Submitted:
07 March 2025
Posted:
10 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
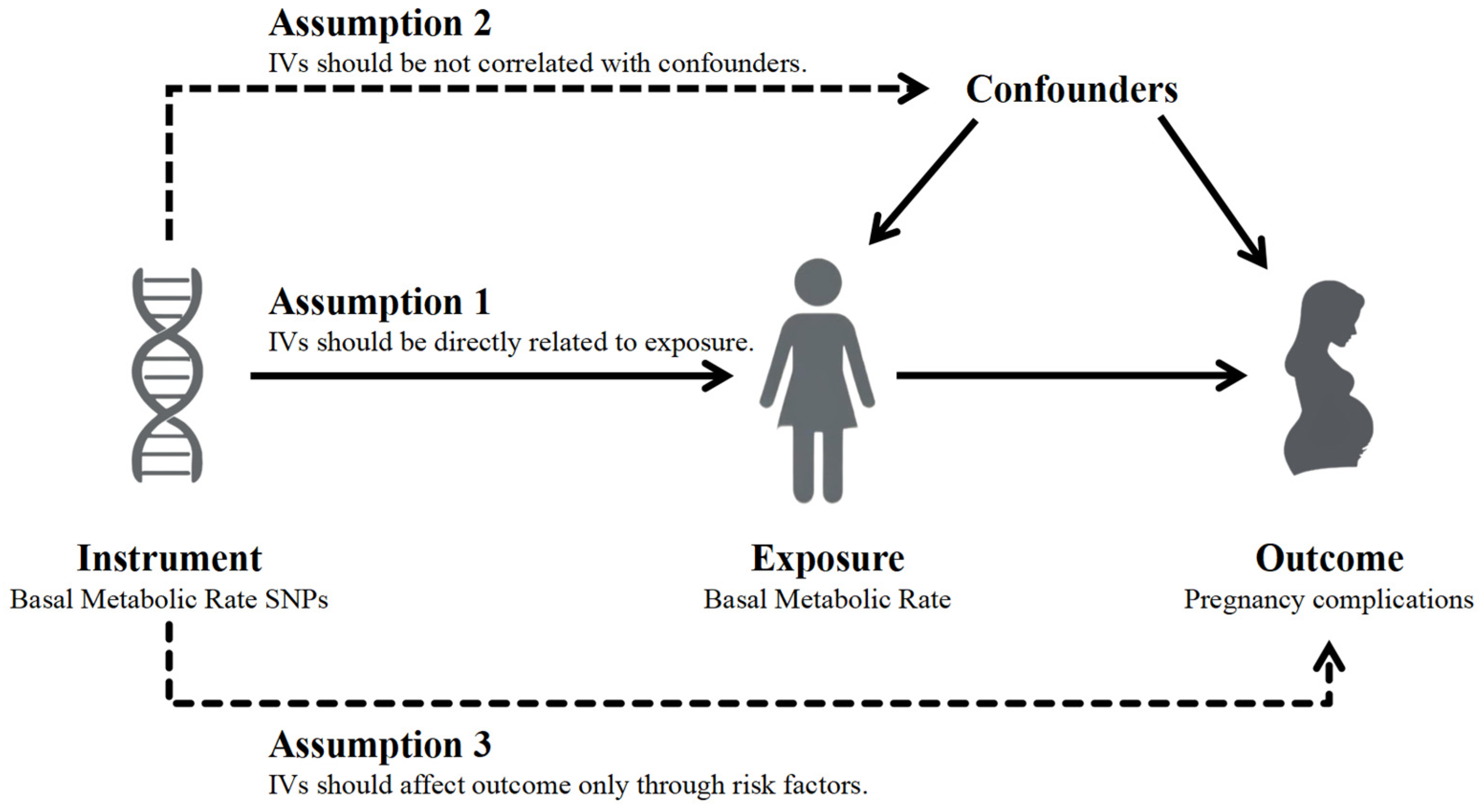
2.2. Selection of Instrumental Variables
2.3. Mendelian Randomization and Sensitivity Analysis
2.4. Multivariable Mendelian Randomization
2.5. Statistical Analysis
3. Results
3.1. Selected Instrumental Variables
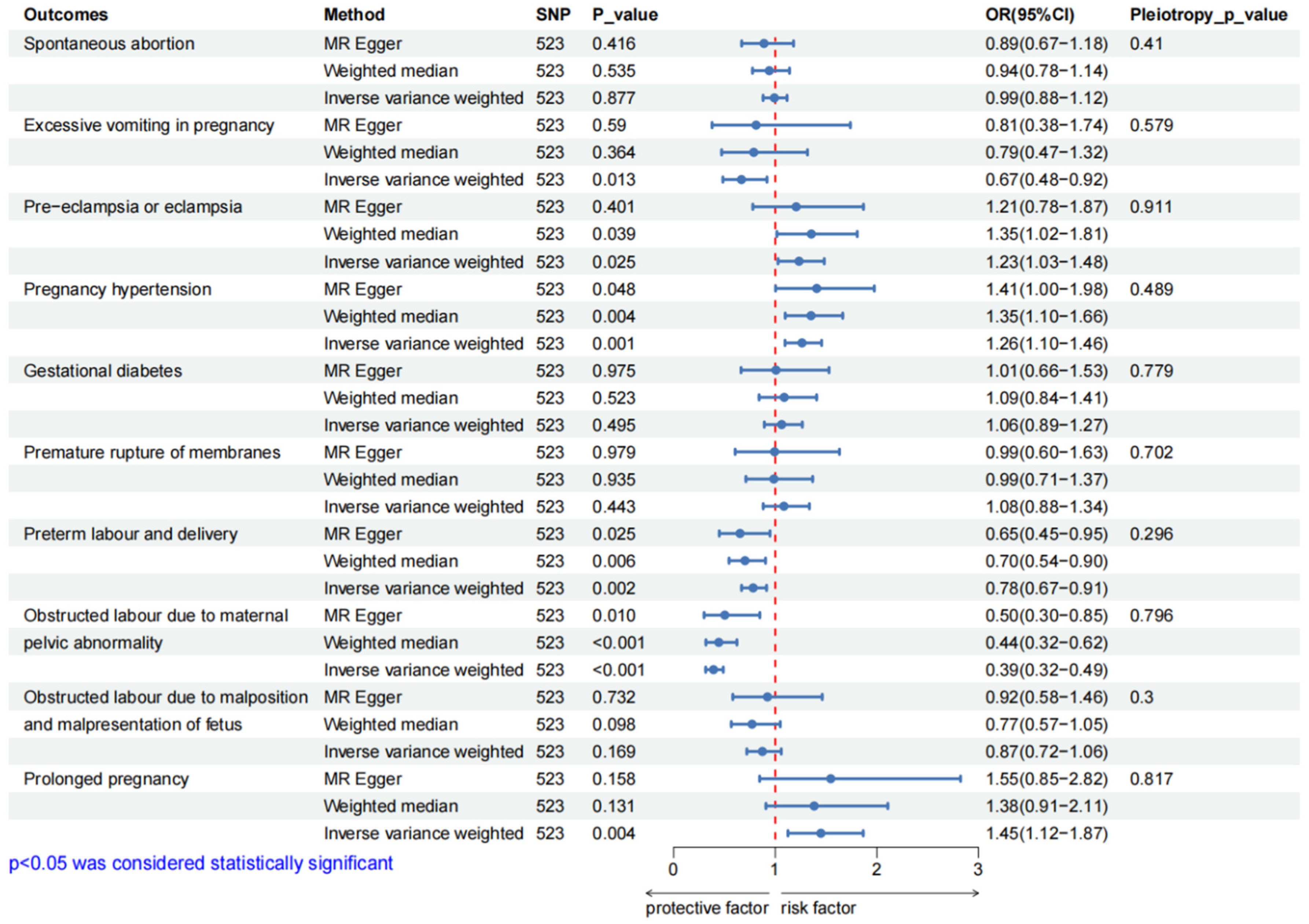
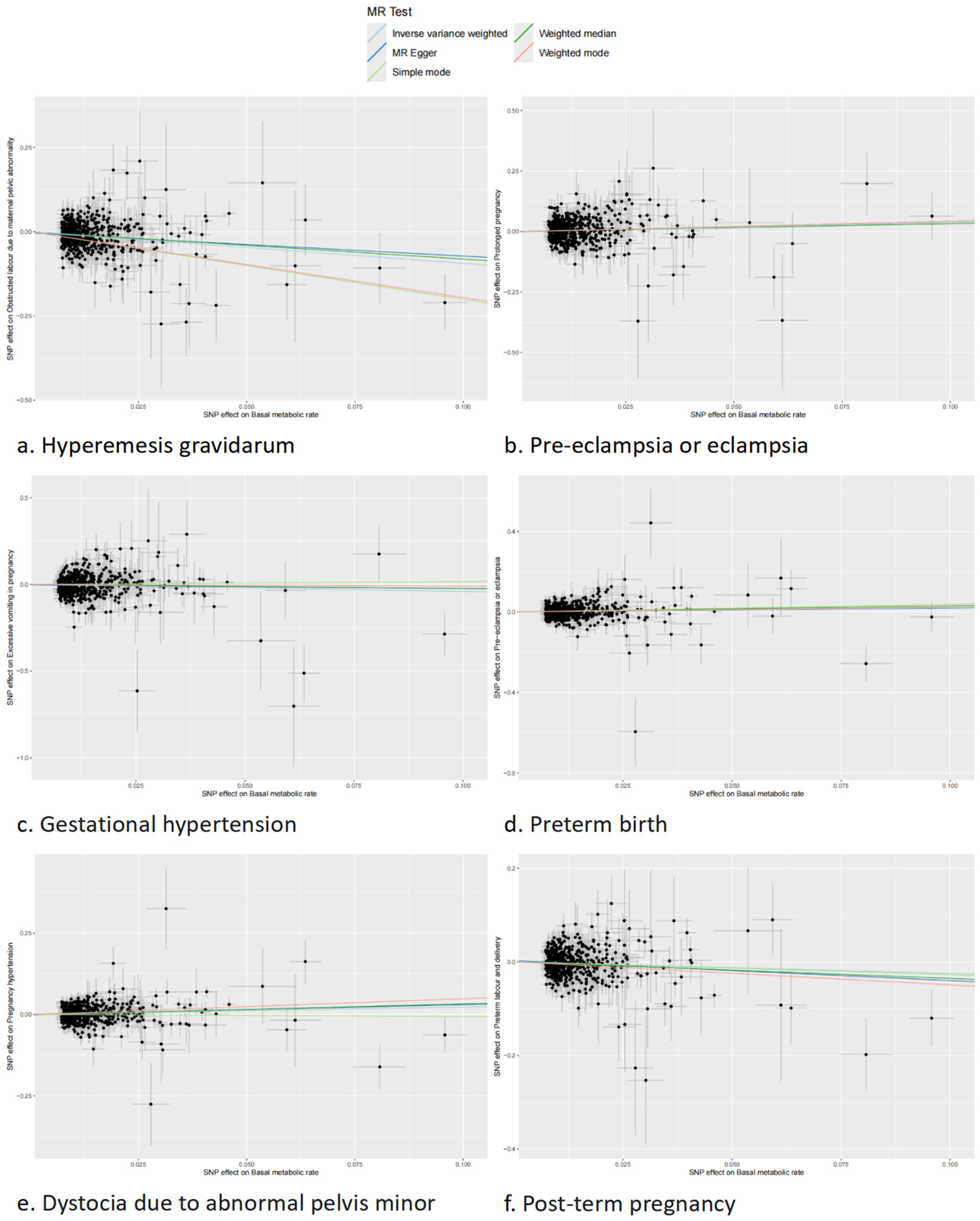
3.2. Causal Effect of BMR on Pregnancy Complications
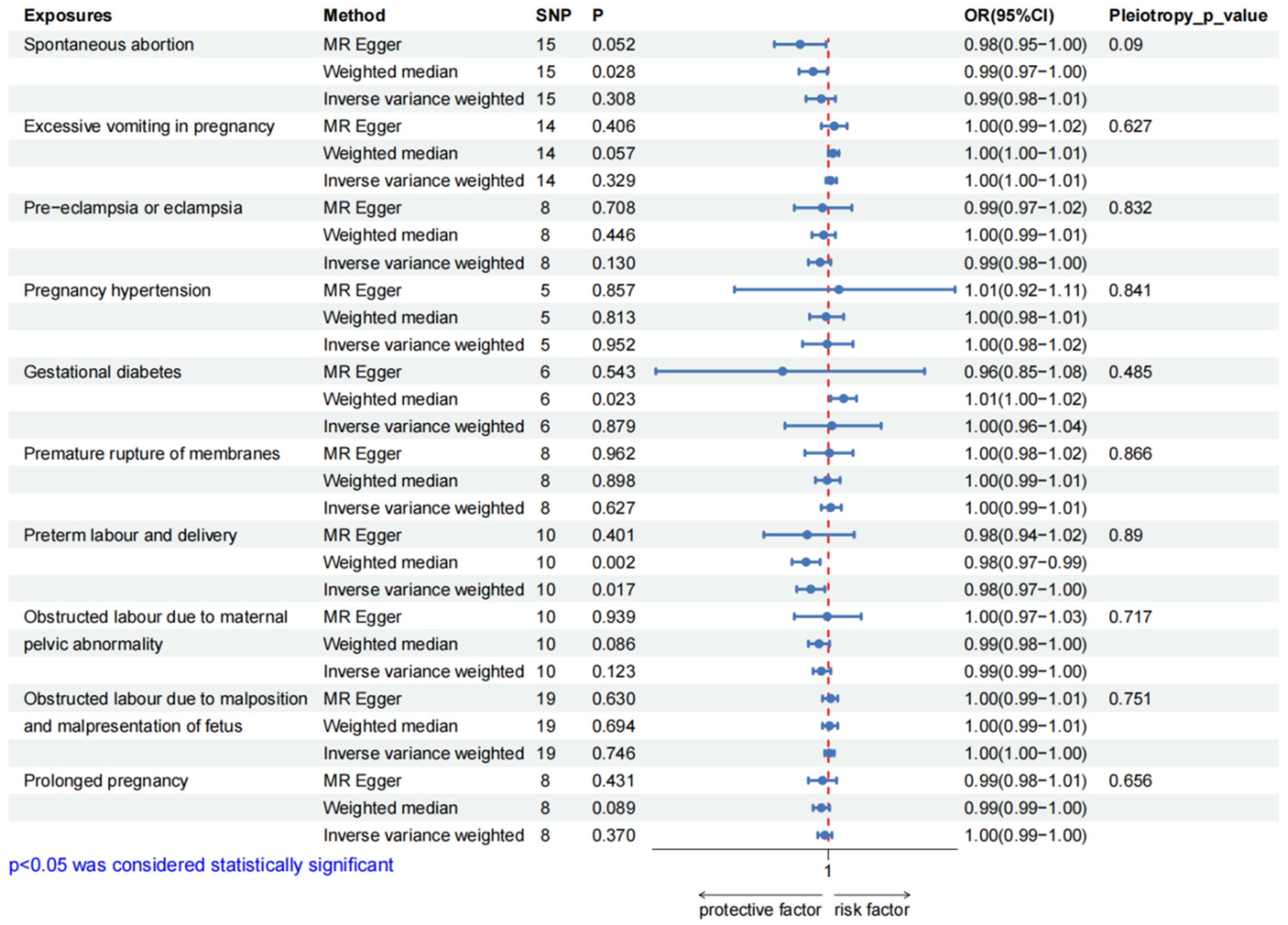
3.3. Causal Effect of Pregnancy Complications on BMR
3.4. Confounding Factors
3.5. Multivariable Mendelian Randomization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMR | Basal Metabolic Rate |
| MR | Mendelian Randomization |
| GWAS | Genome-Wide Association Atudies |
| MVMR | Multivariate Mendelian randomization |
| HG | Hyperemesis Gravidarum |
| PE | Preeclampsia |
| TEF | Thermic Effect of Food |
| GDM | Gestational Diabetes Mellitus |
| HDP | Hypertensive Disorders of Pregnancy |
| SNP | Single Nucleotide Polymorphisms |
| IV | Instrumental Variable |
| BMI | Body Mass Index |
| IVW | Inverse Variance Weighting |
| MR-PRESSO | MR Pleiotropy Residual Sum and Outlier |
| OR | Odds Ratio |
| CI | Confidence Interval |
References
- Shetty, P. Energy requirements of adults. Public Health Nutr. 2005;8(7A):994-1009. [CrossRef]
- Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr. 2005;82(5):941-948. [CrossRef]
- Kim, B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18(2):141-144. [CrossRef]
