive ductal carcinoma (ATCC, Manassas, VA, USA) Breast tumor cell lines from pleural effusions. This cell line is estrogen receptor (ER), progesterone receptor (PR), and E-cadherin negative, p53 mutated, lacking the growth factor receptor HER2, commonly used as a study model of triple-negative breast cancer [
18]. MDA-MB-231 cells, when implanted in xenografts TNBC mouse model into lymph nodes as described below. The MCF-7 is a human metastatic breast cancer cell line (adenocarcinoma) dependent on estrogen, progesterone, and glucocorticoid receptors (ATCC, Manassas, VA, USA) [
19]. MDA-MB-231 and MCF-7 cell lines were cultured in complete media [DMEM supplemented with 10% (v/v) FBS, 100 units/mL of penicillin, 40 µg/mL of streptomycin, and 1 µg/mL of amphotericin B (Sigma-Aldrich Co.), supplemented with 1.6 g/L of glucose. Cell culture media were changed twice a week, incubating at 37 °C under 80% humidity and an atmosphere of 5% CO
2 to confluency. Cells were then trypsin-treated, counted using a hemocytometer chamber, and used for subsequent assays. Cell cultures and other procedures were performed in class II biological safety cabinets.
2.2. Cell Viability and Apoptosis Determination in Cell Cultures
Cell viability was determined colorimetrically with the MTT method. Briefly, cell cultures grew in 96-well flat-bottomed plates with DMEM medium with FBS for 24 h, incubated in the presence or the absence of the indicated amounts of CDPs for 24 h at 37°C with 5% CO2. To determine cell viability, MTT (50 mg/mL) in PBS was added to each well and incubated for 4 h at 37°C. Finally, 100 uL of 2-propanol/1M HCl (19:1, v/v) was added to dissolve the formazan crystals, and the absorbance was measured at 595 nm using a microplate reader (BioTek Instruments).
Necrosis and apoptosis were evaluated in cell cultures incubated in DMEM medium with FBS for 4 h with CDPs treatment. DMSO was used as a control at the same concentration to dissolve the CDPs. Following incubation, cells were collected by centrifugation at 2,000 × g for 10 min. The pellet was suspended in 20 uL and incubated with annexin V and propidium iodide (PI) (Dead Cell Apoptosis Kit; Molecular Probes, Invitrogen Life Technologies). Fluorescence was immediately quantified by FACS using an Accuri-C6 Flow Cytometer (BD Biosciences). At least 20,000 cellular events were used for calculations.
2.3. Wound Closure Migration Assay
MDA-MB-231 and MCF-7 cell lines were grown in a 95% confluent monolayer, and three wounds were made per plate with a sterile pipette tip in assays by triplicate. Plates were washed with PBS twice and fresh complete medium; the CDPs, MTX, and combined treatments were placed for 24 and 48 h, and photographs were taken every 24 h to subsequently quantify the wound area using the ImageJ software (NIH).
2.4. Invasion Assay
MDA-MB-231 or MCF-7 cell lines were cultured in a transwell chamber previously coated with Matrigel (Corning, Life Sciences) with a complete DMEM medium. Co-culture was carried out using Raw 264.7 macrophages seeded in the lower chamber with complete DMEM medium and treatments with CDPs or MTX at 0.01 and 0.005 mg/mL concentrations, respectively. The transwell insert was placed into a 24-well plate with 0.2% (w/v) crystal violet containing PBS and incubated for 5 minutes; the transwell insert was washed with any remaining crystal violet from the membrane. The membrane was dried and photographed using a microscope; multiple images were taken with a 10x or 20x objective to capture a representative field of view [
20].
2.5. Multicellular Microspheroids
MDA-MB-231 or MCF-7 cell lines were cultured (2 x10
5 cells) under non-stick conditions (0.6% agarose) in complete DMEM medium for 14 days with periodic medium changes until the spheroids reached a size of 40-50 µm [
21]. Once treatment with CDPs (0.1 mg/mL), CDPs+cd (0.1 mg/mL), or CDPs-MTX containing (0.1 mg/mL CDPs, 0.05 mg/mL MTX) was applied. Photographs were taken at 72 h of treatment, and a cell viability test was performed on the remaining cells using an MTT reduction assay. MTT, 50 mg/mL in PBS, was added to each well and incubated for 4 h at 37°C. Finally, 100 µL of 2-propanol/1M HCl (19:1, v/v) was added to dissolve the formazan crystals, and the absorbance was measured at 595 nm using a microplate reader (BioTek Instruments).
2.6. Western Blot
Confluent MCF-7 and MDA-MB-231 cells treated with 0.1 mg/ml of CDPs incubated for 15 min, 1 h, and 4 h were harvested after trypsin treatment and washed twice with PBS, subsequently centrifuged at 5000 × g at 4 °C for 10 min and resuspended in RIPA lysis buffer. The cell suspension or tumor tissue was lysed by three cycles of sonication at low intensity (20 kHz, 5 W) for 30 s each at 4 °C (Hielscher-LS24 Ultrasound Technol). The protein extract free of cellular debris was obtained by centrifugation at 7500 × g for 15 min, and the protein concentration was determined by the Bradford method (BioRad). Protein extracts were separated on 10% (SDS-PAGE) polyacrylamide gels and transferred to PVDF membranes (Millipore, Billerica, MA, USA) in a BioRad transfer chamber at 15 volts for 45 min. Briefly, PVDF membranes were incubated with TBS-T (Tris-HCL 10 mM; NaCl 0.9%; tween-20 0.1%, dry milk 5%, pH 7.8). They were washed three times with TBS-T for 6 min each and incubated with primary antibodies dissolved in TBS-T at the concentration suggested by the manufacturer. Antibodies were used: anti-AKT, anti-p-AKT (Ser 473), anti-mTOR, anti-p-mTOR, anti-S6K, anti-p-S6K, anti-Vimentin; anti-Gab1, anti-FOX01, anti-p-FOX01, and anti-b-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA and Cell Signaling Technology, MA, USA). All primary antibodies (1:5,000 dilution) were incubated for 12 h at 4°C and subsequently three washes by 6 min with TBS-T were performed and then incubated with anti-mouse secondary HRP-conjugated antibody (BioRad, CA, USA) at a concentration of 1:10,000 in TBS-T for 2 h; membranes were washed as above and developed with Supersignal West Pico Luminol (Pierce; Thermo Fisher Scientific, Waltham, MA, USA). Then, images were captured using a ChemiDoc™ MP System (Bio-Rad). Assays were conducted at least three times, and representative images are shown. The Image J software (NIH) was used to quantify image band intensities.
