1. Introduction
Kaposiform Hemangioendothelioma (KHE) is a rare, locally aggressive vascular benign neoplasm [
1] with histopathological features that resemble Kaposi’s Sarcoma, usually occurring in early infancy or, often, in the first decade of life, with few cases seen in adult life [
2].
There are no studies that report the incidence or prevalence of this neoplasm.
It usually involves skin or retroperitoneum, less commonly develops in the head and neck region [
3,
4] or in the soft tissues of extremities and the trunk and rarely occurs in the mediastinum [
5].
The KHE was first designated by Zuckerberg and collaborators in 1993 as an entity distinct from infantile hemangioma (IH) due to its locally invasive growth, aggressive course, and “Kaposi-like focal appearance”. [
6]
Currently, there is a lack of literature specifically addressing the incidence of KHE. In fact, atypical or asymptomatic small KHE may be mistakenly identified as unusual variants of IH or other vascular anomalies. As a result, the true prevalence and occurrence of KHE are most likely higher than those reported in the limited published studies. Overall, the cause of KHE remains largely unclear. Nearly all cases of KHE arise without any obvious trigger. In rare instances, the signs and symptoms of KHE may worsen following trauma or infections.[
7]
A slight male predominance has been observed in the development of KHE, and the age of onset is generally distributed with a peak within the first year of life, during which approximately 90% of KHE cases become apparent. Moreover, around 50% of skin lesions are noticeable or detectable at birth. [
8,
9]
Unlike what happens to juvenile hemangioma, KHE seldom undergoes spontaneous regression; regional lymphatic metastases are rare, while distance metastases have never been described [
5].
In 70% of cases and especially in the presence of particularly extensive lesions [
10], kaposiform hemangioendothelioma may be associated with Kasabach-Merritt syndrome (KMS), characterized by severe thrombocytopenia (usually less than 30,000 platelets per micro-liter) and coagulopathy by consumption. It has been hypothesized that KMS is associated with an abnormal platelet activation and aggregation related to interaction with endothelial tumor cells, resulting in local platelet sequestration and clotting factor consumption [
11]; others have advanced the hypothesis that KMS should be brought back to the structure of the small vessels present in the neoplasm, which are thin and twisting, thus making the flow of blood turbulent [
5].
Kasabach Merritt Syndrome is refractory to platelet transfusions and has a mortality of 20% [
12], associated with life-threatening haemorrhages.
Histopathologic features of kaposiform hemangioendothelioma include the presence of spindle cell clusters with few cellular or mitotic abnormalities, numerous vascular fissure spaces, absence of nuclear atypia, microtrrombies and emosiderin deposits [
1].
Several treatments have been used to manage KHE, including surgery, arterial embolization, physical compression, laser, radiotherapy and medical therapy. Although many different treatment modalities have been used, the most effective one is complete surgical excision. Medical therapy has been proposed for lesions that can not be completely excised or when Kasabach-merritt Phenomenon (KMP) is present; the two most commonly used medical therapies are steroids and interferon.
Superficial soft tissues are best treated by wide local excision with clear surgical margins.
Prognosis depends on chronicity of lesions and complications (severity of form and association with Kasabach-Merritt Syndrome).