- Ng JCM, Schooling CM. Effect of basal metabolic rate on lifespan: a sex-specific Mendelian randomization study. Sci Rep. 2023;13(1):7761. Published 2023 May 12. [CrossRef]
- Zhao P, Han F, Liang X, et al. Causal Effects of Basal Metabolic Rate on Cardiovascular Disease: A Bidirectional Mendelian Randomization Study. J Am Heart Assoc. 2024;13(1):e031447. [CrossRef]
- Han F, Hu F, Wang T, et al. Association Between Basal Metabolic Rate and All-Cause Mortality in a Prospective Cohort of Southern Chinese Adults. Front Physiol. 2022;12:790347. Published 2022 Jan 4. [CrossRef]
- Wang Q, Richardson TG, Sanderson E, et al. A phenome-wide bidirectional Mendelian randomization analysis of atrial fibrillation. Int J Epidemiol. 2022;51(4):1153-1166. [CrossRef]
- Most J, Dervis S, Haman F, Adamo KB, Redman LM. Energy Intake Requirements in Pregnancy. Nutrients. 2019;11(8):1812. Published 2019 Aug 6. [CrossRef]
- Durnin, JV. Energy requirements of pregnancy. Diabetes. 1991;40 Suppl 2:152-156. [CrossRef]
- Dinu M, Napoletano A, Giangrandi I, et al. Exploring basal metabolic rate and dietary adequacy in twin pregnancies: the VENERE study. Nutr Metab (Lond). 2024;21(1):99. Published 2024 Dec 2. [CrossRef]
- Chihara H, Otsubo Y, Yoneyama Y, et al. Basal metabolic rate in hyperemesis gravidarum: comparison to normal pregnancy and response to treatment. Am J Obstet Gynecol. 2003;188(2):434-438. [CrossRef]
- Martin A, O'Sullivan AJ, Brown MA. Body composition and energy metabolism in normotensive and hypertensive pregnancy. BJOG. 2001;108(12):1263-1271. [CrossRef]
- Adane AA, Tooth LR, Mishra GD. Pre-pregnancy weight change and incidence of gestational diabetes mellitus: A finding from a prospective cohort study. Diabetes Res Clin Pract. 2017;124:72-80. [CrossRef]