2.7. Orthotopic Model of Metastatic Breast Cancer
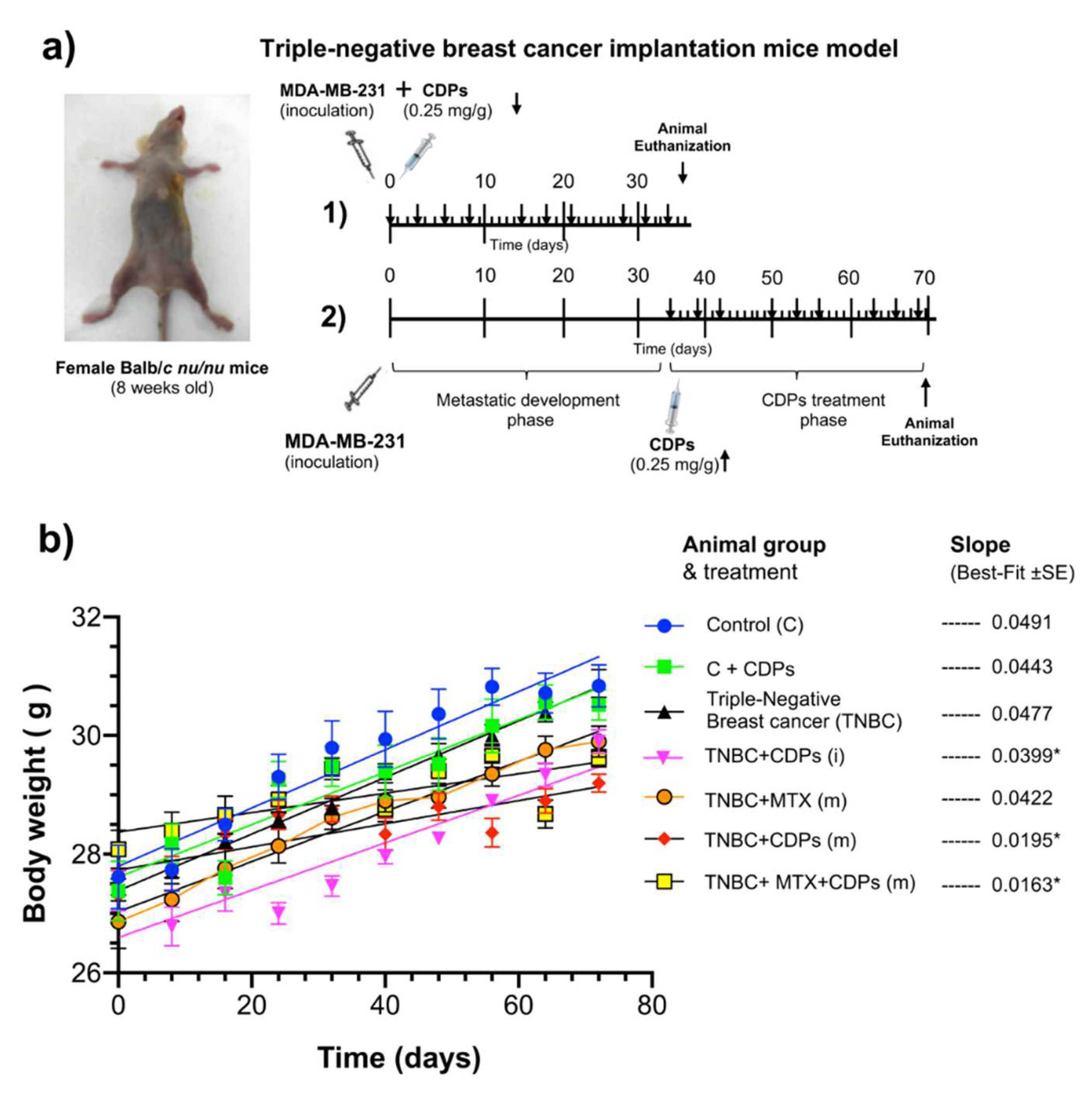
Immunosuppressed Balb/c nu/nu female mice aged 8 weeks were adapted for 15 days in a pathogen-free environment with a standard diet and drinking water ad libitum. The cages, water, food, and bedding were autoclaved. Animal manipulation was authorized by the Institutional Animal and Use Committee (IACUC) from the Universidad Michoacana de San Nicolás de Hidalgo (IIIQB-UMSNH-IACUC-2022-35). Experiments complied with standard guidelines for the welfare of animals following the Institutional Committee and recommendations of the Mexican Official Regulations for the Use and Care of Animals (NOM 062-ZOO-1999; Ministry of Agriculture, Mexico). Animal handling, feeding, and care were done by trained personnel under the NIH guide for the care and use of laboratory animals.
Once the adaptation period had concluded with 100% survival of the mice, 1 x105 MDA-MB-231 metastatic breast cancer cells suspended in Matrigel were inoculated by direct injection into the nearest breast fat pad to the right armpit. Mice were sedated with ketamine (ket)/xylazine (xyl) solution intraperitoneally (IP) (80 mg/kg ket and 10-15 mg/kg xyl) and kept on a thermal mat during sedation to prevent hypothermia. The mice’s weight was monitored, and the longest diameter “a” and the shortest diameter “b” of the tumor were measured with a caliper; the tumor volume was determined using the tumor volume formula (TV) = (0.4) (ab2), “a” was the long diameter and “b” the short diameter of the visual tumor. The treatment consisted of 0.25 mg/kg of body weight of CDPs (0.1 mg/kg CDPs) via the IP or 0.05 mg/kg of MTX in physiological saline by IP via or in CDPs+MTX (0.1 mg/kg CDPs, 0.05 mg/kg MTX) combined treatment. The CDPs treatments were carried out by three rounds of administration, each consisting of three doses administered every 3 days (two days between each administration) with a week of rest between each administration round, summing nine doses per mouse. The animal groups each of five mice were evaluated as follows: healthy mice without tumor and treatment (C); healthy mice without tumor administered with the CDPs (C+CDPs); mice with TNBC tumor and without treatment (TNBC); mice with tumor and treatment with CDPs from cell inoculation in early stage [TNBC+CDPs(i)]; mice with tumor and treatment with CDPs at 35th-day post-inoculation in advanced stage [TNBC+CDPs (m)]; advanced-stage tumor-bearing and MTX-treated mice (TNBC+MTX(m)); mice with tumor and combined CDPs+MTX treatment (TNBC+CDPs+MTX(m)); each group with n=5.
2.8. Animal Euthanasia and Biological Samples
All mice were injected with a lethal dose of sodium pentobarbital intraperitoneally (100-150 mg/Kg body weight) as recommended by IIQB/UMSNH/IACUC and NOM 062-ZOO-1999. The tumors were removed, whole blood was obtained by cardiac puncture, and collected in a microtube with EDTA anticoagulant. The liver, lungs, spleen, kidneys, and femur of both legs were also removed and weighed. Blood hematocrit (Hct) was determined in a capillary tube with heparin by centrifugation at 3500 rpm for 5 min, and Hemoglobin (Hb) was determined by the Hct value divided by 3.3. The whole blood was centrifuged at 3500 rpm for 10 min to separate the blood serum. Subsequently, the enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were determined using a Fuji Dry-Chem NX 700 Fujifilm equipment.