2. Case Report
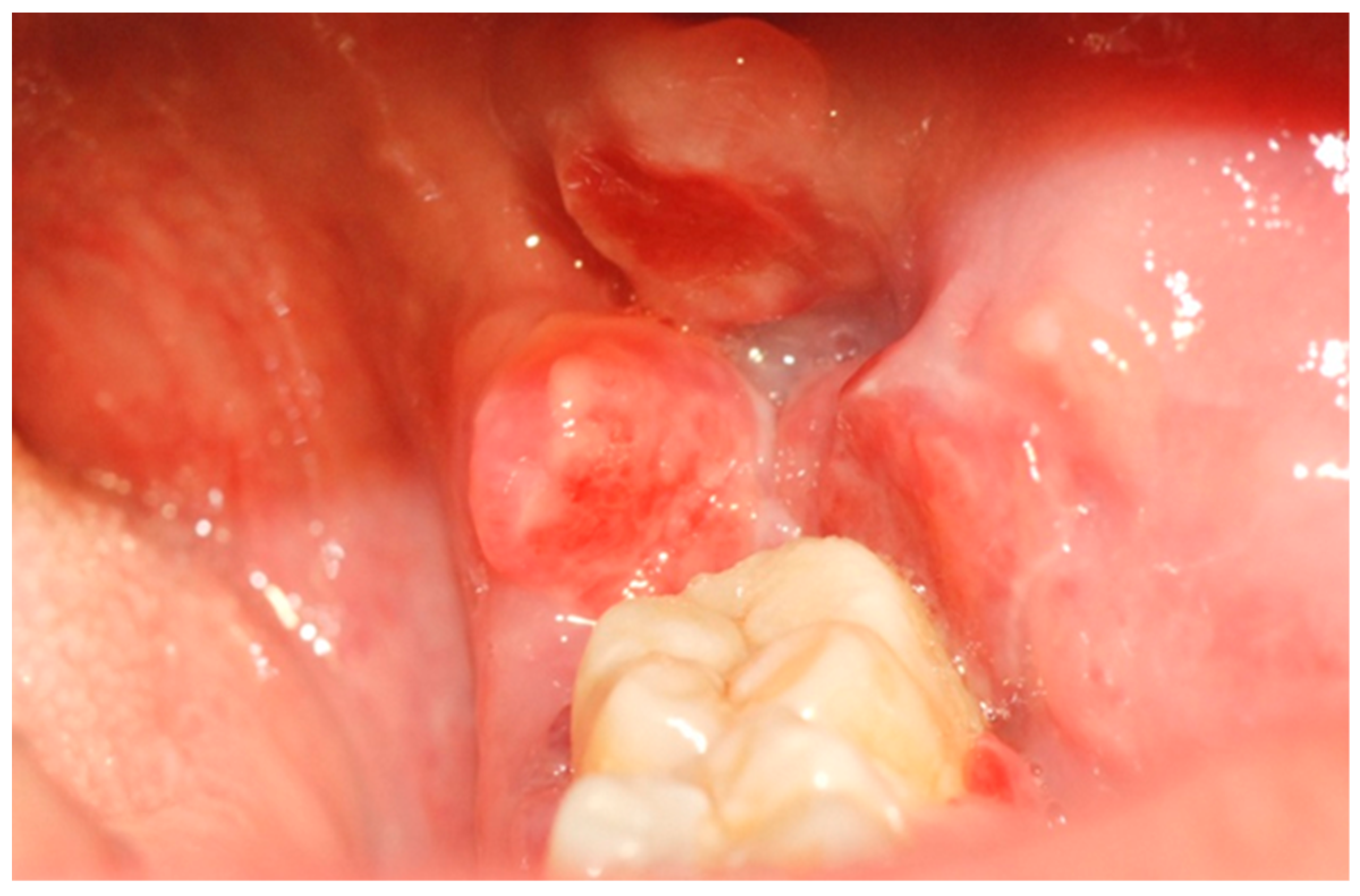
A 39-year-old Caucasian woman was referred to the Department of Oral and Maxillofacial Sciences of Polyclinic Umberto I, “Sapienza” University of Rome, for the presence of an exophytic and erythematous lesion located at the left retromolar trigonum (
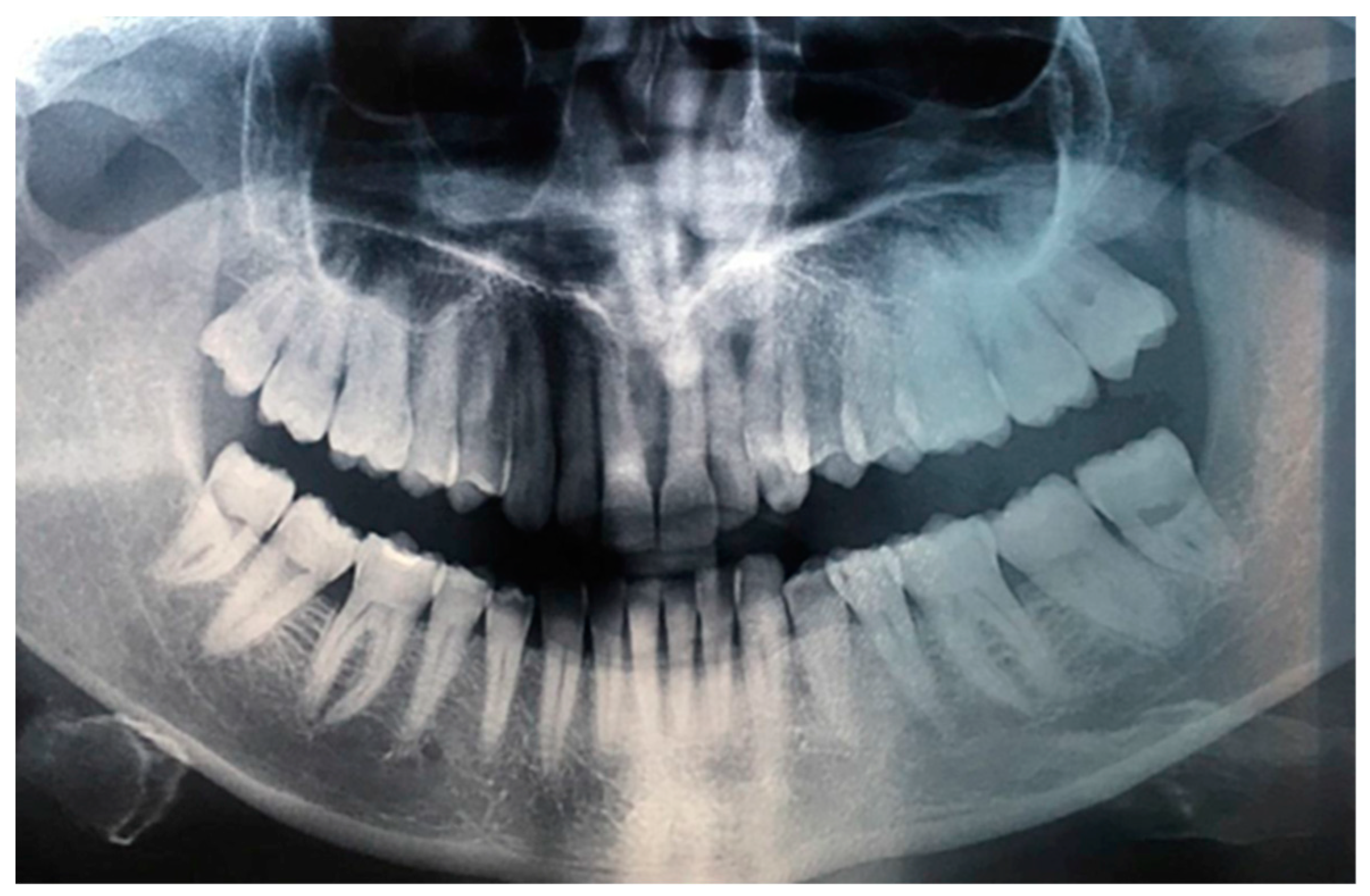
Figure 1), occurring since about 2 months, associated with a radiolucency evidenced by orthopanoramics (
Figure 2).
During the anamnestic data collection, the patient reported being affected by Systemic Lupus Erythematosus in treatment with Deltacortene (Prednisone) and Endoxan (Cyclophosphamide), Hypothyroidism, treated with Eutirox (Sodium Levotiroxin), and Gastroesophageal Reflux, treated with Antra (Omeprazole). She denied having ever consumed tobacco or alcoholic beverages in a habitual manner and reported an allergy to Augmentin.
First a heamatochemistry analysis to evaluate coagulation parameters and a cardiological screening were performed. Then, after having viewed the results, it was decided to perform a scalpel incisional biopsy of the lesion in September 2015.
Before proceeding with local infiltration anesthesia using mepivacaine combined with a vasoconstrictor at a 1:100,000 concentration, a swab soaked in a lidocaine-based topical anesthetic was applied approximately two minutes in advance.
As per procedure, the tissue was sent to an anatomopathologist for a histological evaluation.