- Richmond RC, Davey Smith G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb Perspect Med. 2022;12(1):a040501. Published 2022 Jan 4. [CrossRef]
- Henry, CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8(7A):1133-1152. [CrossRef]
- Burgess S, Thompson SG. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat Med. 2011;30(11):1312-1323. [CrossRef]
- Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783-1802. [CrossRef]
- Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880-1906. [CrossRef]
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [CrossRef]
- Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304-314. [CrossRef]
- Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181(4):251-260. [CrossRef]
- Randall JC, Winkler TW, Kutalik Z, et al. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9(6):e1003500. [CrossRef]
- Richardson TG, Sanderson E, Palmer TM, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020;17(3):e1003062. Published 2020 Mar 23. [CrossRef]
- McCartney DL, Min JL, Richmond RC, et al. Genome-wide association studies identify 137 genetic loci for DNA methylation biomarkers of aging. Genome Biol. 2021;22(1):194. Published 2021 Jun 29. [CrossRef]
- Randall JC, Winkler TW, Kutalik Z, et al. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9(6):e1003500. [CrossRef]
- Ali N, Mahmood S, Manirujjaman M, et al. Hypertension prevalence and influence of basal metabolic rate on blood pressure among adult students in Bangladesh [published correction appears in BMC Public Health. 2017 Sep 22;17(1):736. 10.1186/s12889-017-4709-6.]. BMC Public Health. 2017;18(1):58. Published 2017 Jul 25. [CrossRef]