2.9. Histological Analysis of Tissues and Bones
After euthanasia, the organs were dissected, and the tumors, lungs, and liver were utilized for histological analysis by observing tumor abscesses. The tumor, lungs, and liver were fixed in 4% paraformaldehyde and remained until dehydration. The samples were subjected to a dehydration process of 4 h at different concentrations of ethanol and xylene and embedded in paraffin. Tissue sections of 4 μm were cut and placed on slides for subsequent staining with H&E. Photographs were taken under the optic microscopy and recorded using an Accu-Scope EXC-120 LED microscope camera at magnifications of 4x, 10x, 40x, and 100x across tissue sections.
For bone histology, the lower limbs of the mice were dissected, removing skin and muscle tissue in the femurs. Femurs were fixed in paraformaldehyde and treated with 3 mL decalcification solution (10% EDTA, pH 7.4) for four weeks. Weight loss in femurs was determined by normalizing total animal weight over the weight of the femur during the decalcification procedure. For histology of bone slides, samples were dehydrated and underwent sequential ethanol treatments with increasing concentrations (80–100% ethanol), followed by xylene treatment and paraffin infiltration (Hycel) in two cycles before embedding in histological molds. The femurs were sectioned longitudinally to 5 μm-thick slices using a rotary HM 355S microtome (Thermo Fisher Scientific). To assess bone resorption, the presence of osteoclasts and osteoblasts in the bone sections were stained with hematoxylin and eosin (H&E).
2.10. Statistical Analysis
All data were evaluated using analysis of variance (ANOVA), and significant differences at (p< 0.05) were determined using Tuke’s, Bonferroni, or t-student posthoc tests using GraphPad Prism 6.0 software (GraphPad Software).
4. Discussion
CDPs (from the diketopiperazines family) have emerged as promising candidates for anticancer therapy due to their structural stability, high selectivity, and ability to efficiently inhibit key cancer-related pathways [
23]. Previously, the cytotoxic and apoptotic effects of the bacterial CDPs have been demonstrated in various cancer cell lines [
10,
11,
12,
15,
22]; in addition, their impact on breast cancer lines MCF-7 and MDA-MB-231 cells was described, triggering apoptosis in both cell lines in a concentration-dependent manner [
12].
Several specific CDPs have exhibited potent anticancer activity; cyclo(Phe-Pro) inhibits the growth of HT-29, MCF-7 and HeLa cells [
24], cyclo(Tyr-Cys) inhibits cervical carcinoma cells HT-29 and MCF7 [
25], cyclo(L-Leu-D-Arg) shows cytotoxicity against MDA-MB-231 cells [
26]. Diketopiperazines such as fumitremorgin C analogs have shown potential in overcoming multidrug resistance, including inhibiting the breast cancer resistance protein (BCRP), a key efflux transporter responsible for reducing intracellular drug accumulation. Moreover, the novel diketopiperazine-based compound HLY838 functions as an O-GlcNAc transferase (OGT) inhibitor, potentiating the anti-tumor effects of CDK9 inhibitors by downregulation c-Myc and E2F1 expression [
27]. Similarly, Verticillin A, a diketopiperazine-derived compound, suppresses c-Met phosphorylation and its downstream Ras/Raf/MEK/ERK signaling pathway, significantly reducing colon cancer cell metastasis [
28]. Collectively, these findings highlight the cyclopeptides such as CDPs as anticancer agents. Bacterial CDPs have demonstrated significant potential as anti-metastasic agents by targeting key proteins involved in epithelial-mesenchymal transition (EMT) and metastasis. In the murine melanoma model, CDPs derived from the
P. aeruginosa bacterium significantly reduce the expression of critical EMT markers, including MMP-1, E-cadherin, N-cadherin, HIF-1a, Vimetin, and CK-1; indicating that CDPs may impact cancer cell migration and invasion [
13].
In this work, functional assays further confirmed the anti-metastatic effects of P. aeruginosa CDPs were carried out.
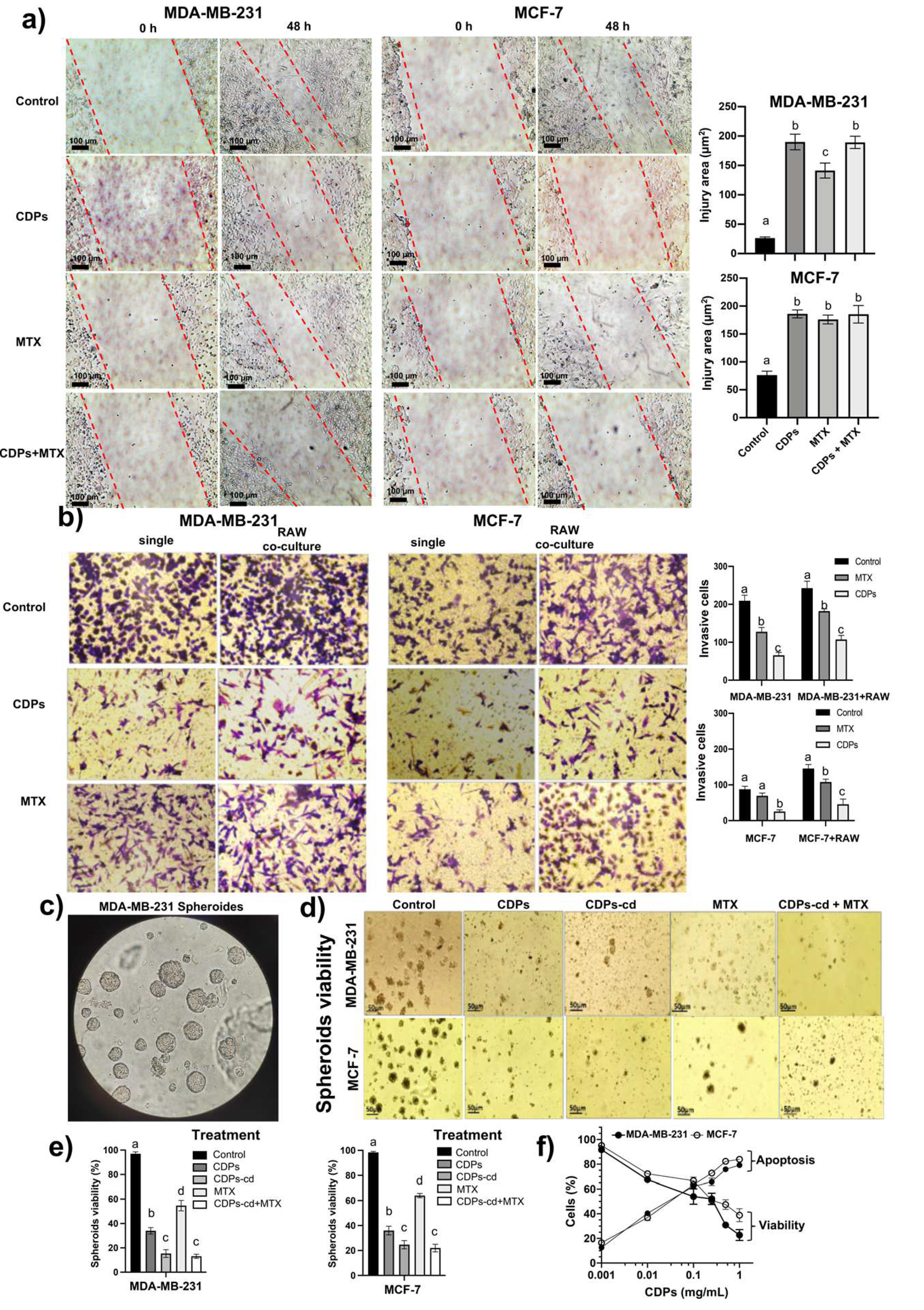
Wound healing assays in monolayers of cell lines cultures of the triple-negative MDA-MB-231and luminal MCF7 breast cancer lines revealed that CDPs-treated cancer cells exhibited a wound area twice as large as the control and 1.5 times that of cells treated with methotrexate (MTX). MDA-MB-231 cells displayed higher migratory capacity than the MCF7 cells in all conditions (
Figure 1a).