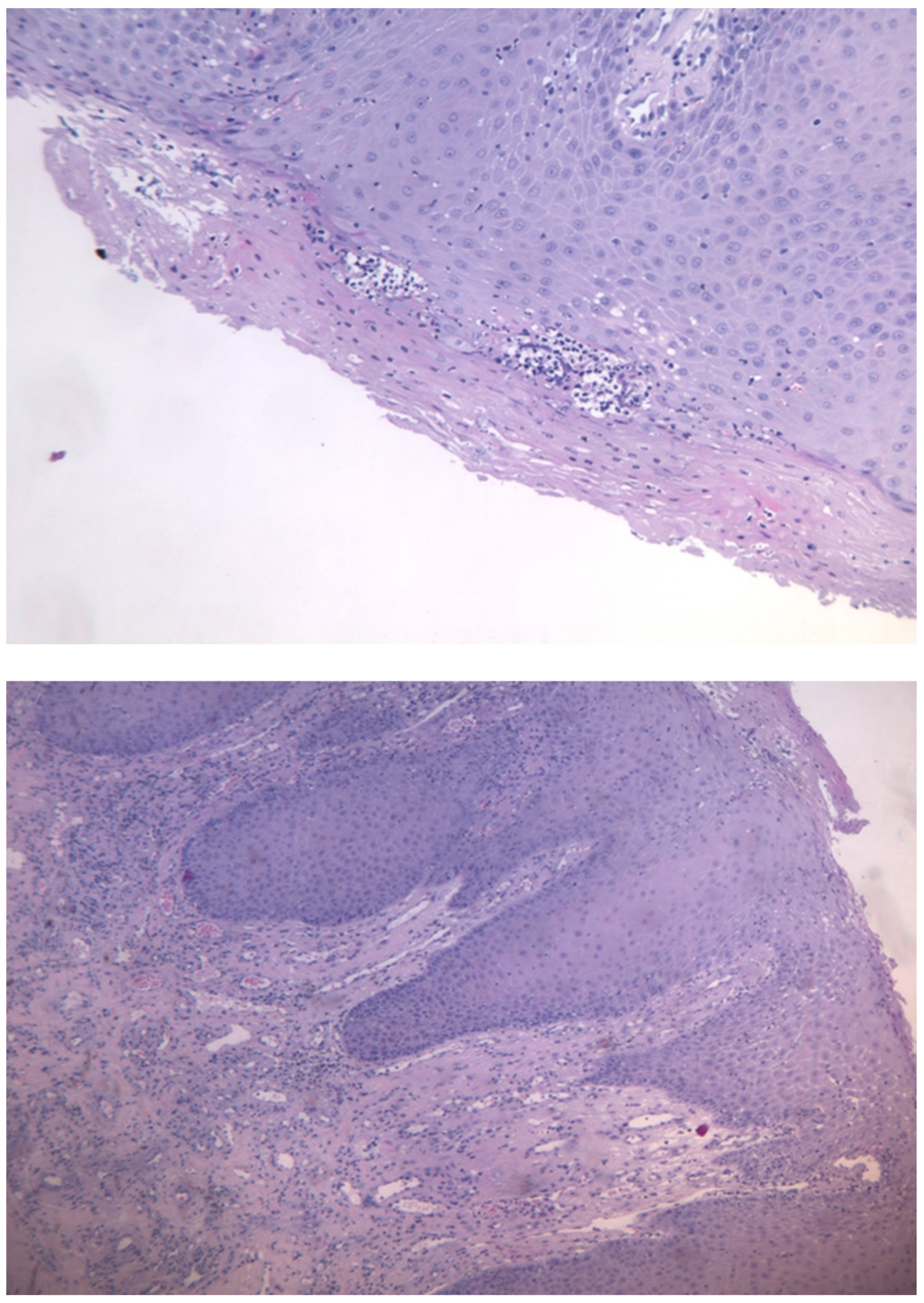
The histological examination described the presence of oral mucosa, partly covered by malpighian epithelium with mild hyperorthokeratosis, papillomatosis and neutrophil granulocytic exocytosis, and partly extensively ulcerated, with abundant granulation tissue in the submucosa, in absence of figures referable to neoplasia. (
Figure 3a,b)
In November 2015, it was decided to repeat the scalpel excisional biopsy of the le-sion with the help of a CO2 laser, together with the extraction of the dental element 3.8 because of its possible traumatic action.
The histological aspect described by the anatomopathologist’s report was that of a partially ulcerated oral mucous membrane, partly covered by malpighian epithelium with mild hyperparakeratosis, acanthosis, focal spongiosis, papillomatosis, and lym-phocytic and neutrophil granulocytic exocytosis. In the corion there were numerous capillary vascular structures (CD31 +, CD34 +, actin MS +, actin ML +), coated by en-dothelial cells sometimes swollen, sometimes spindle,with indistinct limits, weakly eo-sinophilic cytoplasm and round nucleus. These vascular structures were organized in a lobular/glomeruloid type growth pattern and were present also in the muscle tissue in-cluded in the withdrawal. There was also an inflammatory infiltrate mainly of neu-trophil granulocytes and fibrin thrombi in the context of capillary lumens. This mor-phological and immunohistochemical description was compatible with kaposiform hemangioendothelioma. The case was discussed at the HEAD-NECK tumor board and it was decided collectively to proceed with a surgical treatment in order to extend the tumor resection margins.