- King, JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271-291. [CrossRef]
- Luke A, Adeyemo A, Kramer H, Forrester T, Cooper RS. Association between blood pressure and resting energy expenditure independent of body size. Hypertension. 2004;43(3):555-560. [CrossRef]
- Li R, Zhou C, Ye K, Chen H, Peng M. Identification of genes involved in energy metabolism in preeclampsia and discovery of early biomarkers. Front Immunol. 2025;16:1496046. Published 2025 Feb 4. [CrossRef]
- Polito A, Fabbri A, Ferro-Luzzi A, et al. Basal metabolic rate in anorexia nervosa: relation to body composition and leptin concentrations. Am J Clin Nutr. 2000;71(6):1495-1502. [CrossRef]
- Snodgrass JJ, Leonard WR, Sorensen MV, Tarskaia LA, Mosher MJ. The influence of basal metabolic rate on blood pressure among indigenous Siberians. Am J Phys Anthropol. 2008;137(2):145-155. [CrossRef]
- Jena MK, Sharma NR, Petitt M, Maulik D, Nayak NR. Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta. Biomolecules. 2020;10(6):953. Published 2020 Jun 24. [CrossRef]
- Maciak S, Sawicka D, Sadowska A, et al. Low basal metabolic rate as a risk factor for development of insulin resistance and type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001381. [CrossRef]
- Owu DU, Antai AB, Udofia KH, Obembe AO, Obasi KO, Eteng MU. Vitamin C improves basal metabolic rate and lipid profile in alloxan-induced diabetes mellitus in rats. J Biosci. 2006;31(5):575-579. [CrossRef]