Transwell chamber assays using Matrigel and co-culture with macrophages revealed that tumor-associated macrophages (TMAs) enhanced cancer cell invasion by promoting Matrigel degradation and membrane penetration. However, treatment of the TNBC line with CDPs significantly reduced the number of invasive cells in both monoculture and co-culture conditions, suggesting their potential as metastatic agents. This inhibitory effect was comparable to MTX, which has been evaluated in combination therapies for metastatic disease, including bone metastases (
Figure 1b). These findings align with previous reports indicating that TAMs play a crucial role in tumor progression by secreting chemokines and growth factors that facilitate cancer cells proliferation, migration and survival [
29]. Moreover, TAMs have been implicated in modulating drug resistance, further supporting their relevance as therapeutic targets. The ability of the bacterial CDPs to disrupt TAMs cancer cell interactions highlights their potential dual role in inducing apoptosis and inhibiting metastasis.
The multicellular microspheroid model contains subpopulations of cancer stem cells (CSCs) [
30] exhibiting self-renewal capacity and therapy resistance. Our microspheroids assay with MDA-MB-231 and MCF-7 cells demonstrated distinct phenotypic differences between the two lines. MCF-7 spheroids were more compact and enormous, likely due to their epithelial phenotype and high E-cadherin production [
31]. At the same time, the MDA-MB-231 microspheroids were more susceptible to disintegration and more heterogeneous, reflecting their mesenchymal phenotype. Notably, the CDPs treatment significantly reduced spheroids size, number, and viability in both breast cell lines compared to untreated controls and MTX-treated, also inducing apoptosis at concentrations around 0.02 mg/mL for 4 h of CDPs treatment (
Figure 1c-f). These results underscore the potential of bacterial CDPs as effective anti-metastatic agents by targeting both tumor cell invasion and CSC-associated resistance.
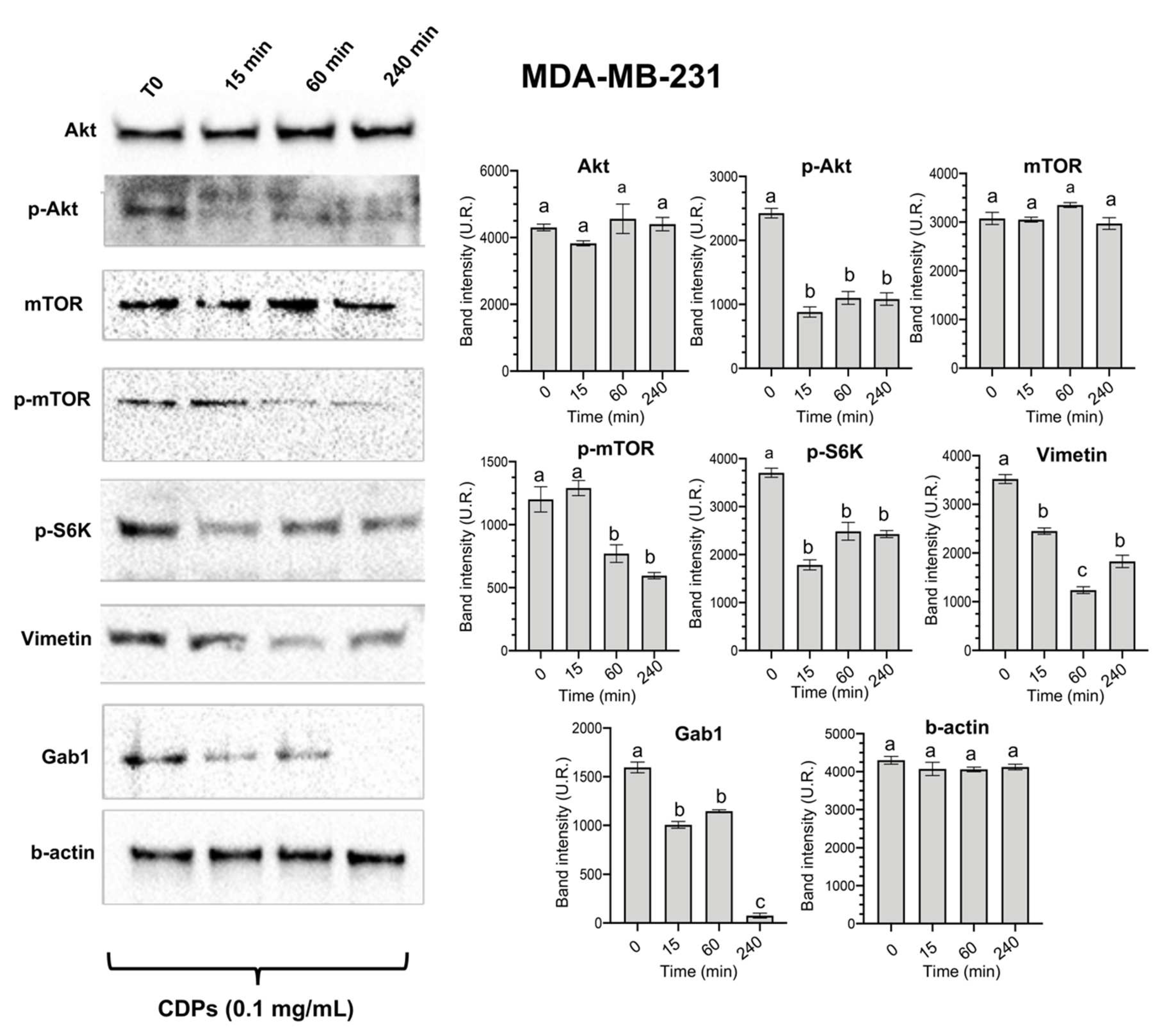
Previous studies from our group have demonstrated in cancer cell lines the involvement of the PI3K/AKT/mTOR pathway in the cytotoxic and proliferative effects of the bacterial CDPs [
10,
11,
13]. Based on these findings, we analyzed key components of this pathway in the triple-negative MDA-MB-231 cell line of breast cancer, observing a significant decrease in the phosphorylation levels of AKT, mTOR, and S6K (
Figure 2). This reduction in protein expression is associated with decreased cell viability, impaired tumor growth, and induction of apoptosis, corroborating previous observations [
12]. Additionally, our results revealed a notable decrease in the expression of Vimentin and Gab1 following treatment with CDPs, further supporting the role of these molecules in targeting metastatic and invasive pathways (
Figure 2). Gab1 overexpression has been linked to enhanced breast cancer metastasis [
32]. At the same time, Vimetin is a well-established marker of EMT and is consistently upregulated during cancer progression and metastasis [
33]. Therefore, the observed downregulation of Gab1 and Vimetin suggests that the CDPs reduce the invasive and migratory capacities in the MDA-MB-231 line, which may translate into decreased tumor aggressiveness.