The patient was then sent to the Department of Maxillo-Facial Surgery.
3. Discussion
The “Kaposiform hemangioendothelioma” term was introduced by Zukerberg et al. in 1993 (9), for similar histological aspects that this tumor has with capillary he-mangioma and Kaposi’s sarcoma.
Zhou and collaborators provided evidence of a somatic translocation between chromosomes 13 and 16, specifically at bands 13q14 and 16p13.3, detected in 10% of metaphase cells within KHE lesions, alongside the presence of normal cells in the kar-yotype. However, it is crucial to highlight that, although GNAQ mutations have been identified in KHE, it remains uncertain whether they play a causal role or emerge sec-ondarily within the tumor.[
13]
The manifestations of KHE are diverse, ranging from skin lesions with a wide va-riety of appearances to deep masses without visible skin signs. In fact, in most patients, KHE presents as a single soft tissue mass with skin findings that vary from an ery-thematous papule, plaque, or nodule to a firm, purplish, and solid tumor. However, about 12% of patients show no skin involvement.[
14]
Complications in individuals with KHE are frequent. The seriousness of these complications largely relies on the patient’s age, the dimensions of the lesion, its site, its spread into deep tissues and vital organs, and any related blood disorders. It is ad-visable for healthcare providers to stay alert to possible complications and the risk factors that might signal future issues.
Complications like KMP manifests with an estimated incidence ranging from 42% to 71%. Thrombocytopenia is typically severe, with a median platelet count of 21 × 10⁹/L at the initial onset of KMP. KHE lesions associated with KMP exhibit progressive enlargement and purpura. KMP can result in considerable pain and secondary hem-orrhaging. [
15]
There are about 50 cases of KHE described in the head and neck region, and of these, cases in the oral cavity are extremely rare. Among the few intraoral cases re-ported, all patients were male, the age ranged from 2 to 21 years, and the lesions ranged from 1 to 3 cm in size. (11-13) Our patient is a 39-year-old adult woman, and the tumor was located in the posterior mandible, presenting a different location from other previously reported intraoral cases. It is likely that KHE can occur in any area of the soft tissues in the mouth. Clinically, KHE may mimic reactive and benign vascular lesions such as pyogenic granulomas and infantile hemangioma, but a histopathologi-cal evaluation (preferably supplemented by immunohistochemistry) is necessary for a definitive diagnosis. [
10]
This case presented as an exophytic mass on the alveolar ridge, associated with an osteolytic defect in the underlying alveolar bone. Histologically, KHE is composed of nodules of ovoid endothelial cells and vascular structures. KHE is characterized by areas of ovoid cells, glomeruloid endothelial proliferation, and areas of lymphangi-omatosis. Cellular atypia and mitotic figures are rare. [
16]
This locally aggressive tumor rarely involves the head and neck region and is very rarely found in adult subjects. DeFatta et al. in 2005 reported a case of Kaposiform Hemangioendothelioma in a 3 year old male localised in the left hard and soft palate. The patient was completely asymptomatic, with no pain, dysphagia, odynophagia, or difficulties in breathing and speaking. The lesion was subjected to excisional biopsy and the histopathological examination described the presence of endothelial cells with abundant eosinophilic cytoplasm, round shaped and oval-shaped blood vessels, spin-dle neoplastic endothelial cells that formed elongated vascular spaces [
17].