- Taousani E, Savvaki D, Tsirou E, et al. Regulation of basal metabolic rate in uncomplicated pregnancy and in gestational diabetes mellitus. Hormones (Athens). 2017;16(3):235-250. [CrossRef]
- Vikanes A, Grjibovski AM, Vangen S, Gunnes N, Samuelsen SO, Magnus P. Maternal body composition, smoking, and hyperemesis gravidarum. Ann Epidemiol. 2010;20(8):592-598. [CrossRef]
- Fang Y, Liu J, Mao Y, et al. Pre-pregnancy body mass index and time to pregnancy among couples pregnant within a year: A China cohort study. PLoS One. 2020;15(4):e0231751. Published 2020 Apr 23. [CrossRef]
- Shachar BZ, Mayo JA, Lee HC, et al. Effects of race/ethnicity and BMI on the association between height and risk for spontaneous preterm birth. Am J Obstet Gynecol. 2015;213(5):700.e1-700.e7009. [CrossRef]
- Awonuga AO, Merhi Z, Awonuga MT, Samuels TA, Waller J, Pring D. Anthropometric measurements in the diagnosis of pelvic size: an analysis of maternal height and shoe size and computed tomography pelvimetric data. Arch Gynecol Obstet. 2007;276(5):523-528. [CrossRef]
- Ribeiro MM, Andrade A, Nunes I. Physical exercise in pregnancy: benefits, risks and prescription. J Perinat Med. 2021;50(1):4-17. Published 2021 Sep 6. [CrossRef]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241-247. [CrossRef]
- Forsum E, Löf M. Energy metabolism during human pregnancy. Annu Rev Nutr. 2007;27:277-292. [CrossRef]
| Phenotype | GWAS * ID | PMID */ Consortium |
SNPs * | Simple size | Case | Control | |
| Spontaneous abortion | Finngen_R12_O15_ABORT_SPONTAN | FinnGen | 16379138 | 222446 | 23167 | 199279 | |