Weight loss is often one of the earliest detectable symptoms of cancer disease and its progression, leading to severe muscle wasting, which significantly contributes to morbidity and mortality. This condition is primarily driven by the increased production of cytokines and other pro-inflammatory molecules secreted by the immune system to inhibit tumor progression [
34,
35]. In our orthotopic model of metastatic breast cancer, body weight was monitored throughout the treatment period. While the overall pattern of weight gain remained consistent across groups, a transient weight loss was observed in animals treated with CDPs during the initial weeks of administration (
Figure 3b). Nevertheless, this effect did not persist; no behavioral changes or signs of deteriorating health were observed, suggesting that the temporary weight loss did not indicate toxicity or systemic distress. Although the volume and weight of the tumors generated in the TNBC group were significantly observed (
Figure 4), they did not significantly modify the weight of the animals (
Figure 3b). Notably, the administration of CDPs in mice prevented the generation of large and heavy tumors, observing that 40-60% of animals in the TNBC+CDPs(m) and TNBC+CDPs+MTX(m) did not show tumors (
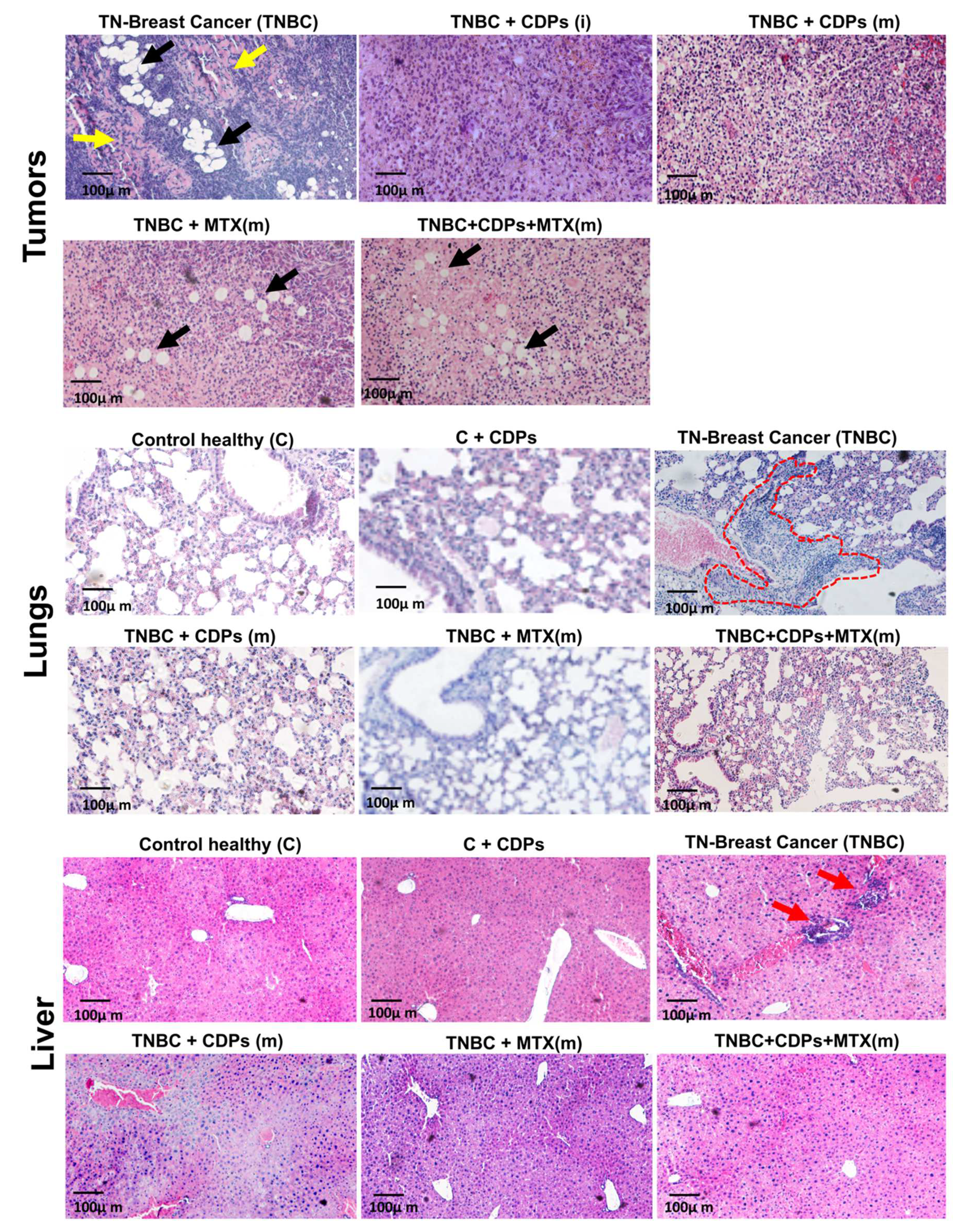
Figure 4). However, when the tumoral tissue of the mice groups was dissected and histologically analyzed, a significant presence of fibrosis and fat drops was observed in the tumors of the TNBC mice group; which was reverted in the CDPs-treated animals (
Figure 6); interestingly the MTX administration, although positively contributed to diminishing fibrosis areas, this drug provokes an increased accumulation of fat drops. Developing bone marrow adipose tissue (BMAT) and fat drops in other organs is a normal physiological process; however, excessive fat accumulation may have a significant pathological implication, such as cancer [
36].