Rekhi et al. in 2011 documented a case of Kaposiform hemangioendothelioma (KHE) in the right tonsil of a 2-year-old child, fortunately not associated with KMP, who presented with a clinical history of episodes of tonsillitis and ipsilateral neck swelling from birth. During one of the clinical episodes, the patient experienced acute dysphagia and dyspnea, and was radiologically diagnosed with a peritonsillar abscess. Upon reviewing the histopathology slides, a “kaposiform” pattern featuring slit-like, crescentic capillaries with platelet thrombi, eosinophilic bodies, and prominent areas of lymphangiomatosis helped distinguish it from a juvenile hemangioma.[
18]
Vashi et al. in 2016 described the case of a 38-year-old man with a mass on the left lower surface of the tongue, increased in size over a 6-month period, with mild, but asymptomatic traumatic bleeding episodes. The histological examination obtained by an incision biopsy led to the diagnosis of kaposiform hemangioendothelioma and the lesion was subsequently excised [
19]. Lima Morais et al. in 2021 described the case of a 10-year-old patient with a soft tissue swelling about 2 cm in diameter in the right pos-terior mandible. Histopathologic examination revealed a vascular proliferation per-meated by inflammatory cells and fascicles of spindle-to-ovoid cells,like in Sarcoma of Kaposi and the patient had to extract involved teeth [
20]. Since KHE is a very uncom-mon tumor, knowledge about its treatment is not very well known. It depends upon the size of the neoplasia, its surgical accessibility, and whether or not KMP is present; the treatment of choice, whenever possible, is certainly represented by surgical exci-sion [
19,
20,
21].
Furthermore, in the absence of any medical contraindications or a history of drug allergy, sensitivity, or reaction to vasoconstrictors (e.g., epinephrine), incorporating a vasoconstrictor facilitates improved hemostasis and extends the anesthesia’s duration. Notably, regardless of the method employed for local anesthetic delivery, it is essential to aspirate before injection to avoid inadvertent intravascular administration of both the anesthetic and vasoconstrictor. Additionally, to maximize patient comfort during the biopsy, we recommend applying a topical anesthetic with a cotton swab for one minute before puncturing the mucosa with the needle.
An incisional biopsy method is recommended when sampling sizeable lesions and those suspected of being malignant. Orosco and colleagues conducted a comprehensive study of positive surgical margins in the ten most common solid tumors, using data from the National Cancer Data Base, and identified oral cancer as the tumor most fre-quently exhibiting positive margins in both sexes.[
22]
4. Conclusions
The correct diagnosis of KHE presents a significant challenge due to its rarity, es-pecially within the oral cavity, which primarily affects children and adolescents. Fur-thermore, there are no cases documented in the literature of KHE in adult women as observed in our case. Prompt diagnosis and intervention are crucial since, given its bi-ological behavior, the lesion requires a comprehensive clinical, radiographic, histolog-ical, and immunohistochemical evaluation.
Funding
This work was also supported by the Italian Ministry of Health with “Current Research funds”.
Informed Consent Statement
The study was conducted in accordance with the Declaration of Helsinki. It does not require approval by the Institutional Review Board or Ethics Committee since the current Regulation of the Ethics Committee of the Higher Institute of Health stipulates that projects with epidemiological, medico-social and evaluative contents need evaluation, approval and monitoring of trial protocols only if they contain personal data according to the legislative decrees on clinical trials and function of the ethics committees (decreto legislativo 24 giugno 2003, n.211; decreto ministeriale 8 febbraio 2013). The official definition of “personal data” is given by the National Data Protection Authority (Garante per la Protezione dei Dati Personali,
https://www.garanteprivacy.it/home/diritti/cosa-intendiamo-per-dati-personali—Regolamento (UE) 2016/679 art.9 (accessed on 07 May 2023). The term “personal data” includes information about first and last names, images, social security codes, IP addresses and licence plate numbers. The images and radiographs in the manuscript are anonymized for this reason. The patient’s parents signed informed consent forms and were given authorization to anonymize the case documentation for scientific purposes.
Data Availability Statement
The authors thank the patients’ families for their availability and sensitivity in allowing us to share their experience and their path in this diagnosis.
Acknowledgments
The authors thank the patients’ families for their availability and sensitivity in allowing us to share their experience and their path in this diagnosis.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| KHE |
Kaposiform Hemangioendothelioma |
| IH |
Juvenile Hemangioendothelioma |
| KMP |
Kasabach – Merritt Phenomenon |
| KMS |
Kasabach – Merritt Syndrome |
References
- Croteau SE, Liang MG, Kozakewich HP, Alomari AI, Fishman SJ, Mulliken JB, Trenor CC 3rd. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J Pediatr. 2013 Jan;162(1):142-7.
- Deraedt K, Vander Poorten V, Van Geet C, Renard M, De Wever I, Sciot R. Multifocal kaposiform haemangioendothelioma. Virchows Arch. 2006 Jun;448(6):843-6.
- Chung MT, Chen CH, Chiu CH, Yang CP, Hsueh C, Jaing TH (2003) Successful nonoperative therapy for Kaposiform hemangioendothelioma involving the neck: report of 1 case. Otolaryngol Head Neck Surg 129:605–607.