| Excessive vomiting in pregnancy | Finngen_R12_O15_EXCESS_VOMIT_PREG | FinnGen | 16379549 | 241235 | 3329 | 237906 | |
| Pregnancy complications | Pre-eclampsia or eclampsia | Finngen_R12_O15_PRE_OR_ECLAMPSIA | FinnGen | 16379723 | 268898 | 9717 | 259181 |
| Pregnancy hypertension | Finngen_R12_O15_HYPTENSPREG | FinnGen | 16379784 | 282064 | 20405 | 261659 | |
| Gestational diabetes | Finngen_R12_GEST_DIABETES | FinnGen | 16379784 | 282064 | 18581 | 263483 | |
| Premature rupture of membranes | Finngen_R12_O15_MEMBR_PREMAT_RUPT | FinnGen | 16379429 | 231594 | 10408 | 221186 | |
| Preterm labour and delivery | Finngen_R12_O15_PRETERM | FinnGen | 16379340 | 226330 | 11405 | 214925 | |
| Obstructed labour due to maternal pelvic abnormality | Finngen_R12_O15_LABOUR_PELVIC_ABNORM | FinnGen | 16379297 | 221238 | 6313 | 214925 | |
| Obstructed labour due to malposition and malpresentation of fetus | Finngen_R12_O15_LABOUR_MALPOS | FinnGen | 16379249 | 225409 | 10484 | 214925 | |
| Prolonged pregnancy | Finngen_R12_O15_PREG_PROLONGED | FinnGen | 16379383 | 228235 | 7049 | 221186 | |
| Exposure factor | Basal metabolic rate | ukb-b-16446 | UKB * | 9851867 | 454874 | — | — |
| Confounding factors | Body mass index | ieu-a-94 | GIANT * | 2736876 | 60586 | — | — |
| Body fat percentage | ukb-e-23099_MID | UKB * | 11904531 | 1535 | — | — | |
| Triglycerides | ieu-b-111 | 32203549 | 12321875 | 441016 | — | — | |
| Type 2 diabetes | ebi-a-GCST90018926 | 34187551 | 24167560 | 490089 | 38841 | 451248 | |
| Female height | ieu-a-97 | GIANT * | 2748546 | 73137 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