In breast cancer patients, alterations in leukocyte composition, particularly in the neutrophil-to-lymphocyte ratio (NRL), have been documented. NLR is widely recognized as a prognostic biomarker in cancer, with elevated levels correlating with worse prognosis, increased tumor aggressiveness, and reduced overall survival [
37]. The findings suggest that changes in leukocyte distribution may have significant clinical implications in breast cancer progression. In our study, alterations in leukocyte composition were also observed in mice with TNBC. NLR analysis revealed that the TNBC group exhibited the highest value (NLR=8).
In contrast, the groups TNBC+CDPs(i) (NLR=3) and TNBC+ CDPs+MTX(m) (NLR=3.5) showed significantly lower values, suggesting a potential reduction in tumor aggressiveness following CDPs treatment. Anemia is also common among cancer patients [
38]. In our hematological analysis, hemoglobin levels showed improvement in animals treated with CDPs (
Figure 5d), indicating a potential benefit of these molecules in mitigating cancer-associated hematological alterations.
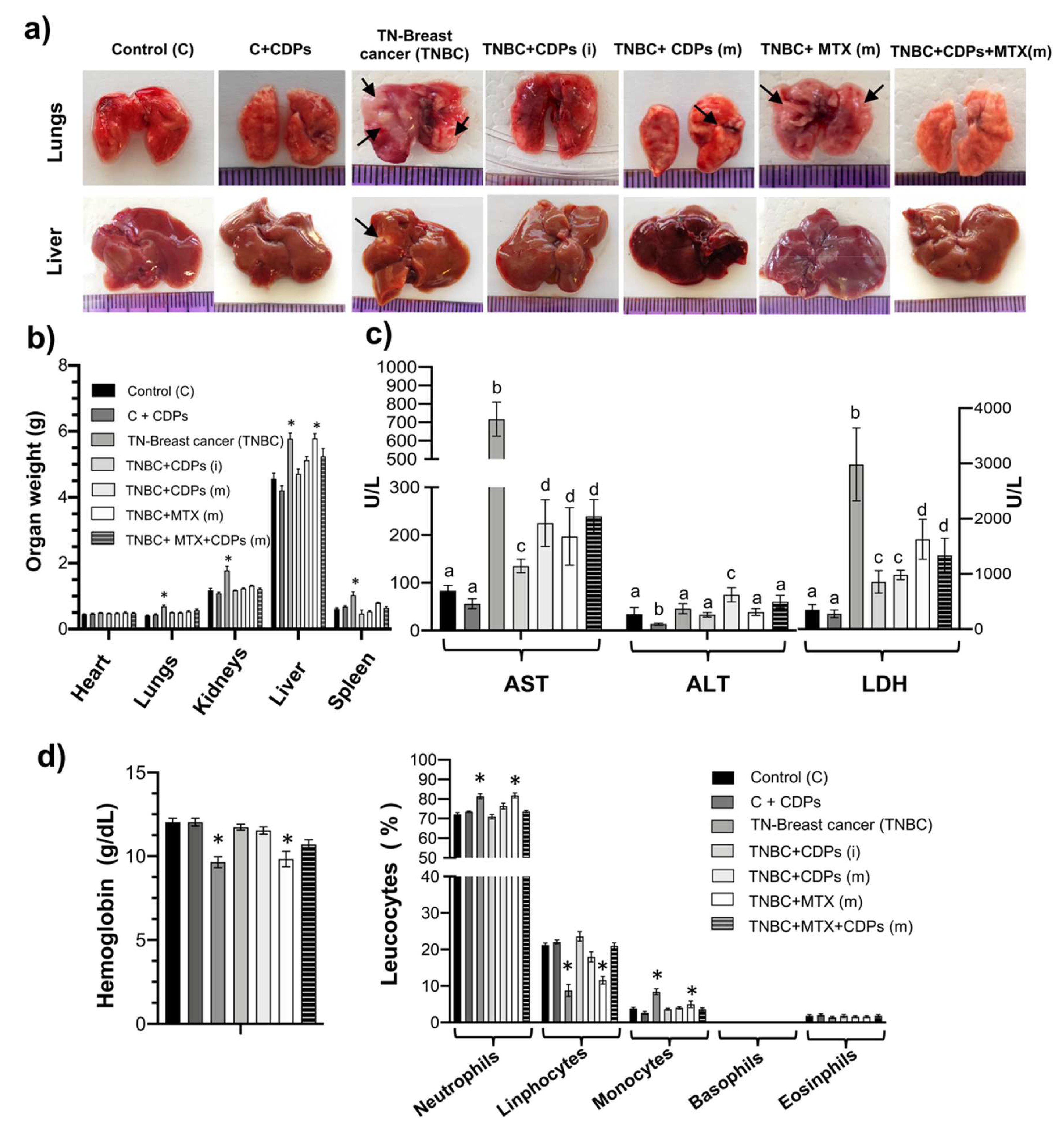
Liver metastasis is common in patients with breast cancer. Previous studies have described that liver function is poor in 92% of patients diagnosed with breast cancer liver metastasis (BCLM) [
39]. Moreover, ALT, AST, GGT, AP, and LDH levels are significantly higher in patients with BCLM. The determination of AST, ALT, and LDH showed a drastic increase in the group with TNBC without treatment (
Figure 5c). The presence of metastatic foci was observed in the TNBC group of animals (
Figure 5a); consistent with these observations, an increase in the weight of the lungs and liver organ was also observed (
Figure 5b). Histological analysis of lung and liver in mice revealed that the CDPs treatment inhibited malign cells and tumor focus (
Figure 6). These findings suggest that the animals treated with CDPs presented levels of AST, ALT, and LDH and organs similar to the control without disease, indicating a positive anti-tumor capability associated with the CDPs treatment.