- Tello MA, Shields G, Gadre SA, Ryan M. Pathology quiz case 2. Diagnosis: Kaposiform hemangioendothelioma (KHE). Arch Otolaryngol Head Neck Surg. 2004;130:991 Diag 993–994.
- Lyons LL, North PE, Mac-Moune Lai F, Stoler MH, Folpe AL, Weiss SW. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559–568.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancyand childhood. An aggressive neoplasm associated with Kasabach-Merritt syndromeand lymphangiomatosis. Am J Surg Pathol 1993;17:321–328.
- Ji Y, Yang K, Peng S, Chen S, Xiang B, Xu Z, et al. Kaposiform haemangioendothelioma: clinical features, complications and risk factors for Kasabach-Merritt phenomenon. Br J Dermatol. 2018;179(2):457–463. [CrossRef]
- Adams DM, Brandão LR, Peterman CM, Gupta A, Patel M, Fishman S, et al. Vascular anomaly cases for the pediatric hematologist oncologists—An interdisciplinary review. Pediatr Blood Cancer. 2018;65(1):e26716. [CrossRef]
- Mahajan P, Margolin J, Iacobas I. Kasabach-Merritt Phenomenon: Classic Presentation and Management Options. Clin Med Insights Blood Disord. 2017;10:1179545X17699849. [CrossRef]
- Schmid S, Holzmann D, Stallmach T, Gysin C..Kaposiform hemangioendothelioma arising in the ethmoid sinus of an 8-year-old girl with severe epistaxis. Head Neck. 2006 Aug;28(8):761-4Birchler MT.
- Hall GW. Kasabach-Merritt syndrome: pathogenesis and management. Br J Haematol. 2001;112:851–62.
- Liu XH, Li JY, Qu XH, Yan WL, Zhang L, Yang C, Zheng JW. Treatment of kaposiform hemangioendothelioma and tufted angioma. Int J Cancer. 2016 Oct 1;139(7):1658-66.
- Zhou S, Wang L, Panossian A, Anselmo D, Wu S, Venkatramani R. Refractory Kaposiform Hemangioendothelioma associated with the chromosomal translocation t(13;16)(q14;p13.3) Pediatr Dev Pathol. 2016;19(5):417–420. [CrossRef]
- Gruman A, Liang MG, Mulliken JB, Fishman SJ, Burrows PE, Kozakewich HPW, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52(4):616–622. [CrossRef]
- Rodriguez V, Lee A, Witman PM, Anderson PA. Kasabach-Merritt Phenomenon. J Pediatr Hematol Oncol. 2009;31(7):522–526. [CrossRef]
- Sreenivasan BS, Ambooken M, Radhakrishna M, Sebastian J. An intraoral epitheloid hemangioendothelioma masquerading clinically as pyogenic granuloma. Iran J Med Sci. 2015;40:185–9.
- DeFatta RJ, Verret DJ, Adelson RT, Gomez A, Myers LL. Kaposiform hemangioendothelioma: case report and literature review. Laryngoscope. 2005 Oct;115(10):1789-92. Review.
- Rekhi B, Sethi S, Kulkarni SS, Jambhekar NA. Kaposiform hemangioendothelioma in tonsil of a child associated with cervical lymphangioma: a rare case report. World J Surg Oncol. 2011;23:57. [CrossRef]
- Vashi P, Abboud E, Bier-Laning C, Gupta D.Adult-onset Kaposiform hemangioendothelioma of the tongue: case report and review of the literature. Curr Oncol. 2016 Oct;23(5):e517-e520.
- Morais TML, Sánchez-Romero C, Ribeiro L, Faé DS, Verner FS, de Almeida OP, de Aquino SN. Kaposiform Hemangioendothelioma of the Oral Cavity: A Rare Tumor with an Unusual Location. Head Neck Pathol. 2021 Dec;15(4):1421-1425.
- Yang H, Wang J, Song L, Zou H. Intraosseous epithelioid haemangioendothelioma of the mandible: A case report and literature review. Med (Baltim) 2019;98:e16572.
- Shanti RM, O’Malley BW Jr. Surgical Management of Oral Cancer. Dent Clin North Am. 2018 Jan;62(1):77-86. Epub 2017 Oct 16. PMID: 29126495. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).