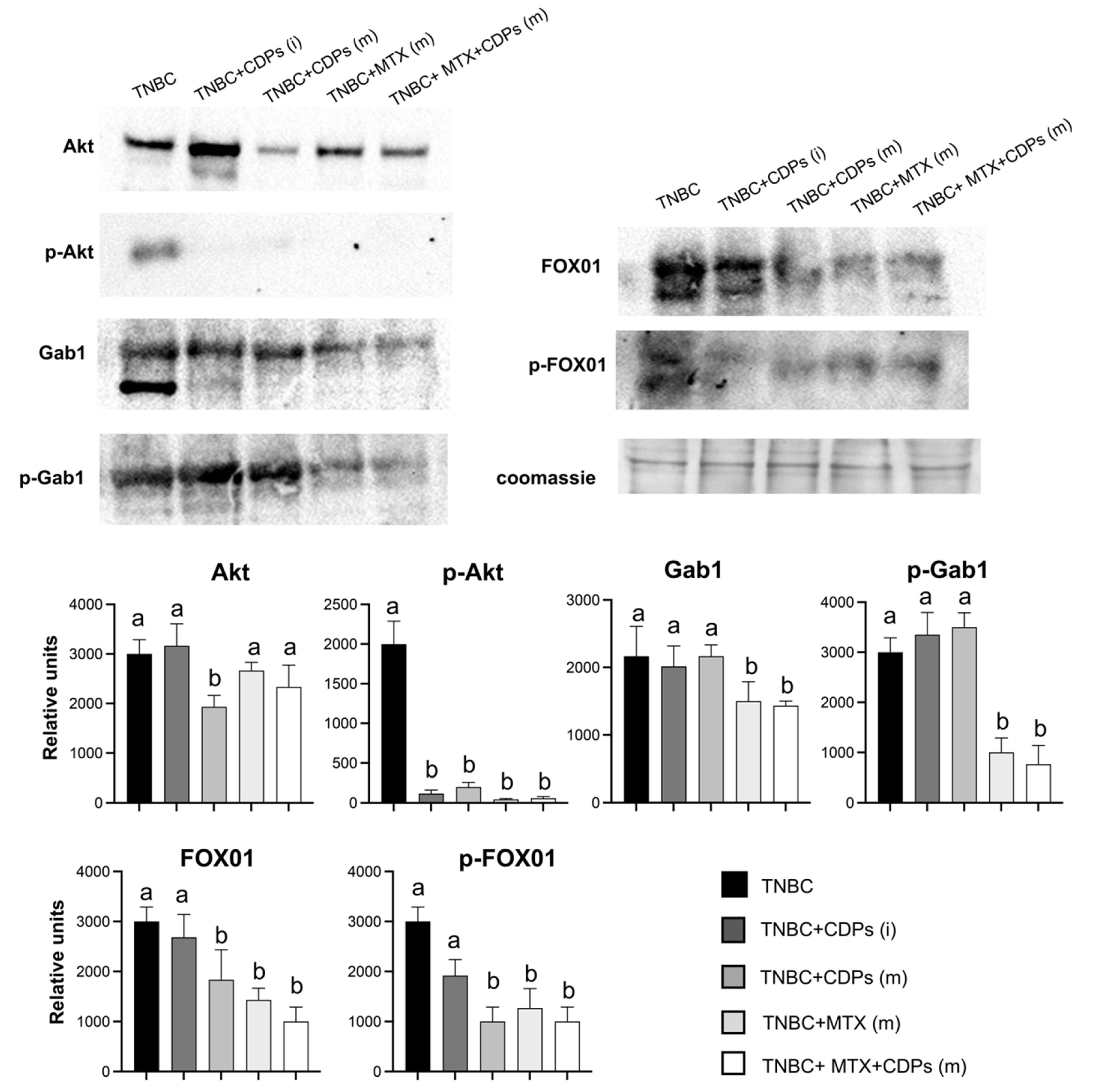
On the other hand, critical tumorigenic markers were repressed in the TNBC CDPs-treated mice. A significant reduction in p-AKT levels was observed in tumors from rodents subjected to treatment (
Figure 7). Notably, TNBC animals treated with CDPs also exhibited a decrease in total AKT protein levels, suggesting a potential disruption of the PI3K/AKT/mTOR signaling pathway in tumors associated with the CDPs administration.
AKT is pivotal in regulating FOX01 phosphorylation, a key factor in cell cycle regulation and apoptosis. Under normal conditions, AKT phosphorylates FOX01, excluding it from the nucleus and suppressing its transcriptional activity. However, when FOX01 remains dephosphorylated, it is translocated to the nucleus, activating apoptosis-related genes and cell cycle arrest [
40].
FOX01, a member of the Forkhead box family of transcription factors, is known to regulate cell cycle arrest, autophagy, and apoptosis. Its tumor-suppressive role has been reported in several malignancies, including breast cancer, where its dysregulation is frequently observed [
41]. In our study, tumors from TNBC animals CDPs-treated exhibited reduced phosphorylation of FOX01, supporting that CDPs inhibit tumor progression (
Figure 7). These findings align with our previous transcriptomic analysis in HeLa cells treated with CDPs, where genes involved in the FOX01 signaling pathway, including GADD45A and SGK1 were upregulated [
14]. Data presented here suggest a differential regulation of FOX01 by CDPs in the triple-negative breast cancer model.
Contrary to observations in the MDA-MB-231 cell line (
Figure 2), Gab1 levels remained unchanged in response to CDPs treatment. However, a notable decrease in both total Gab1 and phosphorylated Gab1 (p-Gab1) levels was detected in tumors from the TNBC+CDPs+MTX(m) and TNBC+MTX(m) groups (
Figure 7). Given that Gab1 acts as a key integrator of multiple signaling pathways involved in cell survival, proliferation, angiogenesis, and invasion [
42]. The Gab1 downregulation suggests a potential mechanism through which CDPs, particularly in combination with MTX, inhibit tumor progression and metastasis.
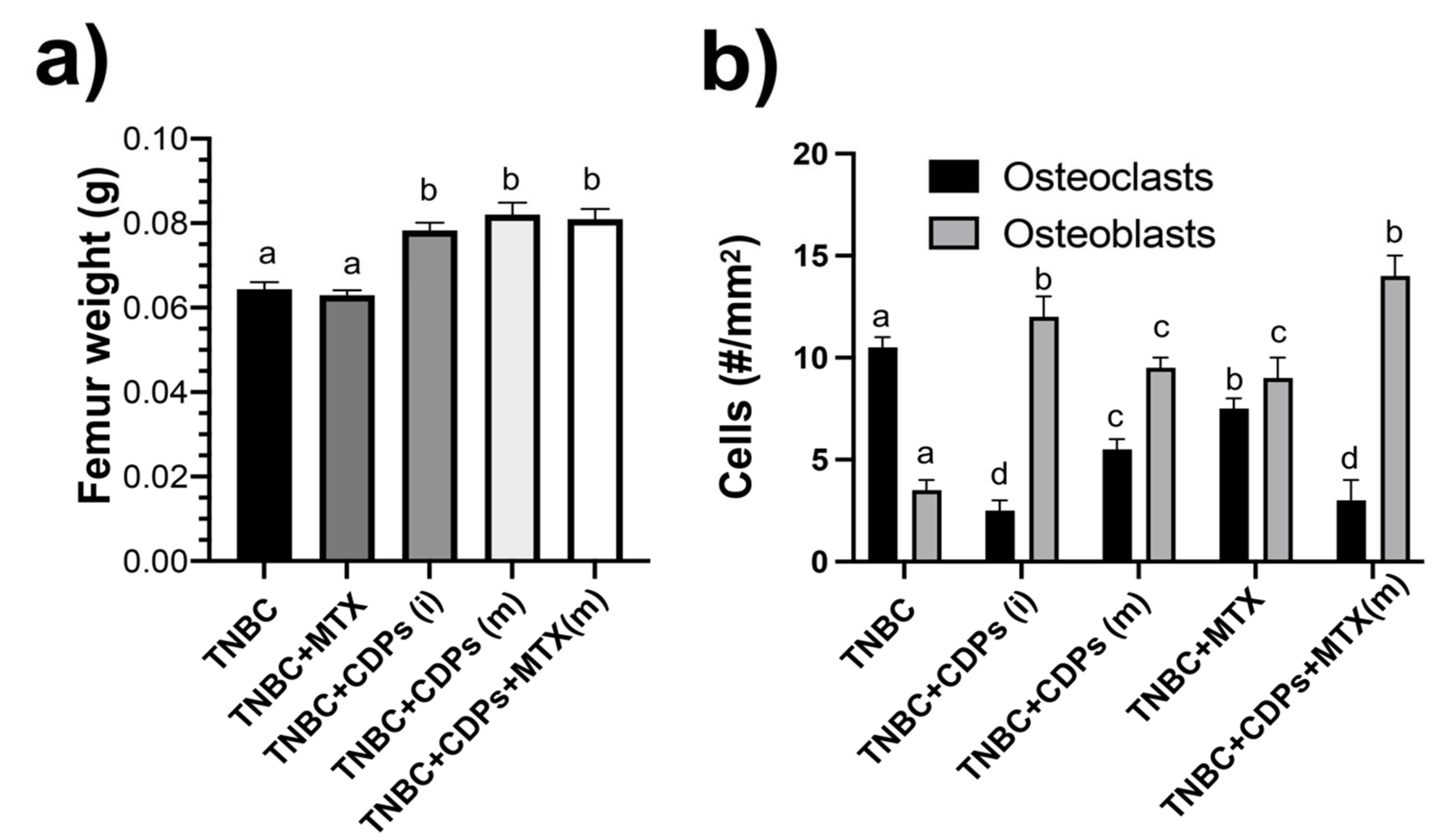
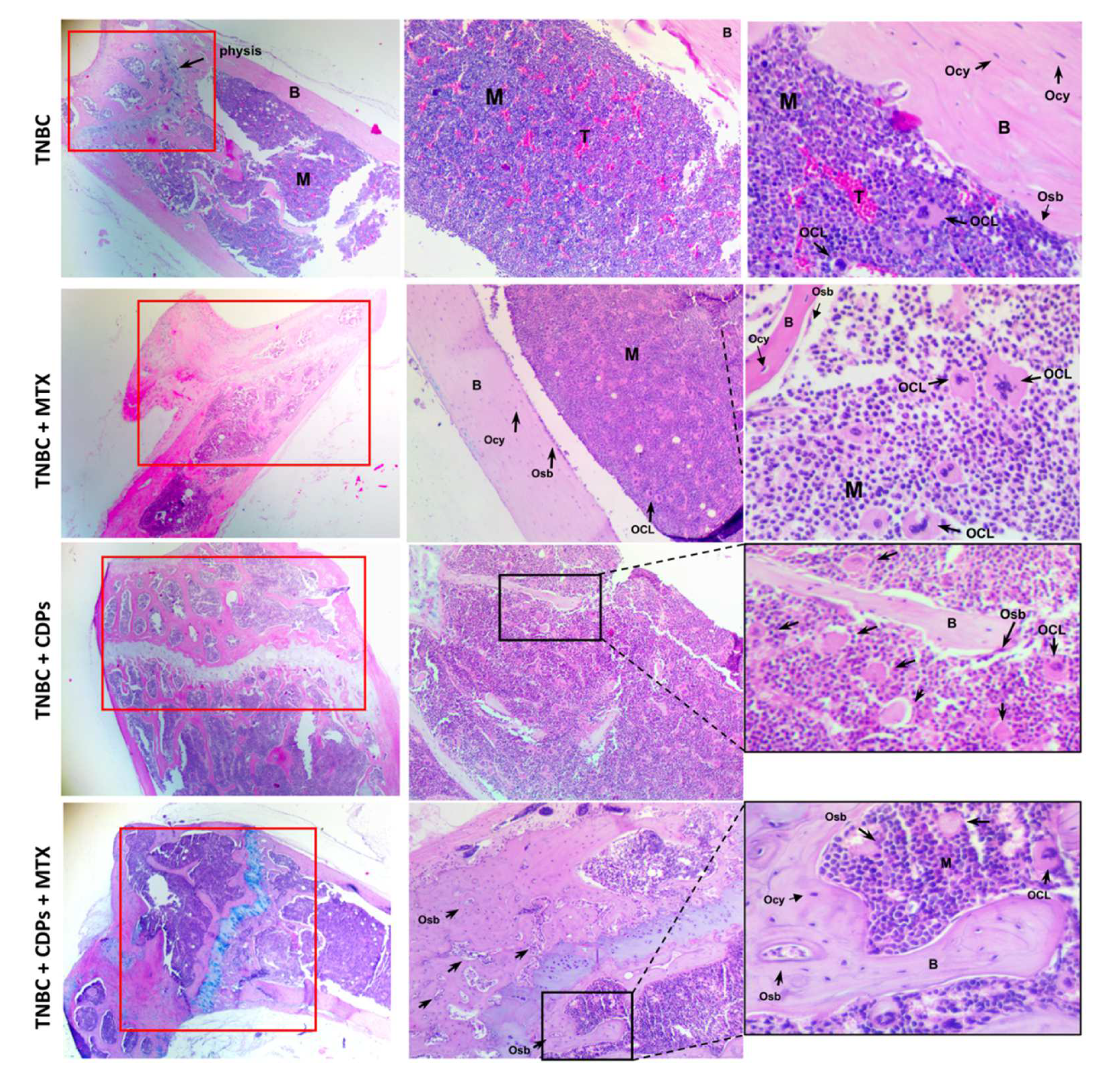
Femora weight determination showed less weight in the TNBC and TNBC+MTX(m) mice groups than in the CDPs-treated (
Figure 8a). Histological analysis of the femur in mice revealed that in the TNBC group zones of invasion of tumoral cells in the bone marrow (B), which were not observed in the femurs of mice submitted to treatment with CDPs or MTX (
Figure 9). Additionally, the treatment with CDPs and the combination of CDPs+MTX led to decrease of osteoclasts and increase in the number of osteoblasts (Figs. 8b and 9). A similar proportion of osteoblasts and osteoclasts was observed in the group treated with MTX alone. These cells play a crucial role in osteogenesis by producing and depositing the organic bone matrix [
43]. Osteoclasts are specialized cells that participate in bone modeling, calcium homeostasis, and hematopoiesis modulation bone healing; they also are essential cells related to the tumor microenvironment in bone metastases in BC [
44], which inhibition can contribute to patients’ recovery and increase life span and quality of life. Another characteristic observed was the increase of the apophysis (growth bone zone) in the head of the femur in all the mice submitted to CDPs/MTX treatments. These findings suggest a potential pro-osteogenic effect of CDPs.